Abstract
Study Design
Prospective
Objectives
To determine the optimum gonadotropin releasing hormone (GnRH) dose to identify dysfunction of the hypothalamic-pituitary-gonadal axis in men with spinal cord injury (SCI).
Setting
Metropolitan Area Hospitals, New York and New Jersey, USA
Methods
SCI men [16 hypogonadal (HG=serum testosterone <12.1 nmol/L) & 14 eugonadal (EG)] and able-bodied (AB) men (27 HG & 11 EG) were studied. GnRH (10, 50, 100 μg) was randomly administered intravenously on three separate visits. Blood samples were collected post-GnRH for serum luteinizing hormone (LH) and follicular stimulating hormone (FSH).
Results
HG and EG men had a similar proportion of clinically acceptable gonadotropin responses to all three GnRH doses. The incremental gonadotropin responses to GnRH were not significantly different across the groups. However, in the SCI-HG group GnRH 100 μg resulted in the greatest integrated FSH response, and in the SCI-EG group, GnRH 50 μg resulted in the greatest integrated LH response compared with the AB groups. A consistent, but not significant, absolute increase in gonadotropin release was observed in the SCI groups at all GnRH doses.
Conclusion
Lower doses of GnRH did not improve the ability to identify clinical dysfunction of the hypothalamic-pituitary-gonadal axis. However, the absolutely higher SCI-HG FSH response to GnRH 100 μg and higher SCI-EG LH response to GnRH 50 μg, along with higher gonadotropin release at all GnRH doses, albeit not significant, suggests a hypothalamic-pituitary dysfunction in persons with SCI.
Keywords: gonadotropins, pituitary, hypothalamus, testosterone, luteinizing hormone, follicle stimulating hormone
Introduction
Paralysis and associated physical inactivity in persons with spinal cord injury (SCI) result in muscle atrophy that will reduce resting and total daily energy expenditure. In the absence of sufficient restriction of caloric intake, adiposity and adverse metabolic consequences may ensue.1,2 Compared to the general male population, men with SCI are observed to have a elevated prevalence of low serum testosterone levels, and hypogonadism appears to increase with each advancing decade of life.3 Loss of muscle mass and gain of adiposity would be anticipated to negatively impact general health due to adverse metabolic changes, impair physical function, reduce social integration, and decrease quality of life.4-6 In persons with SCI, dysfunction of the hypothalamic-pituitary-gonadal axis may result in depression of serum testosterone levels that would be anticipated to worsen the adverse body composition changes that occur as a consequence of paralysis and immobilization, as well as increase the likelihood of developing the adverse metabolic sequelae of SCI.7-11 Testosterone deficiency is also associated with fatigue, depression and other negative mood alterations.12-14 In addition, one possible etiology for reduced semen quality is dysfunction of the hypothalamic–pituitary–testicular axis.15-17 Thus expanding our understanding of the occurrence of a dysfunctional hypothalamic-pituitary-gonadal axis may raise awareness and the ability to identify, diagnose, and treat this disorder.
The gonadotropin releasing hormone (GnRH) stimulation test has been routinely used in clinical practice to determine the integrity of the hypothalamic-pituitary-gonadal axis. In the conventional approach, GnRH 100 μg is administered intravenously and blood is collected for gonadotropin levels at 30 and/or 60 minutes after provocative stimulus. The original work that determined the most appropriate dose of GnRH to administer for clinical purposes was performed about 40 years ago, and subsequently there has been little interest to re-visit the dose of GnRH in an attempt to further elucidate the function of the hypothalamic-pituitary-gonadal axis in various clinical conditions that may be associated with central dysregulation.
Our group reported that the hypothalamic-pituitary-gonadal axis is generally intact when evaluated by standard challenge testing in persons with spinal cord injury (SCI); albeit, the absolute gonadotropin response to GnRH 100 μg was higher in persons with SCI who were classified as “responders” (i.e., adequate response by conventional clinical criteria) to provocative pituitary stimulation compared to healthy able-bodied controls who were classified as “responders”.18 The testicular response to a conventional dose of human chorionic gonadotropin (hCG; 4000 IU) or with lower doses of hCG (400 and 1000) in those with SCI was generally appropriate, regardless of whether the participants had low or normal serum testosterone levels.18,19 Of note, whether an individual with SCI was hypogonadal or eugonadal was not predictive of whether the person would respond with an appropriate clinical response to the standard stimulation dose of hCG. The authors speculated that because peripheral stimulation to hCG was essentially normal in those with SCI that a more subtle central problem due to insufficient hypothalamic drive to gonadotropin release should be considered.
The incidence of men with SCI who have low levels of testosterone (T) is reported to be between 40 and 60%, based on varying populations studied and different threshold values for T concentration to define hypogonadism.3,8,11,20 The etiology of hypogonadism is most likely multi-factorial and includes general health 21, nutrition22, medication usage (especially psychotropic agents and opioids) 23-26, alcohol consumption 27,28, level,and completeness and duration of neurological lesion, possibly directly related to the degree of adiposity.29 Despite its etiology, T deficiency, as a relative or an absolute condition, may influence the tendency to lose lean tissue and gain fat mass, predispose to adverse carbohydrate 30-33 and lipid metabolism 30,31,34,35, and lower energy expenditure.6,36,37
Impotence and infertility are also common conditions among men with SCI.38-42 Of the many possible explanations for poor semen quality15, one possible etiology is dysfunction of the hypothalamic-pituitary-testicular axis.16,17,43 Early reports that employed provocative stimulation of the axis to assess testicular function have been inconclusive with regard to subsequent production of testosterone, luteinizing hormone (LH), and follicle stimulating hormone (FSH).41,44 Huang et al.45 found significantly elevated LH responses to GnRH in a subgroup of participants with SCI compared to controls; 53% (16 of 30) of participants with SCI had exaggerated LH responses and 20% (6 of 30) participants had elevated FSH responses. The apparent discrepancies among reports could, at least in part, be attributed to varying factors in subject selection, including health and nutrition parameters, medication effects, and level and duration of injury effects, or, simply to differences in methodology employed for provocative stimulation.
Provocative testing of the pituitary with GnRH is, by convention, performed at a standard dose. However, depending on the pathophysiology, it may be hypothesized that more subtle forms of hypothalamic-pituitary-gonadal axis dysfunction may be unmasked by the administration of GnRH doses that are lower than those conventionally employed and, thereby, permitting better differentiation between hypogonadal and eugonadal status or, possibly, SCI and able-bodied individuals. As such, this study was designed to evaluate the integrity of the hypothalamic-pituitary-gonadal axis in hypogonadal and eugonadal men with SCI and able-bodied controls of similar gonadal status by provocative stimulation of the pituitary with GnRH at three doses: the conventional dose level (100 μg) and two lower doses (10 μg and 50 μg).
Methods
Study cohort
Participants were recruited from the James J. Peters VA Medical Center (JJPVAMC), Bronx, NY, and the Kessler Institute of Rehabilitation (KIR), West Orange, NJ. Otherwise healthy men between the ages of 18 and 65 with chronic SCI (duration of injury >1 year) who were assumed to have normal sexual and hypothalamic-pituitary-testicular function prior to injury or neurologically intact able-bodied men were considered for eligibility. Exclusion criteria included the following conditions: acute illness; active thyroid disease; medications for depression, mood changes or any nervous condition; centrally acting high blood pressure medications (i.e., guanethidine, reserpine, methyldopa, β-adrenergic blockers, clonidine, etc.); medications for gastrointestinal disorders; medication for heart disease; medications for seizures (i.e., phenytoin or barbiturates); epilepsy; congestive heart failure; anti-cancer medications; antibiotics; pain medications; hormones (other than replacement doses); history of pituitary or testicular surgery; or less than 18 or older than 65 years of age. Abstinence from alcohol containing beverages was required for 48 hours prior to performing the study procedures.
Procedures
This was a prospective, open-label, randomized, and parallel group investigation to differentiate between normal and abnormal pituitary function in persons with SCI. Stimulation tests were performed to test pituitary function with GnRH to assess the release of gonadotropins (LH, FSH). Three doses of GnRH (e.g., 10, 50, or 100 μg) were administered on separate days in a random order (Figure 1). For each stimulation test, a dose response curve was obtained from serial blood draws that were collected between 8 am and 9 am for determination of plasma FSH and LH; serum concentrations of T were obtained for the determination of hypogonadal or eugonadal status.46-48 The GnRH stimulation test was performed on each subject in 3 visits on non-consecutive days over several weeks. For the GnRH stimulation test, an intravenous line was placed in an antecubital vein for serial blood draws in heparinized tubes at time 0, and 15, 30, 60, 120, and 180 minutes following stimulation. Blood samples were centrifuged, plasma separated from formed blood cell elements, and the plasma frozen at -20°C prior to hormone determination for serum T, plasma LH and FSH concentrations.
Figure 1.
Schematic representation of the timeline for the administration of gonadotropin releasing hormone (GnRH) and blood collection. On three separate visits, a dose of GnRH was administered (e.g., 10 μg, 50 μg, 100 μg) in random order.
Laboratory Analysis
The serum T concentration was determined in duplicate by radioimmunoassay, in accordance with the manufacturer's guidelines (ICN Biomedicals, Inc., Costa Mesa, CA). The sensitivity for T assay was 0.08 ng/ml; the intra-assay coefficient of variation (CV) were 9.6, 8.1, and 7.8% for T concentrations of 0.9, 7.0, and 20 ng/ml, respectively; the inter-assay CV were 8.6, 9.1, and 8.4% for T concentrations of 0.7, 6.0, and 16 ng/ml, respectively; the 2 standard deviation normal range for adult males is 2.8-8.8 ng/ml. Plasma FSH and LH levels were determined in duplicate by immunoradiometric assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The sensitivity for the FSH assay was 0.06 mIU/ml; the intra-assay CV were 3.8, 2.4, and 3.8% for FSH concentrations of 1.6, 10, and 73 mIU/ml, respectively; the inter-assay CV were 5.7, 4.0, and 5.0% for FSH concentrations of 4.6, 11, and 38 mIU/ml, respectively; the 95% range for adult males is 1.1-13.5 mIU/ml. The sensitivity of the LH assay was 0.15 mIU/ml; the inter-assay CV were 1.6, 1.2, and 1.0% for LH concentrations of 9, 16, and 22 mIU/ml, respectively; the inter-assay CV were 7.1, 2.6, and 3.4% for LH concentrations of 2, 9, and 24 mIU/ml, respectively; the 95% range for adult males is 0.4-5.7 mIU/ml.
Statistical Analyses
To characterize sub-group responses, independent variables for neurological injury (SCI vs. AB) and gonadal status [hypogonadal (HG) vs. eugonadal (EG)] were concatenated to produce 4 categorical groups [Able-Bodied Eugonadal (AB-EG); Able-Bodied Hypogonadal (AB-HG); SCI-Eugonadal (SCI-EG); SCI-Hypogonadal (SCI-HG)]. Separate factorial analysis of variance (ANOVA) was performed to identify group differences for demographic (age, height, weight, BMI, DOI) and baseline laboratory values at each study visit (T, LH, FSH). Separate single factor (GnRH dose) ANOVA were performed within each group (AB-EG, AB-HG, SCI-EG, SCI-HG) to determine if baseline (Time 0) concentrations of FSH or LH were different. Values are expressed as group mean ± SD.
For each GnRH dose (10, 50, or 100 μg), a separate single factor [concatenated group: AB-EG; AB-HG; SCI-EG; SCI-HG] mixed-model ANOVA with repeated measures on time (0, 15, 30, 60, 120, and 180 minutes) was performed to identify significant differences in the respective hormone concentrations. Significant time main effects were explored using pairwise comparisons within each model. To facilitate a more inclusive analysis of significant interaction effects within each GnRH dose, the area under the curve (AUC; integrated response) was calculated using GraphPad software (Prism 6.0, La Jolla, CA) for each group's FSH and LH response. AUC was calculated as the sum of the rise above the baseline value for each time point and group. The effects of increasing dose on the total stimulated responses were analyzed using a single factor [concatenated group: AB-EG; AB-HG; SCI-EG; SCI-HG] ANOVA with repeated measures on dose (10, 50, or 100 μg). Bonferroni's post-hoc tests were performed to identify group differences to the respective doses.
To evaluate the efficacy of each stimulation dose to produce a standard gonadotropin response, the respective subject responses (e.g., time) to each stimulation dose for FSH and LH were characterized as a binary categorical variable (e.g., “responder” or “nonresponder”) based on whether a greater than 2-fold increase in plasma LH level and a greater than 50% increase in plasma FSH level from the baseline concentration were achieved.49 Chi-square analyses were performed to identify group differences for the percentage of responders at each time point and stimulation dose. Chi-square analyses were then performed to identify the percentage of participants who achieved a positive FSH and LH response to the respective doses at each time point (e.g., diagnostic agreement). With consideration for the standard clinical blood collection times (e.g., 30 and 60 minutes post-stimulation injection)49, chi-square analyses were performed to identify the percentage of responders at the 30 and 60-minute time points at the standard dose (100 μg) and middle dose (50 μg). An a priori level of significance was set at p≤0.05. Statistical analyses were completed using IBM SPSS Statistics 21 (IBM, Armonk, NY, USA).
Statement of ethics
The research protocol was approved by the Institutional Review Boards of the James J. Peters Veterans Affairs Medical Center and the Kessler Foundation. Written informed consent was obtained from each subject prior to study participation. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Results
Demographic Information and Baseline Blood Values
Demographic data for participants and baseline gonadotropin values in the AB-EG, AB-HG, SCI-EG, and SCI-HG groups are provided (Table 1). The groups were well matched for demographic characteristics and there were no differences in distribution in the SCI sub-groups for the proportion of individuals with paraplegia or tetraplegia. Statistical differences for baseline serum T values (p<0.0001) for gonadal status (HG vs. EG) in the SCI and AB groups were by study design. The mean plasma FSH and LH concentrations for the baseline visits are provided prior to the administration of GnRH. With exception of the baseline visit for the plasma FSH concentrations prior to the administration of GnRH 100 μg, the other FSH and LH values were not significantly different between the EG and HG groups. Within each group, the respective plasma FSH or LH concentrations for each study visit were not statistically different at the baseline blood collection. The mean plasma FSH (normal range: 0.9-15.0 mIU/ml) and LH (normal range: 1.3-13.0) concentrations for all groups were within the normal ranges for adult males.
Table 1. Characteristics of the Study Groups.
| Spinal Cord Injury | Able-Bodied | ||||
|---|---|---|---|---|---|
| Eugonadal | Hypogonadal | Eugonadal | Hypogonadal | p value | |
|
|
|||||
| n | 16 | 14 | 11 | 27 | - |
| Age (years) | 33 ± 7 | 41 ± 10 | 36 ± 7 | 36 ± 9 | 0.06 |
| Height (m) | 1.79 ± 0.08 | 1.78 ± 0.07 | 1.82 ± 0.10 | 1.76 ± 0.08 | NS |
| Weight (kg) | 88.1 ± 20.1 | 81.7 ± 15.2 | 87.6 ± 11.3 | 83.6 ± 14.0 | NS |
| BMI (kg/m2) | 27.3 ± 4.5 | 25.4 ± 4.0 | 26.4 ± 3.3 | 26.8 ± 3.8 | NS |
| DOI (years) | 11 ± 7 | 12 ± 10 | - | - | NS |
| Paraplegia/Tetraplegia (n) | 11/5 | 10/4 | - | - | NS |
| Total Testosterone (nmol/l) | 17.4 ± 4.4 | 7.4 ± 3.8 *,† | 16.7 ± 4.3‡ | 7.7 ± 2.3¶ | <0.0001 |
| Luteinizing Hormone (mIU/ml) | |||||
| GnRH 10 μg | 1.4 ± 1.0 | 2.0 ± 1.6 | 2.5 ± 1.6 | 2.5 ± 2.7 | NS |
| GnRH 50 μg | 1.6 ± 1.4 | 2.1 ± 2.5 | 3.3 ± 2.6 | 2.5 ± 5.4 | NS |
| GnRH 100 μg | 3.3 ± 3.0 | 2.0 ± 2.1 | 2.8 ± 1.9 | 5.2 ± 8.9 | NS |
| Follicle Stimulating Hormone (mIU/ml) | |||||
| GnRH 10 μg | 1.4 ± 1.1 | 2.0 ± 2.2 | 2.9 ± 2.7 | 3.6 ± 3.3 | NS |
| GnRH 50 μg | 1.6 ± 1.6 | 1.7 ± 2.2 | 3.2 ± 3.9 | 3.2 ± 3.6 | NS |
| GnRH 100 μg | 2.7 ± 2.6 | 1.8 ± 1.8$ | 2.3 ± 2.8 | 5.0 ± 3.7 # | <0.01 |
Data are presented as group mean ± SD. Abbreviations: BMI=body mass index; DOI= duration of injury; GnRH= gonadotropin releasing hormone; NS= not significant; kg= kilograms; nmol/l= nanomoles per liter; mIU/ml= milli-international units per milliliter; μg= micrograms.
p<0.0001: SCI-Eugonadal vs. SCI-Hypogonadal;
p<0.0001: AB-Eugonadal vs. SCI-Hypogonadal;
p<0.0001: AB-Eugonadal vs. SCI-Hypogonadal;
p<0.0001: AB-Eugonadal vs AB-Hypogonadal;
p<0.01: SCI-Hypogonadal vs. AB-Hypogonadal;
p<0.05: SCI-Hypogonadal vs. SCI-Eugonadal.
GnRH Provocative Stimulation
Provocative testing with GnRH was performed at 3 concentrations (i.e., 10, 50 and 100 μg). Significant group main effects were not observed for plasma FSH or LH responses at any dose. However, significant time main effects were observed for plasma FSH or LH responses at each dose (Figure 2), indicating that the respective stimulations produced a change in the corresponding hormone concentration. Post-hoc analyses revealed that FSH and LH concentrations at each time point after administration of the GnRH doses were significantly greater (p<0.05) than their respective baseline concentration (Time 0). After administration of GnRH 50 and 100 μg for FSH, the 30, 60 and 120-minute post-injection time point concentrations were also significantly greater than the 15-minute time point (p<0.01, Figure 2). After administration of GnRH 50 and 100 μg, the 30-minute post-GnRH time point for LH was the only one that was significantly greater than the 15-minute time point (p<0.01, Figure 2). A significant group × time interaction effect was observed after administration of GnRH 50 μg for FSH and was observed after administration of GnRH 50 and 100 μg for LH.
Figure 2.
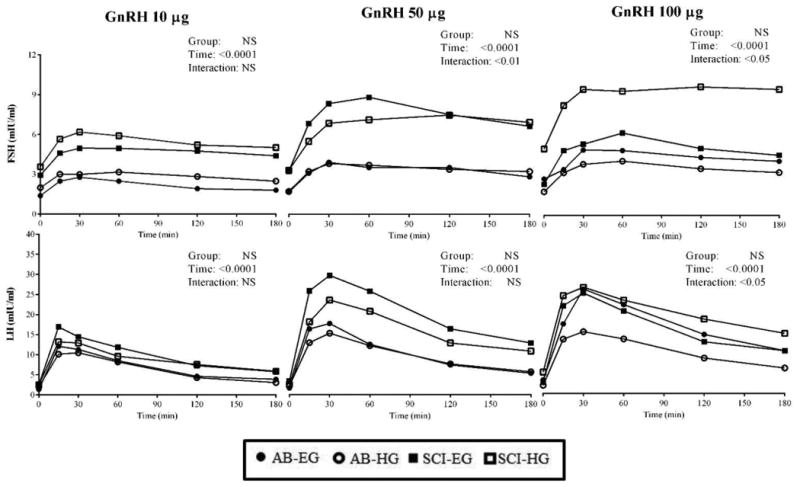
Gonadotropin responses to GnRH stimulation at 3 doses by group.
| GnRH Dose | 10 μg | 50 μg | 100 μg | ||||||||||||||||
| Time (Minutes) | 0 | 15 | 30 | 60 | 120 | 180 | 0 | 15 | 30 | 60 | 120 | 180 | 0 | 15 | 30 | 60 | 120 | 180 | |
| FSH (mIU/ml) |
AB-EG | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 4 | 4 | 3 | 3 |
| AB-HG | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | |
| SCI-EG | 3 | 5 | 5 | 5 | 5 | 5 | 4 | 8 | 11 | 10 | 8 | 7 | 3 | 7 | 7 | 9 | 6 | 5 | |
| SCI-HG | 3 | 4 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 5 | 6 | 5 | 4 | 5 | 6 | 6 | 7 | 7 | |
| LH (mIU/ml) |
AB-EG | 1 | 7 | 6 | 4 | 2 | 2 | 1 | 11 | 12 | 8 | 5 | 3 | 3 | 14 | 22 | 17 | 13 | 10 |
| AB-HG | 2 | 8 | 8 | 8 | 4 | 2 | 3 | 9 | 10 | 9 | 6 | 5 | 2 | 12 | 12 | 11 | 8 | 5 | |
| SCI-EG | 2 | 12 | 9 | 8 | 5 | 4 | 3 | 19 | 20 | 18 | 11 | 10 | 2 | 21 | 20 | 17 | 10 | 8 | |
| SCI-HG | 3 | 8 | 7 | 5 | 5 | 5 | 5 | 11 | 15 | 17 | 9 | 10 | 9 | 17 | 20 | 20 | 21 | 20 | |
Analyses for Area under the Curve Responses to Graded GnRH Stimulation
Within each dose, the omnibus model to test for group differences was significant for FSH at the 10 and 50 μg (p<0.05, respectively) and 100 μg (p<0.001) doses (Figure 3); post-hoc analyses failed to reveal the presence of any subgroup differences. However, at 100 μg, the integrated FSH response in the SCI-HG group was significantly greater than the response in the AB-HG group (*p<0.01) and trending toward a significance difference compared to the AB-EG group († p=0.07) (Figure 3). Within each group, no statistical differences demonstrated an increasing dose effect on the FSH responses to stimulation. For LH, the within dose group comparisons were not statistically significant after administration of GnRH 10 or 100 μg, but did achieve significance after administration of GnRH 50 μg (p<0.01); post-hoc analyses revealed that the integrated LH response in the SCI-EG group was significantly greater than the response in the AB-HG group (*p<0.01) and trending toward a significance difference compared to the AB-EG group († p=0.07) (Figure 3). Within each group, a statistically significant dose effect on the integrated LH responses were observed for the AB-EG and SCI-EG (p<0.05) groups, but not for the SCI-HG and AB-HG, groups that approached significance. The SCI-EG and AB-EG had significantly greater integrated LH responses after administration of GnRH 50 and 100 μg, respectively compared to GnRH 10 μg (‡ p<0.05).
Figure 3.
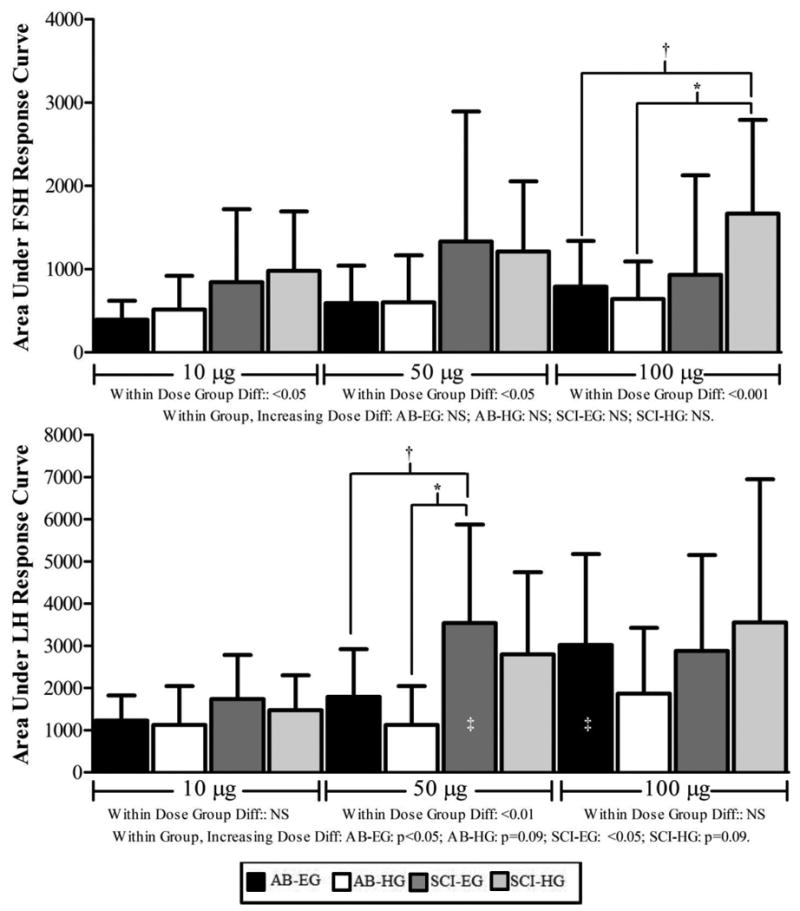
FSH and LH area under the curve responses by dose of GnRH (e.g., 10 μg, 50 μg, 100 μg). Results are provided from the omnibus model to determine the presence of significant group differences within each GnRH dose for FSH (top panel) and LH (bottom panel). The dose of GnRH administered is provided beneath each set of bars. Post-hoc tests that revealed the presence of significant group differences within each dose and hormone (e.g., FSH or LH) are provided with brackets and the corresponding level of significance (*p<0.01, † p=0.07). Results from the omnibus model to determine the presence of significant AUC responses to GnRH dose within group are labeled beneath the FSH (top panel) and LH (bottom panel) graphical presentations. Post-hoc tests revealing the presence of significant increases in AUC responses for LH are provided on the corresponding bar against which the change occurred compared to the 10 μg dose (‡ p<0.05). NS= not significant; AB-EG= able-bodied eugonadal; AB-HG: able-bodied hypogonadal; SCI-EG: spinal cord injury eugonadal; SCI-HG: spinal cord injury hypogonadal.
Clinically Significant Responses to GnRH Dose Stimulation
Within each GnRH dose (10, 50 and 100 μg) and time point (15, 30, 60, 120 and 180 minutes), chi-square analyses were performed to determine if the groups (e.g., AB-EG, AB-HG, SCI-EG, SCI-HG) differed in the proportion of individuals who achieved a clinically acceptable FSH or LH response (Table 2). For all doses and time points, there were no significant differences in the proportion of responders among groups. When categorizing individuals by whether or not they achieved acceptable clinical responses for both FSH and LH at the respective time points within each dose, the groups did not differ on the proportion of diagnostic agreement for gonadotropins. In comparing the effect of increasing GnRH dose at specific time points (e.g., 30 and 60 minutes) within each group, chi-square analyses revealed that diagnostic agreement for an acceptable clinical response at the 30 or 60 minute time points after administration of GnRH 50 μg vs 100 μg were not significantly different for proportion of clinically acceptable responses for both gonadotropins among the groups, except for the AB-HG group that had a greater diagnostic agreement after administration of GnRH 50 μg compared to GnRH 100 μg. There did not appear to be any indication that age, body mass, level of injury, or duration of SCI contributed to these findings.
Table 2. Percentage of individuals within each group achieving the clinically significant response for FSH or LH during GnRH stimulation at increasing dose concentrations.
| GnRH 100 μg | Time Point (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 30 | 60 | 120 | 180 | ||||
| Critical Response Achieved | FSH | 50% ↑ from BL | Control- Eugonadal | 11 | 45% | 82% | 64% | 55% | 55% |
| Control- Hypogonadal | 27 | 59% | 63% | 74% | 67% | 59% | |||
| SCI- Eugonadal | 16 | 69% | 69% | 75% | 75% | 56% | |||
| SCI- Hypogonadal | 14 | 71% | 86% | 79% | 79% | 71% | |||
| LH | 2-fold ↑ from BL | Control- Eugonadal | 11 | 82% | 100% | 91% | 73% | 18% | |
| Control- Hypogonadal | 27 | 81% | 85% | 81% | 70% | 48% | |||
| SCI- Eugonadal | 16 | 75% | 75% | 75% | 56% | 56% | |||
| SCI- Hypogonadal | 14 | 86% | 79% | 79% | 64% | 50% | |||
| Diagnostic Agreement | Control- Eugonadal | 27% | 82% | 55% | 55% | 18% | |||
| Control- Hypogonadal | 59% | 63% | 67% | 63% | 44% | ||||
| SCI- Eugonadal | 63% | 56% | 69% | 50% | 44% | ||||
| SCI- Hypogonadal | 64% | 71% | 64% | 50% | 43% | ||||
| GnRH 50 μg | Time Point (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 30 | 60 | 120 | 180 | ||||
| Critical Response Achieved | FSH | 50% ↑ from BL | Control- Eugonadal | 11 | 64% | 100% | 82% | 82% | 55% |
| Control- Hypogonadal | 27 | 59% | 78% | 78% | 63% | 67% | |||
| SCI- Eugonadal | 16 | 81% | 94% | 100% | 100% | 81% | |||
| SCI- Hypogonadal | 14 | 71% | 86% | 79% | 86% | 79% | |||
| LH | 2-fold ↑ from BL | Control- Eugonadal | 11 | 91% | 91% | 91% | 64% | 64% | |
| Control- Hypogonadal | 27 | 89% | 89% | 85% | 63% | 59% | |||
| SCI- Eugonadal | 16 | 94% | 100% | 94% | 75% | 63% | |||
| SCI- Hypogonadal | 14 | 86% | 93% | 93% | 79% | 64% | |||
| Diagnostic Agreement | Control- Eugonadal | 55% | 73% | 82% | 64% | 45% | |||
| Control- Hypogonadal | 52% | 70% | 67% | 44% | 37% | ||||
| SCI- Eugonadal | 75% | 94% | 94% | 75% | 63% | ||||
| SCI- Hypogonadal | 64% | 79% | 71% | 71% | 50% | ||||
| GnRH 10 μg | Time Point (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 30 | 60 | 120 | 180 | ||||
| Critical Response Achieved | FSH | 50% ↑ from BL | Control- Eugonadal | 11 | 64% | 91% | 73% | 36% | 27% |
| Control- Hypogonadal | 27 | 56% | 44% | 56% | 52% | 52% | |||
| SCI- Eugonadal | 16 | 63% | 63% | 56% | 63% | 50% | |||
| SCI- Hypogonadal | 14 | 64% | 79% | 71% | 57% | 64% | |||
| LH | 2-fold ↑ from BL | Control- Eugonadal | 11 | 82% | 82% | 73% | 55% | 45% | |
| Control- Hypogonadal | 27 | 78% | 89% | 52% | 37% | 26% | |||
| SCI- Eugonadal | 16 | 75% | 63% | 56% | 56% | 44% | |||
| SCI- Hypogonadal | 14 | 79% | 71% | 71% | 50% | 43% | |||
| Diagnostic Agreement | Control- Eugonadal | 55% | 73% | 64% | 27% | 18% | |||
| Control- Hypogonadal | 44% | 41% | 30% | 30% | 22% | ||||
| SCI- Eugonadal | 56% | 56% | 50% | 56% | 31% | ||||
| SCI- Hypogonadal | 50% | 57% | 50% | 36% | 36% | ||||
Data are presented as percentage of responders by group. GnRH=gonadotropin releasing hormone; FSH=follicle stimulating hormone; LH=luteinizing hormone; BL=baseline; μg=micrograms.
Discussion
The question could be raised as to whether individuals with SCI have a more subtle abnormality in central regulation that may be unmasked by a nuanced approach to provocative testing of the hypothalamic-pituitary axis with GnRH. Provocative stimulation of the hypothalamic-pituitary axis with conventional and lower doses of GnRH revealed novel and potentially clinically significant findings in persons with SCI. This work adds further evidence to support the concept that there is central dysfunction in the regulation of the hypothalamic-pituitary axis in persons with SCI because of the heightened pituitary sensitivity to GnRH, regardless of gonadal status. Compared to the AB controls, a greater integrated FSH response in the SCI-HG group after stimulation with GnRH 100 μg and greater integrated LH response in the SCI-EG group after stimulation with GnRH 50 μg suggests that hypothalamic GnRH may be globally depressed in persons with SCI. The current study also revealed that a similar percentage of respondents achieved the clinically acceptable responses of circulating FSH or LH after GnRH stimulation at the standard post-injection collection time points (e.g., 30 and 60 minutes), regardless of gonadal status. Similarly, the agreement in identifying individuals with acceptable dual gonadotropin responses—that is, FSH or LH responses achieved to the same dose of GnRH that met clinical criteria of “normal”—was inconsistent, and without regard to GnRH dose or time of blood collection; however, the highest agreement in obtaining an acceptable gonadotropin response to stimulation was observed after the administration of GnRH 50 μg.
In a prior study, about 75% of the participants in the SCI group, and a similar percent in the AB group, had a clinically acceptable gonadotropin response to standard provocative GnRH pituitary stimulation (“responders”).18 Of note, the SCI responder group had a significantly increased FSH release at several time points compared to the AB responder group; the average and integrated LH release in the SCI responder group approached significance compared with that of the AB-responder group.18 Because serum T responses to standard hCG stimulation testing was not significantly different for able-bodied or SCI men, regardless of gonadal status, it was suggested that the preponderance of hypogonadism identified in men with SCI is probably associated with hypothalamic-pituitary dysfunction, and not end-organ failure.19
While not significant, possibly in part due to the relatively small group sizes, the stimulated absolute FSH mean responses in those with SCI, regardless of gonadal status, were consistently higher than those of the AB groups (Figure 2), especially after administration GnRH 100 μg (Figure 3, top). Although not as striking, the stimulated absolute mean LH responses in those with SCI, regardless of gonadal status, were consistently higher than those of the able-bodied groups (Figure 1), especially after administration of GnRH 50 μg (Figure 3, bottom). One must be cautious not to overstate the importance of this observation in the absence of a statistical difference, but it is tempting to recognize this finding because it was consistent throughout the entire range of GnRH doses administered and regardless of gonadal status in the SCI groups. If one were to speculate, this absolutely higher gonadotropin response to exogenous GnRH may suggest a central GnRH deficiency.
The possible etiologies for the observed dysfunction of hypothalamic-pituitary-gonadal axis may include, but are not limited to, the following considerations. The feedback loop between the periphery (e.g., testis) and central organs (e.g., hypothalamus and/or pituitary) may be disrupted due to interruption of spinal circuits and/or altered hormonal feedback mechanisms, resulting in perturbed regulation of the axis. Inhibin B, a gonadal peptide, acts to suppress FSH release.50-52 By severing the nervous innervation to the testes, it may be postulated that normal diurnal patterns for the release and circulation of testosterone and/or inhibin may be perturbed, resulting in altered gonadotropin release.53,54 Another possibility is that prescribed or illicit medications, such as psychotropic agents that elevate prolactin levels and/or opioids that suppress LH releasing factor, may impair central function.23-26,28,55-57
Schally et al. isolated a porcine pituitary peptide that, if injected into animals or humans, caused a rise in serum LH and FSH concentrations.58 After this discovery, the majority of the studies that lead to the acceptance of the conventional dose of GnRH for the provocative stimulation of the hypothalamic-pituitary axis were performed in the 1970s when GnRH [or, as then referred to, luteinizing releasing hormone (LRH)] initially became available to investigators. The original work established the preferred route of delivery (intravenous vs. intravascular vs. subcutaneous bolus administration) 59-61 and dose of GnRH for the optimum response for routine clinical testing.62-65 Wollesen et al. administered a wide dosage range of GnRH (0-3,000 μg) to 16 healthy men to determine the pituitary gonadotropin release to dose and time after administration;62 the smallest dose of GnRH that resulted in a significant response from normal saline administration was 1.58 ug for LH and 20 μg for FSH, with the maximum response determined to be greater than 3,000 μg; the authors recommended a 100 μg dose of GnRH with sample collection at 30 and 45 minutes after administration, which would capture both the peak LH and FSH responses, and put forth 95% confidence intervals in normal men for the gonadotropin responses: 400-800% of the mean baseline value for serum LH and 100-200% for serum FSH. de Kretser and colleagues compared the 25 to 100 μg dose of GnRH and recommended the 100 μg dose because of the rise in FSH was minimal or absent at the lower doses.63 Testing a GnRH dose range of 1.56 to 450 μg, Newton et al. observed that GnRH 100 μg dose produced a maximal response in the majority of healthy individuals.64 These investigators also administered GnRH to 96 female patients with secondary amenorrhea, 5 patients with primary amenorrhea, and 8 patients with pituitary disorders and concluded that the magnitude and duration of the pituitary response was not of additional diagnostic value.64 In an effort to identify the “appropriate dose” of GnRH, Schonau-Jorgensen et al. intramuscularly administered GnRH 50, 100, and 200 μg and determined serum gonadotropin concentrations sequentially, and found that the 200 μg dose delivered intramuscularly was equivalent to the 100 μg dose delivered intravenously.65
Patients in the general population with idiopathic oligozoospermia have been reported to have basal levels of LH that are within the normal range. Of note, in the work presented the basal LH levels were in the normal range in participants with SCI. Thus, because it is well appreciated that a proportion of persons with SCI have been reported to have reduced sperm counts due to several possible etiologies,15,43 a combined peripheral and central abnormality cannot be excluded at least in a subgroup of those with SCI.
The unique approach of our incremental GnRH stimulation protocol has provided provocative observations on the sensitivity of the pituitary and the time-course of gonadotropin responses. The apparent heightened absolute release of FSH to standard GnRH stimulation in men with SCI who were HG, as well as heightened release of LH in EG men with SCI, was also observed. These observations suggest central dysregulation of the hypothalamic-pituitary axis in men with SCI despite basal and conventionally stimulated plasma gonadotropins levels within the normal range, and regardless of serum testosterone levels.8,16,41
Limitations
The major limitation of this work is the relatively small number of participants in each of the four sample groups. It is tempting to hypothesize that a study with more participants in each of our four subgroups may have yielded additional findings that would have reached significance. Our study group comprised of community dwelling individuals who may have had chronic medical conditions requiring outpatient management. The nutritional status, alcohol consumption and/or opioid usage were not specifically evaluated, although pain medications were an exclusion criterion for study participation. The likelihood for clinically significant malnutrition was low, but in subsets of our participants excessive chronic alcohol consumption or opioid use may have contributed to the presence of the central dysregulation of hypothalamic-pituitary function. However, every effort was made to exclude individuals with known medical conditions that could confound the results of this study.
Conclusion
Our findings demonstrate that graded stimulation of the hypothalamic-pituitary axis with three doses of GnRH (10, 50 and 100μg) was shown to have a modest dose-response effect. Individuals with SCI who had HG and EG status had a similar percentage of clinically acceptable gonadotropin responses to the administration of GnRH 50 and 100μg. The standard dose of GnRH (100 μg) stimulated the greatest FSH response in the SCI-HG group, which may be an important clinical feature if the individual is being evaluated for infertility, while GnRH 50 or 100 μg were equally potent to stimulated LH release in the SCI-HG group. The higher integrated FSH response to GnRH 100 μg in the SCI-HG group and the higher integrated LH response in the SCI-EG group to GnRH 50 μg, in association with increased gonadotropin release at all GnRH doses in SCI compared to AB groups, albeit this latter observation was not significant, is further evidence to support the presence of a hypothalamic-pituitary dysfunction in the persons with SCI. Even a subtle dysfunction of the hypothalamic-pituitary-gonadal axis may be associated with reduced circulating levels of testosterone and predisposition to adverse body composition and accompanying metabolic changes, as well as potential undesirable effects on the psyche and deleterious effects on spermatogenesis. Thus, screening of the serum testosterone concentration should be performed on a regular and routine basis in persons with SCI with the consideration of initiating hormone replacement therapy in those with low values and no contraindications to this intervention.
Acknowledgments
The research reported in this article was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service. This work was funded by the Veteran Affairs Rehabilitation Research & Development National Center of Excellence for the Medical Consequences of Spinal Cord Injury (B2648-C, B4162-C & B9212-C, B2020-C). The authors with to thank the James J. Peters Veterans Affairs Medical Center, Bronx, NY, and the Kessler Institute for Rehabilitation, West Orange, NJ, for their support.
Grant Sources: Veteran Affairs Rehabilitation Research and Development Service (#B2468-C, #B4162-C, #B9212-C, #B2020C) and the James J. Peters VA Medical Center.
Footnotes
Conflicts of Interest: None to declare
Clinical Trial Registration Number: NCT00223860
References
- 1.Sedlock DA, Laventure SJ. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia. 1990;28(7):448–454. doi: 10.1038/sc.1990.60. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77(2):371–378. doi: 10.1093/ajcn/77.2.371. [DOI] [PubMed] [Google Scholar]
- 3.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. 2014;37(1):32–39. doi: 10.1179/2045772313Y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman WA, Spungen AM. Body composition in aging: adverse changes in able-bodied persons and in those with spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6(3):22–36. [Google Scholar]
- 5.Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23(1):48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr The relationship between total body potassium and resting energy expenditure in individuals with paraplegia. Arch Phys Med Rehabil. 1993;74(9):965–968. [PubMed] [Google Scholar]
- 7.Bauman WA, Spungen AM, Flanagan S, Zhong YG, Alexander LR, Tsitouras PD. Blunted growth hormone response to intravenous arginine in subjects with a spinal cord injury. Horm Metab Res. 1994;26(3):152–156. doi: 10.1055/s-2007-1000798. [DOI] [PubMed] [Google Scholar]
- 8.Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil. 1992;71(6):328–332. doi: 10.1097/00002060-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11(1):109–140. [PubMed] [Google Scholar]
- 10.Schopp LH, Clark M, Mazurek MO, et al. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(8):678–684. doi: 10.1097/01.phm.0000228617.94079.4a. quiz 685-677. [DOI] [PubMed] [Google Scholar]
- 11.Clark MJ, Schopp LH, Mazurek MO, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87(9):758–767. doi: 10.1097/PHM.0b013e3181837f4f. [DOI] [PubMed] [Google Scholar]
- 12.Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl. 1992;13(4):297–304. [PubMed] [Google Scholar]
- 13.Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. How to recognize late-onset hypogonadism in men with sexual dysfunction. Asian J Androl. 2012;14(2):251–259. doi: 10.1038/aja.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khera M. Patients with testosterone deficit syndrome and depression. Archivos espanoles de urologia. 2013;66(7):729–736. [PubMed] [Google Scholar]
- 15.Linsenmeyer TA, Perkash I. Infertility in men with spinal cord injury. Arch Phys Med Rehabil. 1991;72(10):747–754. [PubMed] [Google Scholar]
- 16.Kikuchi TA, Skowsky WR, El-Toraei I, Swerdloff R. The pituitary-gonadal axis in spinal cord injury. Fertil Steril. 1976;27(10):1142–1145. doi: 10.1016/s0015-0282(16)42130-8. [DOI] [PubMed] [Google Scholar]
- 17.Cortes-Gallegos V, Castaneda G, Alonso R, Arellano H, Cervantes C, Parra A. Diurnal variations of pituitary and testicular hormones in paraplegic men. Arch Androl. 1982;8(3):221–226. doi: 10.3109/01485018208987044. [DOI] [PubMed] [Google Scholar]
- 18.Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM. Provocative stimulation of the hypothalamic-pituitary-testicular axis in men with spinal cord injury. Spinal Cord. 2016;54(11):961–966. doi: 10.1038/sc.2016.50. [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM. Testicular responses to hCG stimulation at varying doses in men with spinal cord injury. Spinal Cord. 2017;55(7):659–663. doi: 10.1038/sc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PMR. 2011;3(10):929–932. doi: 10.1016/j.pmrj.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Grossmann M, Matsumoto AM. A Perspective on Middle-Aged and Older Men with Functional Hypogonadism: Focus on Holistic Management. J Clin Endocrinol Metab. 2017;102(3):1067–1075. doi: 10.1210/jc.2016-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergendahl M, Veldhuis JD. Altered pulsatile gonadotropin signaling in nutritional deficiency in the male. Trends Endocrinol Metab. 1995;6(5):145–159. doi: 10.1016/1043-2760(95)00081-r. [DOI] [PubMed] [Google Scholar]
- 23.Aloisi AM, Aurilio C, Bachiocco V, Biasi G, Fiorenzani P, Pace MC, et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology. 2009;34(Suppl 1):S162–168. doi: 10.1016/j.psyneuen.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3(5):377–384. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer A, Herz A. Endocrine actions of opioids. Horm Metab Res. 1984;16(8):386–397. doi: 10.1055/s-2007-1014801. [DOI] [PubMed] [Google Scholar]
- 26.Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65(12):1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- 27.Noth RH, Walter RM., Jr The effects of alcohol on the endocrine system. Med Clin North Am. 1984;68(1):133–146. doi: 10.1016/s0025-7125(16)31246-9. [DOI] [PubMed] [Google Scholar]
- 28.Bannister P, Losowsky MS. Ethanol and hypogonadism. Alcohol Alcohol. 1987;22(3):213–217. [PubMed] [Google Scholar]
- 29.Sullivan SD, Nash MS, Tefera E, Tinsley E, Blackman MR, Groah S. Prevalence and Etiology of Hypogonadism in Young Men with Chronic Spinal Cord Injury: A Cross-Sectional Analysis From Two University-Based Rehabilitation Centers. PMR. 2016;pii: S1934-1485(16):31169–8. doi: 10.1016/j.pmrj.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 31.Bauman WA, Adkins RH, Spungen AM, Kemp BJ, Waters RL. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord. 1998;36(1):13–17. doi: 10.1038/sj.sc.3100513. [DOI] [PubMed] [Google Scholar]
- 32.Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- 33.Traish AM. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):313–322. doi: 10.1097/MED.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30(10):697–703. doi: 10.1038/sc.1992.136. [DOI] [PubMed] [Google Scholar]
- 35.Monroe AK, Dobs AS. The effect of androgens on lipids. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):132–139. doi: 10.1097/MED.0b013e32835edb71. [DOI] [PubMed] [Google Scholar]
- 36.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43(8):574–579. doi: 10.1055/s-0031-1280797. [DOI] [PubMed] [Google Scholar]
- 37.Wade GN. Sex steroids and energy balance: sites and mechanisms of action. Ann N Y Acad Sci. 1986;474:389–399. doi: 10.1111/j.1749-6632.1986.tb28029.x. [DOI] [PubMed] [Google Scholar]
- 38.Munro D, Horne HW, Jr, Paull DP. The effect of injury to the spinal cord and cauda equina on the sexual potency of men. N Engl J Med. 1948;239(24):903–911. doi: 10.1056/NEJM194812092392401. [DOI] [PubMed] [Google Scholar]
- 39.Horne HW, Paull DP, Munro D. Fertility studies in the human male with traumatic injuries of the spinal cord and cauda equina. N Engl J Med. 1948;239(25):959–961. doi: 10.1056/NEJM194812162392504. [DOI] [PubMed] [Google Scholar]
- 40.Bors E, Engle ET, Rosenquist RC, Holliger VH. Fertility in paraplegic males; a preliminary report of endocrine studies. J Clin Endocrinol Metab. 1950;10(4):381–398. doi: 10.1210/jcem-10-4-381. [DOI] [PubMed] [Google Scholar]
- 41.Ver Voort SM. Infertility in spinal-cord injured male. Urology. 1987;29(2):157–165. doi: 10.1016/0090-4295(87)90145-2. [DOI] [PubMed] [Google Scholar]
- 42.Beretta G, Chelo E, Zanollo A. Reproductive aspects in spinal cord injured males. Paraplegia. 1989;27(2):113–118. doi: 10.1038/sc.1989.17. [DOI] [PubMed] [Google Scholar]
- 43.Perkash I, Martin DE, Warner H, Blank MS, Collins DC. Reproductive biology of paraplegics: results of semen collection, testicular biopsy and serum hormone evaluation. J Urol. 1985;134(2):284–288. doi: 10.1016/s0022-5347(17)47126-6. [DOI] [PubMed] [Google Scholar]
- 44.Nance PW, Shears AH, Givner ML, Nance DM. Gonadal regulation in men with flaccid paraplegia. Arch Phys Med Rehabil. 1985;66(11):757–759. [PubMed] [Google Scholar]
- 45.Huang TS, Wang YH, Chiang HS, Lien YN. Pituitary-testicular and pituitary-thyroid axes in spinal cord-injured males. Metabolism. 1993;42(4):516–521. doi: 10.1016/0026-0495(93)90112-2. [DOI] [PubMed] [Google Scholar]
- 46.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 47.Winters SJ. Diurnal rhythm of testosterone and luteinizing hormone in hypogonadal men. J Androl. 1991;12(3):185–190. [PubMed] [Google Scholar]
- 48.Gupta SK, Lindemulder EA, Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol. 2000;40(7):731–738. doi: 10.1177/00912700022009486. [DOI] [PubMed] [Google Scholar]
- 49.Bhasin S, Jameson JL. Disorders of the testes and male reproductive system. In: Braunwald E, Fauci AS, Hauser SL, Kasper DL, Jameson JL, Longo DL, editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill Education; 2005. p. 2188. [Google Scholar]
- 50.Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22(3):253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- 51.Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. Pituitary actions of ligands of the TGF-beta family: activins and inhibins. Reproduction. 2006;132(2):207–215. doi: 10.1530/rep.1.01073. [DOI] [PubMed] [Google Scholar]
- 52.Iliadou PK, Tsametis C, Kaprara A, Papadimas I, Goulis DG. The Sertoli cell: Novel clinical potentiality. Hormones. 2015;14(4):504–514. doi: 10.14310/horm.2002.1648. [DOI] [PubMed] [Google Scholar]
- 53.Tenover JS, Bremner WJ. Circadian rhythm of serum immunoreactive inhibin in young and elderly men. J Gerontol. 1991;46(5):M181–184. doi: 10.1093/geronj/46.5.m181. [DOI] [PubMed] [Google Scholar]
- 54.Chong YH, Pankhurst MW, McLennan IS. The Daily Profiles of Circulating AMH and INSL3 in Men are Distinct from the Other Testicular Hormones, Inhibin B and Testosterone. PLoS One. 2015;10(7):e0133637. doi: 10.1371/journal.pone.0133637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ajmal A, Joffe H, Nachtigall LB. Psychotropic-induced hyperprolactinemia: a clinical review. Psychosomatics. 2014;55(1):29–36. doi: 10.1016/j.psym.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 Suppl 1):S12–18. doi: 10.1016/j.amjmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Schooling CM, Au Yeung SL, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57. doi: 10.1186/1741-7015-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, Redding TW, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43(2):393–399. doi: 10.1016/0006-291x(71)90766-2. [DOI] [PubMed] [Google Scholar]
- 59.Arimura A, Spies HG, Schally AV. Relative insensitivity of rhesus monkeys to the LH-releasing hormone (LH-RH) J Clin Endocrinol Metab. 1973;36(2):372–374. doi: 10.1210/jcem-36-2-372. [DOI] [PubMed] [Google Scholar]
- 60.Redding TW, Kastin AJ, Gonzales-Barcena D, Coy DH, Coy EJ, Schalch DS, et al. The half-life, metabolism and excretion of tritiated luteinizing hormone-releasing hormone (LH-RH) in man. J Clin Endocrinol Metab. 1973;37(4):626–631. doi: 10.1210/jcem-37-4-626. [DOI] [PubMed] [Google Scholar]
- 61.Mortimer CH, Besser GM, Goldie DJ, Hook J, McNeilly AS. The TSH, FSH and prolactin responses to continuous infusions of TRH and the effects of oestrogen administration in normal males. Clin Endocrinol (Oxf) 1974;3(2):97–103. doi: 10.1111/j.1365-2265.1974.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 62.Wollesen F, Swerdloff RS, Odell WD. LH and FSH responses to luteinizing releasing hormone in normal fertile women. Metabolism. 1976;25(11):1275–1285. doi: 10.1016/s0026-0495(76)80011-x. [DOI] [PubMed] [Google Scholar]
- 63.de Kretser DM, Burger HG, Hudson B, Keogh The pituitary-testicular response to luteinising hormone releasing hormone administration to normal men. Aust N Z J Med. 1975;5(3):227–230. doi: 10.1111/j.1445-5994.1975.tb04573.x. [DOI] [PubMed] [Google Scholar]
- 64.Newton JR, Kilpatrick MJ, Pike JM, Collins WP. An evaluation of the diagnostic value of synthetic luteinizing hormone releasing hormone. Acta Endocrinol (Copenh) 1975;80(3):417–428. doi: 10.1530/acta.0.0800417. [DOI] [PubMed] [Google Scholar]
- 65.Schonau-Jorgensen F, Kampmann JP, Micic S, Roos J, Johnsen SG. LH and FSH in serum after intramuscular administration of LH/FSH-releasing hormone in normal and hypogonadal men. Acta Endocrinol. 1975;78(1):1–10. doi: 10.1530/acta.0.0780001. [DOI] [PubMed] [Google Scholar]


