Abstract
Although non-steroidal anti-inflammatory drugs can alleviate menstrual pain, about 18% of women with dysmenorrhea are unresponsive, leaving them and their physicians to pursue less well-studied strategies. The goal of this review is to provide a background for treating menstrual pain when first-line options fail. Research on menstrual pain and failure of similar drugs in the antiplatelet category has suggested potential mechanisms underlying non-steroidal anti-inflammatory drug resistance. Based on these mechanisms, alternative options may be helpful for refractory cases. This review also identifies key pathways in need of further study to optimize menstrual pain treatment.
Keywords: Adenomyosis, Endometriosis, Menstrual Pain, Non-steroidal Anti-inflammatory Drugs, Oral Contraception, Primary Dysmenorrhea, Secondary Dysmenorrhea
Introduction
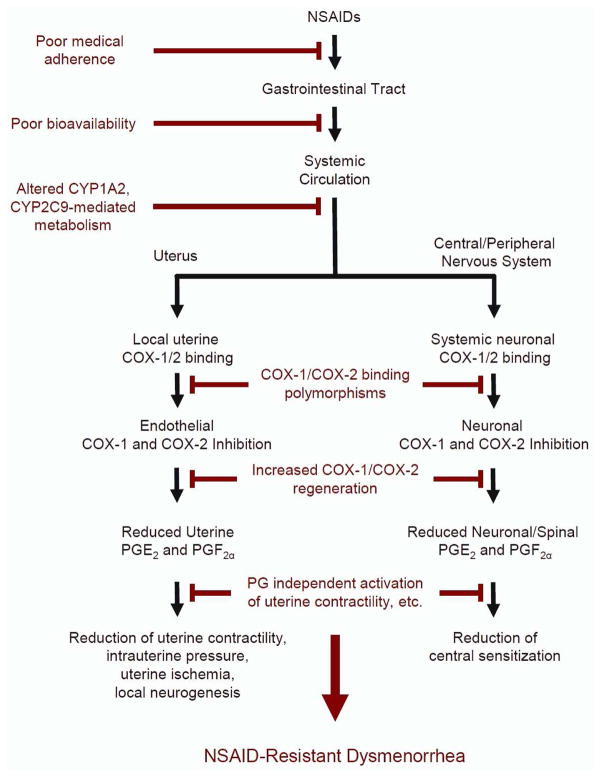
The scope of the clinical problem of menstrual pain was effectively communicated by former First Lady Michelle Obama, when she tweeted, “Why are girls still missing so many days of school because of their menstrual cycles?”1 Too many women hide this personal stigma, and experience a physical and psychological burden of frequent, severely painful cramps occurring over several days every month, persisting for decades. The transcultural impact of this problem was highlighted when Chinese Olympic medalist Fu Yuanhui acknowledged that menstrual pain affected her Olympic swimming performance.2 The etiology of menstrual pain remains inadequately characterized,3 and this limited scientific understanding hinders adequate treatment for women who are unresponsive to first-line options including non-steroidal anti-inflammatory drug (NSAID) therapy. To optimize the management of menstrual pain, further studies of its pathophysiology are needed. This review summarizes current scientific knowledge and associated critical gaps in menstrual pain unresponsive to NSAIDs (Figure 1).
Figure 1.
A proposed pathway examining NSAID-resistant dysmenorrhea. Many complex mechanisms contribute to the development of NSAID-resistant dysmenorrhea. NSAIDs normally reduce menstrual pain via the suppression of peripheral and systemic prostaglandins and corresponding downstream effects (shown in black). Elements on the left branch highlight uterine mechanisms while the right branch highlights central and peripheral neural mechanisms. Various physiological factors, ranging from poor medical adherence to the involvement prostaglandin-independent cascades, may disrupt NSAIDs’ efficacy to ameliorate menstrual pain and promote NSAID resistance (shown in red).
Epidemiology of NSAID-resistant dysmenorrhea
Menstrual pain, also known as dysmenorrhea, is common and affects nearly half of reproductive age women.4–6 Before the advent of NSAID therapy, it was observed that 10% of high school girls in Los Angeles missed classes because of dysmenorrhea.7 The development of NSAIDs in 1969 heralded a new era of pain management, and over-the-counter availability of this medication class in 1983 held the promise of resolving dysmenorrhea for many women. Indeed, for most women, NSAIDs are effective for treating dysmenorrhea as demonstrated by a meta-analysis of 35 randomized controlled trials.8 However, dysmenorrhea still causes 10–20% of U.S. female high school students to miss class during their menses.9,10 This phenomenon is also seen internationally,11 with menstrual pain-induced absenteeism occurring at similar or greater rates.12–14 Further, a review of 51 different clinical trials found that 18% of women report minimal or no relief of menstrual pain with NSAIDs.15 This failure to relieve pain suggests multiple pathological mechanisms may contribute to treatment unresponsiveness. Clarifying these mechanisms is an obvious critical need in gynecological research.
What causes menstrual pain?
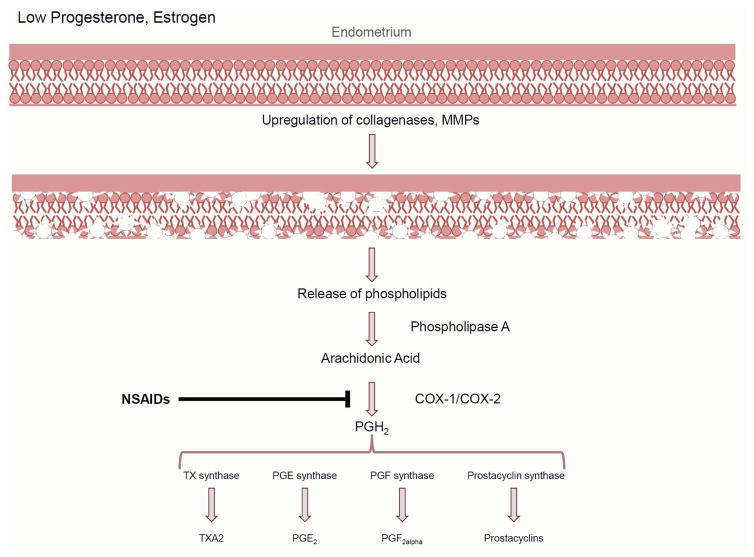
Preclinical research studies suggest prostaglandin-dependent mechanisms drive dysmenorrhea in a majority of women (reviewed by Maia et al. 2005).16 The start of menstruation is marked by the simultaneous decrease in circulating progesterone and estradiol, initiating increased transcription of endometrial collagenases, matrix metalloproteinases (MMPs), and inflammatory cytokines (Figure 2). Upregulated MMPs specifically target and break down endometrial tissue, freeing phospholipids from the cellular membrane. Uterine phospholipases convert available phospholipids to arachidonic acid, which is then synthesized into prostaglandins (PG), prostacyclins, and thromboxane-2a via cyclooxygenase (COX)-1 and -2. Notably, COX-2 expression is highest during menses.16 Although it is unclear whether increased COX-2 expression occurs in dysmenorrhea, the end products PGE2 and PGF2α are elevated in the menstrual effluent in dysmenorrheic women when compared to healthy controls.17,18
Figure 2.
The production of prostaglandins via the onset of menstruation. Decreased progesterone and estrogen levels at the end of the luteal phase initiate a cascade that results in the breakdown of the endometrial tissues, the release of cellular phospholipids, and the subsequent production of prostaglandins. COX: cyclooxygenase, MMP: matrix metalloproteinases, NSAIDs: non-steroidal anti-inflammatory drugs, PG: prostaglandin, TX: thromboxane.
The identification of elevated PGE2 and PGF2α in dysmenorrhea has supported the strategy of inhibiting COX-2 with NSAIDs to treat menstrual pain. Non-specific NSAIDs (e.g. those listed in Table 1) bind to both COX-1 and COX-2 to inhibit prostaglandin synthesis. More selective NSAIDs known as COX-2 inhibitors alleviate menstrual pain by specifically inhibiting COX-2 activity. Unlike COX-1, which is constitutively expressed, COX-2 is upregulated by stimuli associated with inflammation19 and during progesterone withdrawal,20,21 thus making COX-2 inhibitors an appropriate alternative to non-specific NSAIDs.
Table 1.
Commonly used NSAIDs and concentrations that inhibit COX activity in blood.
| NSAID | COX-1 IC50 (μM) | COX-2 IC50 (μM) | COX-1: COX-2 IC50 Ratio* |
|---|---|---|---|
| Diclofenac | 0.26 | 0.01 | 0.05 |
| Aspirin | 4.45 | 13.88 | 3.12 |
| Ketorolac | 0.27 | 0.18 | 0.68 |
| Naproxen | 32.01 | 28.19 | 0.88 |
| Ibuprofen | 5.90 | 9.90 | 1.69 |
Ratios greater than 1 indicate drug is more selective for COX-1. Ratios lesser than 1 indicate drug is more selective for COX-2.
Although it is possible that prostaglandins could excite nociceptors and cause pain, it is believed that prostaglandins indirectly cause cramping pain by stimulating uterine contractility.22 Preclinically, we have recently confirmed that PGF2α administration increases uterine contractility and elicits visceral pain.23 Conversely, drugs that inhibit prostaglandin synthesis, such as ibuprofen24 and naproxen, 25 reduce uterine contractility in dysmenorrheic women. These findings suggest that prostaglandins increase uterine contractility and produce cramping pain via temporary elevations in uterine pressure.22 Since not all women with dysmenorrhea have alterations in uterine pressure,26 other mechanisms might contribute to menstrual pain. For example, impaired uterine perfusion has been observed in dysmenorrhea27; ischemia may also cause cramping pain. In our mouse model of dysmenorrhea, impaired uterine perfusion and hypoxemia also occurred.23 Although these studies collectively suggest physiological mechanisms underlying dysmenorrhea, they fail to clarify why some women do not respond to NSAIDs.
Anatomical factors
A subset of women with dysmenorrhea, particularly those with delayed presentation after menarche, may harbor separate contributing anatomical factors such as endometriosis, leiomyoma, or adenomyosis; these cases are examples of ‘secondary dysmenorrhea’ that could underlie NSAID resistance. Undoubtedly, surgical interventions for these structural issues address dysmenorrhea. For example, in a meta-analysis, laparoscopic excision of endometriosis was shown to reduce menstrual pain.28 The molecular contributions of anatomical factors to secondary dysmenorrhea are limited. Immunohistological studies investigating endometriosis demonstrated that lesions have increased COX-2 expression29, which led to corresponding increased prostaglandins30 and aromatase activity.31 Ectopic endometrium from adenomyosis patients expressed increased levels of transient receptor potential vanilloid 1 (TRPV1 – a pain signaling protein) and oxytocin receptor.32 Gene expression of myometrial regulators myostatin and MMP14 from leiomyoma biopsies were positively correlated to severe dysmenorrhea.33 These in vitro studies provide insight into mechanisms that promote secondary dysmenorrhea, but more research is needed to unmask the complex pathophysiology associated with these anatomical factors.
The causal contribution of anatomical factors to dysmenorrhea, particularly those that exhibit NSAID unresponsiveness, is unclear. A meta-analysis has estimated as many as 29% of dysmenorrheic women may have moderate to severe endometriosis.34 However, since many women do not undergo laparoscopic evaluation, it is difficult to identify the proportion of women with NSAID-resistant dysmenorrhea who have endometriosis. A small clinical study found that among 31 women with NSAID-resistant dysmenorrhea, 35% had endometriosis.35 In a larger study (n=654), 25% of participants with NSAID-resistant dysmenorrhea had ultrasound or magnetic resonance imaging suggestive of endometriosis.36 Conversely, it is important to note that dysmenorrhea symptoms are nonspecific for endometriosis,37 and NSAIDs can be effective in relieving some cases of menstrual pain in women with endometriosis.38,39 In one observational study of leiomyomas, 70% of women with fibroids used NSAIDs and 51% reported a reduction in symptoms.40 It is uncertain whether NSAIDs are useful for adenomyosis.41 Since it is unknown whether anatomical factors contribute to NSAID unresponsiveness, further research is needed to determine whether treatment strategies targeting anatomical factors are sufficient for addressing the causes of NSAID-resistant dysmenorrhea.
Molecular mechanisms
Therapeutic alternatives for NSAID-resistant dysmenorrhea will be developed quicker once mechanistic characterization progresses. NSAIDs collectively elicit non-specific inhibition of COX isoforms (Table 1). COX-1 and COX-2 are homologous, share 63% identical amino acid sequences and have a similar catalytic binding site.19 Although NSAIDs bind non-selectively to both COX isoforms, they vary in isoform-specific inhibition. As seen in Table 1, NSAIDs such as aspirin and ibuprofen are more selective for COX-1, while diclofenac preferentially targets COX-2.42 Genetic polymorphisms have been shown to disrupt COX-1 inhibition with aspirin. For example, Ulehlova et al demonstrated that COX-1 polymorphism rs10306114 was correlated with high platelet aggregation in aspirin-resistant individuals.43 Although multiple single nucleotide polymorphisms (SNPs) that contribute to aspirin resistance have been identified, they have only been replicated in some studies and remain an active area of research (reviewed by Weng and colleagues).44 Although there are no documented COX polymorphisms directly associated with NSAID binding, there are several COX SNPs within the promoter regions that may alter NSAID efficacy.45 Notably, rs20417 is a SNP in the promoter region of COX-2 associated with aspirin resistance.41 Further research is needed to determine if the identified SNP have a transcriptional effect contributing to NSAID-resistant dysmenorrhea.
Another molecular factor that contributes to treatment resistance is drug bioavailability. The drug formulation alongside an individual’s metabolic profile may alter the efficacy of both antiplatelet and NSAID therapy. One study found a significant relationship between total naproxen serum levels and a reduction in rheumatoid arthritis symptoms46; the range of oral dosages used (250mg, 500mg, 1500mg), however, makes it difficult to determine whether variable absorption significantly contributed to inadequate pain relief. Other mechanisms affecting NSAID metabolism could also greatly impact COX inhibition. Cytochrome P450 (CYP) enzymes, specifically CYP1A2, CYP2C8, and CYP2C9, are responsible for metabolizing NSAIDs. CYP “gain of function” variants are associated with increased metabolism, resulting in decreased drug effect.47 For example, the CYP2C9*2/*2 polymorphism was associated with increased total clearance of celecoxib and diclofenac.48 More research is necessary to determine if other gain-of-function variants exist and alter NSAID metabolism.
Other molecular contributors to NSAID-resistant dysmenorrhea
In addition to COX and prostaglandin-mediated pathways, other molecular mechanisms could drive NSAID-resistant dysmenorrhea. Leukotrienes, a class of eicosanoids synthesized via 5-lipoxygenase, should be considered candidate mediators,49 as their increased expression is found in the endometrium,50 urine,51 and menstrual effluent52 of women with dysmenorrhea. However, leukotriene receptor inhibition did not successfully alleviate menstrual pain.53,54 Another potential COX-independent mechanism is the platelet activating factor (PAF) pathway. PAF mediates inflammatory states unaffected by NSAIDs and is elevated in the menstrual effluent of women with NSAID-resistant dysmenorrhea.52 Alterations in PAF synthesis have been found in women with endometriosis.55,56 In a mouse model, we have recently confirmed a PAF receptor agonist is capable of increasing uterine hypercontractility and impairing perfusion, causing uterine hypoxemia and pain.23 The effects on uterine physiology were blocked with a PAF receptor antagonist in our mouse model, but PAF-targeting treatments have not yet been conducted in women with dysmenorrhea. Additional research is needed to elucidate the possible roles of leukotrienes and PAF in NSAID-resistant dysmenorrhea.
Peripheral and central sensitization within dysmenorrhea
The aforementioned molecules are readily implicated in mechanisms that would increase peripheral nerve sensitivity. Prostaglandins can sensitize primary afferents57 via the modulation of tetrodotoxin-resistant sodium channels58 and TRPV1 receptors.59 Local neurogenesis is another element of peripheral sensitization, and has been demonstrated to contribute to secondary dysmenorrhea.60–62,32 However, the role of local neurogenesis in NSAID-resistant dysmenorrhea has not yet been demonstrated.
Alternatively, wide-spread increases in pain sensitivity known as central sensitization could contribute dysmenorrhea.63 Although it has not been demonstrated directly, evidence of central sensitization within dysmenorrhea includes increased referred pain,64 and heightened experimentally evoked thermal, ischemic, muscular, and pressure pain sensitivity.65–68 Dysmenorrheic women also exhibit altered grey matter volume in key cortical regulatory pain regions.69–71 Since NSAIDs are not known to affect central sensitization,72 further research is needed to confirm whether dysfunctional central sensitization occurs in NSAID-resistant dysmenorrhea.
Mechanisms driving peripheral or central sensitization could also lead to increased referred pain. In rat models, uterine inflammation led to neurogenic plasma extravasation the abdominal musculature and adjacent organs.73,74 Although some women with dysmenorrhea may also have superficial abdominal muscular pain, it is not predictive of endometriosis.75 Thus, it remains unclear whether women with abdominal muscle cramps during menses are more or less likely to respond to NSAIDs.
The importance of medical adherence
Medication adherence likely contributes to NSAID-resistant dysmenorrhea. A quarter to half of dysmenorrheic women do not take the correct medication or dosage.10,12 Side effects associated with NSAIDs such as gastrointestinal discomfort also limit medication adherence.8 Along with medication type, dosage, and side effects, the timing of NSAID administration may affect efficacy. Notably, biochemical analyses demonstrated that naproxen administration prior to initiating the COX-2 cascade results in nearly complete suppression of prostaglandin synthesis; attempting to block synthesis afterwards only produced a gradual and incomplete suppression.76 However, a single, but underpowered trial, comparing menstrual pain relief between prophylactic versus abortive treatment with ibuprofen did not find a difference.77 It is possible that differences in prophylactic use of naproxen and ibuprofen could be due to different preferential binding to COX-1 and COX-2 (Table 1). Aside from this trial, clinical investigators have not sufficiently investigated prophylactic NSAIDs use prior to the onset of menses. Although an educational trial regarding prophylaxis did demonstrate increased patient knowledge, reduction of menstrual pain was not evaluated.78
Treatments for NSAID-resistant dysmenorrhea
Until it can be determined why some women with dysmenorrhea are unresponsive to NSAIDs, it is essential that clinicians be aware of adequate alternative treatments. Below, we present a list of candidate pharmacological and non-pharmacological treatments previously investigated for use in dysmenorrhea. We have noted where generic medications are available, but insurance coverage for off-label use needs to be considered in terms of patient costs.
Hormone-based Treatments
Hormonal treatments, specifically oral contractive pills (OCPs), are widely used for NSAID-resistant dysmenorrhea.22,79,80 OCPs thin the endometrial lining, resulting in reduced COX-2 and prostaglandin production.16,81 The bulk of research examining OCPs and dysmenorrhea focuses on the effect of different hormonal regimens and combinations. A systematic review suggested continuous regimens are generally more effective at reducing dysmenorrhea symptoms than cyclic regimens.82 Cyclic regimens often improve dysmenorrhea, but studies have rarely found differences between different hormone combinations.83 Nomegestrol acetate/17β-estradiol was more effective in treating menstrual pain when compared to drospirenone/ethinylestradiol oral contraceptive.84 A comparison of 20μg ethinyl estradiol/150μg desogestrel to 20μg ethinyl estradiol/100μg levonorgestrel suggested each improved dysmenorrhea similarly (23 and 26% of women respectively).85 Combination OCPs with estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel both reduced experienced time of dysmenorrhea pain by four days, but significant differences between the regimens were not observed.86 A systematic review concluded that levonorgestrel-releasing intrauterine devices are as effective as OCPs at alleviating menstrual pain.87 A critical limitation of the above studies of comparing hormonal regimens and combinations in primary dysmenorrhea is that they have not specifically evaluated their utility in NSAID-resistant dysmenorrhea.
Hormonal treatments are also used for women with secondary dysmenorrhea unresponsive to NSAIDs and do not wish to undergo surgery. A randomized placebo-controlled trial demonstrated that OCPs were an effective treatment for secondary dysmenorrhea associated with endometrosis.88 Continuous OCP regimens improve dysmenorrhea better than cyclical regimens after surgery for endometriosis89, although there are concerns that the estradiol component of OCPs could exacerbate endometriosis.90 In any case, hormonal suppression is still recommended for treatment of dysmenorrhea in current consensus guidelines.91
Other studies on secondary dysmenorrhea treatment have focused on gonadotropin-releasing hormone (GnRH) agonists. A randomized placebo-controlled trial showed GnRH agonist leuprolide almost completely eliminated menstrual pain in 44 patients with suspected endometriosis.92 Although effective in treating secondary dysmenorrhea, GnRH agonist-induced reduction of estrogen promotes bone density loss over time.93,94 Pairing GnRH agonists with ‘add-back’ or replacement estrogen therapy95–97 or utilizing low GnRH agonist dosages98 are capable of alleviating menstrual pain associated with endometriosis without bone loss. The utilization of these drugs is recommended by the American Society for Reproductive Medicine guidelines only after laparoscopic diagnosis of endometriosis given these risks.99 Alongside its side-effect profile, patients may find monthly injections of GnRH agonists inconvenient.
A recent review has suggested that oral progestins may be a better first-line option for menstrual and pelvic pain associated with endometriosis.90 Oral progestins such as norethindrone acetate and dienogest, target the progesterone receptor, and have regulatory approval for endometriosis. A randomized placebo-controlled trial demonstrated that dienogest reduced dysmenorrhea in women with endometriosis.100 Dienogest was also as effective in reducing menstrual pain when compared to the GnRH agonist leuprolide.101 An open-label study found norethindrone acetate was as effective at reducing menstrual pain as OCPs.102 Despite their efficacy, it is important to consider the frequent irregular bleeding associated with oral progestins.103 Although a meta-analysis supports oral progestin usage for endometriosis,104 it remains to be investigated whether it is an effective empirical option for NSAID-resistant dysmenorrhea.
Another class of hormonal treatment used for secondary dysmenorrhea is aromatase inhibitors.105 Aromatase is an enzyme that is expressed in the ovarian follicle and endometriotic stromal cells and converts androgens to estrogen.106 Aromatase inhibitors, primarily used to reduce endometriomas107 and myomas108 in women, may be beneficial for secondary dysmenorrhea by rendering patients amenorrheic. Due to concern regarding its effects on bone mineral density and other adverse side effects, add-back regimens may be necessary.109 Further research is needed to determine if aromatase inhibitors are appropriate of NSAID-resistant dysmenorrhea.
Surgical Interventions
Although excision of endometriotic lesions are routinely recommended,99 some symptomatic patients that do not have identified anatomical factors following diagnostic surgical evaluation may benefit from alternative surgical strategies. Laparoscopic uterine nerve ablation (LUNA) and laparoscopic presacral neurectomy (PSN) are two surgical interventions historically employed for the treatment of secondary dysmenorrhea (reviewed by Proctor, Lathe and colleagues).110,111 However, a large multi-site randomized controlled trial conducted by Daniels and colleagues determined that LUNA for chronic pelvic pain did not have a significant effect on dysmenorrhea, regardless of time accrued following surgery,112 and has led to this procedure largely being abandoned. However, this trial and many of the other negative trials did not study the effects of LUNA or PSN in NSAID-resistant dysmenorrhea in women without chronic pelvic pain and endometriosis.
Clinical trials have examined the efficacy of surgical interventions on primary dysmenorrhea. A double-blinded randomized controlled trial of LUNA demonstrated menstrual pain relief in half of women with primary dysmenorrhea.113 A trial comparing LUNA and LUNA plus PSN reported 69% and 73% of primary dysmenorrhea patients respectively had improvements in menstrual pain.114 Chen et al found that 77% of primary dysmenorrhea patients benefited from PSN.115 Although it is unknown whether these patients with primary dysmenorrhea were NSAID-resistant, it is quite possible that surgery was performed since NSAID management was not feasible. Thus, further research is needed to clarify the utility of LUNA and PSN as treatments for NSAID-resistant dysmenorrhea, particularly in the absence of endometriosis and chronic pelvic pain.
Vasodilators
Another potential treatment for dysmenorrhea is sildenafil citrate. Sildenafil specifically blocks cyclic guanosine monophosphate degradation, thus promoting smooth muscle relaxation in the uterus and surrounding blood vessels.116 In a randomized placebo-controlled trial, sildenafil reduced menstrual pain in women with primary dysmenorrhea.117 Similar to sildenafil, nitric oxide (NO) donor drugs also promote vasodilation and myometrial muscle relaxation, and are capable of reducing menstrual pain. Transdermal nitroglycerin or glyceryl trinitrate administration on the first day of menstruation was sufficient to reduce reported menstrual pain for the duration of menses.118,119 Glyceryl trinitrate and nitroglycerin are available as generic medications. A limiting factor of glyceryl trinitrate and similar vasodilators are their side effects that impair tolerability including headaches.120 Therefore, the utility of glyceryl trinitrate or other vasodilators for NSAID-resistant dysmenorrhea remains to be determined.
Calcium Channel Blockers
Calcium channel blockers, available as generic medications, are primarily indicated to treat hypertension by reducing contractility in vascular smooth muscle and cardiac muscles; they also inhibit uterine contractions in pregnant and non-pregnant women.121 Observational studies from the late seventies demonstrated that 20–40mg of calcium channel blocker nifedipine provided menstrual pain relief but was associated with side effects such as tachycardia, flushing, and headache.122,123 These findings are supported in a controlled trial showing that 14 out of 19 patients obtained menstrual pain relief with nifedipine.124 Although one research study suggested efficacy of nifedipine in women that unresponsive to salicylates,125 future research is needed to establish efficacy for women unresponsive to NSAIDs.
Vasopressin and Oxytocin Receptor Antagonists
Vasopressin and oxytocin, hormones known to stimulate myometrial contractions, have also been implicated in primary dysmenorrhea.126 There is conflicting evidence, however, on the effects of vasopressin/oxytocin receptor antagonists on dysmenorrhea. Several studies have shown that vasopressin-induced contractions in dysmenorrheic women were reduced by vasopressin/oxytocin receptor antagonists atosiban127,128 and SR49059.129 In contrast, Valentin and colleagues demonstrated that when compared to healthy controls, dysmenorrheic women did not show elevated levels of vasopressin and that the intravenous administration of atosiban did not attenuate menstrual pain or uterine contractility.130 It is important to note that the Valentin study administered atosiban intravenously after menses onset, while the Brouard study administered SR49059 orally at least 4 hours prior to menses onset. Thus, more evidence is needed to examine how the time and type of administration impacts the efficacy of vasopressin/oxytocin receptor antagonists on NSAID-resistant dysmenorrhea.
Anti-spasmodics
Although infrequently used in the United States, anti-spasmodics such as hyoscine butylbromide are used globally to treat abdominal pain, including menstrual pain. Hyoscine butylbromide is an anticholinergic drug that targets muscarinic receptors to relax smooth muscle.131 In the United States, a similar drug, hyoscyamine sulfate is available as a generic medication. Common adverse effects include dry mouth, constipation and dizziness. Although it is frequently prescribed for visceral spasms, it is not FDA indicated for dysmenorrhea.
In a double-blind crossover study, Kemp and colleagues demonstrated that hyoscine butylbromide was just as effective as aspirin in treating dysmenorrhea.132 Questionnaire-based studies have shown that women used hyoscine butylbromide to self-treat their dysmenorrhea with a similar frequency as paracetamol and NSAIDs.133–136 A randomized controlled trial compared a combination of an anti-spasmodic (drotaverine) and an NSAID (aceclofenac) versus aceclofenac alone, and found the combination provided superior pain relief for primary dysmenorrhea.137 Since the addition of drotaverine provided better pain relief than aceclofenac alone, these results support the use of an adjunct anti-spasmodic to treat refractory menstrual pain. These findings also suggest that muscle spasm pain in dysmenorrhea may contribute to NSAID-resistant pain.
Complementary and Non-Pharmacological Medical Treatments
Herbal and dietary supplements have been proposed as alternative treatments for dysmenorrhea. Although many varieties are currently used to treat dysmenorrhea, inconsistencies between various studies make it difficult to determine the efficacy of supplements (reviewed by Pattinitum and colleagues).138 Ginger, the most commonly reported effective remedy in randomized controlled trials, only reduced pain 1.5 cm on a 10 cm visual analog scale.139 Thus more high-quality trials demonstrating superior effectiveness of herbal and dietary supplements are needed to provide viable options for patients unresponsive to NSAIDs.
Many non-pharmacological remedies for dysmenorrhea have been investigated. Limited evidence suggests acupuncture,140 hot water bottles,141 yoga,142 massage,143 physiotherapy,144 and exercise145 may be helpful for menstrual pain, but as with many traditional pharmaceuticals, effects have not been consistently repeated or verified with large randomized controlled trials. In contrast, transcutaneous electrical nerve stimulation (TENS) has been shown to reduce menstrual pain in several randomized146–148 and observational149,150 trials. Since trans-abdominal application of TENS has no effect on uterine contractility,25 TENS may affect associated abdominal muscle contractility instead. The role of abdominal muscle cramping in dysmenorrhea would be consistent with the utility of antispasmodic agents described above. The findings obtained with TENS are consistent with the hypothesis that prostaglandin-independent pathways contribute to dysmenorrhea, and suggest that the attenuation of these alternative pathways may be effective.
Future Directions
As mentioned above, most studies investigating various treatments for dysmenorrhea have not examined the prevalence of NSAID resistance amongst their participants. Since dysmenorrhea patients may choose treatments based on preference rather than previous NSAID treatment failure, the overall efficacy of treatments for NSAID-resistant dysmenorrhea is unknown. Validated electronic tools which track menstrual pain and the use of rescue medication151 would be useful for clinical trials. It is likely that multiple phenotypes of dysmenorrhea exist reflecting different underlying causes. However, since the abandonment of classifying spasmodic and congestive menstrual pain phenotypes,152 a replacement classification scheme has not been popularly accepted, and should possibly be reconsidered for the diagnosis for NSAID-resistant dysmenorrhea.
Pharmacological and gene assays could help identify forms of NSAID-resistant dysmenorrhea that may respond to alternative treatment strategies. A similar research strategy has revolutionized the understanding of aspirin resistance observed in antiplatelet therapy. The utilization of ex-vivo assays that detect mechanisms of aspirin resistance has led to the identification of polymorphisms,43,44 absorption impairments,153 or other factors that limit drug bioavailability.154, 46 The translation of these tests for NSAID-resistant pain could similarly clarify why some patients are unresponsive and provide avenues for adequate therapeutic development.
Conclusion
A significant proportion of women who suffer from dysmenorrhea obtain no relief from NSAIDs. Opportunities to characterize NSAID resistance with diagnostic testing and enroll women with resistance phenotypes into novel clinical trials have not been pursued. We suggest that future studies explore molecular targets that could explain resistance and evaluate novel therapies in these patients. Given that cyclooxygenases are implicated in other acute (e.g., muscle soreness, inflammation, burn pain) and chronic pain conditions (e.g., migraine, arthritis) studying the mechanisms of NSAID resistance has the broad potential to improve pain relief in patients with multiple types of refractory pain conditions.
Prior treatment algorithms suggest that symptomatic patients with NSAID-resistant dysmenorrhea that do not respond to OCPs undergo diagnostic laparoscopic examination 22,79. Recent consensus guidelines suggest trials of levonorgestrel-releasing intrauterine devices, with surgery being the last diagnostic and therapeutic option.91 Although surgery for symptomatic patients is often effective and recommended155–157, some patients may be not willing to undergo surgery. For these patients, until research establishes the underlying mechanisms, some of the options described here could partially ameliorate their unremitting monthly pain.
Acknowledgments
We would like to thank Dr. Gerald Gebhart for his assistance with the manuscript.
Financial Support: This research was supported by NICHD HD081709, NIDDK DK100368, and NorthShore University Health System.
Footnotes
Disclosure: F.F.T. was a consultant for AbbVie Pharmaceuticals. The remaining authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Folabomi A. OLADOSU, Evanston IL, USA, Department of Obstetrics and Gynecology, NorthShore University HealthSystem & Pritzker School of Medicine University of Chicago.
Frank F. TU, Evanston IL, USA, Department of Obstetrics and Gynecology, NorthShore University HealthSystem & Pritzker School of Medicine University of Chicago.
Kevin M. HELLMAN, Evanston IL, USA, Department of Obstetrics and Gynecology, NorthShore University HealthSystem & Pritzker School of Medicine University of Chicago.
References
- 1.@FLOTUS44. —The First Lady on the barriers to girls’ education. Apr, 2016. Why are girls still missing so many days of school because of their menstrual cycles? [Google Scholar]
- 2.Feng E. Uninhibited Chinese Swimmer, Discussing Her Period, Shatters Another Barrier. New York Times. http://www.nytimes.com/2016/08/17/world/asia/china-fu-yuanhui-period-olympics.html?_r=0. Published August 16, 2016.
- 3.Berkley KJ, McAllister SL. Don’t dismiss dysmenorrhea! Pain. 2011;152(9):1940–1941. doi: 10.1016/j.pain.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Zondervan KT, Yudkin PL, Vessey MP, et al. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract. 2001;51(468):541–547. [PMC free article] [PubMed] [Google Scholar]
- 5.Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol. 2013;209(5):422.e.1–422.e10. doi: 10.1016/j.ajog.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grace VM, Zondervan KT. Chronic pelvic pain in New Zealand: prevalence, pain severity, diagnoses and use of the health services. Aust N Z J Public Health. 2004;28(4):369–375. doi: 10.1111/j.1467-842x.2004.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldwasser M. Primary Dysmenorrhea: A Local Manifestation of A Constitutional Disease and Its Treatment. Cal West Med. 1938;48(6):418–421. [PMC free article] [PubMed] [Google Scholar]
- 8.Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001751.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68(5):661–664. [PubMed] [Google Scholar]
- 10.O’Connell K, Davis AR, Westhoff C. Self-treatment patterns among adolescent girls with dysmenorrhea. J Pediatr Adolesc Gynecol. 2006;19(4):285–289. doi: 10.1016/j.jpag.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med. 2013;10(2):e1001388. doi: 10.1371/journal.pmed.1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillen TI, Grbavac SL, Johnston PJ, Straton JA, Keogh JM. Primary dysmenorrhea in young Western Australian women: prevalence, impact, and knowledge of treatment. J Adolesc Health. 1999;25(1):40–45. doi: 10.1016/s1054-139x(98)00147-5. [DOI] [PubMed] [Google Scholar]
- 13.Ozerdogan N, Sayiner D, Ayranci U, Unsal A, Giray S. Prevalence and predictors of dysmenorrhea among students at a university in Turkey. International Journal of Gynecology & Obstetrics. 2009;107(1):39–43. doi: 10.1016/j.ijgo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz MI. Primary dysmenorrhea among Mexican university students: prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):73–77. doi: 10.1016/j.ejogrb.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Owen PR. Prostaglandin synthetase inhibitors in the treatment of primary dysmenorrhea. Outcome trials reviewed. Am J Obstet Gynecol. 1984;148(1):96–103. doi: 10.1016/s0002-9378(84)80039-3. [DOI] [PubMed] [Google Scholar]
- 16.Maia H, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol. 2005;21(1):57–61. doi: 10.1080/09513590500099602. [DOI] [PubMed] [Google Scholar]
- 17.Chan WY. Prostaglandins and nonsteroidal antiinflammatory drugs in dysmenorrhea. Annu Rev Pharmacol Toxicol. 1983;23:131–149. doi: 10.1146/annurev.pa.23.040183.001023. [DOI] [PubMed] [Google Scholar]
- 18.Lundström V, Green K. Endogenous levels of prostaglandin F 2α and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. American journal of obstetrics and gynecology. 1978;130(6):640–646. doi: 10.1016/0002-9378(78)90320-4. [DOI] [PubMed] [Google Scholar]
- 19.Vane JR, Bakhle YS, Botting RM. CYCLOOXYGENASES 1 AND 2. Annual review of \ldots. 1998;38(1):97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 20.Marx SG, Wentz MJ, MacKay LB, et al. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. Journal of Histochemistry & Cytochemistry. 2006;54(6):623–639. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 21.Tamura I, Taketani T, Lee L, et al. Differential effects of progesterone on COX-2 and Mn-SOD expressions are associated with histone acetylation status of the promoter region in human endometrial stromal cells. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):E1073–E1082. doi: 10.1210/jc.2010-2489. [DOI] [PubMed] [Google Scholar]
- 22.Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108(2):428–441. doi: 10.1097/01.AOG.0000230214.26638.0c. [DOI] [PubMed] [Google Scholar]
- 23.Hellman KM, Yu PY, Oladosu FA, et al. The Effects of Platelet-Activating Factor on Uterine Contractility, Perfusion, Hypoxia, and Pain in Mice. Reproductive Sciences. 2017 doi: 10.1177/1933719117715122. 1933719117715122. [DOI] [PubMed] [Google Scholar]
- 24.Milsom I, Andersch B. Effect of ibuprofen, naproxen sodium and paracetamol on intrauterine pressure and menstrual pain in dysmenorrhoea. Br J Obstet Gynaecol. 1984;91(11):1129–1135. doi: 10.1111/j.1471-0528.1984.tb15089.x. [DOI] [PubMed] [Google Scholar]
- 25.Milsom I, Hedner N, Mannheimer C. A comparative study of the effect of high-intensity transcutaneous nerve stimulation and oral naproxen on intrauterine pressure and menstrual pain in patients with primary dysmenorrhea. American journal of obstetrics and gynecology. 1994;170(1):123–129. doi: 10.1016/s0002-9378(94)70396-5. [DOI] [PubMed] [Google Scholar]
- 26.Woodbury RA, Torpin R. Myometrial physiology and its relation to pelvic pain. J Am Med Assoc. 1947;134(13):1081–1085. doi: 10.1001/jama.1947.02880300023007. [DOI] [PubMed] [Google Scholar]
- 27.Dmitrović R. Transvaginal color Doppler study of uterine blood flow in primary dysmenorrhea. Acta Obstet Gynecol Scand. 2000;79(12):1112–1116. doi: 10.1034/j.1600-0412.2000.0790121112.x. [DOI] [PubMed] [Google Scholar]
- 28.Pundir J, Omanwa K, Kovoor E, Pundir V, Lancaster G, Barton-Smith P. Laparoscopic Excision Versus Ablation for Endometriosis-associated Pain: An Updated Systematic Review and Meta-analysis. J Minim Invasive Gynecol. 2017;24(5):747–756. doi: 10.1016/j.jmig.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Human reproduction. 2001;16(3):561–566. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- 30.Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertility and sterility. 2004;81(5):1351–1356. doi: 10.1016/j.fertnstert.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 31.Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. The Journal of Clinical Endocrinology & Metabolism. 1997;82(2):600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 32.Nie J, Liu X, Guo S-W. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. American journal of obstetrics and gynecology. 2010;202(4):346–e1. doi: 10.1016/j.ajog.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Tsigkou A, Reis FM, Ciarmela P, et al. Expression levels of myostatin and matrix metalloproteinase 14 mRNAs in uterine leiomyoma are correlated with dysmenorrhea. Reproductive Sciences. 2015;22(12):1597–1602. doi: 10.1177/1933719115592710. [DOI] [PubMed] [Google Scholar]
- 34.Johannesson U, de Boussard CN, Brodda Jansen G, Bohm-Starke N. Evidence of diffuse noxious inhibitory controls (DNIC) elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain. 2007;130(1–2):31–39. doi: 10.1016/j.pain.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Stavroulis AI, Saridogan E, Creighton SM, Cutner AS. Laparoscopic treatment of endometriosis in teenagers. Eur J Obstet Gynecol Reprod Biol. 2006;125(2):248–250. doi: 10.1016/j.ejogrb.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Ragab A, Shams M, Badawy A, Alsammani MA. Prevalence of endometriosis among adolescent school girls with severe dysmenorrhea: A cross sectional prospective study. Int J Health Sci (Qassim) 2015;9(3):273–281. [PMC free article] [PubMed] [Google Scholar]
- 37.Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 38.Kauppila A, Puolakka J, Ylikorkala O. Prostaglandin biosynthesis inhibitors and endometriosis. Prostaglandins. 1979;18(4):655–661. doi: 10.1016/0090-6980(79)90033-9. [DOI] [PubMed] [Google Scholar]
- 39.Kauppila A, Rönnberg L. Naproxen sodium in dysmenorrhea secondary to endometriosis. Obstet Gynecol. 1985;65(3):379–383. [PubMed] [Google Scholar]
- 40.Jacoby VL, Jacoby A, Learman LA, et al. Use of medical, surgical and complementary treatments among women with fibroids. European journal of obstetrics, gynecology, and reproductive biology. 2014;182:220–225. doi: 10.1016/j.ejogrb.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streuli I, Dubuisson J, Santulli P, de Ziegler D, Batteux F, Chapron C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother. 2014;15(16):2347–2360. doi: 10.1517/14656566.2014.953055. [DOI] [PubMed] [Google Scholar]
- 42.Cryer B, Feldman M. Cyclooxygenase-1 and Cyclooxygenase-2 Selectivity of Widely Used Nonsteroidal Anti-Inflammatory Drugs. The American Journal of Medicine. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 43.Ulehlova J, Slavik L, Kucerova J, Krcova V, Vaclavik J, Indrak K. Genetic Polymorphisms of Platelet Receptors in Patients with Acute Myocardial Infarction and Resistance to Antiplatelet Therapy. Genetic Testing and Molecular Biomarkers. 2014;18(9):599–604. doi: 10.1089/gtmb.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng Z, Li X, Li Y, Lin J, Peng F, Niu W. The association of four common polymorphisms from four candidate genes (COX-1, COX-2, ITGA2B, ITGA2) with aspirin insensitivity: a meta-analysis. PLoS ONE. 2013;8(11):e78093. doi: 10.1371/journal.pone.0078093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agúndez JA, Blanca M, Cornejo-García JA, García-Martin E. Pharmacogenomics of cyclooxygenases. Pharmacogenomics. 2015;16(5):501–522. doi: 10.2217/pgs.15.6. [DOI] [PubMed] [Google Scholar]
- 46.Hundal O, Rugstad HE, Husby G. Naproxen free plasma concentrations and unbound fractions in patients with osteoarthritis: relation to age, sex, efficacy, and adverse events. Ther Drug Monit. 1991;13(6):478–484. doi: 10.1097/00007691-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Kirchheiner J, Brockmoller J. Clinical consequences of cytochrome P450 2C9 polymorphisms. Clinical Pharmacology & Therapeutics. 2005;77(1):1–16. doi: 10.1016/j.clpt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro H, Singer P, Ariel A. Beyond the classic eicosanoids: Peripherally-acting oxygenated metabolites of polyunsaturated fatty acids mediate pain associated with tissue injury and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2016;111:45–61. doi: 10.1016/j.plefa.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Rees MC, DiMarzo V, Tippins JR, Morris HR, Turnbull AC. Leukotriene release by endometrium and myometrium throughout the menstrual cycle in dysmenorrhoea and menorrhagia. J Endocrinol. 1987;113(2):291–295. doi: 10.1677/joe.0.1130291. [DOI] [PubMed] [Google Scholar]
- 51.Harel Z, Lilly C, Riggs S, Vaz R, Drazen J. Urinary leukotriene (LT) E(4) in adolescents with dysmenorrhea: a pilot study. J Adolesc Health. 2000;27(3):151–154. doi: 10.1016/s1054-139x(00)00123-3. [DOI] [PubMed] [Google Scholar]
- 52.Nigam S, Benedetto C, Zonca M, Leo-Rossberg I, Lübbert H, Hammerstein J. Increased concentrations of eicosanoids and platelet-activating factor in menstrual blood from women with primary dysmenorrhea. Eicosanoids. 1991;4(3):137–141. [PubMed] [Google Scholar]
- 53.Fujiwara H, Konno R, Netsu S, et al. Efficacy of montelukast, a leukotriene receptor antagonist, for the treatment of dysmenorrhea: a prospective, double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):195–198. doi: 10.1016/j.ejogrb.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Harel Z, Riggs S, Vaz R, Flanagan P, Harel D. The use of the leukotriene receptor antagonist montelukast (Singulair) in the management of dysmenorrhea in adolescents. J Pediatr Adolesc Gynecol. 2004;17(3):183–186. doi: 10.1016/j.jpag.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Simoni J, Simoni G, Lox CD, McGunegle DE, Feola M. Cytokines and PAF release from human monocytes and macrophages: effect of hemoglobin and contaminants. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(3):525–534. doi: 10.3109/10731199409117880. [DOI] [PubMed] [Google Scholar]
- 56.Hemmings R, Miron P, Falcone T, Bourque J, Lepage N, Langlais J. Platelet-activating factor acetylhydrolase activity in peritoneal fluids of women with endometriosis. Obstet Gynecol. 1993;81(2):276–279. [PubMed] [Google Scholar]
- 57.Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. The role of arachidonic acid oxygenation products in pain and inflammation. Annual review of immunology. 1984;2(1):335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 58.England S, Bevan S, Docherty R. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. The Journal of physiology. 1996;495(2):429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP 1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular pain. 2005;1(1):3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokushige N, Markham R, Russell P, Fraser I. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Human Reproduction. 2005;21(3):782–787. doi: 10.1093/humrep/dei368. [DOI] [PubMed] [Google Scholar]
- 61.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294(1):R162–R171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertility and sterility. 2010;94(2):730–737. doi: 10.1016/j.fertnstert.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Human Reproduction Update. 2015;21(6):762–778. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- 64.Arendt-Nielsen L, Madsen H, Jarrell J, Gregersen H, Drewes AM. Pain evoked by distension of the uterine cervix in women with dysmenorrhea: evidence for central sensitization. Acta obstetricia et gynecologica Scandinavica. 2014;93(8):741–748. doi: 10.1111/aogs.12403. [DOI] [PubMed] [Google Scholar]
- 65.Iacovides S, Baker FC, Avidon I, Bentley A. Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. The Journal of Pain. 2013;14(10):1066–1076. doi: 10.1016/j.jpain.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. The Clinical journal of pain. 2002;18(3):180–190. doi: 10.1097/00002508-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Slater H, Paananen M, Smith AJ, et al. Heightened cold pain and pressure pain sensitivity in young female adults with moderate-to-severe menstrual pain. Pain. 2015;156(12):2468–2478. doi: 10.1097/j.pain.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 68.Iacovides S, Avidon I, Baker F. Women with dysmenorrhoea are hypersensitive to experimentally induced forearm ischaemia during painful menstruation and during the pain-free follicular phase. European Journal of Pain. 2015;19(6):797–804. doi: 10.1002/ejp.604. [DOI] [PubMed] [Google Scholar]
- 69.As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. PAIN®. 2012;153(5):1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tu C-H, Niddam DM, Yeh T-C, et al. Menstrual pain is associated with rapid structural alterations in the brain. PAIN®. 2013;154(9):1718–1724. doi: 10.1016/j.pain.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 71.Liu P, Yang J, Wang G, et al. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. European Journal of Pain. 2016;20(4):512–520. doi: 10.1002/ejp.753. [DOI] [PubMed] [Google Scholar]
- 72.Okkerse P, van Amerongen G, de Kam ML, et al. The use of a battery of pain models to detect analgesic properties of compounds: a two-part four-way crossover study. Br J Clin Pharmacol. 2017;83(5):976–990. doi: 10.1111/bcp.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1592–1601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 74.Wesselmann U, Lai J. Mechanisms of referred visceral pain: uterine inflammation in the adult virgin rat results in neurogenic plasma extravasation in the skin. Pain. 1997;73(3):309–317. doi: 10.1016/S0304-3959(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 75.Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 Pt 1):223–230. doi: 10.1097/AOG.0b013e318223fed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duggan KC, Walters MJ, Musee J, et al. Molecular Basis for Cyclooxygenase Inhibition by the Non-steroidal Anti-inflammatory Drug Naproxen. The Journal of biological chemistry. 2010;285(45):34950–34959. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan WY, Dawood MY, Fuchs F. Prostaglandins in primary dysmenorrhea. Comparison of prophylactic and nonprophylactic treatment with ibuprofen and use of oral contraceptives. Am J Med. 1981;70(3):535–541. doi: 10.1016/0002-9343(81)90576-3. [DOI] [PubMed] [Google Scholar]
- 78.Chiou M-H, Wang H-H, Yang Y-H. Effect of systematic menstrual health education on dysmenorrheic female adolescents’ knowledge, attitudes, and self-care behavior. Kaohsiung J Med Sci. 2007;23(4):183–190. doi: 10.1016/S1607-551X(09)70395-X. [DOI] [PubMed] [Google Scholar]
- 79.Harel Z. Dysmenorrhea in adolescents and young adults: an update on pharmacological treatments and management strategies. Expert Opin Pharmacother. 2012;13(15):2157–2170. doi: 10.1517/14656566.2012.725045. [DOI] [PubMed] [Google Scholar]
- 80.Latthe PM, Champaneria R. Dysmenorrhoea. BMJ Clin Evid. 2014;2014 [PMC free article] [PubMed] [Google Scholar]
- 81.Bieglmayer C, Hofer G, Kainz C, Reinthaller A, Kopp B, Janisch H. Concentrations of various arachidonic acid metabolites in menstrual fluid are associated with menstrual pain and are influenced by hormonal contraceptives. Gynecological endocrinology. 1995;9(4):307–312. doi: 10.3109/09513599509160464. [DOI] [PubMed] [Google Scholar]
- 82.Edelman A, Micks E, Gallo MF, Jensen JT, Grimes DA. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014;(7):CD004695. doi: 10.1002/14651858.CD004695.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong CL, Farquhar C, Roberts H, Proctor M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009;(4):CD002120. doi: 10.1002/14651858.CD002120.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Witjes H, Creinin MD, Sundström-Poromaa I, Martin Nguyen A, Korver T. Comparative analysis of the effects of nomegestrol acetate/17 β-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea. The European Journal of Contraception & Reproductive Health Care. 2015;20(4):296–307. doi: 10.3109/13625187.2015.1016154. [DOI] [PubMed] [Google Scholar]
- 85.Winkler UH, Ferguson H, Mulders JaPA. Cycle control, quality of life and acne with two low-dose oral contraceptives containing 20 microg ethinylestradiol. Contraception. 2004;69(6):469–476. doi: 10.1016/j.contraception.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 86.Petraglia F, Parke S, Serrani M, Mellinger U, Römer T. Estradiol valerate plus dienogest versus ethinylestradiol plus levonorgestrel for the treatment of primary dysmenorrhea. International Journal of Gynecology & Obstetrics. 2014;125(3):270–274. doi: 10.1016/j.ijgo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 87.Imai A, Matsunami K, Takagi H, Ichigo S. Levonorgestrel-releasing intrauterine device used for dysmenorrhea: five-year literature review. Clin Exp Obstet Gynecol. 2014;41(5):495–498. [PubMed] [Google Scholar]
- 88.Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertility and sterility. 2008;90(5):1583–1588. doi: 10.1016/j.fertnstert.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 89.Muzii L, Di Tucci C, Achilli C, et al. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(2):203–211. doi: 10.1016/j.ajog.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 90.Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107(3):533–536. doi: 10.1016/j.fertnstert.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Burnett M, Lemyre M. Primary Dysmenorrhea Consensus Guideline. Journal of Obstetrics and Gynaecology Canada. 2017;39(7):585–595. doi: 10.1016/j.jogc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 92.Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51–58. doi: 10.1016/s0029-7844(98)00341-x. [DOI] [PubMed] [Google Scholar]
- 93.Dawood MY, Lewis V, Ramos J. Cortical and trabecular bone mineral content in women with endometriosis: effect of gonadotropin-releasing hormone agonist and danazol. Fertility and sterility. 1989;52(1):21–26. doi: 10.1016/s0015-0282(16)60782-3. [DOI] [PubMed] [Google Scholar]
- 94.Dodin S, Lemay A, Maheux R, Dumont M, Turcot-lemay L. Bone mass in endometriosis patients treated with GnRH agonist implant or danazol. Obstetrics & Gynecology. 1991;77(3):410–415. [PubMed] [Google Scholar]
- 95.Leather A, Studd J, Watson N, Holland E. The prevention of bone loss in young women treated with GnRH analogues with“ add-back” estrogen therapy. Obstetrics & Gynecology. 1993;81(1):104–107. [PubMed] [Google Scholar]
- 96.Hornstein MD, Surrey ES, Weisberg GW, Casino LA, et al. Group LA-BS. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Obstetrics & Gynecology. 1998;91(1):145–148. doi: 10.1016/s0029-7844(97)00620-0. [DOI] [PubMed] [Google Scholar]
- 97.Zupi E, Marconi D, Sbracia M, et al. Add-back therapy in the treatment of endometriosis-associated pain. Fertility and sterility. 2004;82(5):1303–1308. doi: 10.1016/j.fertnstert.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 98.Tahara M, Matsuoka T, Yokoi T, Tasaka K, Kurachi H, Murata Y. Treatment of endometriosis with a decreasing dosage of a gonadotropin-releasing hormone agonist (nafarelin): a pilot study with low-dose agonist therapy (“draw-back” therapy) Fertility and sterility. 2000;73(4):799–804. doi: 10.1016/s0015-0282(99)00636-6. [DOI] [PubMed] [Google Scholar]
- 99.Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Strowitzki T, Faustmann T, Gerlinger C, Seitz C. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2010;151(2):193–198. doi: 10.1016/j.ejogrb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Human Reproduction. 2010;25(3):633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]
- 102.Al-Jefout M, Nawaiseh N. Continuous Norethisterone Acetate versus Cyclical Drospirenone 3 mg/Ethinyl Estradiol 20 μg for the Management of Primary Dysmenorrhea in Young Adult Women. J Pediatr Adolesc Gynecol. 2016;29(2):143–147. doi: 10.1016/j.jpag.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Zigler RE, McNicholas C. Unscheduled vaginal bleeding with progestin-only contraceptive use. American Journal of Obstetrics & Gynecology. 2017;216(5):443–450. doi: 10.1016/j.ajog.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68(3):393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- 105.Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98(6):1370–1379. doi: 10.1016/j.fertnstert.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Attar E, Bulun S. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Human reproduction update. 2006;12(1):49–56. doi: 10.1093/humupd/dmi034. [DOI] [PubMed] [Google Scholar]
- 107.Agarwal AK, Garg R, Ritch A, Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83(981):478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gurates B, Parmaksiz C, Kilic G, Celik H, Kumru S, Simsek M. Treatment of symptomatic uterine leiomyoma with letrozole. Reprod Biomed Online. 2008;17(4):569–574. doi: 10.1016/s1472-6483(10)60246-5. [DOI] [PubMed] [Google Scholar]
- 109.Berlanda N, Somigliana E, Viganò P, Vercellini P. Safety of medical treatments for endometriosis. Expert Opin Drug Saf. 2016;15(1):21–30. doi: 10.1517/14740338.2016.1121991. [DOI] [PubMed] [Google Scholar]
- 110.Proctor M, Latthe P, Farquhar C, Khan K, Johnson N. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD001896.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Latthe P, Proctor M, Farquhar C, Johnson N, Khan K. Surgical interruption of pelvic nerve pathways in dysmenorrhea: a systematic review of effectiveness. Acta obstetricia et gynecologica Scandinavica. 2007;86(1):4–15. doi: 10.1080/00016340600753117. [DOI] [PubMed] [Google Scholar]
- 112.Daniels J, Gray R, Hills RK, et al. Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. JAMA. 2009;302(9):955–961. doi: 10.1001/jama.2009.1268. [DOI] [PubMed] [Google Scholar]
- 113.Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. The Journal of reproductive medicine. 1987;32(1):37–41. [PubMed] [Google Scholar]
- 114.Juang C-M, Chou P, Yen M-S, Horng H-C, Twu N-F, Chen C-Y. Laparoscopic uterosacral nerve ablation with and without presacral neurectomy in the treatment of primary dysmenorrhea: a prospective efficacy analysis. J Reprod Med. 2007;52(7):591–596. [PubMed] [Google Scholar]
- 115.Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet Gynecol. 1997;90(6):974–977. doi: 10.1016/s0029-7844(97)00484-5. [DOI] [PubMed] [Google Scholar]
- 116.Nehra A, Colreavy F, Khandheria B, Chandrasekaran K. Sildenafil citrate, a selective phosphodiesterase type 5 inhibitor: urologic and cardiovascular implications. World journal of urology. 2001;19(1):40–45. doi: 10.1007/pl00007091. [DOI] [PubMed] [Google Scholar]
- 117.Dmitrovic R, Kunselman AR, Legro RS. Sildenafil citrate in the treatment of pain in primary dysmenorrhea: a randomized controlled trial. Human Reproduction. 2013;28(11):2958–2965. doi: 10.1093/humrep/det324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ali A, Bipozzi MA, Burgos RA, et al. Transdermal nitroglycerine in the management of pain associated with primary dysmenorrhoea: a multinational pilot study. The Transdermal Nitroglycerine/Dysmenorrhoea Study Group. The Journal of international medical research. 1997;25(1):41–44. doi: 10.1177/030006059702500106. [DOI] [PubMed] [Google Scholar]
- 119.Moya RA, Moisa CF, Morales F, Wynter H, Ali A, Narancio E. Transdermal glyceryl trinitrate in the management of primary dysmenorrhea. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;69(2):113–118. doi: 10.1016/s0020-7292(00)00185-5. [DOI] [PubMed] [Google Scholar]
- 120.Facchinetti F, Sgarbi L, Piccinini F, Volpe A. A comparison of glyceryl trinitrate with diclofenac for the treatment of primary dysmenorrhea: an open, randomized, cross-over trial. Gynecological Endocrinology. 2002;16(1):39–43. [PubMed] [Google Scholar]
- 121.Fenakel K, Lurie S. The use of calcium channel blockers in obstetrics and gynecology; a review. European journal of obstetrics, gynecology, and reproductive biology. 1990;37(3):199–203. doi: 10.1016/0028-2243(90)90025-v. [DOI] [PubMed] [Google Scholar]
- 122.Andersson KE, Ulmsten U. Effects of nifedipine on myometrial activity and lower abdominal pain in women with primary dysmenorrhoea. British journal of obstetrics and gynaecology. 1978;85(2):142–148. doi: 10.1111/j.1471-0528.1978.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 123.Sandahl B, Ulmsten U, Andersson KE. Trial of the calcium antagonist nifedipine in the treatment of primary dysmenorrhoea. Archives of gynecology. 1979;227(2):147–151. doi: 10.1007/BF02103289. [DOI] [PubMed] [Google Scholar]
- 124.Mondero NA. Nifedipine in the treatment of dysmenorrhea. J Am Osteopath Assoc. 1983;82(9 Suppl):704–708. [PubMed] [Google Scholar]
- 125.Ulmsten U. Calcium blockade as a rapid pharmacological test to evaluate primary dysmenorrhea. Gynecol Obstet Invest. 1985;20(2):78–83. doi: 10.1159/000298977. [DOI] [PubMed] [Google Scholar]
- 126.Akerlund Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Progress in brain research. 2002;139:359–365. doi: 10.1016/s0079-6123(02)39030-7. [DOI] [PubMed] [Google Scholar]
- 127.Akerlund M. Can primary dysmenorrhea be alleviated by a vasopressin antagonist? Results of a pilot study. Acta obstetricia et gynecologica Scandinavica. 1986;66(5):459–461. doi: 10.3109/00016348709022055. [DOI] [PubMed] [Google Scholar]
- 128.Liedman R, Grant L, Igidbashian S, et al. Intrauterine pressure, ischemia markers, and experienced pain during administration of a vasopressin V1a receptor antagonist in spontaneous and vasopressin-induced dysmenorrhea. Acta obstetricia et gynecologica Scandinavica. 2006;85(2):207–211. doi: 10.1080/00016340500495082. [DOI] [PubMed] [Google Scholar]
- 129.Brouard R, Bossmar T, Fournié-Lloret D, Chassard D, \AAkerlund M. Effect of SR49059, an orally active V1a vasopressin receptor antagonist, in the prevention of dysmenorrhoea. BJOG: An International Journal of Obstetrics & Gynaecology. 2000;107(5):614–619. doi: 10.1111/j.1471-0528.2000.tb13302.x. [DOI] [PubMed] [Google Scholar]
- 130.Valentin L, Sladkevicius P, Kindahl H, Broeders A, Marsal K, Melin P. Effects of a vasopressin antagonist in women with dysmenorrhea. Gynecologic and obstetric investigation. 2000;50(3):170–177. doi: 10.1159/000010319. [DOI] [PubMed] [Google Scholar]
- 131.Pomeroy A, Rand M. Anticholinergic effects and passage through the intestinal wall of N-butylhyoscine bromide. Journal of Pharmacy and Pharmacology. 1969;21(3):180–187. doi: 10.1111/j.2042-7158.1969.tb08224.x. [DOI] [PubMed] [Google Scholar]
- 132.Kemp J. “Buscopan” in spasmodic dysmenorrhoea. Current medical research and opinion. 1972;1(1):19–25. doi: 10.1185/03007997209111141. [DOI] [PubMed] [Google Scholar]
- 133.Moawed S. Indigenous practices of Saudi girls in Riyadh during their menstrual period. 2001 [PubMed] [Google Scholar]
- 134.Ogunfowokan AA, Babatunde OA. Management of primary dysmenorrhea by school adolescents in ILE-IFE, Nigeria. The Journal of School Nursing. 2010;26(2):131–136. doi: 10.1177/1059840509349723. [DOI] [PubMed] [Google Scholar]
- 135.Aziato L, Dedey F, Clegg-Lamptey JNA. Dysmenorrhea management and coping among students in Ghana: A qualitative exploration. Journal of pediatric and adolescent gynecology. 2015;28(3):163–169. doi: 10.1016/j.jpag.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Enck P, Koehler U, Weigmann H, Mueller-Lissner S. Abdominal pain, cramping or discomfort impairs quality of life in women: An internet-based observational pilot study focussing on impact of treatment. Zeitschrift für Gastroenterologie. 2017;55(03):260–266. doi: 10.1055/s-0043-100022. [DOI] [PubMed] [Google Scholar]
- 137.Pareek A, Chandurkar NB, Patil RT, Agrawal SN, Uday RB, Tambe SG. Efficacy and safety of aceclofenac and drotaverine fixed-dose combination in the treatment of primary dysmenorrhoea: a double-blind, double-dummy, randomized comparative study with aceclofenac. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):86–90. doi: 10.1016/j.ejogrb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 138.Pattanittum P, Kunyanone N, Brown J. Dietary supplements for dysmenorrhoea. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD002124.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen CX, Barrett B, Kwekkeboom KL. Efficacy of Oral Ginger (Zingiber officinale) for Dysmenorrhea: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2016;2016:6295737. doi: 10.1155/2016/6295737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith CA, Armour M, Zhu X, Li X, Lu ZY. Acupuncture for dysmenorrhoea. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD007854.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chaudhuri A, Singh A, Dhaliwal L. A randomised controlled trial of exercise and hot water bottle in the management of dysmenorrhoea in school girls of Chandigarh, India. Indian J Physiol Pharmacol. 2013;57(2):114–122. [PubMed] [Google Scholar]
- 142.Yang N-Y, Kim S-D. Effects of a Yoga Program on Menstrual Cramps and Menstrual Distress in Undergraduate Students with Primary Dysmenorrhea: A Single-Blind, Randomized Controlled Trial. J Altern Complement Med. 2016;22(9):732–738. doi: 10.1089/acm.2016.0058. [DOI] [PubMed] [Google Scholar]
- 143.Azima S, Bakhshayesh HR, Kaviani M, Abbasnia K, Sayadi M. Comparison of the Effect of Massage Therapy and Isometric Exercises on Primary Dysmenorrhea: A Randomized Controlled Clinical Trial. J Pediatr Adolesc Gynecol. 2015;28(6):486–491. doi: 10.1016/j.jpag.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Ortiz MI, Cortés-Márquez SK, Romero-Quezada LC, Murguía-Cánovas G, Jaramillo-Díaz AP. Effect of a physiotherapy program in women with primary dysmenorrhea. Eur J Obstet Gynecol Reprod Biol. 2015;194:24–29. doi: 10.1016/j.ejogrb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 145.Brown J, Brown S. Exercise for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(2):CD004142. doi: 10.1002/14651858.CD004142.pub2. [DOI] [PubMed] [Google Scholar]
- 146.Lauretti GR, Oliveira R, Parada F, Mattos AL. The New Portable Transcutaneous Electrical Nerve Stimulation Device Was Efficacious in the Control of Primary Dysmenorrhea Cramp Pain. Neuromodulation. 2015;18(6):522–526. doi: 10.1111/ner.12269. discussion 522–527. [DOI] [PubMed] [Google Scholar]
- 147.Wang S-F, Lee J-P, Hwa H-L. Effect of transcutaneous electrical nerve stimulation on primary dysmenorrhea. Neuromodulation. 2009;12(4):302–309. doi: 10.1111/j.1525-1403.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 148.Dawood MY, Ramos J. Transcutaneous electrical nerve stimulation (TENS) for the treatment of primary dysmenorrhea: a randomized crossover comparison with placebo TENS and ibuprofen. Obstet Gynecol. 1990;75(4):656–660. [PubMed] [Google Scholar]
- 149.Schiøtz HA, Jettestad M, Al-Heeti D. Treatment of dysmenorrhoea with a new TENS device (OVA) J Obstet Gynaecol. 2007;27(7):726–728. doi: 10.1080/01443610701612805. [DOI] [PubMed] [Google Scholar]
- 150.Kaplan B, Rabinerson D, Lurie S, Peled Y, Royburt M, Neri A. Clinical evaluation of a new model of a transcutaneous electrical nerve stimulation device for the management of primary dysmenorrhea. Gynecol Obstet Invest. 1997;44(4):255–259. doi: 10.1159/000291539. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen AM, Arbuckle R, Korver T, et al. Psychometric validation of the dysmenorrhea daily diary (DysDD): a patient-reported outcome for dysmenorrhea. Quality of Life Research. 2017:1–15. doi: 10.1007/s11136-017-1562-0. [DOI] [PubMed] [Google Scholar]
- 152.Webster SK, Martin HJ, Uchalik D, Gannon L. The menstrual symptom questionnaire and spasmodic/congestive dysmenorrhea: Measurement of an invalid construct. Journal of behavioral medicine. 1979;2(1):1–19. doi: 10.1007/BF00846559. [DOI] [PubMed] [Google Scholar]
- 153.Grosser T, Fries S, Lawson JA, Kapoor SC, Grant GR, FitzGerald GA. Drug Resistance and Pseudoresistance: An Unintended Consequence of Enteric Coating Aspirin. Circulation. 2013;127(3):377–385. doi: 10.1161/CIRCULATIONAHA.112.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eikelboom JW, Hankey GJ. Overexpression of the Multidrug Resistance Protein-4 Transporter in Patients Undergoing Coronary Artery Bypass Graft Surgery. JAC. 2011;58(7):762–764. doi: 10.1016/j.jacc.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 155.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Human reproduction. 2005;20(10):2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 156.Practice Bulletin no. 114: Management of Endometriosis. Obstetrics & Gynecology. 2010;116(1):223–236. doi: 10.1097/AOG.0b013e3181e8b073. [DOI] [PubMed] [Google Scholar]
- 157.Falcone T, Lebovic DI. Clinical management of endometriosis. Obstetrics & Gynecology. 2011;118(3):691–705. doi: 10.1097/AOG.0b013e31822adfd1. [DOI] [PubMed] [Google Scholar]