Abstract
Environmental endocrine disruptors (EEDs) are often consequences of human activity; however, the effects of EEDs are not limited to humans. A primary focus over the past ~30 years has been on chemical EEDs, but the repercussions of non-chemical EEDs, such as artificial light at night (LAN), are of increasing interest. The sensitivity of the circadian system to light and the influence of circadian organization on overall physiology and behavior make the system a target for disruption with widespread effects. Indeed, there is increasing evidence for a role of LAN in human health, including disruption of circadian regulation and melatonin signaling, metabolic dysregulation, cancer risk, and disruption of other hormonally-driven systems. These effects are not limited to humans; domesticated animals as well as wildlife are also exposed to LAN, and at risk for disrupted circadian rhythms. Here, we review data that support the role of LAN as an endocrine disruptor in humans to be considered in treatments and lifestyle suggestions. We also present the effects of LAN in other animals, and discuss the potential for ecosystem-wide effects of artificial LAN. This can inform decisions in agricultural practices and urban lighting decisions to avoid unintended outcomes.
Keywords: Light at night, Circadian rhythms, Environmental endocrine disruptor, Metabolism, Cancer, Wildlife
1. Introduction
Industrialization and urbanization have been beneficial for the prosperity and health of people, but have also introduced novel threats to wildlife and humans. Environmental endocrine disruptors (EEDs), which alter hormone homeostasis often to the detriment of organisms, are one consequence of human activity. EEDs are a growing concern over the past ~30 years. Although primary focus has been directed to the effects of chemicals found in plasticizers, pharmaceuticals, and pesticides, non-chemical sources such as light at night (LAN) can also interfere with the endocrine system. Low levels of LAN are nearly ubiquitous in the modern world [1,2]. Because evolution of life has occurred under dark nights over millions of years, and animals have only been exposed to artificial LAN for about 100 years, it is not surprising to discover that LAN likely perturbs circadian organization.
The daily light-dark cycles produced by the earth’s rotation are a central influence over organismal behavior. The most salient cyclic behavior is sleep, but many other behavioral and physiological processes follow a daily cyclic pattern as well. Daylight is essential for regulating daily activity patterns in many animals; some animals are active at night, while it is beneficial to be active during the day for others. In addition, core body temperature also follows a daily rhythm in endotherms [3]. Virtually all life has internalized the environmental light-dark cycles in the form of circadian rhythms. Circadian rhythms are endogenous biological rhythms with periods of about 24 h. Circadian rhythms persist in the absence of environmental cues [4]; however, organisms use environmental cues, such as light, to entrain their circadian rhythms precisely to the 24-hour solar day [5].
Entraining circadian rhythms to the solar day allows individuals to synchronize with environmental conditions and display appropriate behaviors and physiological responses. Endogenous circadian rhythms are present in virtually all living organisms, including bacteria, plants, invertebrates, and vertebrates. Again, light is the most effective entraining agent, or zeitgeber. In many vertebrates, light stimulates intrinsically photosensitive retinal ganglion cells, which depolarize and synapse directly onto neurons in the suprachiasmatic nucleus (SCN) of the hypothalamus. The master biological clock is located within the SCN where approximately 20,000 neurons maintain a transcriptional autoregualtory feedback loop. The molecular mechanism of the circadian clock has been reviewed in detail elsewhere [6]. This autoregulatory loop is the primary mechanism driving circadian rhythms; however, there is increasing evidence of additional processes, including posttranslational modifications [7] and cAMP signaling [8], that are also essential for maintenance. Time-of-day information, based on light intensity, is then relayed from the SCN to other brain regions, as well as to peripheral tissues, stimulating appropriate responses.
In vertebrates, in addition to the molecular clock, circadian rhythmicity is also influenced by the nightly secretion of melatonin from the pineal gland. Light stimulates clock gene transcription in the SCN, which sends GABAergic inhibitory signals through the paraventricular nucleus (PVN) of the hypothalamus. These PVN neurons then send projections through the intermediolateral cell column (IML), which stimulates norepinephrine release from the superior cervical ganglion (SCG). Norepinephrine then activates melatonin synthesis and secretion from the pineal gland [9]. In this way, light has an inhibitory effect on melatonin secretion, and the onset of dark triggers melatonin secretion. Melatonin has a negative feedback effect on clock gene transcription in the SCN, and is important for circadian rhythmicity [10,11].
The circadian clock directly induces a cyclic hormonal rhythm in endocrine tissues. Human serum cortisol concentrations, and corticosterone in many other vertebrates, fluctuate daily, with the highest concentrations in the early morning, within 30–45 minutes of waking in diurnal species [12,13]. Serum thyroid-stimulating hormone (TSH) follows a 24-hour profile, with a maximum between 0200 and 0400 h and a nadir between 1600 and 2000 h [14,15]. Furthermore, melatonin influences several endocrine pathways, including the stress and reproductive axes [16], and also signals to adipose tissue and influences body weight [17]. Many endocrine tissues are also innately cyclic via endogenous expression of clock genes. Therefore, disrupted circadian rhythms can have broad physiological outcomes through several pathways.
The circadian system is vulnerable to aberrant lighting outside the solar day due to its high sensitivity to light. Exposure to constant bright light can greatly disrupt or completely abolish circadian rhythms [18], but brief durations of bright light, or reduced light levels, are also disruptive. Just a brief pulse of light can transiently induce expression of Period 1 (Per1), a core clock gene, and phase shift the molecular clock [19]. In Siberian hamsters, just one 30 minute pulse of light during the dark phase was sufficient to activate the neurons of the SCN [20]. Furthermore, very low levels of LAN are also capable of disrupting the clock. The rhythmic expression of three essential clock genes, Per1, Per2, and cryptochrome 2 (Cry2) were attenuated by exposure to just 5 lux of light [21], a level ubiquitous in urban/suburban areas. In addition, light differentially affects secretion of melatonin as a function of the time of day. In humans, peak melatonin secretion occurs between midnight and 0400 h, and exposure to light at night during this time inhibits melatonin secretion for the entire night [22,23]. Light at night, therefore, can be disruptive at multiple levels of circadian circuitry.
Whereas bright levels of light at night are experienced occasionally, low levels of light at night are fairly ubiquitous. Forty lux of light is the approximate level of light commonly emitted from electronic devices including cellular phones held approximately 30 cm from the face, and therefore is a common exposure level for humans. Five lux of light is approximately 5 times brighter than moonlight and is comparable to levels of light pollution around urban centers [2]; thus, 5 or more lux is a common level of exposure for humans and many other animals. Light can directly alter endocrine signaling from circadian dysregulation or disrupted or dampened melatonin production, or indirectly through inflammatory responses or elevated circulating stress hormones. We will discuss these mechanisms in relation to the consequences of LAN exposure below.
This review will describe many epidemiological and basic science studies investigating the role of LAN in circadian disruption and physiological outcomes. Epidemiological and clinical results refer to diurnal humans, whereas most basic science research is conducted in nocturnal rodents. Diurnal (day-active) and nocturnal (night-active) species’ locomotor activity profiles are opposite from one another, however, the underlying mechanisms of the molecular clock are highly conserved between diurnal and nocturnal species. The structural and molecular components of the SCN are similar; however, some downstream components of the system can vary between nocturnal and diurnal animals [24]. Importantly, the effects of light on entraining circadian rhythms, as well as the photic inhibition of melatonin, are highly similar between nocturnal and diurnal animals. In addition, many of the behavioral effects of circadian dysregulation are similar between diurnal and nocturnal rodents [25,26]. LAN often disrupts sleep in diurnal animals, and thus the resulting effects cannot be attributed to circadian dysregulation independently from sleep disturbances. Therefore, using nocturnal animals in studies of LAN allows the isolation of the effects of circadian dysregulation in the absence of alterations in sleep.
2. Effects of Light at Night on Human Health
The broad endocrine effects that result from LAN exposure can have many physiological outcomes to human health. Most studies investigate the effects of LAN on disruption of metabolic processes, resulting in obesity or diabetes, and cancer incidence. Additionally, altered hormonal signaling from LAN can result in elevated stress and reproductive abnormalities. In this section we will discuss each of these physiological outcomes in relation to human health.
2.1 Obesity and Metabolic Disorders
Obesity has become an epidemic in our modern world, with global obesity rates in adults nearly double what they were in 1980. More than 2 in 3 adults and 1 in 6 children and adolescents are considered obese in the United States [27]. Obesity is a leading risk factor for type 2 diabetes, heart disease, high blood pressure, stroke, fatty liver disease, osteoarthritis, and some types of cancers. An estimate of the economic cost of obesity in the U.S. in 2008 was approximately $147 billion/year [28]. Thus, obesity is a major detriment to both human health and the economy.
A notable trend in night shift workers is an overall higher incidence of obesity [29] and metabolic syndrome compared with individuals who do not participate in night shift work. In a simulated study of night shift work in humans, more than one night of shift work reduced the total daily energy expenditure by ~3%, indicating metabolic dysregulation [30]. Furthermore, Danish nurses who work night shifts have a higher risk of diabetes compared with those working day shifts [31]. Activity during typical sleeping hours, and conversely sleeping during waking hours, presents an assortment of behavioral alterations that could lead to weight gain, including the time of day food is consumed, the type of food consumed, and changes in overall activity level. However, an additional factor of growing interest is the aberrant exposure to light during natural sleeping hours. A recent population-level study correlates global levels of LAN with obesity rates. In this model, LAN explains 70% of the variation in prevalence rates of overweight and obese individuals, while controlling for other lifestyle characteristics, such as food consumption [32]. In addition, a study investigating type 2 diabetes risk in night shift workers that separates early and late chronotypes, reported that individuals with a late chronotype had the highest diabetes risk when working daytime schedules, and conversely, individuals with an early chronotype had the highest risk when working night shifts [33]. These data support a role for circadian disruption in the metabolic dysregulation associated with shift work.
Animal models of shift work also support the idea that circadian misalignment contributes to metabolic dysregulation. Several rodent studies have been conducted to elaborate on LAN as a contributing factor in metabolic dysregulation. Mice exposed to lighting regimes mimicking shift work had impaired glucose tolerance [34]. Rats exposed to LAN also had impaired glucose tolerance, and the effect was time- intensity- and wavelength-dependent [35]. A number of studies report increased overall body mass when exposed to dim light at night (dLAN) [36,37], and returning mice to dark nights reverses the effect [21]. Exposing mice to dLAN for just two weeks reduced energy expenditure and increased carbohydrate over fat oxidation, resulting in an overall increase in body mass. These mice simultaneously maintained similar activity levels and daily food intake as mice housed in dark nights [37], indicating altered metabolic function. In addition, mice exposed to dLAN shifted a portion of their normal nocturnal food intake to day-time. In this study, mice increased body mass despite equivalent caloric intake to mice experiencing dark nights [36]. Therefore, animal studies support epidemiological observations of LAN as a contributing component to weight gain and metabolic dysregulation in humans.
Behavioral changes in humans engaging in shift work that might lead to weight gain do not simultaneously explain the resulting metabolic dysregulation. Circadian dysregulation induces several physiological effects that could contribute to metabolic alterations. Continuous exposure to LAN disrupts circadian clock function in islet cells through impairment in the amplitude, phase, and inter-islet synchrony of clock transcriptional oscillations. This leads to diminished glucose-stimulated insulin secretion [38]. This could be one potential mechanism by which circadian dysregulation can predispose to islet failure in type 2 diabetes mellitus. Constant light is also a known stressor, increasing overall circulating glucocorticoids [39,40]. Chronic increases in circulating glucocorticoids can lead to weight gain. However, exposure to dim levels of LAN does not necessarily increase circulating glucocorticoids [41,21]; therefore, weight gain associated with exposure to dLAN is likely not the result of increased glucocorticoids. Exposure to LAN is also associated with an increase in inflammation [21], and inflammation and obesity have long been associated [42]. However, it is uncertain whether inflammation is the effector or, more likely, the response to obesity. Consequently, there must be another contributing factor to the changes in metabolism associated with LAN.
LAN suppresses melatonin levels [43], and therefore could contribute to metabolic alteration. Circulating melatonin drives daily rhythms of plasma leptin and modulates glucose homeostasis [44], demonstrating a role of melatonin in metabolic regulation. Melatonin supplementation improved the obesity phenotype in Zucker diabetic fatty rats [45,46], and decreased weight gain in response to a high fat diet in Sprague Dawley rats [47,48]. Furthermore, the sympathetic nervous system has principle control over white adipose tissue, and strongly influences lipolysis in mammals [49]. Melatonin receptors interact with sympathetic nervous system connectivity with white adipose tissue [50], presenting a potential mechanism for melatonin’s interaction with adiposity, however this concept remains unstudied. Photoperiodic Syrian hamsters in long-day conditions develop obesity, and this phenotype is completely reversed when the animals are exposed to short-day conditions. The reversal of weight gain occurs in the absence of an initial decrease in food intake, and is instead attributed to an increase in energy expenditure [51]. Short day and long day phenotypes are heavily dependent on the duration of melatonin signaling. Therefore, the decrease in melatonin signaling resulting from exposure to LAN could be a mechanism for an increase in adiposity without an increase in caloric intake. In addition, in Wistar rats under normal dark night conditions, melatonin reduced weight gain. However, a caveat to this study was that this occurred without altering overall metabolic activity, and was likely due instead to an increase in nocturnal activity [52]. Additionally, the strains of mice used in many studies of LAN and body weight produce very low levels of melatonin, while still showing metabolic dysregulation. Therefore, an alternative mechanism to blunted melatonin likely contributes to the metabolic effects seen with LAN exposure in mice.
The circadian system is also involved in metabolic regulation independent of melatonin rhythms. Several peripheral tissues involved in metabolic homeostasis have rhythmic expression of clock genes, and rhythmicity is disrupted with circadian dysregulation. Circadian disruption decreased rhythmic expression in all but one clock gene in the liver of mice exposed to dLAN [21], and in humans, circadian dysregulation altered clock gene expression in adipose tissue [53]. Clock-gene mutant mice have reduced insulin production, impaired glucose tolerance, and develop obesity [54]. In another study, disruption of either of two major clock genes, circadian locomotor output cycles kaput (Clock) or brain and muscle ARNT-like 1 (Bmal1) led to hypoinsulinemia and diabetes [55]. Within the past 20 years, orexin neurons in the lateral hypothalamus were discovered, and they provide a critical link between circadian rhythms and metabolic homeostasis. Orexin promotes wakefulness and food-seeking behavior in animals in response to ghrelin signaling [56]. Orexin neuron activity maintains a circadian rhythm, with elevated activity during the night in nocturnal animals, and elevated activity during the daytime in diurnal animals [57]. Likewise, ghrelin, along with several other hormones involved in metabolic regulation, also display a circadian rhythm [58,59,60]. Orexin neurons are also influenced by the SCN via indirect pathways, and ablation of the SCN eliminates orexin rhythmicity [61]. Consequently, metabolic homeostasis is intimately tied with circadian rhythms via orexin signaling. Therefore, circadian disruption in the SCN is likely to alter orexin signaling, and thus alter hunger via resulting disruption of leptin/ghrelin homeostasis. This is a likely pathway contributing to metabolic dysregulation that results from circadian disruption.
2.2. Light at night in endocrine-related cancers
A link between exposure to light at night and cancer risk was first hypothesized in 1987 [62]. In more recent years, shift work has been listed as a risk factor for cancer, and the incidence of cancer in shift workers has been the focus of numerous epidemiological studies. In a population-based study, the overall cancer risk was increased for prostate, colon, bladder, rectum, pancreas, and lung cancers among men who ever worked at night compared with men who never worked at night [63]. Women night shift workers have increased risk for ovarian, breast, squamous cell carcinoma, and malignant melanoma [64].
The increased risk of breast cancer in night shift workers has been of particular focus in epidemiological and rodent studies. Meta-analyses of epidemiological studies investigating breast cancer risk among women chronically exposed to LAN found an average increase of about 50% [65,66]. Nurses who had worked at least three nights per month had a significantly increased risk of breast cancer. This risk increased with the number of years spent working night shifts; risk was increased as much as 36% when shift work occurred over a 30 year period [67]. In another study in nurses, a significant increase in risk was discovered when women worked at least 6 consecutive night shifts per month in just 5 years [68]. This suggests a cumulative effect of shift work, where both an increase in consecutive nights and duration over time are both contributing factors. In addition, global levels of lighting were compared with the regional incidence of breast cancer. Mean illumination levels at night from 164 countries were correlated with breast cancer rates, while also considering relevant socioeconomic data that could explain the variation in cancer rates. This study found a strong correlation between levels of light at night and breast cancer rates [69].
Studies correlating shift work with other types of cancers are limited. A 19% increase in risk for prostate cancer was reported in men who regularly worked full-time rotating shifts when compared with men who had never worked full-time rotating shifts [70]. In addition, a study conducted in Spain reports a 37% increased risk of prostate cancer in men who worked over 28 years of shift work [71]. Likewise, an increased risk of prostate cancer was also reported in pilots and flight crews who engaged in transmeridian flights [72]. Women working rotating night shifts of at least three consecutive nights over 15 years had an increased risk of colorectal cancer, but a subsequent study indicates a short duration of sleep might be the contributing factor as opposed to light [73,74].
Animal studies that directly address light at night in cancer initiation are rare. Instead, available studies focus on the role of light or circadian dysregulation in tumor progression, and use xenografts or implanted cells. In addition, the majority of studies focus on circadian dysregulation by shifted light cycles, confounding the influence of light itself in tumorigenesis. Nevertheless, available studies indicate a direct role of light in cancer progression. Bright light intensities in rats with human breast cancer xenografts increased the rate of tumor progression [75]. Likewise, LAN had a positive effect on tumor growth-rate in mice inoculated with 4T1 [76], and LAN slowed the effects of therapy in breast cancer [77].
The suppression of rhythmic melatonin seen with LAN exposure likely contributes to tumor-progression. In 1978, Cohen and colleagues pointed out a role of the pineal gland in the etiology and treatment of breast cancer [78]. The inhibition of melatonin or pinealectomy has tumor-enhancing effects [79], and conversely administration of melatonin has tumor-reducing effects [80,81,82]. Also, melatonin receptors MT1 and MT2 are present in human xenografts of breast cancer, further supporting the ability of melatonin to directly influence tumor cells [75].
Melatonin is also a strong anti-inflammatory agent, and accordingly, exposure to dLAN exaggerates inflammatory responses. Mice exposed to 4 weeks of dLAN increased body temperature and elevated pro-inflammatory cytokine expression in microglia following lipopolysaccharide (LPS) administration [83]. LPS acts as an endotoxin and elicits strong immune responses when administered to animals. Dim LAN also exacerbated the inflammatory phenotype, with elevated TNFα and MAC1 gene expression in white adipose tissue, present under high fat diet conditions [84]. Many humans in the developed world are already exposed to increased inflammation due to diets high in fats, and therefore light exposure at night could be compounding the inflammatory response. A relationship between inflammation and tumor progression is not a new concept. Several inflammatory proteins such as IL-1, IL-6, and inflammatory cytokines promote tumor progression [85,86,87]. Therefore, the increased inflammation under artificial LAN could contribute to a tumor-promoting environment.
In addition to its anti-inflammatory properties, melatonin also has anti-estrogenic effects. This is an important consideration in estrogen-dependent tumors such as breast cancer. Melatonin can interact with estrogen receptor α [75], leading to decreased signaling of estrogens. Conversely, suppressed melatonin can increase estrogen/progesterone signaling, and therefore suppressed melatonin promotes estrogen-dependent tumor growth by increasing signaling of estrogens. Epidemiological studies support suppressed melatonin’s role in tumor promotion. In one study, there was decreased melatonin and an increased estradiol concentration in women engaging in shift work [88]. In another study, there is a significant inverse relationship between plasma melatonin concentrations and estrogen-receptor positive breast cancer [89]. Hence, increased e signaling of estrogens is a likely mechanism behind the role of dLAN and subsequent melatonin suppression in hormone-dependent tumor progression.
Lastly, the oncostatic properties of LAN could arise from the direct regulation of the cell cycle by the circadian clock. In rodents, 7% of clock-controlled genes regulate cell proliferation or apoptosis that mediate responses to DNA damage [90]. Furthermore, specific transcription factors with a role in the cell cycle such as cyclin b1, cdc2 kinase, c-Myc, p53, caspases, and cyclins, are regulated by clock genes [91,92]. Mice with mutations in clock genes show an increase in tumor development [93]. Therefore, disruption of the circadian clock from LAN could directly affect the cell cycle, and thus cell proliferation and apoptosis, leading to tumorigenesis. The initiation and progression of cancer are thus likely caused by a combination of suppressed melatonin and direct disruption of the cell cycle.
2.3. Other Disorders of the Endocrine System
Metabolic disorders and cancer are the most well-studied health effects of circadian dysregulation. However, clock disruption is also associated with several other hormonal systems. Upregulation of estrogenic signaling via suppression of melatonin was discussed in relation to estrogen-dependent cancers, but the canonical role of estrogens in female reproduction is also pertinent. Circadian dysregulation has been linked with reproductive dysfunction and subfertility in humans. In fact the circadian clock system is integrated into all aspects of the female hypothalamus-pituitary-gonad axis of the endocrine system. The clock is necessary to regulate neuroendocrine control of pituitary hormone gene expression and secretion. The ovaries of vertebrates, from fish to mammals, display cyclic clock gene expression [94,95]. The mammalian ovary, throughout follicle development, is regulated by a number of biological rhythms. The core clock proteins BMAL1, CLOCK, CRY1, CRY2, PER1, and PER2 all exhibit daily cyclic expression throughout follicle development [95], and circadian timing is involved in ovarian steroid hormone biosynthesis and secretion, ovulation, implantation, and parturition.
In addition to circadian clock gene involvement, melatonin also plays a major role in female reproductive function. Many studies indicate normal cyclic activity of melatonin has a positive effect on female reproduction [96]. Melatonin is produced in granulosa cells, cumulus oophorous, and the oocyte, which all contribute melatonin to the follicular fluid. Melatonin concentration in the follicular fluid is higher than in blood, and it acts to protect the oocyte from oxidative stress. There is a lack of studies that directly test the effects of light or circadian dysregulation on reproductive fitness. However, given the necessity of both rhythmic clock gene expression and cyclic melatonin in successful follicular development, light is likely to have negative consequences on reproduction.
The effect of LAN on the hypothalamus-pituitary-adrenal (HPA) axis depends on the exposure. Constant exposure to LAN activates the HPA axis, increasing blood glucocorticoid concentrations. Even short durations of bright light exposure at night can significantly increase glucocorticoids [39,40]. In humans an exposure to 40 lux of short wavelength light upon waking increased levels of cortisol [97]. Because cortisol is important for wakefulness, when timed properly, this could be beneficial. However, light presented earlier could inappropriately increase cortisol levels and increase arousal during sleep. In contrast, dim LAN (5 lux) does not increase circulating glucocorticoids in mice, and therefore effects seen with dLAN exposure such as weight gain and increased immune response are independent of increased glucocorticoids [21,36,41,98]. Thus, the level of the light is important in considering physiological outcomes.
3. Agricultural Implications of Light at Night
It is important recognize that although humans are the source of LAN, we are not the only recipients of its effects. Agricultural animals are often housed in rural settings, but the pervasiveness of light pollution does not exclude them from LAN exposure. Perhaps fertility could be improved in livestock and fisheries if dark nights are assured. Conversely, because data indicate LAN can induce an obese phenotype, exposure might assist in increasing the size of livestock animals via metabolic dysregulation. The growth system is an additional target of LAN not previously discussed in relation to human health. Growth factors (growth hormone (GH), insulin-dependent growth factor 1 (IGF1), and IGF1 receptor) in vertebrates display daily rhythms, and the response to growth hormone administration is dependent on the time of day. In teleost fish, the strongest effect from GH administration was observed when given at mid-darkness [99]. Administration of GH in the middle of the dark period significantly reduced pituitary GH and enhanced Igf1 expression in the liver. In addition, melatonin promotes bone growth [100], and suppressed melatonin and circadian rhythmicity could inhibit the growth axis. Therefore, the effects of LAN in agriculture could increase body weight due to metabolic dysregulation, but also could inhibit growth via the endocrine growth axis. An additional consideration is in developmental exposure to LAN. Although developmental research in this area is sparse, exposure to dLAN (3 lux) during development prevented increases in songbird mass [101]. Growth is extremely important during development and a strong indicator of overall health. LAN exposures could have different effects during development compared with adulthood. Further work is needed to parse out the effects of LAN specific to agricultural-relevant species. In addition to considerations in human health, the physiological effects of LAN could have implications on best practices in livestock and fisheries management.
4. Ecological Consequences of Light at Night
Many wildlife populations tend to be away from urban centers. However, as made clear by the singing birds in the morning and the deer crossing signs on the highway, wildlife is also pervasive in urban/suburban settings, and therefore is also vulnerable to the effects of artificial lighting. Indeed, several studies indicate artificial LAN alters behavior and physiology in wild species. In great tits (Parus major), there is a strong dose-dependent effect of LAN, in which the onset of activity was increasingly advanced, and overall nighttime activity was increased with higher light intensities at night. In addition, increased intensities of LAN also decreased melatonin levels, suggesting artificial LAN disrupts normal day/night behavior via suppressed melatonin [102]. Additionally, while controlling for additional time of day cues, LAN remained positively correlated with corticosterone and negatively correlated with estrone levels in female blackbirds [103]. European blackbirds exposed to very low levels of LAN (0.3 lux), which is pervasive in urban, suburban, and even rural areas, exited their photorefractory period nearly one month earlier than birds exposed to dark nights [104]. These birds were monitored for a second year, and in the second year birds exposed to dLAN showed no sign of reproductive activity, suggesting that even low levels of LAN might suppress the signal to exit the seasonal photorefractory period. If this is the case, then dLAN has the potential to severely limit fitness in these animals. In both the previous study by Dominoni and colleagues, as well as a study conducted in Florida scrub-jays by Schoech and coauthors, birds in an urban/suburban habitat breed earlier than birds in their native habitat. This alone indicates circadian dysregulation from LAN plays a role in the early exit from photorefraction. In addition, male Florida scrub-jays exposed to LAN had a depressed concentration of luteinizing hormone (LH) and females had low testosterone concentrations. Estradiol levels were reduced in both sexes and the typical correlation between T and E2 levels was disrupted [105]. These studies strongly suggest disruption at each level of the HPG from exposure to LAN.
Whereas the majority of studies in wildlife species have been conducted in birds, studies indicate mammalian effects as well. The nocturnal mouse lemur (Microcebus murinus) was exposed to light that mimicked streetlight (~50 lux) at night for 5 weeks and compared with lemurs exposed to light simulating moonlight (0.3 lux). Lemurs exposed to LAN had significantly decreased urinary concentrations of 6-sulfatoxymelatonin [106], melatonin’s major urinary metabolite, which has previously been closely correlated with plasma melatonin levels in blood [107]. Lemurs exposed to LAN also increased testis size and plasma T concentration just 2 weeks after entering light treatment, indicating premature sexual recrudescence [106]. It remains unspecified whether non-laboratory mammals are also affected by exposure to LAN, and the repercussions to their overall fitness and the fitness of the ecosystem have not been determined.
An additional reproductive consideration in wildlife arises because many vertebrates are seasonal breeders, mating only during the spring and summer. Thus, individuals must calculate the optimal time to breed so spermatogenesis, territorial defense, migration, or any other time-consuming adaptations can be developed prior to the onset of the breeding season. Consequently, seasonally breeding vertebrate animals often must detect and respond to environmental cues that accurately signal, well in advance, the arrival or departure of seasons favoring reproductive success. The annual changes in day length serve as a precise reference for the time of year, and the principal physiological mediator of day length is melatonin. To make appropriate physiological and behavioral modification necessary to initiate or terminate breeding at the correct time of year, animals must be able to discern long days from short days. Short durations of melatonin secretion signal long-days, and conversely, long durations of melatonin signal short days. Short days reverse the long-day obese phenotype in hamsters through an increase in energy expenditure [51]. LAN, through the suppression of melatonin signaling, could thus alter energy expenditure and reverse the appropriate weight-loss in short day conditions. Laboratory studies strongly suggest that exposure to dLAN is sufficient to block adaptive short-day responses [108] and desynchronize seasonal reproduction in wild mammals [109].
The effects to wildlife presented thus far have focused on disruption to endocrine systems. However, this is in no way an exhaustive account of the consequences of anthropogenic artificial LAN on wildlife populations. LAN can also alter behaviors in animals, which can reduce fitness both independently and in combination with adverse effects to physiology. In many urban and suburban areas, sky brightness resulting from urban sky glow is greater than nights with a full moon [2]. Because natural lunar cycles alone exert dramatic effects on predator-prey interactions, then artificial LAN could have equal, if not more dramatic changes on ecological dynamics. Indeed, artificial lighting exerts strong effects on foraging behavior and predation [110]. The precise mechanistic basis for such changes in foraging behaviors remains elusive, but foraging behavior is under neuroendocrine regulation through ghrelin signaling. Peripheral and central ghrelin induces food foraging, hoarding, and intake in Siberian hamsters [111,112]. An interaction between ghrelin and natural melatonin rhythms might contribute to these behavioral changes induced by LAN [113]. Melatonin regulates food intake in mammals [114], thus changes in melatonin and/or other physiological signals resulting from light exposure may alter foraging behavior.
As noted, LAN disrupts clock function, which leads to elevated body mass and body fat in laboratory animals [21]. Although gaining body fat with reduced foraging effort and food intake in response to light pollution may seem beneficial to free living animals, there seem to be significant potential fitness costs. Timing of food intake is shifted by exposure to just 5 lux of light each night for 4 weeks, which could influence predator-prey dynamics in the wild. Predator-prey interactions are important determinants of many decisions made by animals, ranging from foraging behavior to mate choice [reviewed in 115,116]. It is well established that dynamics of predator-prey interactions change as a function of ambient light levels [117–119]. Independent of circadian regulation, light drives individuals to make activity decisions either directly by changing the risk of being seen by a predator (Predation Risk Hypothesis [115]) or indirectly by altering prey availability and thus changing the payoff of foraging during times of high illumination (Foraging Efficiency Hypothesis [118]). These ideas are not mutually exclusive; LAN has both direct and indirect effects [120]. Thus, changes in illumination levels affect not only the behaviors of predators, but also the behaviors of their prey, potentially resulting in large-scale ecosystem changes [121].
Migratory species are also influenced by light. The magnetic compass of migratory birds might be partially light-dependent. Retinal neurons that express cryptochromes have high activity when night-migratory birds perform magnetic compass orientation [122]. Furthermore, many observations of birds being disoriented or entrapped by lighted structures at night have been reported [123,124,125]. Contrary to the mammalian effects of light at night, magnetic orientation in birds seems to be most disrupted by red wavelengths of light [126], and least affected by light in the blue/green spectrum. Disrupted migration in birds can lead to altered predator/prey interactions, altered reproduction, or mortality.
Invertebrates also are not exempt from the effects of artificial LAN. One study in moths indicates exposure to artificial LAN reduces sex pheromone production and also alters the chemical composition of the pheromones in females. This could make the females less attractive to the males and negatively impact reproduction [127]. Although the health of moths may not be of great concern to some, the repercussions to the ecosystem are poorly understood. Artificial LAN can also alter immigration/emigration through local regions based on either repulsion or attraction to light [128]. Combined with adverse effects to reproductive physiology, this alone could have great negative outcomes to reproductive fitness in wildlife, and further work is necessary to elucidate and limit effects of LAN on ecosystems.
5. Conclusions
We reviewed the evidence of endocrine disruption via exposure to LAN in human health, agriculture, and wildlife. The full spectrum of effects is still to be determined, but the consequences are increasingly apparent. Data on the effects of LAN on human health are on the rise, and can likely be applied in agricultural practices as well. Wildlife is not excluded from deleterious effects, and exposure to LAN likely provokes a fitness cost. Therefore, LAN should be considered in urban planning and choice of lighting options. With the evidence available, we can assert that LAN has endocrine disrupting properties, and the effects of LAN should be considered in human health, agriculture, and wildlife management.
Figure 1.
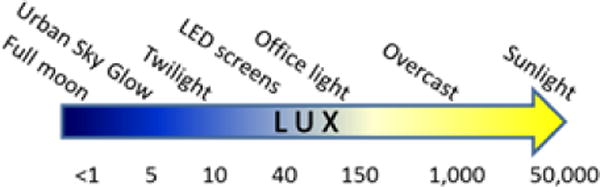
Approximate levels of light emission from common sources.
Figure 2.
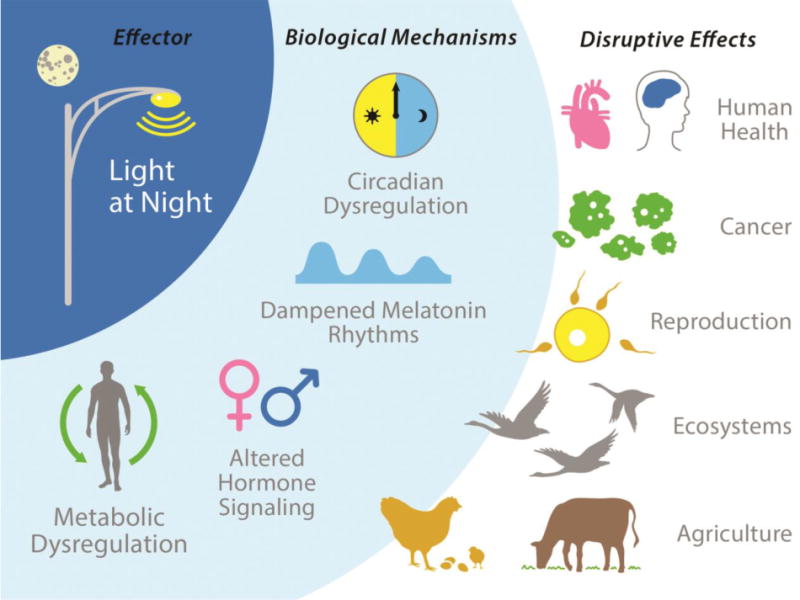
Exposure to light at night interferes with several biological mechanisms and disrupts endocrine signaling.
Highlights.
Artificial light at night disrupts endocrine signaling in humans and wildlife
Effects of artificial light at night in humans could translate to agricultural livestock
Artificial light at night is an environmental endocrine disruptor
Acknowledgments
This review was supported by the National Institutes of Health [R21CA202745 and R01NS092388].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Cinzano P, Falchi F, Elvidge CD. The first world atlas of artificial night sky brightness. Mon Not R Astron Soc. 2001;328:689–707. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falchi F, Cinzano P, Duriscoe D, Kyba C, Elvidge CD, Baug K. The new world atlas of artificial night sky brightness. Sci Adv. 2016;10 doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colin J, Timbal J, Boutelier C, Houdas Y, Siffre M. Rhythm of the rectal temperature during a 6-month free-running experiment. J Appl Physiol. 1968;25:170–176. doi: 10.1152/jappl.1968.25.2.170. [DOI] [PubMed] [Google Scholar]
- 5.Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Dekker, Inc; 1999. pp. 149–180. [Google Scholar]
- 6.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarroch EE. Suprachiasmatic nucleus and melatonin: reciprocal interactions and clinical correlations. Neurology. 2008;71:594–598. doi: 10.1212/01.wnl.0000324283.57261.37. [DOI] [PubMed] [Google Scholar]
- 10.Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- 11.Arendt J. Melatonin. Clin Endocrinol. 1988;29:205–229. doi: 10.1111/j.1365-2265.1988.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 12.Orth DN, Island DP, Liddle GW. Experimental alteration of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. J Clin Endocrinol Metab. 1967;27:549–555. doi: 10.1210/jcem-27-4-549. [DOI] [PubMed] [Google Scholar]
- 13.Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Patel YC, Burger HG. Serum thyrotropin (TSH) in pituitary and/or hypothalamic hypothyroidism: Normal or elevated basal levels and paradoxical responses to thyrotropin-releasing hormone. J Clin Endocrinol Metab. 1973;37:190–196. doi: 10.1210/jcem-37-2-190. [DOI] [PubMed] [Google Scholar]
- 15.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJM. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrionol Metab. 2008;93:2300–2306. doi: 10.1210/jc.2007-2674. [DOI] [PubMed] [Google Scholar]
- 16.Tamura H, Takasaki A, Miwa I, Taniquchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Suqino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Gan L, Luo D, Sun C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res. 2017;62 doi: 10.111/jpi.12383. [DOI] [PubMed] [Google Scholar]
- 18.Arvanitogiannis A, Stewart J, Amir S. Conditioned stimulus control in the circadian system: Two tales tell one story. J Biol Rhythms. 2000;15:292–293. doi: 10.1177/074873000129001378. [DOI] [PubMed] [Google Scholar]
- 19.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 20.Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J Neurosci. 2013;33:13081–13087. doi: 10.1523/JNEUROSCI.5734-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28:262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touitou Y, Fevre M, Lagoguey M, Carayon A, Bogdan A, Reinberg A, Beck H, Cesselin F. Age- and mental health-related circadian rhythms of plasma levels of melatonin, prolactin, luteinizing hormone and follicle-stimulating hormone in man. J Endocrinol. 1981;91:467–475. doi: 10.1677/joe.0.0910467. [DOI] [PubMed] [Google Scholar]
- 23.Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. doi: 10.1016/j.lfs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- 25.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinol. 2011;36:1062–1069. doi: 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Fonken L, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms. 2012;27:319–327. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff. 2009;28:822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 29.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Meab Disord. 2003;27:1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 30.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP., Jr Impact of circadian misalignment on energy metabolism during simulated nightshift work. PNAS. 2014;111:17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen AB, Stayner L, Hansen J, Andersen AJ. Night shift work and incidence of diabetes in the Danish nurse cohort. Occup Environ Med. 2016;73:262–268. doi: 10.1136/oemed-2015-103342. [DOI] [PubMed] [Google Scholar]
- 32.Rybnikova NA, Haim A, Portnov BA. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int J Obes. 2016;40:815–823. doi: 10.1038/ijo.2015.255. [DOI] [PubMed] [Google Scholar]
- 33.Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care. 2015 doi: 10.2337/dc15-0302. DOI: https://doi.org/10.2337/dc15-0302. [DOI] [PMC free article] [PubMed]
- 34.Figueiro MG, Radetsky L, Plitnick B, Rea MS. Glucose tolerance in mice exposed to light-dark stimulus patterns mirroring dayshift and rotating shift schedules. Sci Reports. 2017;7 doi: 10.1038/srep40661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opperhuizen AL, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia. 2017 doi: 10.1007/s00125-017-4262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. PNAS. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borniger JC, Maurya SK, Periasamy M, Nelson RJ. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol Int. 2014;31:917–925. doi: 10.3109/07420528.2014.926911. [DOI] [PubMed] [Google Scholar]
- 38.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2013;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson AL, Downs CT. Light interference and melatonin affects digestion and glucocorticoid metabolites in striped mouse. Biol Rhythm Res. 2015;6:929–939. [Google Scholar]
- 41.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugden DS. Melatonin biosynthesis in the mammalian pineal gland. Experientia. 1989;45:922–928. doi: 10.1007/BF01953049. [DOI] [PubMed] [Google Scholar]
- 44.Chakir I, Dumont S, Pevet P, Ouarour A, Challet E, Vuillez P. Pineal melatonin is a circadian time-giver for leptin rhythm in Syrian hamsters. Front Neurosci. 2015;9 doi: 10.3389/fnins.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan D, Reiter RJ, Agil A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res. 2013;55:416–423. doi: 10.1111/jpi.12089. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez-Aranda A, Fernandez-Vasquez G, A-Serrano MM, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zucker diabetic fatty rats. J Pineal Res. 2014;57:103–109. doi: 10.1111/jpi.12147. [DOI] [PubMed] [Google Scholar]
- 47.Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, Casteilla L, Penicaud L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinol. 2003;144:5347–5352. doi: 10.1210/en.2003-0693. [DOI] [PubMed] [Google Scholar]
- 48.Puchalski SS, Green JN, Rasmussen DD. Melatonin effect on rat body weight regulation in response to high-fat diet at middle age. Endocrine. 2003;21:163–167. doi: 10.1385/ENDO:21:2:163. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen NLT, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2014;6:R375–R386. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 51.Wade GN, Bartness TJ. Seasonal obesity in Syrian hamsters: effects of age, diet, photoperiod, and melatonin. Am J Physiol. 1984;247:R328–334. doi: 10.1152/ajpregu.1984.247.2.R328. [DOI] [PubMed] [Google Scholar]
- 52.Terron MP, Delgado-Adamez J, Pariente JA, Barriga C, Paredes SD, Rodriguez AB. Melatonin reduces body weight gain and increases nocturnal activity in male Wistar rats. Physiol Behav. 2013;118:8–13. doi: 10.1016/j.physbeh.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 54.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinzemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami Ki, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 57.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 58.Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 59.Ruiter M, La Fleur SE, Van Heijningen C, Van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 60.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, Mignot E. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27:619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 62.Stevens RG. Electric power use and breast cancer: a hypothesis. Am J Epidemiol. 1987;125:556–561. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- 63.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 64.Lie J, Andersen A, Kjaerheim K. Cancer risk among 43,000 Norwegian nurses. Scand J Work Environ Health. 2007;33:66–73. [PubMed] [Google Scholar]
- 65.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, Huang S, Chen J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37:197–206. doi: 10.1016/j.canep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 68.Lie J, Kjuus H, Zienolddiny S, Haugen A, Stevens RG, Kjaerheim Kristina. Night work and breast cancer risk among Norwegian nurses: Assessment by different exposure metrics. Am J Epidemiol. 2011;173:1272–1279. doi: 10.1093/aje/kwr014. [DOI] [PubMed] [Google Scholar]
- 69.Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol Int. 2008;25:65–81. doi: 10.1080/07420520801921572. [DOI] [PubMed] [Google Scholar]
- 70.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 71.Papantoniou K, Castano-Vinyals G, Espinosa A, Aragones N, Perez-Gomez B, Burgos J, Gomez-Acebo I, Llorca J, Peiro R, Jimenez-Moleon JJ, Arredondo F, Tardon A, Pollan M, Kogevinas M. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137:1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 72.Rafnsson V, Hrafnkelsson J, Tulinius H. Incidence of cancer among commercial airline pilots. Occup Environ Med. 2000;57:175–179. doi: 10.1136/oem.57.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schernhammer ES, Laden F, Speizer FE, Willet WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Nat Cancer Ins. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 74.Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841–847. doi: 10.1002/cncr.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. Melatonin-depleted blood from premenopausal woman exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 76.Schwimmer H, Metzer A, Pilosof Y, Szyf M, Machnes ZM, Fares F, Harel O, Haim A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol Int. 2014;31:144–150. doi: 10.3109/07420528.2013.842925. [DOI] [PubMed] [Google Scholar]
- 77.Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, Blask DE, Hill SM. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014;74:4099–4110. doi: 10.1158/0008-5472.CAN-13-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen M, Lippman M, Chabner B. Role of the pineal gland in aetiology and treatment of breast cancer. Lancet. 1978;14:814–816. doi: 10.1016/s0140-6736(78)92591-6. [DOI] [PubMed] [Google Scholar]
- 79.Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 1981;41:4432–4436. [PubMed] [Google Scholar]
- 80.Anisimov VN, Popovich IG, Zabezhinski MA. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997;18:1549–1553. doi: 10.1093/carcin/18.8.1549. [DOI] [PubMed] [Google Scholar]
- 81.Cini G, Coronnello M, Mini E, Neri B. Melatonin’s growth-inhibitory effect on hepatoma AH 130 in the rat. Cancer Lett. 1998;125:51–59. doi: 10.1016/s0304-3835(97)00480-1. [DOI] [PubMed] [Google Scholar]
- 82.Cos S, Fernandez R, Guezmes A, Sanchez-Barcelo EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–4390. [PubMed] [Google Scholar]
- 83.Fonken LK, Weil ZM, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun. 2013;34:159–163. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Inflammation associated with a high-fat diet in male mice. Endocrinol. 2013;154:3817–3825. doi: 10.1210/en.2013-1121. [DOI] [PubMed] [Google Scholar]
- 85.Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–4687. [PubMed] [Google Scholar]
- 86.Tricot G. New insights into role of microenvironment in multiple myeloma. Lancet. 2000;355:248–250. doi: 10.1016/S0140-6736(00)00019-2. [DOI] [PubMed] [Google Scholar]
- 87.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, Carrascal T, Walsh P, Reznikov LL, Kim SH, Novick D, Rubinstein M, Dinarello CA. IL-18 regulates IL-1β-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. PNAS. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schernhammer ES, Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer. 2004;90:941–943. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danforth DN, Tamarkin L, Mulvihill JJ, Bagley CS, Lippman ME. Plasma melatonin and the hormone-dependency of human breast cancer. J Clin Oncol. 1985;3:941–948. doi: 10.1200/JCO.1985.3.7.941. [DOI] [PubMed] [Google Scholar]
- 90.Fu L, Lee CC. The circadian clock: Pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 91.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 92.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 93.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan ZA, Yumnamcha T, Rajiv C, Sanjita Devi H, Mondal G, Devi SD, Bharali R, Chattoraj A. Melatonin biosynthesizing enzyme genes and clock genes in ovary and whole brain of zebrafish (Danio rerio): Differential expression and a possible interplay. Gen Comp Endocrinol. 2016;233:16–31. doi: 10.1016/j.ygcen.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Wiggins G, Legge M. Cyclic variation of cellular clock proteins in the mouse estrous ovary. J Reprod Infertil. 2016;17:192–198. [PMC free article] [PubMed] [Google Scholar]
- 96.Tamura H, Takasaki A, Taketani T, Tanabe M, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N. Melatonin and female reproduction. J Obstet Gynaecol Res. 2014;40:1–11. doi: 10.1111/jog.12177. [DOI] [PubMed] [Google Scholar]
- 97.Figueiro MG, Rea MS. Short-wavelength light enhances cortisol awakening response in sleep-restricted adolescents. Int J Endocrinol. 2012;2012:301935. doi: 10.1155/2012/301935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costa LS, Rosa PV, Fortes-Silva R, Sanchez-Vazquez FJ, Lopez-Olmeda JF. Daily rhythms of the expression of genes from the somatotropic axis: The influence on tilapia (Oreochromis niloticus) of feeding and growth hormone administration at different times. Comp Biochem Physiol C Toxicol Pharmacol. 2015;181–182:27–34. doi: 10.1016/j.cbpc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Maria S, Witt-Enderby PA. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res. 2014;56:115–125. doi: 10.1111/jpi.12116. [DOI] [PubMed] [Google Scholar]
- 101.Raap T, Casasole G, Costantini D, AbdElgawad H, Asard H, Pinxten R, Eens M. Artificial light at night affects body mass but not oxidative status in free-living nestling songbirds: an experimental study. Sci Rep. 2016;6:35626. doi: 10.1038/srep35626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Jong M, Ouyang JQ, Da Silva A, van Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Philos Trans R Soc Lond B Biol Sci. 2015 doi: 10.1098/rstb.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Russ A, Reitemeier S, Weissmann A, Gottschalk J, Einspanier A, Klenke R. Seasonal and urban effects on the endocrinology of a wild passerine. Ecol Evol. 2015;5:5698–5710. doi: 10.1002/ece3.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dominoni DM, Quetting M, Partecke J. Long-term effects of chronic light pollution on seasonal functions of European blackbirds (Turdus merula) PLoS ONE. 2013 doi: 10.1371/journal.pone.0085069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schoech SJ, Bowman R, Hahn TP, Goymann W, Schwabl I, Bridge ES. The effects of low levels of light at night upon the endocrine physiology of western scrub-jays (Aphelocoma californica) J Exp Zool A Ecol Genet Physiol. 2013;319:527–538. doi: 10.1002/jez.1816. [DOI] [PubMed] [Google Scholar]
- 106.Le Tallec T, Thery M, Perret M. Melatonin concentrations and timing of seasonal reproduction in male mouse lemurs (Microcebus murinus) exposed to light pollution. J Mammal. 2016;97:753–760. [Google Scholar]
- 107.Baskett JJ, Cockrem JF, Antunovich TA. Sulphatoxymelatonin excretion in older people: relationship to plasma melatonin and renal function. J Pineal Res. 1998;24:58–61. doi: 10.1111/j.1600-079x.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 108.Ikeno T, Weil ZM, Nelson RJ. Dim light at night disrupts the short-day response in Siberian hamsters. Gen Comp Endocrinol. 2014;197:56–64. doi: 10.1016/j.ygcen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Robert KA, Lesku JA, Partecke J, Chambers B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. PNAS. 2015 doi: 10.1098/rspb.2015.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown JS, Kotler BP, Smith RJ, Wirtz WO. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia. 1988;76:408–415. doi: 10.1007/BF00377036. [DOI] [PubMed] [Google Scholar]
- 111.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 112.Thomas MA, Ryu V, Bartness TJ. Central ghrelin increases food foraging/hoarding that is blocked by GHSR antagonism and attenuates hypothalamic paraventricular nucleus neuronal activation. Am J Physiol Integr Comp Physiol. 2016;310:R275–R285. doi: 10.1152/ajpregu.00216.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarlow EM, Hau M, Anderson DJ, Wikelski M. Diel changes in plasma melatonin and corticosterone concentrations in tropical Nazca boobies (Sula granti) in relation to moon phase and age. Gen Comp Endocrin. 2003;133:297–304. doi: 10.1016/s0016-6480(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 114.Nelson RJ, Drazen DL. Melatonin mediates seasonal adjustments in immune function. Reprod Nutr Dev. 1999;39:383–398. doi: 10.1051/rnd:19990310. [DOI] [PubMed] [Google Scholar]
- 115.Mougeot F, Bretagnolle V. Predation risk and moonlight avoidance in nocturnal seabirds. J Avian Biol. 2000;31:376–386. [Google Scholar]
- 116.Lima SL. Putting predators back into behavioral predator-prey interactions. Trends Ecol Evol. 2002;17:70–75. [Google Scholar]
- 117.Morrison DW. Lunar phobia in the neotropical fruit bat, Artibeus jamaicensus (Chiroptera: Phyllostomidae) Anim Behav. 1978;26:852–855. [Google Scholar]
- 118.Clarke JA. Moonlight’s influence on predator/prey interactions between short eared owls (Asio flammeus) and deer mice (Peromyscus maniculatus) Behav Ecol Sociobiol. 1983;13:205–209. [Google Scholar]
- 119.Daly M, Behrends PR, Wilson MI, Jacobs LF. Behavioural modulation of predation risk: Moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim Behav. 1992;44:1–9. [Google Scholar]
- 120.Imber MJ. Behaviour of petrels in relation to the moon and artificial lights. Notornis. 1975;22:302–306. [Google Scholar]
- 121.Longcore T, Rich C. Ecological light pollution. Front Ecol Environ. 2004;2:191–198. [Google Scholar]
- 122.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dick MH, Donaldson W. Fishing vessel endangered by crested auklet landings. Condor. 1978;80:235–236. [Google Scholar]
- 124.Ogden LJE. Collision course: the hazards of lighted structures and windows to migrating birds. Toronto Canada: World Wildlife Fund Canada and Fatal Light Awareness Program; 1996. [Google Scholar]
- 125.Wiese FK, Montevecchi WA, Davoren GK. Seabirds at risk around offshore oil platforms in the North-west Atlantic. Mar Pollut Bull. 2001;42:1–18. doi: 10.1016/s0025-326x(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 126.Wiltschko W, Munro U, Ford H, Wiltschko R. Magnetic inclination compass: A basis for the migratory orientation of birds in the Northern and Southern Hemisphere. Experientia. 1993;49:167–170. [Google Scholar]
- 127.Van Geffen KG, Groot AT, Van Grunsven RHA, Donners M, Berendse F, Veenendaal EM. Artificial night lighting disrupts sex pheromone in a noctuid moth. Ecol Entomol. 2015;40:401–408. [Google Scholar]
- 128.Gaston KJ, Bennie J. Demographic effects of artificial nighttime lighting on animal populations. Environ Rev. 2014;22:323–330. [Google Scholar]


