Abstract
Pegylated liposomal doxorubicin (PLD) has a good safety profile, but long-term use has been associated with development of squamous cell carcinoma of the tongue and oral cavity (SCCTO) in some patients. The study objective was to estimate the prevalence of oral leukoplakia, a known precursor of SCCTO, in patients with ovarian cancer and long-term PLD use.
After approval of the institutional review board, medical record of 114 patients who were treated with PLD at our institution between January 2010 and December 2016 were retrospectively reviewed. All those patients have been referred for routine monitoring of oral mucositis every time before administration by a dentist. The patient characteristics included in the evaluation were age, smoking and drinking habits, the PLD dose and schedule, and presence or absence of oral leukoplakia and SCCTO at each oral examination. The relationships of the incidence of oral leukoplakia and patient characteristics were analyzed.
The median total PLD dose was 160 (range 40–1550) mg/m2. Oral leukoplakia was seen in 6 (5.3%) patients. The median PLD dose, at the time of oral leukoplakia diagnosis, was 685 (range 400–800) mg/m2. SCCTO was not found. Univariate analysis revealed that age, Brinkman index, and habitual drinking were not considered as risk factors for oral leukoplakia, and only total PLD dose (OR, 1.470; 95% CI, 1.19–1.91; P < .001) remained as a significant independent risk factor for oral leukoplakia. The ROC curve analysis indicated that the optimal cutoff value of the total PLD dose to predict development of oral leukoplakia was 400 mg/m2. The sensitivity was 100% and the specificity was 88.8%. No patient discontinued PLD because of oral leukoplakia or SCCTO.
The 2 most important clinical observations were the occurrence of oral leukoplakia in patients with long-term PLD use and that the development of oral leukoplakia was related to a total cumulative dose ≥400 mg/m2. Routine oral surveillance is recommended, particularly when the cumulative total dose exceeds 400 mg/m2.
Keywords: chemotherapy, oral leukoplakia, pegylated liposomal doxorubicin, secondary cancer
1. Introduction
Pegylated liposomal doxorubicin (PLD) is approved for the treatment of Kaposi's sarcoma and recurrent ovarian cancer. PLD has a good safety profile,[1–3] but long-term use has been associated with development of squamous cell carcinoma of the tongue and oral cavity (SCCTO) in some patients.[4–11] Most of those patients were not smokers or excessive alcohol users and were not at risk of developing SCCTO.[4] Consequently, periodic oral evaluations are recommended for long-term PLD patients.[10] Since 2010, all patients treated with PLD at our institution have been referred for routine monitoring of oral mucositis each time before administration by a dentist. SCCTO has not been found in any patients, but oral leukoplakia, a known precursor of SCCTO has.
Oral leukoplakia occurs as a white patch or plaque of questionable risk that cannot be characterized clinically or pathologically as any other known disease.[13] It is not related to the presence or absence of dysplasia, but is considered a premalignant condition arising from chronic irritation of the oral mucosa. Smoking, alcohol consumption, chronic cheek biting, ill-fitting dentures, sharp teeth, syphilitic glossitis, candida infection, and vitamin A and B deficiencies have all been reported to increase the risk of oral leukoplakia.[14] Malignant transformation of oral leukoplakia is estimated to occur in 1% to 20% of patients over a period of 1 to 30 years.[15]
The study objective was to estimate the prevalence of oral leukoplakia in patients with ovarian cancer and long-term PLD use.
2. Materials and methods
The relationship of PLD treatment and the incidence of oral leukoplakia was retrospectively evaluated in a cohort of patients with histologically diagnosed ovarian, fallopian tube, and primary peritoneal cancer. After approval of the institutional review board, a search of electronic medical records identified 141 eligible patients who were treated at Cancer Institute Hospital of Japanese Foundation for Cancer Research between January 2010 and December 2016. Twenty-seven patients were excluded because of the lack of periodic checking for oral mucositis before each administration of PLD. The remaining 114 patients were included in this study. The patient characteristics included in the evaluation were age, smoking and drinking habits, the PLD dose and schedule, and presence or absence of oral leukoplakia and SCCTO at each oral examination. The presence of a smoking habit was “Yes” when the Brinkman index = [(Number of cigarettes smoked per day) × (Number of years smoked)] ≥100 and “No” if <100. The presence of a drinking habit was “Yes” when the patient drank “more than socially” and “No” when otherwise.
The characteristics of patients with and without oral leukoplakia were compared using the Chi-squared test for categorical variables, expressed as medians and range, and Student t test for continuous variables, expressed as means ± standard deviation. The relationships of the incidence of oral leukoplakia and patient characteristics were analyzed by logistic regression with the occurrence of leukoplakia as the dependent variable and patient age, smoking and drinking habits, and the total dose of PLD as explanatory variables. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The optimal cutoff value of the total PLD dose for the prediction of oral leukoplakia was calculated using receiver operating characteristic (ROC) curve analysis. Statistical analysis was performed using R version 3.3.1.[12]P values <.05 were considered statistically significant.
3. Results
The patient characteristics are shown in Table 1. The median age at the initial administration of PLD was 62 (range 25–86) years of age, 21 of 141 patients (18.4%) were smokers, and 47 (41.2%) were more than social drinkers. The median total PLD dose was 160 (range 40–1550) mg/m2. Oral leukoplakia was seen in 6 (5.3%) patients; SCCTO was not found. Univariate analysis revealed that age, Brinkman index, and habitual drinking were not considered as risk factors for oral leukoplakia, and only total PLD dose (OR, 1.470; 95% CI, 1.19–1.91; P < .001) remained as a significant independent risk factor for oral leukoplakia (Table 2). The ROC curve analysis indicated that the optimal cutoff value of the total PLD dose to predict development of oral leukoplakia was 400 mg/m2 (Fig. 1). The sensitivity was 100%, and the specificity was 88.8%. The characteristics of patients with oral leukoplakia are shown in Table 3 and Fig. 2. The median PLD dose, at the time of oral leukoplakia diagnosis, was 685 (range 400–800) mg/m2, PLD was discontinued in 5 of the 6 patients because of disease progression. No patient discontinued PLD because of oral leukoplakia or SCCTO. The distribution of total PLD dose in patients with and without leukoplakia is shown in Fig. 2.
Table 1.
Patient characteristics.
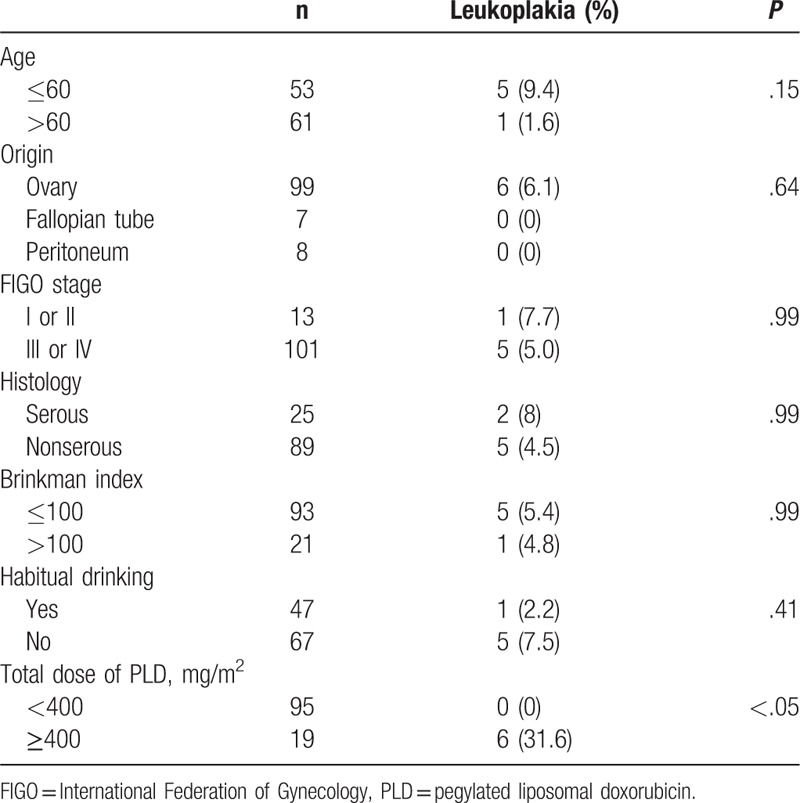
Table 2.
Univariate analysis of risk factors for oral leukoplakia.
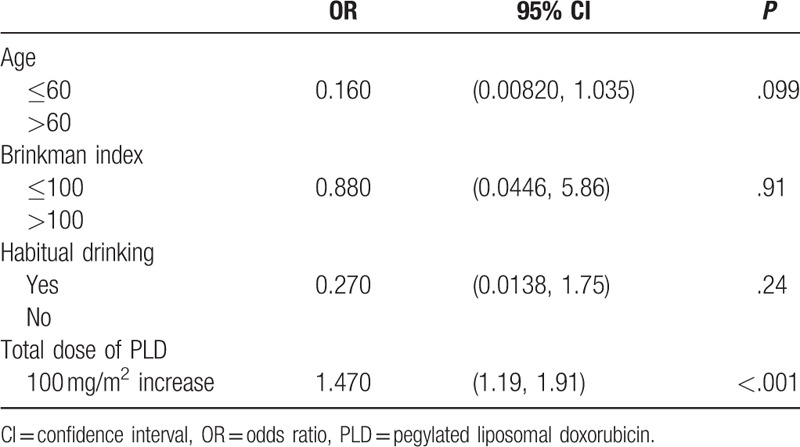
Figure 1.
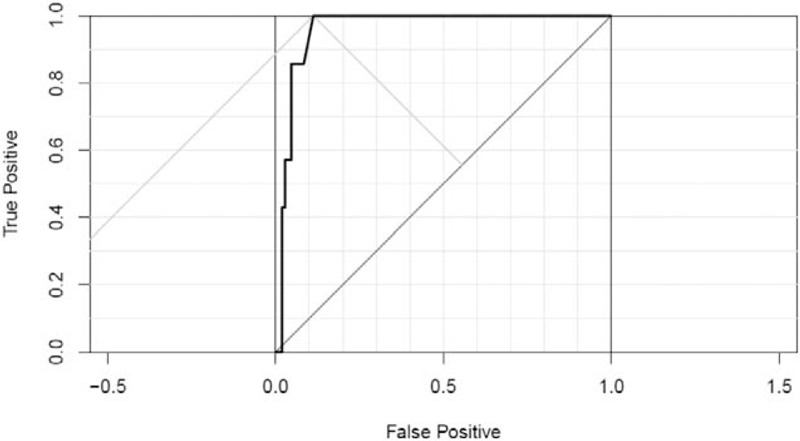
Receiver operating characteristic (ROC) curve analysis.
Table 3.
Characteristics of patients with oral leukoplakia.
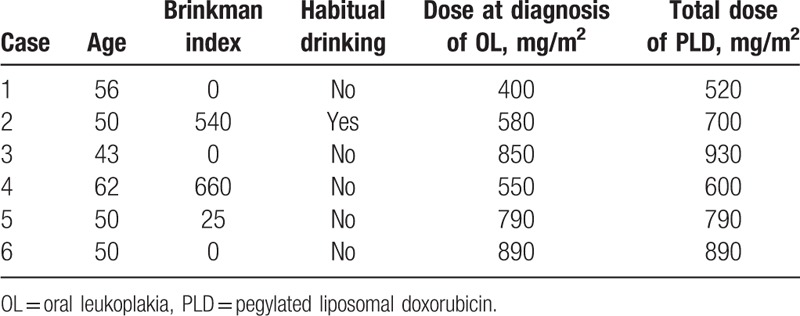
Figure 2.
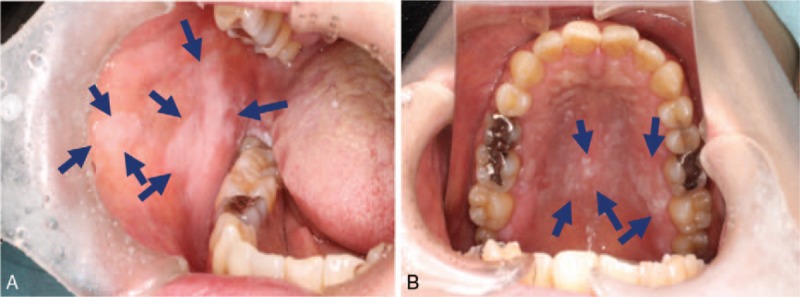
Oral leukoplakia appearing as a white patch.
4. Discussion
The 2 most important clinical observations were the occurrence of oral leukoplakia in patients with long-term PLD use and that the development of oral leukoplakia was related to a total cumulative dose ≥400 mg/m2.
Six of the 19 patients given >400 mg/m2 of PLD (31.6%) and 5 of the 12 (41.7%) given >500 mg/m2 developed oral leukoplakia. As oral leukoplakia had not developed in any patients before PLD administration, the results may imply that PLD administration increased the incidence of oral leukoplakia in dose-dependent manner. Pharmacokinetic studies have shown that PLD has a higher plasma concentration and a much smaller volume of distribution than nonliposomal doxorubicin.[16] Liposomes accumulate in skin and mucous membranes and release doxorubicin and its metabolites over time. The prolonged exposure to doxorubicin is presumed to be the cause of an increased rate of secondary oral malignancies.[4] The limited available information indicates that the secondary oral tumors are less sensitive to conventional doses of radiation therapy than the primary tumors.[10] It is thus important to diagnose and treat SCCTO at an early stage. In general, older age, habitual smoking, and habitual drinking are thought to increase the risk of oral leukoplakia.[14] In this study cohort, general risk factors other than PLD administration were not considered as risk factors for oral leukoplakia. This fact shows that patients with oral leukoplakia in our study are associated with PLD administration. Early detection and management of oral leukoplakia, which is a precursor lesion of SCCTO, may improve the quality of life in patients with recurrent ovarian cancer.
This study was limited by its retrospective design, which is subject to selection bias. Secondly, as no patients developed SCCTO, the results provide little information about the incidence of, and time to develop, SCCTO in patients with oral leukoplakia. A cumulative PLD dose of 1260 to 3000 mg/body has previously been reported as required before developing SCCTO.[10]
In conclusion, the occurrence of 6 cases (5.3%) of oral leukoplakia in a cohort of patients with long-term PLD administration supports a recommendation of routine oral surveillance, especially when the cumulative total dose exceeds 400 mg/m2. Under strict surveillance, patients with oral leukoplakia may continue treatment using PLD.
Footnotes
Abbreviations: CIs = confidence intervals, ORs = odds ratios, PLD = pegylated liposomal doxorubicin, ROC = receiver operating characteristic, SCCTO = squamous cell carcinoma of the tongue and oral cavity.
The authors have no conflicts of interest to disclose.
References
- [1].de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumors). Cancer 2010;116:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin-Carbonero L, Palacios R, Valencia E, et al. Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin. Clin Infect Dis 2008;47:410–7. [DOI] [PubMed] [Google Scholar]
- [3].Andreopoulou E, Gaiotti D, Kim E, et al. Pegylated liposomal doxorubicin HCL (PLD; Caelyx/Doxil): experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann Oncol 2007;18:716–21. [DOI] [PubMed] [Google Scholar]
- [4].Cannon TL, Lai DW, Hirsch D, et al. Squamous cell carcinoma of the oral cavity in nonsmoking women: a new and unusual complication of chemotherapy for recurrent ovarian cancer? Oncologist 2012;17:1541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pezzoli M, Bona Galvagno M, Bongioannini G. Oral squamous cell carcinoma in a patient treated with long-term pegylated liposomal doxorubicin for recurrent ovarian cancer. BMJ Case Rep 2015. 1–3. doi: 1136/bcr-2014-204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ben-David Y, Leiser Y, Kachta O, et al. Does long-term treatment with Doxil@ predispose patients to oral cancer? Int J Clin Oncol 2013;18:554–5. [DOI] [PubMed] [Google Scholar]
- [7].Matsuo K, Blake EA, Yessaian AA, et al. Long-term pegylated liposomal doxorubicin use and oromaxillary squamous cell carcinoma in endometrial cancer. Oncologist 2012;17:1598–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gu P, Wu J, Sheu M, et al. Aggressive squamous cell carcinoma of the oral tongue in a woman with metastatic giant cell tumor treated with pegylated liposomal doxorubicin. Oncologist 2012;17:1596–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonomi MR, Misiukiewicz K, Posner M, et al. Squamous cell carcinoma of the oral tongue in two patients previously exposed to long-term pegylated liposomal doxorubicin. Oncologist 2012;17:1594–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muggia F. Squamous cell carcinomas of the tongue and oral cavity as secondary malignancies: what factors are implicated? Oncologist 2013;18:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Randon G, Nicoletto MO, Milite N, et al. Squamous cell carcinoma of the oral cavity in a woman with a 9-year history of ovarian cancer: is exposure to pegylated liposomal doxorubicin a factor? Oncologist 2014;14:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Available from: https://www.R-project.org/. Accessed May 3, 2017. [Google Scholar]
- [13].Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575–80. [DOI] [PubMed] [Google Scholar]
- [14].Eugene N, James Y, Jeffrey M, et al. Cancer of the Head and Neck. Philadelphia: Saunders; 2003. [Google Scholar]
- [15].Cawson RA, Odell EW. Cawson's Essentials of Oral Pathology and Oral Medicine. London: Elsevier; 2008. [Google Scholar]
- [16].Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet 2003;42:419–36. [DOI] [PubMed] [Google Scholar]


