Abstract
Individuals prone to ethanol overconsumption may have preexisting neurochemical disturbances that contribute to their vulnerability. This study examined the paraventricular nucleus of thalamus (PVT), a limbic structure recently shown to participate in ethanol intake. To identify individuals prone to ethanol overconsumption, we tested Long-Evans rats in behavioral paradigms and found high levels of vertical time (rearing behavior) in a novel activity chamber to be a consistent predictor of subsequent excessive 20% ethanol drinking under the intermittent-access model. Examining neurochemicals in the PVT, we found before ethanol exposure that prone rats with high rearing, compared to non-prone rats, had significantly lower levels of neurotensin (NTS) mRNA and peptide in the posterior (pPVT) but not anterior (aPVT) subregion of the PVT. Our additional finding that ethanol intake has no significant impact on either rearing or NTS levels indicates that these measures, which are different in prone rats before ethanol consumption, remain stable after ethanol consumption. The possibility that NTS directly controls ethanol drinking is supported by our finding that NTS administration specifically suppresses ethanol drinking when injected into the pPVT but not aPVT, with this effect occurring exclusively in higher drinkers that presumably have lower endogenous levels of NTS. Further, an NTS antagonist in the pPVT augments intake in lower drinkers with presumably more endogenous NTS while NTS in the pPVT inhibits novelty-induced rearing that predicts excessive drinking. Together, these results provide strong evidence that low endogenous levels of NTS in the pPVT contribute to an increased propensity toward excessive ethanol drinking.
Keywords: anterior thalamic paraventricular nucleus, intermittent access, prone
INTRODUCTION
Alcohol overconsumption accounts for 5.1% of the burden of disease and injury and 5.9% of all deaths worldwide (World Health Organization, 2015). In considering how to curb or prevent excessive alcohol consumption, it is important to recognize that not all individuals who drink alcohol actually engage in overconsumption. For example, among adult alcohol users in the United States, about 43% binge drink in a given month (Center for Behavioral Health Statistics and Quality, 2015). Similarly, of adult Long-Evans rats given ethanol using the 20% ethanol two-bottle-choice intermittent-access model, about 30% consume ethanol at binge levels (blood ethanol concentration > 80 mg%) and 40% consume at pharmacologically-relevant levels (> 50 mg%) (Barson et al., 2015; McBride et al., 2013; Simms et al., 2008). This separation of excessive and non-excessive drinkers suggests that these subgroups have preexisting neurobiological differences.
To investigate neurochemical differences that may predispose individuals to excessive ethanol intake, we focused on the limbic paraventricular nucleus of thalamus (PVT), which has recently been shown through local neuropeptide injections to promote ethanol drinking (Barson et al., 2015; Barson et al., 2017) but has yet to be examined for its endogenous role in the propensity to drink. The evidence suggests that the neuropeptide-induced increase in drinking is driven by the anterior subregion of the PVT (aPVT) that is associated with arousal via projections to the suprachiasmatic nucleus (Barson et al., 2015; Salazar-Juarez et al., 2002), rather than the posterior subregion (pPVT) that is associated with fear and anxiety via projections to the amygdala and bed nucleus of the stria terminalis (Li and Kirouac, 2008; Li et al., 2010; Penzo et al., 2015). Dysregulation of endogenous neuropeptides and their receptors in individuals prone to excessive drinking has been described in other limbic areas, including the nucleus accumbens, prefrontal cortex, and hypothalamus (Barson et al., 2013; Marinelli et al., 2000). While there are no such studies of the PVT, the neurochemicals known to be transcribed in this nucleus have, through systemic or central injections, each been shown to influence ethanol intake. This nucleus appears to be composed exclusively of projection neurons, many of which contain glutamate that can be identified by the presence of vesicular glutamate transporter 2 (vGLUT2) (Frassoni et al., 1997; Moutsimilli et al., 2008; Myers et al., 2014) but which also contain the neuropeptides enkephalin (ENK), substance P (SP), and neurotensin (NTS) (Arluison et al., 1994). Through microinjections into the aPVT, SP has been shown to promote ethanol drinking (Barson et al., 2017), and through systemic antagonist injections, both ENK and glutamate are also suggested to endogenously enhance excessive drinking (Cozzoli et al., 2014; Simms et al., 2008). In contrast, NTS through systemic injection of an NTS analog has been shown to decrease ethanol intake (Lee et al., 2010), while mice lacking the NTS receptor type 1 (NTS1) or type 2 (NTS2), both of which exist in the PVT (Boudin et al., 1996; Sarret et al., 2003), exhibit the opposite effect (Lee et al., 2010; Lee et al., 2011). Thus, the PVT may be one nucleus where differences in endogenous neurochemicals contribute to an individual’s proclivity for excessive ethanol drinking.
To characterize such neurochemical differences, we sought to use a non-invasive behavioral measure in ethanol-naïve outbred rats to identify those individuals that are prone to excessive ethanol drinking under the 20% intermittent-access model. To date, studies have found anxiety in an elevated plus maze (Spanagel et al., 1995), novelty-seeking in a hole-board apparatus (Manzo et al., 2014), and horizontal locomotor activity in a novel or familiar chamber (Barson et al., 2013; Bisaga and Kostowski, 1993) to predict higher intake, whereas vertical (rearing) behavior in a novel environment failed to be an effective predictor (Bienkowski et al., 2001; Koros et al., 1998; Nadal et al., 2002). Notably, these studies used models of ethanol access, either ad libitum, 8 hours/day, or operant self-administration, that induce levels of consumption (1 – 3 g/kg/day) significantly lower than those produced by the 20% intermittent-access model (4 – 6 g/kg/day), which can attain pharmacological relevance (Barson et al., 2015; Simms et al., 2008). This difference led us to question whether these behavioral measures are in fact good predictors of excessive, pharmacologically-relevant ethanol drinking.
To investigate whether there are preexisting differences in the PVT that make individuals prone to overconsuming ethanol, we had the following three goals: (1) to determine if individuals prone to higher levels of drinking under the 20% ethanol intermittent-access model can be identified using the behaviors established for lower-level drinking; (2) to characterize neurochemical differences in the PVT that may underlie these differences; and (3) to determine if these differences are causal factors in the predisposition to excessive ethanol drinking. We hypothesized that one or several of the neurochemicals transcribed in the PVT are differentially expressed in animals identified as prone versus non-prone to excessive ethanol drinking and that this difference contributes to their vulnerability to this behavior.
MATERIALS AND METHODS (see Appendix for further details)
Subjects
Adult, male Long-Evans rats (N = 163; 201 – 225 g, Charles River Laboratories International, Inc., Kingston, NY, USA and Malvern, PA, USA) were individually housed in an AAALAC-accredited facility, on a 12-hour reversed light/dark cycle (lights off at 0900 h). They were given at least one week to acclimate to the facility and were handled daily prior to the start of experiments. Rats received ad libitum chow (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO, USA) and water throughout the study. Experiments were approved by the Institutional Animal Care and Use Committees of Drexel University College of Medicine and The Rockefeller University and followed the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Protocol
Experiment 1
To identify behaviors that could predict excessive ethanol drinking, ethanol-naïve rats (N = 23) were tested for novelty-induced locomotor activity, novelty-seeking, locomotor activity, and anxiety. One week after the conclusion of these tests, they were given access to 20% ethanol in an intermittent-access paradigm over 9 weeks. The hourly pattern of their drinking was determined during week 4 by measuring intake at 30 min, 1 hr, 2 hr, 4 hr, and 24 hr after the start of daily ethanol access.
To confirm results showing novelty-induced vertical time to be a reliable predictor of ethanol drinking, an additional group of ethanol-naïve rats (N = 20) was tested for novelty-induced locomotor activity and, one week later, given access to 20% ethanol in an intermittent-access paradigm over 4 weeks. This group subsequently received further testing as described in Experiments 5 and 6. (See Appendix for details of behavioral testing and ethanol drinking.)
Experiment 2
To examine differences in PVT neurochemicals in individuals prone to excessive ethanol drinking, ethanol-naïve rats (N = 24) were tested for novelty-induced locomotor activity and then given one week of no testing. They were then sacrificed via rapid decapitation one hour after the start of the dark cycle to examine mRNA levels of NTS, its receptors (NTS1R and NTS2R), and ENK, SP and vGLUT2 in the aPVT and pPVT using quantitative real-time PCR (qRT-PCR) (Table 1). Food was removed one hour prior to sacrifice. (See Appendix for details of quantitative real-time PCR.)
Table 1.
Primer sequences and concentrations used for quantitative real-time polymerase chain reaction in Experiments 2 and 4.
| Primer | Sequence | Concentration |
|---|---|---|
| Cyclophilin-A | 5′-GTGTTCTTCGACATCACGGCT-3′ (forward) 5′-CTGTCTTTGGAACTTTGTCTGCA-3′ (reverse) |
200 nM |
| Enkephalin | 5′-GGACTGCGCTAAATGCAGCTA-3′ (forward) 5′-GTGTGCATGCCAGGAAGTTG-3′ (reverse) |
100 nM |
| Neurotensin | 5′-CATCGAAGGTCAGCAAAGGAA-3′ (forward) 5′-GGTCGTCATCACGCATTTCTC-3′ (reverse) |
100 nM |
| Neurotensin Receptor 1 | 5′-AAGCAGGCACCCTTCATCT-3′ (forward) 5′-GGAGGCTGGATGGTTCTGT-3′ (reverse) |
100 nM |
| Neurotensin Receptor 2 | 5′-GAATGTGCTGGTGTCCTTCGC-3′ (forward) 5′-ACTTGTATTTCTCCCAGGCTG-3′ (reverse) |
100 nM |
| Substance P | 5′-ACCAAATCAAGGAGGCAATG-3′ (forward) 5′-AGCCTTTAACAGGGCCACTT-3′ (reverse) |
200 nM |
| Vesicular Glutamate Transporter 2 | 5′-CGTGAAGAATGGCAGTATGTCTTC-3′ (forward) 5′-TGAGGCAAATAGTGCATAAAATATGACT-3′ (reverse) |
100 nM |
Experiment 3
To determine whether NTS peptide levels are also disturbed in prone individuals, ethanol-naïve rats (N = 24) were tested for novelty-induced locomotor activity. Then, two weeks later, food was removed at the start of the dark cycle and 90 min later the rats were anesthetized and perfused for analysis of NTS peptide in the PVT subregions using immunohistochemistry. (See Appendix for details of immunohistochemistry.)
Experiment 4
To determine if the predictor behavior and NTS are themselves affected by ethanol drinking, thus possibly altering their role in drinking as ethanol exposure increases, rats (N = 32) were given access to 20% ethanol in an intermittent-access paradigm or were maintained on water and chow only (n = 16/group). During the fourth week of ethanol (or water) access, 30 min into the daily access period, when ethanol drinking was found to peak, half of each group was tested for novelty-induced locomotor activity (n = 8/group). During the fifth week, again starting 30 min into daily access and with food removed one hour prior, the other half of this group was sacrificed via rapid decapitation for analysis of gene expression in the PVT subregions using qRT-PCR (n = 8/group). One sample from the qRT-PCR water group was lost prior to testing so data from this animal were not included in the analysis.
Experiment 5
To determine if NTS in the PVT activates local neurons, rats (N = 20), after being given access to 20% ethanol in an intermittent-access paradigm over four weeks (Experiment 1), underwent surgery to receive a brain cannula aimed at the aPVT or pPVT (n = 10/subregion). Approximately one week after the completion of injections to examine effects on ethanol intake (Experiment 6), on a day when the rats would normally receive ethanol, they were injected with vehicle (0.3 μl) or NTS (1.20 nmol) in a between-subject design, food was removed, and they were perfused 90 min later for analysis of c-Fos in the PVT subregions using immunohistochemistry. Cannula placement was confirmed by examining slide-mounted tissue. Due to a loose cannula or poor health, two rats in the aPVT group and one rat from the pPVT group were removed from the study. (See Appendix for details of microinjections, immunohistochemistry, and histology.)
Experiment 6
To establish if higher levels of NTS in the PVT are protective against excessive intake, one group of ethanol-drinking rats (N = 20, previously tested in Experiment 1), one week following cannulation surgery, was injected in the aPVT or pPVT with vehicle counterbalanced against two doses of NTS (0.12 and 1.20 nmol) in a within-subject Latin-square design across three ethanol access days. Following injections, intake of ethanol, food, and water was measured at 30 min, 1 hr, 2 hr, and 4 hr. One rat in the aPVT subgroup was removed prior to microinjection due to a loose cannula. This experiment was then repeated in a second, larger group of ethanol-drinking rats cannulated in the pPVT (N = 24), to determine if the response to NTS (0.12 and 1.20 nmol vs vehicle) differs depending on the baseline level of drinking. One week later, they were tested as before, but were instead injected with the nonselective NTS receptor antagonist SR-142948 (1 and 10 nmol vs. vehicle), to determine if blockade of activity of NTS in the PVT instead promotes excessive intake. Six rats from this group were removed from the study due to a loose cannula or poor health. (See Appendix for details of microinjections and intake measurements.)
Experiment 7
To determine if NTS itself affects novelty-induced vertical time, ethanol-naïve rats (N = 16), one week following cannulation surgery, were injected in the pPVT with vehicle or NTS (1.20 nmol) in a between-subject design and tested for novelty-induced locomotor activity. Following an additional day of acclimation to the activity chamber, they received vehicle counterbalanced against NTS in a between-subject design and were tested for locomotor activity. Two rats were removed from the study due to a loose cannula and data from one additional rat was removed from the novelty-induced locomotor activity test due to a failed injection. (See Appendix for details of microinjections and behavioral testing.)
Statistical Analysis
Linear regression was used to examine the relationship between ethanol intake and blood ethanol concentration (BEC). To analyze drinking across time (Experiment 1), a one-way repeated-measures ANOVA was used and followed up with a Sidak pairwise comparison test. Multiple regression analysis was performed using the enter method. Differences between multiple groups were analyzed by one-way ANOVA, followed-up by Tukey multiple comparison tests when appropriate. Differences between two groups were analyzed by independent-samples t-test with two tails and differences between different conditions for the same group were analyzed by paired-samples t-test with two tails. Correlations were made using the Pearson product-moment correlation coefficient. To examine the effect of microinjected drugs on ethanol intake over time (Experiment 6), a two-way repeated-measures or mixed ANOVA was used, with dose and time as repeated measures and drinking group (high, middle, or low) as a between-subject measure. As drug effects in the first group in Experiment 6 were observed only at 30 min, subsequent analyses in the second group included only 30 min and 1 hr measurements. Significant main effects were followed up with a Sidak pairwise comparison test and significant interaction effects were followed up with a one-way repeated-measures ANOVA and a Sidak pairwise comparison test when appropriate. To analyze the effects of NTS on locomotor activity (Experiment 7), a mixed ANOVA was used, with drug as the between-subjects measure and time as the repeated measure. Significant interaction effects were followed up with independent-samples t-test with two tails. Significance was determined at P < 0.05. Data are reported as mean ± standard error of the mean (S.E.M.).
RESULTS
Experiment 1: Rearing behavior in a novel activity chamber predicts excessive ethanol drinking
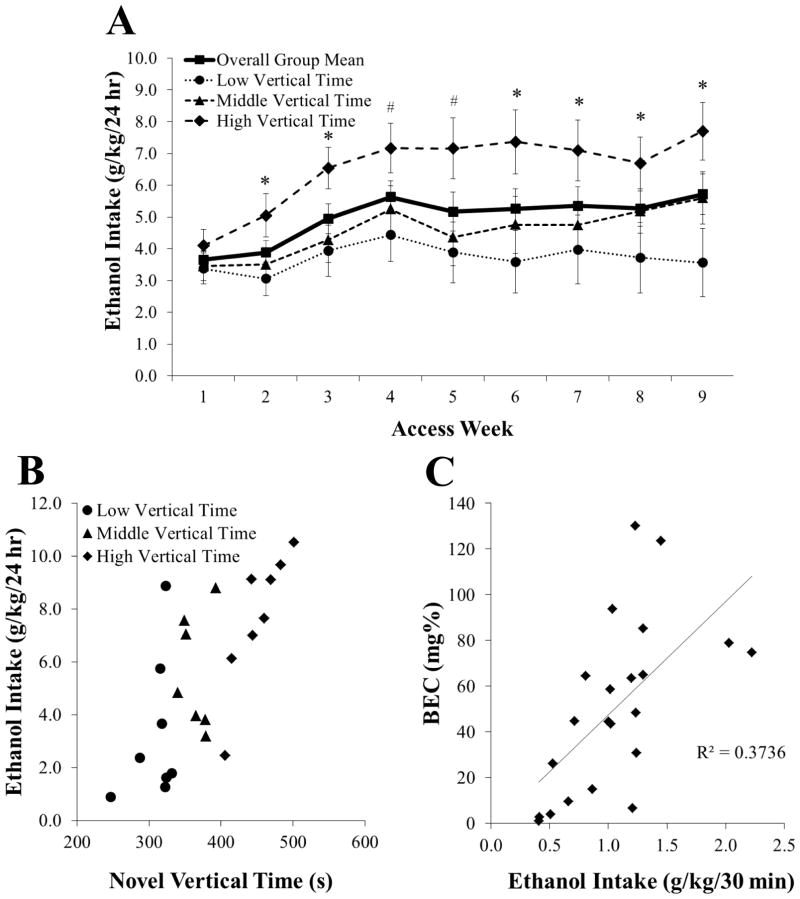
This experiment examined possible behavioral predictors of excessive 20% ethanol drinking under the intermittent-access two-bottle-choice paradigm. In the first group of rats (N = 23), given access to ethanol over 9 weeks, intake averaged 4.9 ± 0.5 g/kg/day, gradually increasing until week 4 when pairwise comparisons between weeks were no longer significant (not significant, NS) (Figure 1A). Peak daily intake occurred during the first 30 minutes of access, when rats consumed 16.6% (1.1 ± 0.1 g/kg) of their daily total. This 30-minute intake significantly predicted BEC levels (R2 = +0.37, P < 0.01), which averaged 50.6 ± 8.0 mg% and went as high as 130 mg% (Figure 1B). Overall, 45% of subjects achieved BECs above 50 mg% and 18% achieved levels above 80 mg%.
Figure 1.
Intermittent access induces pharmacologically-relevant drinking of 20% ethanol in Long-Evans rats over 9 weeks (Experiment 1). A Average weekly drinking in the overall group (N = 23) was stable by week 4 but was already significantly higher by week 2 in rats with high (n = 8) compared to low vertical time (n = 8) in a novel activity chamber. B Rats with high compared to low vertical time in the novel activity chamber showed fairly distinct clustering in their level of ethanol drinking, while rats with middle vertical time failed to form a clear group. C Blood ethanol concentrations (BEC) in the overall group (N = 23) were significantly predicted by the 30-minute intake prior to blood collection. Data are mean ± S.E.M.; *p < 0.05, #p = 0.05 vs. low vertical time group.
With measurements of novelty-induced locomotor activity, novelty-seeking, locomotor activity, and anxiety, multiple regression analysis for average ethanol intake revealed a significant model [F(6, 22) = 2.89, P < 0.05] that accounted for 52% of the variance. The measure of time spent vertical (rearing behavior) during 15 minutes in a novel activity chamber emerged as the only significant predictor of ethanol drinking (P < 0.01) (Table 2). Additional analysis of number of rears made in the novel chamber showed that this measure failed to predict drinking (r2 = 0.04, NS). When sorted by tertile split according to level of vertical time in a novel activity chamber, rats had significantly different levels of average ethanol intake [F(2, 22) = 4.28, P < 0.05], with multiple comparisons revealing that those with high levels of vertical time drank nearly twice as much as those with low levels of vertical time (n = 8/group; 452 ± 12 sec vs. 308 ± 10 sec; 6.5 ± 0.7 g/kg/day vs. 3.7 ± 0.8 g/kg/day, P < 0.05), with this difference evident at every time point after week 1 (P ≤ 0.05) (Figure 1A–B). Similarly, 30-minute ethanol intake was significantly higher in rats with high compared to low levels of vertical time [1.1 ± 0.1 g/kg vs. 0.7 ± 0.1 g/kg; t(13) = 2.66, P < 0.05], leading them to have average BECs of 60 ± 14 mg% compared to 39 ± 18 mg%. Due to a strong negative correlation between ethanol and water drinking (r = −0.76, P < 0.001), average water intake was also significantly predicted by vertical time in a novel activity chamber [F(1, 22) = 9.20, P < 0.01], which accounted for 55% of the variance, while average chow intake was not predicted by this behavior [F(1, 22) = 0.01, NS].
Table 2.
Predictor variables for average 20% ethanol intake consumed in an intermittent-access paradigm over nine weeks. Time spent vertical (rearing behavior) in a novel activity chamber emerged as the only significant predictor of ethanol drinking (Experiment 1, N = 23).
| Predictor Variable | Beta | P |
|---|---|---|
| Novel Locomotor: Vertical Time | 0.56 | 0.01 |
| Novel Locomotor: Horizontal Time | −0.27 | 0.22 |
| Novelty-Seeking | 0.20 | 0.31 |
| Locomotor: Vertical Time | 0.22 | 0.29 |
| Locomotor: Horizontal Time | 0.24 | 0.25 |
| Anxiety | −0.10 | 0.64 |
The ability of rearing behavior to predict level of ethanol drinking was confirmed in a second group of rats (N = 20). Regression analysis here showed that average ethanol intake, this time over 4 weeks, was again significantly predicted by time spent vertical in a novel activity chamber [F(1, 19) = 7.10, P < 0.05], which similarly accounted for 53% of the variance. When these rats were sorted by tertile split, those with high levels of vertical time drank significantly more than those with low levels of vertical time [n = 7/group; 447 ± 12 sec vs. 313 ± 4 sec; 4.9 ± 0.7 g/kg/day vs. 3.5 ± 0.1 g/kg/day; t(12) = 2.16, P < 0.05]. Therefore, vertical time in a novel activity chamber emerged as a strong measure for identifying rats prone to excessive ethanol drinking under the 20% intermittent-access model.
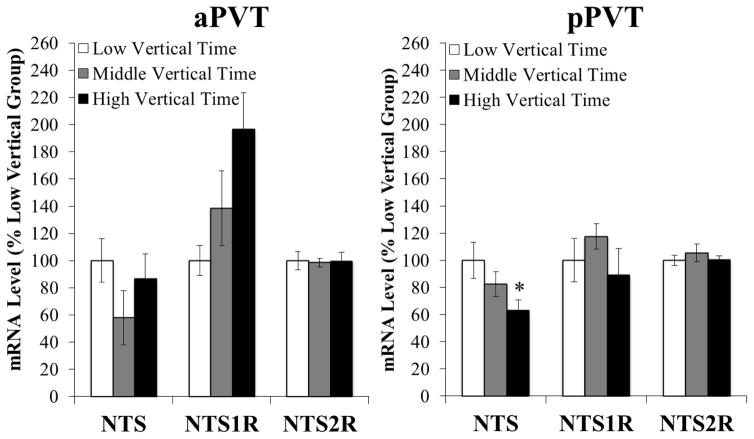
Experiment 2: Neurotensin mRNA in the pPVT is reduced in prone animals with higher vertical time
To examine neurochemicals in the PVT of ethanol-naïve animals prone to consuming excessive ethanol, rats (N = 24) were tested in a novel activity chamber, classified by tertile split according to time spent vertical (n = 8/group), and gene expression was measured in the PVT subregions using qRT-PCR. In the pPVT, significant differences were found in mRNA levels specifically of NTS [F(2, 21) = 3.97, P < 0.05] (Figure 2, right). Multiple comparisons revealed that prone rats with high levels of vertical time (412 ± 20 sec) had significantly lower levels of this neuropeptide in this subregion than those with low levels of vertical time (223 ± 14 sec; P < 0.05). Moreover, gene expression of NTS in the pPVT was significantly, negatively correlated with time spent vertical (r = −0.56, P < 0.01). This is in clear contrast to the aPVT, where no significant group differences were detected for mRNA levels of NTS [F(2, 21) = 2.18, NS] (Figure 2, left). Additional measurements of the NTS receptors in both PVT subregions also failed to reveal any significant differences in mRNA levels across groups, for both NTS1R [pPVT: F(2, 21) = 0.90, NS; aPVT: F(2, 21) = 1.66, NS] and NTS2R [pPVT: F(2, 21) = 0.42, NS; aPVT: F(2, 21) = 0.02, NS], although NTS1R mRNA levels in the aPVT of prone rats were 97% higher than those of non-prone rats (Figure 2, left). Further, no significant group differences in mRNA levels of other neurochemicals were detected in either PVT subregion, including ENK [pPVT: F(2, 21) = 0.48, NS; aPVT: F(2, 21) = 2.68, NS], SP [pPVT: F(2, 21) = 3.22, NS; aPVT: F(2, 21) = 2.29, NS], and vGLUT2 [pPVT: F(2, 21) = 0.51, NS; aPVT: F(2, 21) = 0.73, NS]. These measurements of gene expression prior to ethanol exposure identify NTS mRNA, specifically in the pPVT, as being significantly reduced in rats prone to excessive ethanol drinking under the intermittent-access model.
Figure 2.
Ethanol-naïve rats with high vertical time in a novel activity chamber have low gene expression of neurotensin in the posterior paraventricular thalamus, as assessed by quantitative real-time polymerase chain reaction (Experiment 2, n = 8/group). Target gene expression was quantified relative to cyclophilin using the relative quantification method. Abbreviations: aPVT, anterior paraventricular thalamus; NTS, neurotensin; NTS1R, neurotensin receptor type 1; NTS2R, neurotensin receptor type 2; pPVT, posterior paraventricular thalamus. Data are mean ± S.E.M.; *p < 0.05 vs. low vertical time group.
Experiment 3: Neurotensin peptide levels in the PVT are reduced in prone animals with higher vertical time
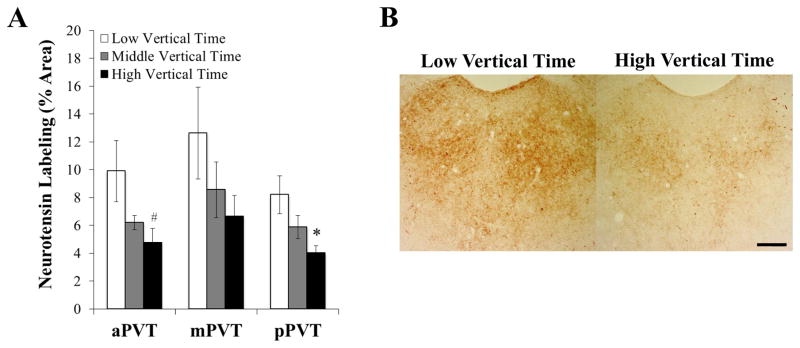
To determine if these qRT-PCR findings could be substantiated with measurements of NTS peptide, ethanol-naïve rats (N = 24) were tested in a novel activity chamber, classified by tertile split according to time spent vertical (n = 8/group), and examined with immunohistochemistry to determine the density of NTS peptide in the PVT as measured by the percentage of area stained. We found immunoreactivity for NTS to be in both cell bodies and fibers throughout this nucleus. In the pPVT, significant group differences were observed in the percent area labeled for NTS peptide [F(2, 21) = 4.61, P < 0.05] (Figures 3A and 3B). Multiple comparisons revealed that prone rats with high levels of vertical time (384 ± 8 sec) compared to low levels (289 ± 4 sec) had significantly reduced levels of this neuropeptide in this subregion (P < 0.05). Also, levels of NTS in the pPVT correlated significantly and negatively with time spent vertical (r = −0.61, P < 0.01). While the percent area labeled for NTS immunoreactivity in the middle PVT (mPVT) was not significantly different between groups [F(2, 21) = 1.63, NS] (Figure 3A), a strong trend for a significant difference was found in the aPVT [F(2, 21) = 3.45, P = 0.05] (Figure 3A), where multiple comparisons again revealed lower peptide levels in rats with high compared to low levels of vertical time (P < 0.05) and NTS levels correlated significantly and negatively with time spent vertical (r = −0.57, P < 0.01). These results confirm those of Experiment 2, showing that levels of NTS peptide in the pPVT prior to ethanol exposure are reduced in rats prone to excessive ethanol drinking under the intermittent-access model.
Figure 3.
Ethanol-naïve rats with high vertical time in a novel activity chamber have lower peptide levels of neurotensin in subregions of the paraventricular thalamus (Experiment 3, n = 8/group). A Rats with high vertical time have a greater percentage of area stained for neurotensin in the posterior paraventricular thalamus (pPVT) and anterior paraventricular thalamus (aPVT) but not the middle paraventricular thalamus (mPVT), as assessed by immunohistochemistry with 3,3′-diaminobenzidine tetrahydrochloride (DAB). B Representative photomicrographs of the posterior paraventricular thalamus, showing fewer cell bodies and less density of fibers containing neurotensin in rats with high compared to low vertical time. Data are mean ± S.E.M.; *p < 0.05, #p = 0.05 vs. low vertical time group. Scale bar = 200 μm.
Experiment 4: Ethanol drinking has no effect on vertical time in a novel activity chamber or levels of neurotensin mRNA
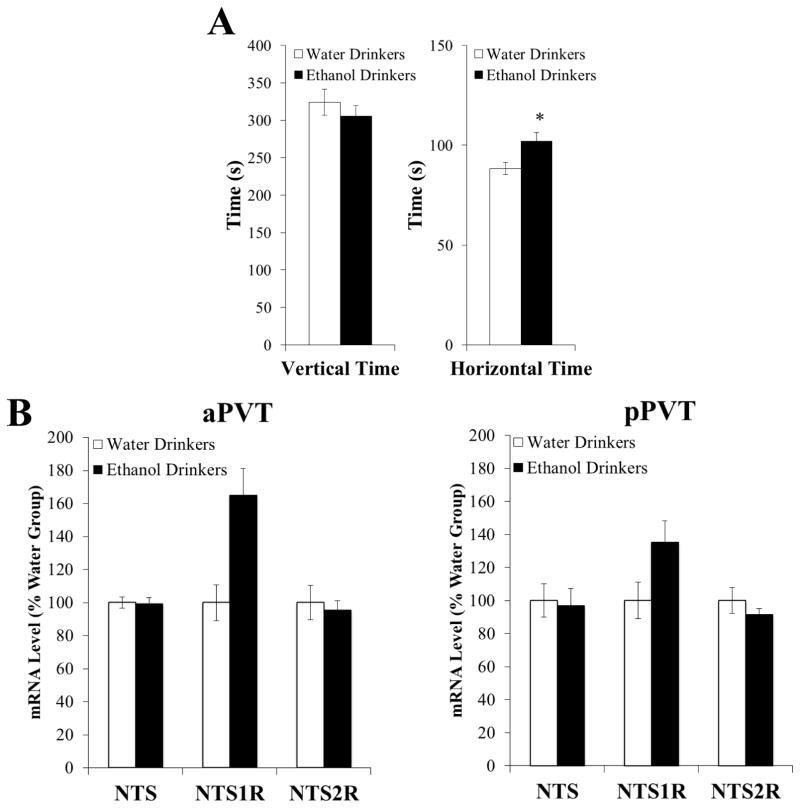
With levels of vertical time being higher and NTS in the pPVT being lower in prone rats before ethanol exposure, this experiment tested whether these measures are themselves altered by the consumption of ethanol. In rats trained to drink 20% ethanol in an intermittent-access paradigm compared to those maintained on water and chow only (n = 8/group), 30 minutes of ethanol drinking (0.9 ± 0.1 g/kg) at the start of daily access, when intake peaks (see Experiment 1), had no significant effect on time spent vertical in a novel activity chamber [t(14) = −0.84, NS], despite increasing time spent moving horizontally [t(14) = 2.52, P < 0.05] (Figure 4A). In an additional group of ethanol and water drinkers (n = 7 – 8/group), 30 minutes of ethanol drinking (1.4 ± 0.4 g/kg) also had no significant impact on mRNA levels of NTS in the pPVT [t(13) = 0.24, NS] or aPVT [t(13) = 0.20, NS], as well as on mRNA levels of NTS1R or NTS2R in the pPVT [NTS1R: t(13) = −1.63, NS; NTS2R: t(13) = 1.06, NS] or aPVT [NTS1R: t(13) = −2.13, NS; NTS2R: t(13) = 0.42, NS] (Figure 4B), although NTS1R levels in the aPVT of ethanol drinkers were 65% higher than those of water drinkers. These results indicate that rearing behavior and NTS expression in the PVT are stable traits in rats prone to drinking excess ethanol, even after they have consumed ethanol in sufficient amounts to affect another behavior, horizontal activity.
Figure 4.
Ethanol drinking does not significantly affect vertical time in a novel activity chamber or levels of neurotensin mRNA (Experiment 4). A Ethanol drinking does not alter time spent vertical in a novel activity chamber despite increasing time spent moving horizontally in the chamber (n = 8/group). B Ethanol drinking does not significantly alter gene expression of neurotensin (NTS), the neurotensin receptor type 1 (NTS1R), or neurotensin receptor type 2 (NTS2R) in the anterior paraventricular thalamus (aPVT) or posterior paraventricular thalamus (pPVT), as assessed by quantitative real-time polymerase chain reaction (n = 8/group). Target gene expression was quantified relative to cyclophilin using the relative quantification method. Data are mean ± S.E.M.; *p < 0.05 vs. water drinkers.
Experiment 5: Neurotensin injection in the PVT activates local neurons
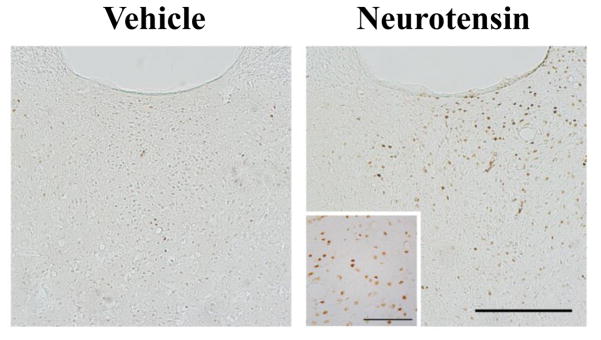
To investigate the effects of NTS in the PVT on the activity of local neurons, rats (N = 17) were injected with NTS (1.2 nmol) in the pPVT or aPVT and these subregions were subsequently examined for expression of c-Fos. Histological examination revealed that pPVT injections were made between bregma −2.92 mm and −3.60 mm, and aPVT injections were between bregma −1.44 mm and −1.92 mm (Figure 6A). Immunohistochemical analysis showed that injection of NTS in the pPVT (n = 5) compared to vehicle (n = 4) produced a significant increase in the number of cells expressing c-Fos in this PVT subregion [t(7) = −3.38, P < 0.05] (Figure 5) while having no effect in the adjacent subregions, the aPVT [t(7) = −0.16, NS] and mPVT [t(7) = −1.95, NS] (Table 3). In contrast, injection of NTS in the aPVT compared to vehicle (n = 4/group) lead to a trend for an increase in the number of Fos+ cells in the aPVT [t(6) = −1.96, P = 0.09] and mPVT [t(6) = −2.03, P = 0.08], and to a significant increase in the pPVT [t(6) = −3.48, P < 0.05] (Table 3). These results show that NTS activates neurons in the PVT, with effects from injections in the pPVT restricted only to this specific subregion.
Figure 6.
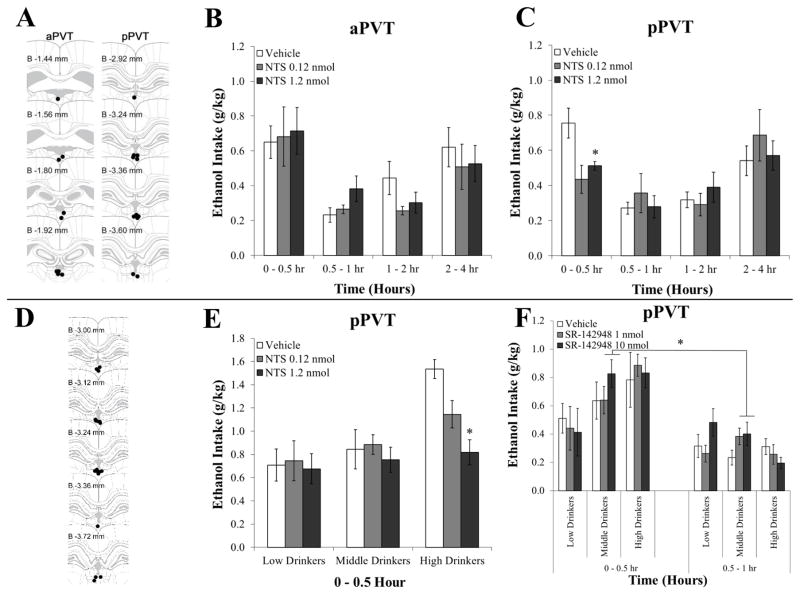
Injection of neurotensin (NTS) in the paraventricular thalamus reduces pharmacologically-relevant ethanol drinking in high drinking animals while an antagonist increases it in lower drinking animals (Experiment 6). A Injection sites (black dots) for animals included in the analysis for the first group, which received injections in the anterior paraventricular thalamus (aPVT, left panels) compared to the posterior paraventricular thalamus (pPVT, right panels) (Experiments 5 and 6). Each subregion served as an anatomical control for the other. B Injection in the aPVT with 0.3 μl NTS (0.12, 1.2 nmol) compared to vehicle did not significantly affect ethanol drinking (n = 9). C Injection in the pPVT with 0.3 μl NTS (0.12, 1.2 nmol) compared to vehicle significantly suppressed ethanol drinking during the first 30 minutes of daily access (n = 10). D Injection sites (black dots) for animals included in the analysis for the second group, which received injections in the pPVT (Experiment 6). E Injection in the pPVT with 0.3 μl NTS (0.12, 1.2 nmol) compared to vehicle significantly suppressed ethanol drinking during the first 30 minutes of daily access only in rats normally drinking high levels of ethanol (n = 6/group). F Injection in the pPVT with 0.3 μl of the nonselective NTS receptor antagonist SR-142948 (1, 10 nmol) compared to vehicle significantly stimulated ethanol drinking only in rats normally drinking moderate levels of ethanol (n = 6/group). Data are mean ± S.E.M.; *p < 0.05 vs. vehicle. Injection site diagrams adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. B = bregma.
Figure 5.
Representative photomicrographs of the posterior paraventricular thalamus, showing an increase in c-Fos+ neurons after local injection of neurotensin (1.2 per 0.3 μl of solution) compared to vehicle (0.3 μl) (Experiment 5). Scale bar in main image = 200 μm; scale bar in inset = 100 μm.
Table 3.
Average number of c-Fos+ neurons per brain slice after injection of neurotensin (1.2 per 0.3 μl of solution) or vehicle (0.3 μl) into the anterior paraventricular nucleus of thalamus (aPVT, n = 8) or posterior paraventricular nucleus of thalamus (pPVT, n = 9) (Experiment 5).
| Injection | aPVT | mPVT | pPVT |
|---|---|---|---|
| aPVT | |||
| Vehicle | 37 ± 10 | 34 ± 19 | 19 ± 11 |
| Neurotensin | 214 ± 90# | 177 ± 68# | 107 ± 23* |
| pPVT | |||
| Vehicle | 23 ± 4 | 10 ± 4 | 18 ± 12 |
| Neurotensin | 24 ± 6 | 36 ± 11 | 230 ± 55* |
Data are mean ± S.E.M.;
p < 0.05,
p = 0.08 or 0.09 vs. vehicle.
Experiment 6: Neurotensin injection in the pPVT reduces ethanol intake in high-drinking animals while an antagonist increases it in lower-drinking animals
To determine if higher levels of NTS in the PVT are protective against excessive intake, one group of rats (N = 20) was trained to drink 20% ethanol in an intermittent-access paradigm and administered NTS (0.12, 1.2 nmol) in the pPVT or aPVT. With injection directly into the pPVT (n = 10), there was a significant main effect of time on ethanol intake [F(3, 27) = 13.49, P < 0.001] and a strong trend for an interaction effect between time and drug dose [F(6, 54) = 2.23, P = 0.05]. Consistent with the peak intake during the first 30 minutes of access in Experiment 1, pairwise comparisons demonstrated that, overall, ethanol intake was significantly greater (P < 0.05) during the 0 – 30 minute period as well as the 2 – 4 hour period than it was during the intervening periods. Notably, for these pPVT injections, tests of the simple effects showed that the interaction between time and drug dose was due to a significant effect during the initial 30-minute period [F(2, 18) = 5.24, P < 0.05], when ethanol drinking after NTS injection compared to vehicle was significantly suppressed by the higher dose (1.2 nmol; P < 0.05) (Figure 6C). This effect in the pPVT was specific to ethanol, with analysis of the intake of simultaneously available food and water revealing no main effects of drug or interaction effects of drug with time for the intake of either food [F(2,18) = 0.59, NS; F(6,54) = 1.44, NS] or water [F(2,18) = 0.57, NS; F(6,54) = 0.52, NS] (data not shown). Thus, during the first 30 minutes of access, ethanol preference was also significantly suppressed by the higher dose of NTS [53 ± 6% vs. 37 ± 7%; t(9) = 2.91, P < 0.05]. In contrast to the pPVT, results from injections into the aPVT (n = 9) showed no significant main effects of NTS and also no interaction effects of drug with time on ethanol intake [F(2,16) = 0.42, NS; F(6,48) = 0.62, NS] (Figure 6B) or on food intake [F(2,16) = 0.57, NS; F(6,48) = 1.89, NS] or water intake [F(2,16) = 0.17, NS; F(6,48) = 2.18, NS] (data not shown). Therefore, the addition of exogenous NTS reduces ethanol intake, specifically in the pPVT, and specifically during the initial, 30-minute period of daily access when this drinking is normally highest.
To determine if the effects of NTS in the pPVT are dependent on level of drinking, that presumably reflects endogenous levels of NTS, a second group of ethanol-drinking rats (N = 18), which were classified by tertile split according to their average weekly drinking under the 20% ethanol intermittent-access paradigm, was administered NTS (0.12, 1.2 nmol) in the pPVT. Histological examination revealed that injections were made between bregma −3.00 mm and −3.72 mm (Figure 6D). Similar to the above findings, there was a significant main effect of drug [F(2, 30) = 5.40, P < 0.01] and of drinking group on ethanol intake [F(2, 15) = 4.38, P < 0.05] and a significant interaction effect between drug and group [F(4, 30) = 3.74, P < 0.05]. Pairwise comparisons confirmed that, in the overall group, ethanol drinking after NTS injection compared to vehicle was significantly suppressed by the higher dose (1.2 nmol; P < 0.01). Notably, tests of the simple effects showed that this effect of drug occurred only in animals normally drinking higher levels of ethanol [F(2, 10) = 10.77, P < 0.01], rather than those drinking moderate [F(2, 10) = 1.81, NS] or low levels [F(2, 10) = 0.06, NS] (n = 6/group) (Figure 6D). Moreover, this effect in the high drinking group showed an interaction with time [F(2, 10) = 7.22, P < 0.05], such that the suppression of ethanol drinking from NTS occurred during the initial 30-minute period [F(2, 10) = 9.35, P < 0.01], along with a suppression of ethanol preference [77 ± 3% vs. 67 ± 5%; t(9) = 2.66, P < 0.05]. Thus, NTS reduced the 30-minute intake of the high-drinking group to the same level as the low-drinking group after vehicle [t(10) = 0.63, NS]. As in the first group of rats, these effects were specific to ethanol, with analyses in both the overall group and the high drinkers revealing no main effects of drug on the intake of food [F(2,34) = 0.66, NS; F(2,10) = 1.17, NS] or water [F(2,34) = 0.47, NS; F(2,10) = 1.77, NS] (data not shown). Thus, the addition of NTS to the pPVT specifically reduces ethanol intake in rats drinking excessive levels of ethanol, which presumably have endogenously lower levels of this peptide.
To determine if the opposite effect, an increase in ethanol intake in lower-drinking animals, occurs after blocking NTS functioning in the pPVT, this second group (N = 18) was next administered the nonselective NTS receptor antagonist SR-142948 (1 and 10 nmol). This time, the drug response was found in the animals normally drinking moderate levels of ethanol. These moderate drinkers showed a significant main effect of drug on ethanol intake [F(2, 10) = 4.94, P < 0.05] but no interaction effect between drug and time [F(2, 10) = 0.93, NS] (Figure 6F). Pairwise comparisons demonstrated that ethanol drinking after SR-142948 injection compared to vehicle was significantly enhanced by the higher dose (10 nmol; P < 0.05). In contrast, there were no effects of this NTS antagonist on ethanol drinking in the animals normally drinking high levels of ethanol [F(2, 10) = 0.18, NS] or low levels [F(2, 10) = 1.90, NS] (Figure 6F). These effects were specific to ethanol, with analyses in both the overall group and moderate drinkers revealing no main effects of drug for the intake of food [F(2,34) = 0.55, NS; F(2,10) = 0.60, NS] or water [F(2,34) = 0.81, NS; F(2,10) = 1.77, NS] (data not shown). Thus, the blockade of endogenous NTS in the pPVT specifically enhances ethanol intake in rats drinking moderate levels of ethanol, which presumably have endogenously higher levels of this peptide.
Experiment 7: Neurotensin injection in the pPVT reduces rearing behavior in a novel activity chamber
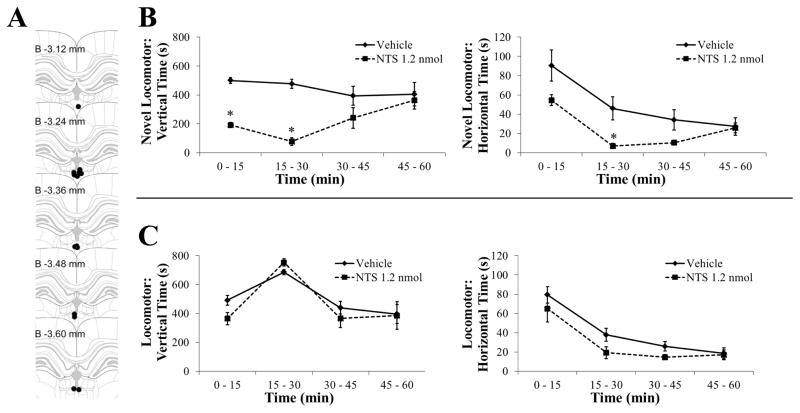
To establish if higher levels of NTS that suppress ethanol intake also reduce the behavior that predicts intake, ethanol-naïve rats (N = 14) were administered NTS (1.2 nmol) or vehicle in the pPVT and tested in an activity chamber in 60-minute sessions. Histological examination revealed that injections were made between bregma -3.12 mm and −3.60 mm (Figure 7A). In a novel chamber, in relation to vertical time, there was a significant main effect of drug [F(1, 11) = 13.68, P < 0.01, ηp2 = 0.55] and a significant interaction effect between drug and time [F(3, 33) = 9.67, P < 0.001]. This interaction effect was due to a significant suppression of rearing behavior in the animals receiving NTS, which occurred during both the first and second 15 minute periods of the test session (P < 0.001) (Figure 7B). In relation to horizontal time, there was only a trend for a main effect of drug [F(1, 11) = 3.63, P = 0.08, ηp2 = 0.25], although there was a significant interaction effect between drug and time [F(3, 33) = 10.55, P < 0.001]. This interaction effect was due to a significant suppression by NTS of horizontal behavior that occurred only during the second 15 minute period of the session (P < 0.05) (Figure 7B). In a familiar chamber, there were no main effects of drug for either vertical time [F(1, 12) = 2.39, NS] or horizontal time [F(1, 12) = 1.99, NS] (Figure 7C). Thus, with NTS in the pPVT having minimal effect on locomotor measures in a familiar environment, the primary locomotor effect of NTS is a suppression of rearing behavior in a novel chamber.
Figure 7.
Injection of neurotensin (NTS) in the posterior paraventricular thalamus reduces rearing behavior in a novel activity chamber (Experiment 7). A Injection sites (black dots) for animals included in the analysis. B In a novel activity chamber, injection in the posterior paraventricular thalamus with 0.3 μl NTS (1.2 nmol, n = 6) compared to vehicle (n = 7) reduced vertical time (left panel) more than it reduced horizontal time (right panel). C In a familiar activity chamber, injection in the posterior paraventricular thalamus with 0.3 μl NTS (1.2 nmol) compared to vehicle (n = 7/group) did not significantly affect vertical time (left panel) or horizontal time (right panel). Data are mean ± S.E.M.; *p < 0.05 vs. vehicle. Injection site diagram adapted from The Rat Brain, 5th edition, G. Paxinos and C. Watson, Copyright 2005, with permission from Elsevier. B = bregma.
DISCUSSION
This study shows for the first time that NTS controls ethanol drinking through actions in the brain. Building on reports with systemic injections and receptor knockouts suggesting that NTS suppresses ethanol consumption (Lee et al., 2010; Lee et al., 2011), the present results identify and characterize a particular brain site where this neuropeptide acts endogenously to affect this behavior. Making use of our new model for identifying with a measure of vertical time (rearing behavior) animals prone to excessive, pharmacologically-relevant drinking, we discovered that these individuals while ethanol naïve have significantly lower endogenous expression and levels of NTS specifically in the pPVT and that raising levels of this peptide in the pPVT not only reverses the predictor behavior but also significantly suppresses ethanol drinking, specifically in high-drinking rats. Moreover, inhibiting the endogenous activity of NTS in the pPVT instead stimulates excessive drinking in lower-drinking rats. These findings focus attention on NTS in the pPVT as a potential target for the treatment and prevention of alcohol use disorders and also on the novel idea that individuals prone to these disorders may be identified by the specific trait(s) represented by rearing behavior.
Our results identify for the first time a behavior that consistently predicts level of ethanol drinking under the 20% ethanol intermittent-access paradigm. They demonstrate that vertical time in a novel open field can identify ethanol-naïve rats that are likely to go on to drink excessive, pharmacologically-relevant levels of ethanol. While this behavior is not immediately analogous to a personality trait in humans, we hypothesize that it represents risk-taking behavior, which is a known predictor in humans of later heavy alcohol use (Goudriaan et al., 2011; MacPherson et al., 2010). Although previous studies with lower-level ethanol drinking have failed to show that the number of rears made by rats is a successful predictor (Bienkowski et al., 2001; Koros et al., 1998; Nadal et al., 2002), the present study using an intermittent-access paradigm that induces pharmacologically-relevant drinking demonstrates, in two separate groups of rats, the effectiveness of a related measure, vertical time in a novel open field. Interestingly, previous studies have shown that rats spending more time rearing in a novel environment have lower levels of serotonin in the medial prefrontal cortex (Antoniou et al., 2008), where blockade of serotonin function promotes ethanol drinking (Blakley et al., 2001). This region, particularly the infralimbic cortex which receives serotonergic input from the raphe nuclei, sends dense projections to the pPVT that are heavier than to the aPVT (Li and Kirouac, 2012) and it has also been implicated in risky decision-making (Zeeb et al., 2015). Thus, afferent projections to the pPVT from the infralimbic cortex may be one pathway at the intersection between rearing behavior and excessive ethanol drinking.
Using this measure of rearing behavior to identify rats prone to excessive drinking, we obtained results suggesting that lower levels of endogenous NTS in a specific subregion of the PVT are permissive of the overconsumption of ethanol. This idea agrees with a previous study of the prefrontal cortex, showing that ethanol-naïve rats selectively bred to be alcohol-preferring (6 g/kg/day) have lower NTS peptide levels than non-preferring rats (1 g/kg/day) (Ehlers et al., 1999). In the present study, prone rats showed lower gene expression of NTS specifically in the pPVT but not the nearby aPVT, and this phenomenon was confirmed by the finding of lower peptide levels of NTS in the pPVT. In the aPVT, prone rats instead showed a trend both for lower peptide levels of NTS and increased mRNA levels of NTS1R, which indicates lower extracellular levels of NTS (Azzi et al., 1996). Further tests are needed to determine the significance of these aPVT results. Together with our evidence that local injection of NTS into the pPVT suppresses vertical time in a novel but not familiar activity chamber, these results suggest that low levels of NTS may be permissive not only of excessive ethanol intake but also of high levels of novelty-induced rearing.
While endogenous NTS may influence both ethanol drinking and rearing behavior, this relationship does not appear to work in the opposite direction. In rats drinking ethanol in the intermittent-access paradigm at a level previously shown to enhance horizontal locomotor activity (Barson et al., 2015), we observed no significant change in either NTS expression or rearing. This lack of change from ethanol agrees with other evidence, showing that chronic ethanol drinking has no impact on NTS levels in other limbic regions, including the nucleus accumbens and frontal cortex (Tajuddin and Druse, 1998). These findings suggest that the lower NTS levels in the pPVT and higher vertical time found in prone rats are stable traits that persist even after they consume excessive levels of ethanol.
With local PVT injections, we discovered that NTS activates neurons in this nucleus and that this effect remains relatively restricted to the site of injection. Previous studies using slice electrophysiology have shown that NTS application has mixed effects, stimulating neurons in some limbic nuclei such as the medial prefrontal cortex and hypothalamus, while having no effect or inhibiting neuronal activity in others like the nucleus accumbens and locus coeruleus (Stowe and Nemeroff, 1991). In the present study, NTS injected into the pPVT increased the number of Fos+ cells only in this subregion, while having no effect in the aPVT or even the adjacent mPVT. The site-specific nature of PVT subregion effects has been demonstrated in other studies, showing that neuropeptides injected into the pPVT induce behaviors which differ significantly from those in the aPVT or adjacent dorsal third ventricle (Barson et al., 2015; Barson et al., 2017). While NTS injection into the aPVT also increased the local number of Fos+ cells, it additionally increased Fos activation in the pPVT, although at half the level of the aPVT. This result suggesting that the aPVT can stimulate the pPVT may reflect a specific characteristic of the aPVT, which has efferent fibers that travel to the pPVT while those of the pPVT do not travel in the reverse direction (Vertes and Hoover, 2008). Thus, with injection of methylene blue dye into the aPVT found not to reach the pPVT (Barson et al., 2015; Barson et al., 2017), it appears that the primary effects of NTS occur locally within the injected PVT subregion and involve the activation of local neurons that then project to other brain areas.
Our evidence that excessive ethanol drinking is curbed by injection of NTS into the pPVT but not the nearby aPVT strengthens the idea that NTS specifically in the pPVT inhibits ethanol drinking and that this subregion differs markedly from the aPVT. Importantly, we found that this added NTS is effective specifically in high-drinking individuals, which presumably have endogenously lower NTS levels in the pPVT than low-drinking individuals, and that blockade with the NTS antagonist SR-142948 elevates ethanol intake specifically in those drinking lower levels of ethanol that presumably have higher NTS levels. The effectiveness of this antagonist only in the moderate-drinking group rather than low-drinking group suggests that other mechanisms may be involved in these lower drinkers. The identification of the pPVT as a primary site of action for the inhibition of drinking is consistent with our recent reports showing that neuroexcitatory neuropeptides injected into this subregion fail to enhance ethanol drinking despite having a strong stimulatory effect in the aPVT (Barson et al., 2015; Barson et al., 2017). Given that the pPVT sends dense projections to the amygdala and bed nucleus of the stria terminalis (Li and Kirouac, 2008), extracellular NTS in this subregion likely produces its behavioral effects through activation of local neurons that project to these emotion-related brain regions. We find that the effects of this peptide in the pPVT occur rapidly, within the first 30 minutes after injection, coinciding with the period of peak ethanol drinking. This is consistent with another study in ethanol-naïve rats, showing that intracerebroventricular NTS reduces food intake only during the first 60 minutes after injection (Cooke et al., 2009), and with our other behavioral results showing NTS in the pPVT to suppress novelty-induced locomotor behaviors only during the first 30 minutes post-injection. While further studies of the pPVT are needed to determine whether endogenous NTS acts pre- or post-synaptically (Boudin et al., 1996) and originates from either intra-PVT retrograde release (Arluison et al., 1994) or afferent innervation (Watts et al., 1987), our findings here suggest that endogenous, extracellular NTS inhibits ethanol drinking via actions specifically in the pPVT.
Together, our results identify for the first time a specific behavior that predicts excessive, pharmacologically-relevant drinking under the 20% ethanol intermittent-access paradigm and a specific brain region where NTS acts both to affect this behavior and to curb this high level of drinking. Adding to clinical evidence linking NTS1R gene polymorphisms to alcohol dependence (Ma et al., 2013), our findings showing site-specific disturbances in endogenous NTS in relation to excessive ethanol drinking and a means of predicting who will engage in this behavior, may encourage further studies of this novel neurochemical target to develop pharmacotherapeutic interventions for implementation through precision medicine.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism under Award Numbers K99/R00AA021782 (J.R.B.) and R01AA12882 and R01AA24798 (S.F.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Authors Contribution
S.P, S.F.L., and J.R.B were responsible for the study concepts and design. S.P., P.B., G.R.C, and J.R.B. collected and analyzed the data. S.P. and J.R.B. drafted the manuscript and prepared the figures. S.F.L. and J.R.B edited and revised the manuscript. All authors critically reviewed the content and approved the final version for publication.
References
- Antoniou K, Papathanasiou G, Papalexi E, Hyphantis T, Nomikos GG, Spyraki C, Papadopoulou-Daifoti Z. Individual responses to novelty are associated with differences in behavioral and neurochemical profiles. Behav Brain Res. 2008;187:462–472. doi: 10.1016/j.bbr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Arluison M, Brochier G, Vankova M, Leviel V, Villalobos J, Tramu G. Demonstration of peptidergic afferents to the bed nucleus of the stria terminalis using local injections of colchicine. A combined immunohistochemical and retrograde tracing study. Brain Res Bull. 1994;34:319–337. doi: 10.1016/0361-9230(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Azzi M, Boudin H, Mahmudi N, Pelaprat D, Rostene W, Berod A. In vivo regulation of neurotensin receptors following long-term pharmacological blockade with a specific receptor antagonist. Brain Res Mol Brain Res. 1996;42:213–221. doi: 10.1016/s0169-328x(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF. Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol Clin Exp Res. 2013;37(Suppl 1):E141–151. doi: 10.1111/j.1530-0277.2012.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Poon K, Ho HT, Alam MI, Sanzalone L, Leibowitz SF. Substance P in the anterior thalamic paraventricular nucleus: promotion of ethanol drinking in response to orexin from the hypothalamus. Addict Biol. 2017;22:58–69. doi: 10.1111/adb.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36:525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Kostowski W. Individual behavioral differences and ethanol consumption in Wistar rats. Physiol Behav. 1993;54:1125–1131. doi: 10.1016/0031-9384(93)90336-e. [DOI] [PubMed] [Google Scholar]
- Blakley GG, Pohorecky LA, Benjamin D. Bidirectional changes in ethanol consumption in rats with site-specific antisense down-regulation of 5-hydroxytryptamine2A receptors in brain. J Pharmacol Exp Ther. 2001;299:277–289. [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. HHS Publication No SMA 15-4927, NSDUH Series H-50. 2015. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. [Google Scholar]
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 2009;17:1135–1143. doi: 10.1038/oby.2008.652. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Strong-Kaufman MN, Tanchuck MA, Hashimoto JG, Wiren KM, Finn DA. The Effect of mGluR5 Antagonism During Binge Drinkingon Subsequent Ethanol Intake in C57BL/6J Mice: Sex- and Age-Induced Differences. Alcohol Clin Exp Res. 2014;38:730–738. doi: 10.1111/acer.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Frassoni C, Spreafico R, Bentivoglio M. Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res. 1997;115:95–104. doi: 10.1007/pl00005689. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and response inhibition as predictors of heavy alcohol use: a prospective study. Alcohol Clin Exp Res. 2011;35:1050–1057. doi: 10.1111/j.1530-0277.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koros E, Piasecki J, Kostowski W, Bienkowski P. Saccharin drinking rather than open field behaviour predicts initial ethanol acceptance in Wistar rats. Alcohol Alcohol. 1998;33:131–140. doi: 10.1093/oxfordjournals.alcalc.a008369. [DOI] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi DS. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol Biochem Behav. 2010;95:235–241. doi: 10.1016/j.pbb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Unal SS, Richelson E, Choi DS. Increased ethanol consumption and preference in mice lacking neurotensin receptor type 2. Alcohol Clin Exp Res. 2011;35:99–107. doi: 10.1111/j.1530-0277.2010.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217:257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212:251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Ma H, Huang Y, Zhang B, Wang Y, Zhao H, Du H, Cong Z, Li J, Zhu G. Association between neurotensin receptor 1 gene polymorphisms and alcohol dependence in a male Han Chinese population. J Mol Neurosci. 2013;51:408–415. doi: 10.1007/s12031-013-0041-5. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcohol Clin Exp Res. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo L, Gomez MJ, Callejas-Aguilera JE, Donaire R, Sabariego M, Fernandez-Teruel A, Canete A, Blazquez G, Papini MR, Torres C. Relationship between ethanol preference and sensation/novelty seeking. Physiol Behav. 2014;133:53–60. doi: 10.1016/j.physbeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Liang T, Edenberg HJ, Lumeng L, Bell RL. Gene expression within the extended amygdala of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Alcohol. 2013;47:517–529. doi: 10.1016/j.alcohol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsimilli L, Farley S, El Khoury MA, Chamot C, Sibarita JB, Racine V, El Mestikawy S, Mathieu F, Dumas S, Giros B, Tzavara ET. Antipsychotics increase vesicular glutamate transporter 2 (VGLUT2) expression in thalamolimbic pathways. Neuropharmacology. 2008;54:497–508. doi: 10.1016/j.neuropharm.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct. 2014;219:1287–1303. doi: 10.1007/s00429-013-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519:455–459. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Juarez A, Escobar C, Aguilar-Roblero R. Anterior paraventricular thalamus modulates light-induced phase shifts in circadian rhythmicity in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R897–904. doi: 10.1152/ajpregu.00259.2002. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol. 2003;461:520–538. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Nemeroff CB. The electrophysiological actions of neurotensin in the central nervous system. Life Sci. 1991;49:987–1002. doi: 10.1016/0024-3205(91)90300-z. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. Effects of chronic ethanol consumption and aging on proenkephalin and neurotensin. Alcohol Clin Exp Res. 1998;22:1152–1160. [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Fact Sheet: Alcohol. 2015. [Google Scholar]
- Zeeb FD, Baarendse PJ, Vanderschuren LJ, Winstanley CA. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology (Berl) 2015;232:4481–4491. doi: 10.1007/s00213-015-4075-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.