Abstract
Background
Siglec-8 is a CD33 subfamily cell surface receptor that is selectively expressed on human eosinophils. Following cytokine-priming, Siglec-8 mAb or glycan ligand binding causes eosinophil apoptosis associated with reactive oxygen species (ROS) production. Most CD33-related Siglecs function as inhibitory receptors, but the ability of Siglec-8 to stimulate eosinophil ROS production and apoptosis suggests that Siglec-8 may instead function as an activating receptor.
Objective
To determine the role of IL-5 priming and to identify the signaling molecules involved in Siglec-8 function for human eosinophils.
Methods
We used a mAb and/or a multimeric synthetic sulfated sialoglycan ligand recognizing Siglec-8, in combination with integrin blocking antibodies, pharmacological inhibitors, phosphoproteomics and western blot analysis, to define the necessity of various proteins involved in Siglec-8 function for human eosinophils.
Results
Cytokine priming was required to elicit the unanticipated finding that Siglec-8 engagement promotes rapid β2-integrin dependent eosinophil adhesion. Also novel was the finding that this adhesion was necessary for subsequent ROS production and apoptosis. Siglec-8-mediated ROS was generated via NADPH oxidase activation, because pretreatment of eosinophils with catalase (an extracellular superoxide scavenger) or NSC23766 (a Rac GTPase inhibitor) completely inhibited Siglec-8 mediated eosinophil apoptosis. Finally, engagement of Siglec-8 on IL-5 primed eosinophils resulted in increased phosphorylation of Akt, p38 and JNK1 that was also β2-integrin dependent; pharmacologic inhibition of these kinases completely prevented Siglec-8-mediated eosinophil apoptosis.
Conclusions
These data demonstrate that Siglec-8 uniquely functions as an activating receptor on IL-5 primed eosinophils via a novel pathway involving regulation of β2-integrin-dependent adhesion, NADPH oxidase and a subset of protein kinases.
Keywords: Eosinophil, Siglec-8, β2-integrin, apoptosis, phosphoproteomics, NADPH oxidase, p38, JNK, Akt
Introduction
Eosinophils are innate immune leukocytes involved in host protection against helminth infections, yet under different circumstances, eosinophils can be pro-inflammatory effector cells through their inappropriate localization, activation, and release of pro-inflammatory substances, contribute to the pathophysiology of disorders including asthma, rhinitis, certain gastrointestinal disorders and atopic dermatitis.1, 2 Current treatments for these conditions include mediator antagonists, glucocorticosteroids, biologics and other anti-inflammatory drugs that reduce allergic cell numbers and inhibit mediator functions, but they are neither fully effective nor curative or disease modifying.3, 4 It thus remains essential to identify additional targets on these and other allergic effector cells for therapeutic exploitation.
Siglecs (sialic acid-binding immunoglobulin-like lectins) are single-pass transmembrane cell surface proteins found predominantly on leukocytes and are characterized by their ability to bind specific sialic acid structures. The Siglec family is divided into two subsets, a highly conserved subset and a rapidly evolving CD33-related subset, the latter of which includes Siglec-8.5, 6 Siglec-8 is an eosinophil-selective surface receptor that contains an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM), thought to be responsible for inhibitory signal transduction.7–9 Previous work has shown that multimeric engagement of Siglec-8 on normal, non-cytokine primed eosinophils causes apoptosis in a caspase-dependent manner.10 However, in IL-5 primed human eosinophils, Siglec-8 engagement induces loss of mitochondrial membrane potential and ROS production, enhances activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway, and results in apoptosis that is instead dependent on mitochondrial damage and ROS production.11–14 This suggests that Siglec-8 may function as an activating receptor.
The intracellular mechanisms and pathways of signaling for most Siglecs are not well understood. Several studies have shown that antibody binding of CD33-related Siglecs leads to rapid phosphorylation of Src family kinases (SFKs) that mediate the recruitment of Src-homology region 2 domain-containing phosphatases such as SHP-1 and SHP-2, presumably to intracellular Siglec ITIM domains.15–19 In an effort to more fully characterize the signaling mechanisms mediated via Siglec-8, we discovered that its engagement on eosinophils leads to a rapid enhancement of eosinophil adhesion that is entirely β2-integrin mediated and requires IL-5 priming. We also show that blocking of β2-integrins in IL-5 primed human eosinophils completely inhibits Siglec-8-mediated apoptosis and ROS production. These responses require the intracellular activation of NADPH oxidase and a subset of protein tyrosine kinases, thus identifying a novel signaling pathway by which Siglec-8 actually functions as an activating receptor.
Materials and Methods
Eosinophil isolation
Written informed consent for blood donation (up to 180 mL) was obtained using an institutional review board–approved protocol at the Northwestern University Feinberg School of Medicine. Eosinophils from mildly allergic and non-allergic donors were purified from peripheral blood using density gradient centrifugation, erythrocyte hypotonic lysis, and CD16 immunomagnetic negative selection (Miltenyi Biotec, San Diego, CA) as described.20 Purity and viability were consistently >95% as determined by flow cytometry and DAPI exclusion (Thermo Scientific, Waltham, MA). Cells were cultured in RPMI 1640 medium containing 10% FCS and antibiotics (“complete medium”, all from Thermo Scientific) with or without 30 ng/ml rhIL-5 (R&D Systems, Minneapolis, MN) for 18–24 hr as indicated.
Drugs and monoclonal antibody (mAb) reagents
LY294002, SB203580, SP600125, NSC23766 were obtained from Selleckchem (Houston, TX), mitoTempo and catalase were obtained from Sigma-Aldrich (St. Louis, MO) and carboxy-PTIO was obtained from Cayman Chemical (Ann Arbor, MI). Fluorochrome-conjugated or unconjugated mouse IgG1 blocking mAbs against human CD11b (clone ICRF44), CD18 (clone TS1/18) and activated CD11b (clone CBRM1/5) as well as an irrelevant mouse IgG1 and human IgG4 control mAbs were obtained from BioLegend (San Diego, CA). Mouse IgG1 anti-Siglec-8 mAb (clone 2C4) was used as previously reported8. Chimeric IgG4 anti-Siglec-8 mAb (clone c2E2, made from clone 2E2 that recognizes the same epitope as 2C410 was generated by molecularly swapping the mouse IgG1 Fc region with human IgG4. In addition, c2E2 F(ab′)2 fragments were generated using immobilized pepsin (Thermo Scientific). Briefly, c2E2 was initially dialyzed overnight against 20 mM sodium acetate and 100 mM sodium chloride (pH 4.5) and then incubated with immobilized pepsin for 4 h at 37°C. The pepsin reaction was stopped by raising the pH to 8.0. Non-digested and Fc fragments were removed using MabSelect Resin (GE Healthcare Life Sciences, Pittsburgh, PA) and size exclusion chromatography. Both c2E2 and c2E2 F(ab′)2 fragments were kindly provided by John Leung and Nenad Tomasevic (Allakos, San Carlos, CA). Clear, flat-bottomed 48-well Falcon tissue culture-treated plates were obtained from ThermoFisher Scientific (Pittsburgh, PA).
Eosinophil adhesion assay
Freshly isolated or rhIL-5 primed eosinophils (5 × 104) were incubated for 1 h at 37°C in complete medium with 2.5 μg/mL mAb 2C4 or IgG1 control in a 48-well tissue culture-treated plate. Changes in morphology were assessed using an Olympus 1X71 CellSens Light Microscope (16x) (Tokyo, Japan). To quantify adhesion, freshly isolated or rhIL-5 primed eosinophils (5 × 104) were incubated with either mAb 2C4, IgG1 control, a specific artificial Siglec-8 ligand (a polyacrylamide polymer decorated with 6′-sulfated sialyl N-acetyl-D-lactosamine [6′-O-Sulfo-3′SLN-PAA]), or a control PAA polymer decorated instead with Lewis X (Lewis X-PAA)21, both kindly provided by Dr. Corwin Nycholat (The Scripps Research Institute, La Jolla, CA) for 2 h at 37°C in complete medium and non-adherent cells were removed by pipetting up and down twice, collected and their numbers determined using a Cellometer AutoX4 Cell Counter (Nexcelom Biosciences, Lawrence, MA). Percent adhesion was calculated by subtracting the number of non-adherent cells from the number of cells added.
Eosinophil degranulation assay
rhIL-5 primed eosinophils (5 × 104) were incubated in complete medium at 37°C with mAb 2C4 or IgG1 control for various time points. Next, eosinophil cationic protein (ECP) levels in supernatants diluted 1:10 with assay buffer were assessed using Mesacup ECP Test (MLB, Woburn, MA) per the manufacturer’s instructions and normalized to ng/mL per 106 cells, with the limit of detection of the assay being 0.125 ng/mL. Percent degranulation was calculated by normalizing to total eosinophil ECP levels.
Eosinophil phosphoproteomics
Freshly isolated or rhIL-5 primed eosinophils (15 × 106) were incubated for 15 min at 37°C in complete medium with mAb 2C4 or IgG1 control, after which cell pellets were collected by centrifugation at 140 × g for 5 min at 4°C. Pellets were solubilized using three freeze-thaw cycles in 8 M urea yielding between 150 – 200 μg total protein for each treatment, as previously described.22 Protein extracts were reduced and alkylated before digestion with trypsin (Promega, Madison, WI). Resulting peptides were desalted using Sep-Pak C18 cartridge chromatography (Waters, Milford, MA). The desalted peptides were fractionated by weak cation exchange chromatography (PolySULFOETHYL A HPLC, PolyLC) and the bound peptides were fractionated by elution with potassium chloride.23 Fractions were combined into 4 pools, dried, and incubated with PHOS-Select Iron Affinity Gel (Sigma-Aldrich). Non-phosphorylated peptides were collected in the unbound material and phosphopeptides were eluted with ammonium hydroxide. The resulting pools of phospho and non-phospho peptides were analyzed by liquid chromatography– mass spectrometry (LC-MS/MS) (Orbitrap Fusion Lumos, Thermo Scientific).
MS Analysis
Raw spectral data were processed using Byonic (Protein Metrics, San Carlos, CA). Spectra were searched against a target and decoy database of curated human proteins (Uniprot, www.uniprot.org, November 5, 2015). Protein identifications were obtained using ProteoIQ (Premier Biosoft). Protein identifications were scored and filtered using a minimum peptide probability of 0.05% and a minimum protein group probability of 0.5%. Gene ontology (GO) analysis of biological processes associated with the identified proteins was performed using DAVID.24, 25 Data are presented by selecting all of the GO categories with p-values less than 0.0001.
Apoptosis assay
rhIL-5 primed eosinophils (2 × 105) were incubated for 18–24 h at 37°C with mAb 2C4, IgG1 control, mAb c2E2, IgG4 control, c2E2 F(ab′)2, 6′-O-Sulfo-3′SLN-PAA or Lewis X-PAA as previously described,8 at which point apoptosis was assessed by means of flow cytometry after FITC-Annexin V (BD Biosciences, San Jose, CA) and DAPI (Thermo Scientific) labeling per the manufacturer’s instructions.
ROS assay
Eosinophil ROS production was measured using the ROS-ID Total ROS detection kit (Enzo Life Sciences, Inc. Farmingdale, NY). rhIL-5 primed eosinophils (5 × 105) were incubated with mAb 2C4, IgG1 control, mAb c2E2, IgG4 control, c2E2 F(ab′)2, 6′-O-Sulfo-3′SLN-PAA or Lewis X-PAA at 37°C for the indicated time points and ROS levels were assessed per the manufacturer’s instructions. Levels of ROS generation were normalized to no treatment group within each experiment.
Flow cytometry
Flow cytometric measurements were performed using a BD LSR II flow cytometer (BD Biosciences), and FlowJo software version 10 (Tree Star, Ashland, OR). In brief, freshly isolated or rhIL-5 primed eosinophils (2 × 105) were incubated for indicated time points with mAb 2C4, IgG1 control, mAb c2E2, IgG4 control, c2E2 F(ab′)2, 6′-O-Sulfo-3′SLN-PAA or Lewis X-PAA at 37°C in complete medium. CD11b surface expression was measured using a PE-conjugated mouse anti-human mAb while levels of CD18 and activated CD11b were detected using FITC-conjugated mAbs.
Stress and apoptosis array
rhIL-5 primed eosinophils (5 × 106) were incubated in complete medium at 37°C with mAb 2C4 or IgG1 control for various time points. Phosphorylated stress and apoptosis proteins were measured using the PathScan Stress and Apoptosis Signaling Antibody Array Kit (Fluorescent Readout) (Cell Signaling, Danvers, MA) per manufacturer’s protocol.
Western blot analysis
rhIL-5 primed eosinophils (5 × 105) were incubated in complete medium at 37°C with mAb 2C4 or IgG1 control for various time points. Next, 2x Laemmli buffer (Bio-Rad, Hercules, CA) was used to isolate total protein from cell lysates, which were then electrophoresed through mini-PROTEAN TGX precast gels and transferred to Immun-Blot low fluorescence PVDF membranes using the Trans-Blot SD semi-dry transfer cell (Bio-Rad), according to the manufacturer’s guidelines. After blocking with Odyssey Blocking Buffer (TBS) (LI-COR, Lincoln, NE), membranes were incubated overnight with various monoclonal or polyclonal antibodies (1:500 – 1:1000 in 5% BSA in TBS-T): phospho-Akt, Akt, phospho-p38, p38, phospho-JNK/SAPK, JNK/SAPK (Cell Signaling). Specific binding of these antibodies was detected with infrared dye (IRDye) 680RD or 800RD-conjugated secondary antibodies (LI-COR) using the Odyssey Imaging System (LI-COR).
Statistical analysis
Data are presented as mean ± SEM unless otherwise indicated. Statistical significance was determined by one-way ANOVA using GraphPad Prism 6.0e. IC50’s were calculated using nonlinear fit, and outliers were determined using the Grubbs test. Values were considered significant at p < 0.05.
Results
Siglec-8 engagement in the presence or absence of IL-5 induces changes in eosinophil morphology and promotes cell adhesion
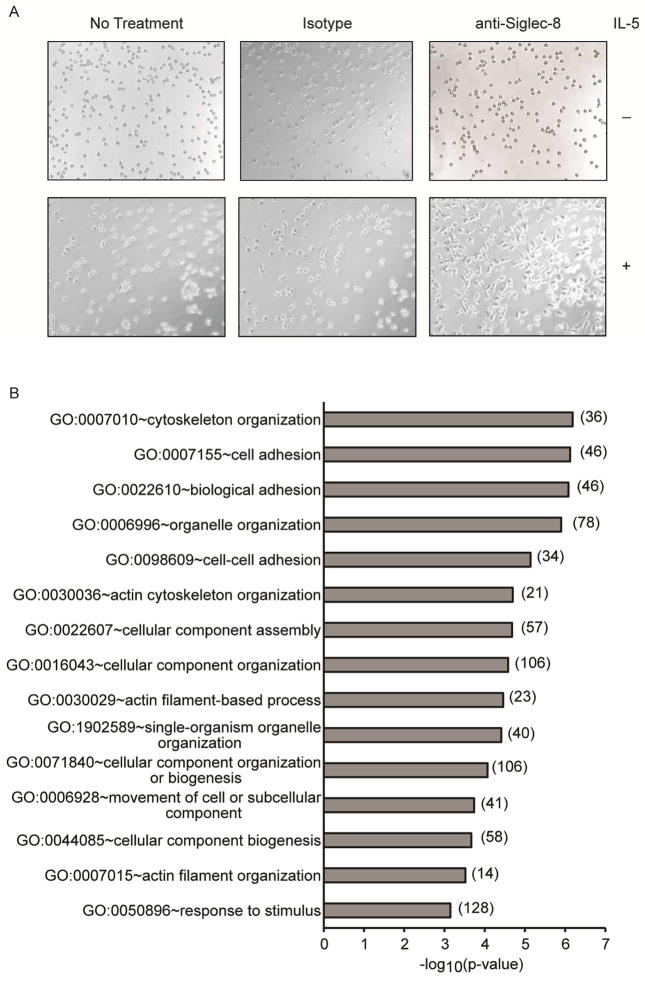
Initial experiments to identify molecules involved in the function of Siglec-8 in human eosinophils led to the fortuitous observation that mAb engagement of Siglec-8 on IL-5 primed eosinophils promoted eosinophil sticking to tissue culture plates. To directly investigate the effect of Siglec-8 engagement on eosinophil adhesion, freshly isolated or IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control for 1 h and morphologic changes were assessed using light microscopy. As shown in Figure 1A, the addition of an antibody to Siglec-8 triggered eosinophil flattening and spreading but only in IL-5-primed cells. Subsequently, analysis of the eosinophil phosphoproteome using LC-MS/MS identified 237 proteins that were differentially phosphorylated following Siglec-8 engagement. A GO analysis revealed that some of these proteins are linked to pathways associated with cell adhesion and cytoskeleton organization (Figure 1B), suggesting that Siglec-8 engagement on IL-5-primed eosinophils activates signaling pathways associated with cell adhesion.
Figure 1. Siglec-8 antibody-induced changes in eosinophil morphology and phosphoproteomics in the presence or absence of IL-5 priming.
(A) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control for 1 h and adhesion and cell spreading was documented using light microscopy. Images are taken at 16× magnification and data are representative of three separate experiments with identical results. (B) Phosphorylated proteins were identified using mass spectrometry in lysates of IL-5 primed human eosinophils cultured with mAb 2C4 or IgG1 control for 15 min. Biological processes associated with Siglec-8 engagement on primary eosinophils were determined using functional enrichment analysis by GO terms as described in the Methods section. Data are from a single experiment and the numbers of identified phosphoproteins within each GO category are indicated.
Siglec-8 engagement activates CD11b/CD18-dependent eosinophil adhesion in an IL-5 dependent manner
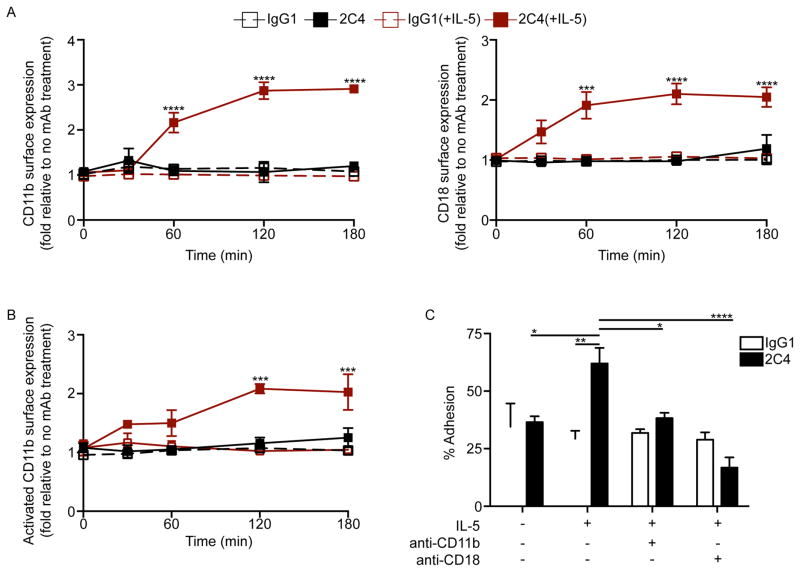
On their surface, eosinophils express a number of adhesion molecules. β2-integrins in particular are known to mediate eosinophil adhesion to various surfaces, and activation of adhesiveness in eosinophils is often associated with rapid upregulation of surface expression of certain β2-integrins.26–28 Indeed, after 60 min and 120 min of incubation with mAb 2C4 or Siglec-8 synthetic ligand respectively, marked upregulation of CD11b and CD18 was detected on the surface of eosinophils and this effect, like adhesion and cell spreading, was dependent on IL-5 priming (Figure 2A and Supplementary Figure 1A). Additionally, 2C4 promoted activation of CD11b (detected using a conformational epitope-specific mAb) that was also dependent on IL-5 priming (Figure 2B). When formally quantified, significantly enhanced adhesion (62 ± 6.8% with 2C4 and 48 ± 1.9% with Siglec-8 synthetic ligand) was observed 2 h following Siglec-8 engagement with IL-5 primed, but not unprimed eosinophils (36.5 ± 5.5% adhesion) and IL-5 priming alone had very little, if any, effect on adhesion (Figure 2C and Supplementary Figure 1B). To determine if adhesion was β2-integrin dependent, IL-5 primed eosinophils were incubated with CD11b or CD18 blocking mAbs, reducing Siglec-8-mediated eosinophil adhesion to 38.3 ± 2.3% and 16.8 ± 4.4% respectively (Figure 2C). These data demonstrate that Siglec-8 engagement triggers a rapid upregulation and activation of eosinophil CD11b and CD11b/CD18 dependent adhesion in IL-5 primed eosinophils but not in non-primed cells. Because eosinophil activation and adhesion can result in degranulation,26, 29 supernatants of IL-5 primed eosinophils were assayed at indicated time points with or without 2C4 for the presence of ECP by ELISA. As shown in Supplementary Figure 2, levels of ECP were no different in the presence or absence of mAb 2C4. These data therefore suggest that Siglec-8-induced adhesion does not result in degranulation or necrosis above background, at least during the time frame studied, and that granule protein release cannot be responsible for secondarily enhancing adhesion.
Figure 2. Siglec-8 engagement causes rapid CD11b/CD18 dependent eosinophil adhesion that requires IL-5 priming.
(A–B) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control and the surface expression of CD11b, CD18 and activated CD11b was assessed at various times for up to three hours. Levels of surface integrins were normalized to no treatment group (CD11b - GMFI was 1214 ± 130 in the presence of IL-5 priming and 1016 ± 312 in the absence of IL-5 priming; CD18 – GMFI was 3996 ± 697 in the presence of IL-5 priming and 3563 ± 399.8 in the absence of IL-5; activated CD11b – GMFI was 747.6 ± 196 in the presence of IL-5 priming and 626.2 ± 188.1 in the absence of IL-5 priming). (C) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control for 2 h. Additionally, overnight IL-5 primed eosinophils were incubated with 10 μg/mL of CD11b or CD18 blocking antibody for 1 h as indicated before the addition of mAb 2C4 or IgG1 control for 2 h. After removal of non-adherent cells, percent eosinophil adhesion was determined. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 (n = 3–5; one-way ANOVA).
Siglec-8-mediated eosinophil ROS production and apoptosis are β2-integrin dependent
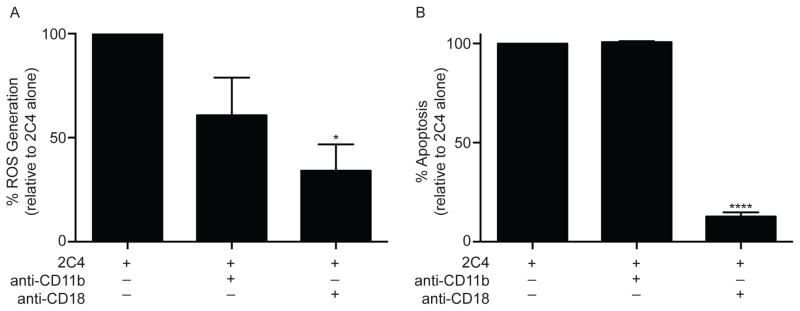
Previously, it was shown that antibody ligation of Siglec-8 on IL-5-primed eosinophils led to ROS production and apoptosis,12 suggesting a downstream activation response, but the intervening pathways contributing to this process remain poorly defined. Given the data in Figures 1 and 2, experiments were performed to determine if adhesion was necessary for ROS production and apoptosis. As shown in Figure 3, Siglec-8-mediated eosinophil ROS production and apoptosis were both significantly inhibited in the presence of CD18 blocking antibody, (≈65% and ≈90% inhibition, respectively), while CD11b blocking antibody only partially (by ≈40%) and non-significantly inhibited ROS generation and had no effect on apoptosis. Taken together, these data demonstrate that Siglec-8-mediated generation of ROS and apoptosis require functional eosinophil β2-integrins.
Figure 3. Siglec-8-mediated eosinophil ROS generation and apoptosis are β2-integrin dependent.
(A) Eosinophils were incubated overnight with IL-5 then 10 μg/mL of CD11b or CD18 blocking antibody was added for 1 h before addition of mAb 2C4 or IgG1 control. ROS was assessed 1 h later and total ROS production was normalized to levels obtained with 2C4 mAb alone (GMFI 418 ± 97) following subtraction of background ROS levels (GMFI 146 ± 75). (B) Eosinophils were incubated as in panel A. Apoptosis was then assessed 18–24 h later and was normalized to levels of cell death seen with mAb 2C4 treatment alone (96 ± 0.13 % apoptosis) following subtraction of baseline apoptosis seen in the no treatment group (34 ± 5 % apoptosis). * p < 0.05; **** p < 0.0001 (n = 3 – 4, one-way ANOVA).
Siglec-8 mAb-mediated effects on integrin-dependent processes, ROS production and apoptosis are independent of Fc binding
Given the unanticipated activation of eosinophils by Siglec-8 engagement with mAb, and given the expression of the Fcγ RIIA isoform on eosinophils,30 it was important to rule out involvement of the Fcγ RIIA following Siglec-8 engagement by mAb. Therefore, in addition to exposure to mAb 2C4 and IgG1 control, we incubated eosinophils with a chimeric human IgG4 anti-Siglec-8 mAb c2E2 or c2E2 F(ab′)2 fragment and show that CD11b upregulation, ROS generation and eosinophil apoptosis are indeed Siglec-8 specific and independent of Fc binding (Supplementary Figure 3).
Siglec-8 engagement leads to NADPH oxidase activation in IL-5-primed eosinophils
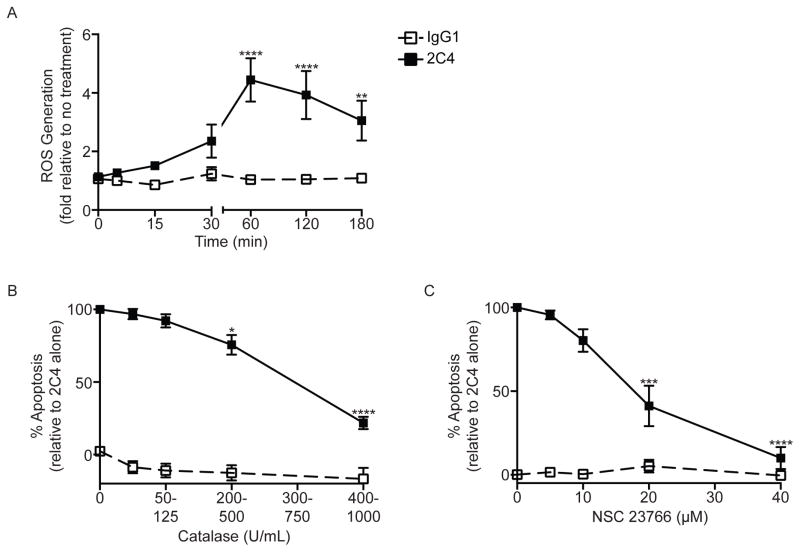
Previously, it was shown that antibody ligation of Siglec-8 on IL-5-primed eosinophils led to ROS production and that this ROS production was necessary for cell death, but the kinetics of ROS generation and its source remain unknown.12 As shown in Figure 4A and Supplementary Figure 4A, following Siglec-8 engagement, there was a time-dependent increase in Siglec-8-mediated eosinophil ROS production that peaked at 60 min with the Siglec-8 mAb and at 180 min with the synthetic Siglec-8 ligand. The source of ROS production that was resulting in apoptosis (Supplementary Figure 4B) following Siglec-8 engagement of IL-5 primed eosinophils was examined using intracellular and extracellular ROS scavengers and a Rac GTPase inhibitor. As shown in Figures 4B and C, Siglec-8 mediated eosinophil apoptosis was inhibited by catalase (extracellular superoxide scavenger) and NSC 23766 (a Rac GTPase inhibitor) at an IC50 of 250 – 625 U/mL and 19.8 μM, respectively. Although mitoTempo (mitochondrial ROS scavenger) and carboxy-PTIO (nitrate/nitrite scavenger) also significantly inhibited Siglec-8-mediated apoptosis (Supplementary Figure 5), these effects were minimal in magnitude (≈10–20% inhibition) compared to catalase and NSC 23766 and required concentrations of scavenger well beyond their reported IC50s.31, 32 Taken together, these data suggest that Siglec-8-mediated ROS generation in IL-5-primed eosinophils is through activation of NADPH oxidase rather than mitochondrial sources.
Figure 4. Siglec-8-mediated eosinophil apoptosis is inhibited by catalase and NSC 23766.
(A) Eosinophils were incubated overnight with IL-5 before the addition of mAb 2C4 or IgG1 control, and total ROS levels were measured at various times for up to 3 h. Total ROS production was normalized to no treatment group (GMFI 79 ± 6) as in Figure 3. (B–C) Eosinophils were incubated overnight with IL-5 then catalase (superoxide scavenger), or NSC23766 (Rac GTPase inhibitor) was added for 1 h before the addition of mAb 2C4 or IgG1 control. Apoptosis was assessed 18–24 h later as in Figure 3. * p < 0.05; ** p < 0.01; **** p < 0.0001 (n = 3–4, one-way ANOVA).
Siglec-8 engagement induces phosphorylation of various intracellular stress proteins in a β2-integrin dependent manner
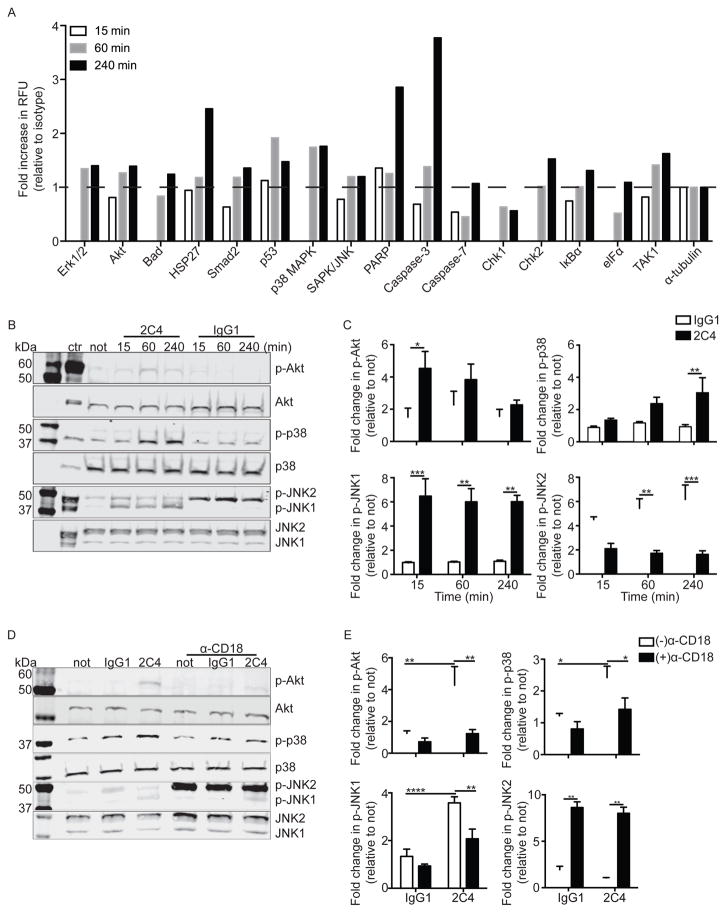
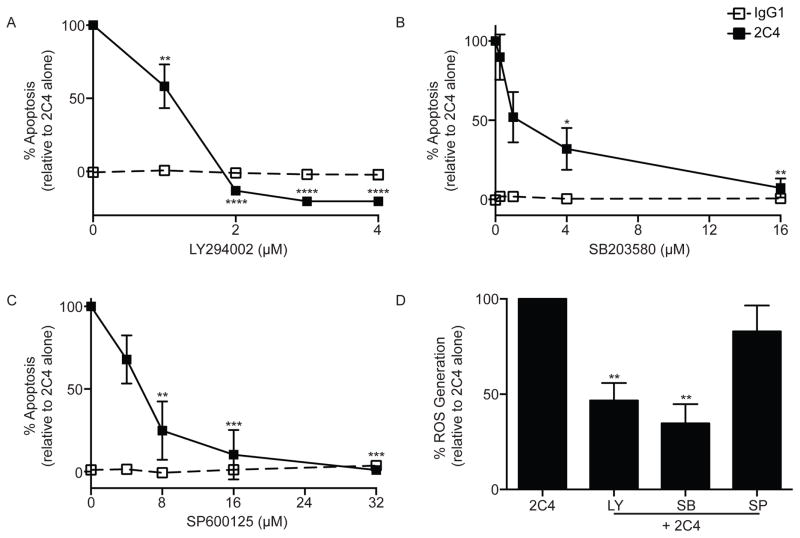
To expand upon data generated in Figures 1B and to identify specific proteins involved in Siglec-8-mediated signaling and eosinophil apoptosis, we utilized a phosphoprotein array that included a range of stress proteins. As shown in Figure 5A, exposure of IL-5 primed eosinophils to mAb 2C4 led to enhanced phosphorylation of heat shock protein 27 (HSP27) and cleavage of Caspase-3 and poly ADP ribose polymerase (PARP), molecules shown to be regulated by PI3K/Akt, p38 mitogen-activated protein kinase (p38) and c-Jun N-terminal kinases (JNK1/2), which in turn can be regulated by β2-integrins and oxidative stress.33, 34 Therefore, subsequent experiments were performed to explore whether Akt, p38 and JNK1/2 are phosphorylated at various time points following Siglec-8 engagement on IL-5-primed eosinophils. As displayed in Figure 5B–E, Siglec-8 engagement led to rapid and statistically significant increases in phosphorylation of Akt, p38 and JNK1, but not JNK2, and phosphorylation of these proteins was prevented by blocking β2-integrins. To establish necessity of these signaling proteins in Siglec-8 function, pharmacological inhibitors were used. As shown in Figures 6A–C, dose dependent decreases in Siglec-8-mediated eosinophil apoptosis were observed, with IC50s of 1 μM for LY294002 (PI3K/Akt pathway inhibitor), 1.1 μM for SB203580 (p38 inhibitor) and 4.3 μM for SP600125 (JNK inhibitor), all close to their published IC50s for their respective targets.35–37 Furthermore, when used at IC50 concentrations, only LY294002 and SB203580 were able to significantly block Siglec-8-mediated ROS generation (Figure 6D), suggesting that Akt and p38 act upstream of ROS production. These data show that Akt, p38 and JNK1 are necessary for Siglec-8 signaling and downstream function in IL-5-primed eosinophils, providing further evidence in support of the conclusion that Siglec-8 functions as an activating receptor.
Figure 5. Siglec-8 engagement induces phosphorylation of various stress proteins in a β2-integrin dependent manner.
(A) Eosinophils were incubated overnight with IL-5 before the addition of mAb 2C4 or IgG1 control for the indicated time points. Changes in phosphorylation of various stress proteins were measured using a phosphoprotein array. Shown are data from one experiment representative of at least two experiments. (B) Eosinophils were treated as in panel A and levels of selected phosphorylated intracellular proteins were measured by western blot at various times as indicated. Levels of total protein were used as loading controls. Shown are data from one experiment representative of at least three experiments. (C) Bar graphs show average quantified levels of phosphorylated proteins from replicate experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 (n = 3–4, one-way ANOVA). (D) Eosinophils were incubated overnight with IL-5 then 10 μg/mL of CD18 blocking antibody was added for 1 h before addition of mAb 2C4 or IgG1 control for 1 h. Levels of selected phosphorylated intracellular proteins were measured by western blot. Levels of total protein were used as loading controls. Shown are data from one experiment representative of at least three experiments. (E) Bar graphs show average quantified levels of phosphorylated proteins from replicate experiments. * p < 0.05; ** p < 0.01; **** p < 0.0001 (n = 3, one-way ANOVA).
Figure 6. Siglec-8-mediated eosinophil apoptosis is inhibited by LY294002, SB203580 and SP600125.
(A–C) Eosinophils were incubated overnight with IL-5 then indicated concentrations of LY294002 (PI3K/Akt inhibitor), SB203580 (p38 inhibitor) or SP600125 (JNK inhibitor) were added for 1 h before the addition of mAb 2C4 or IgG1 control. Apoptosis was assessed 18–24 h later as in Figure 3. (D) Eosinophils were incubated overnight with IL-5 then 1 μM LY294002 (PI3K/Akt inhibitor), 1.1 μM SB203580 (p38 inhibitor) or 4.3 μM SP600125 (JNK inhibitor) was added for 1 h before the addition of mAb 2C4 or IgG1 control. ROS was assessed one hour later as in figure 3. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 (n = 3–4, one-way ANOVA).
Discussion
CD33-related Siglecs are thought to negatively regulate inflammatory responses, but the proteins mediating the downstream functional outcomes following receptor engagement are not fully characterized, especially for Siglec-8.38 The present work extends previous findings that Siglec-8 engagement with mAb or a synthetic ligand induces eosinophil apoptosis in IL-5-activated eosinophils11–14, 21 by showing that Siglec-8 engagement, depending on the priming status of the cell, rapidly induces patterns of phosphoprotein expression consistent with activation of signaling pathways associated with adhesion, further corroborated by finding rapid enhancement of β2-integrin-dependent eosinophil adhesion. Previously, it was demonstrated that in the presence of IL-5, GM-CSF or IL-33 priming, Siglec-8 engagement leads to ROS generation and enhances eosinophil apoptosis, without causing degranulation, the latter differing from what can be seen with briefer exposure to priming cytokines.10, 13, 14, 19 In this study, IL-5 priming was necessary to cause rapid and pronounced Siglec-8 engagement-mediated upregulation of eosinophil surface CD11b/CD18, activation of surface CD11b and adhesion to and spreading on tissue culture plates, processes that were effectively inhibited by CD11b and CD18 blocking mAbs. Although the exact ligand(s) responsible for adhesion in the assay used were not explored, FBS used in the culture media contains numerous proteins, including known ligands for CD11b/CD18 (vitronectin, fibrinogen, periostin and even albumin).39, 40 Therefore, we presume that proteins in the media get coated onto the tissue culture dish surface, providing substrates for CD11b/CD18 eosinophil adhesion.
The finding that Siglec-8 engagement upregulates CD11b/CD18 surface expression, activates CD11b and promotes eosinophil adhesion and spreading differs significantly from data on the mouse neutrophil Siglec-E presented by McMillan et al. showing that engagement of mouse neutrophil Siglec-E can negatively regulate CD11b-dependent neutrophil recruitment to the lung, but has no effect on neutrophil CD11b surface expression or on CD11b-mediated neutrophil adhesion, which is in accordance with the typical notion of Siglecs functioning as inhibitory receptors.41 Whether this occurs in eosinophils via its ITIM or ITSM domain8 remains unknown and is the basis of ongoing studies.
While the requirement for prior IL-5 exposure to allow Siglec-8 engagement to then activate integrins and all the downstream activation steps leading to eosinophil apoptosis is now better delineated, the biochemical events responsible for its IL-5-dependence are less clear. One likely explanation, supported by the literature, is that IL-5 provides pre-activation and repositioning of integrins within cellular microdomains. For example, in freshly isolated blood eosinophils, CD11b/CD18 primarily exists in a bent, inactive conformation. However, IL-5 priming of eosinophils results in a high-activity conformation of CD11b/CD18, enhancing eosinophil adhesion.42, 43 Han et al. showed that IL-5 priming promotes eosinophil polarization, where the nucleus and granules move in opposite directions, forming the nucleopod and the granular compartment. Additionally, IL-5 priming promotes localization and clustering of adhesion receptors to the nucleopod, facilitating “outside-in” signaling of the integrin by efficient coupling to downstream signaling proteins.44 It is therefore tempting to speculate that when eosinophils are exposed to IL-5 family cytokines, eosinophil adhesion and polarization promotes both CD11b/CD18 and Siglec-8 clustering in the nucleopod, bringing Siglec-8 in proximity to CD11b/CD18 and facilitating downstream signaling processes that allow for induction of Siglec-8-mediated eosinophil apoptosis upon Siglec-8 engagement by its ligand.
Several lines of evidence have demonstrated that β2-integrins can regulate the release of ROS from eosinophils (and neutrophils) by activating NADPH oxidase.29, 45, 46 NADPH oxidase is a multi-subunit complex composed of membrane-associated (gp91phox and p22phox) and cystosolic (p40phox, p47phox, p67phox and Rac GTPase) components. Upon cell stimulation, Rac GTPase binds the cytosolic components and promotes membrane translocation and NADPH oxidase activation.47 The present studies show that β2-integrin activation and adhesion are necessary for Siglec-8-mediated eosinophil ROS production and that scavenging of extracellular ROS or inhibition of Rac GTPase activity prevents Siglec-8-mediated apoptosis, but only in IL-5 primed cells. These data are in agreement with work by McMillan et al. showing that Siglec-E on mouse neutrophils positively regulates NADPH oxidase activity and ROS production in a β2-integrin dependent manner.48
Siglec-8-mediated eosinophil apoptosis was inhibited by LY294002 and Siglec-8 engagement led to a time dependent increase in Akt phosphorylation. Similar Siglec-related observations were made in platelets, where Siglec-7-mediated apoptosis was inhibited in the presence of the inhibitor LY294002.49 Additionally, Siglec-E-mediated ROS production in mouse neutrophils required Akt activation.48 Akt is in a family of serine/threonine kinases that play a crucial role in regulating cell metabolism, growth, proliferation and apoptosis in a PI3K-dependent manner.50 A common function attributed to Akt is its ability to inhibit apoptosis by negatively regulating pro-apoptotic molecules and promoting cell-cycle progression by controlling energy metabolism.51 However, Nogueira et al. challenge this dogma and show that activation of Akt can promote cell apoptosis by increasing oxidative stress. They report that activated Akt induces ROS-dependent apoptosis of mouse fibroblasts by increasing oxygen consumption and impairing function of ROS scavengers.52 The present data is in agreement with this report and suggests that Akt activation following Siglec-8 engagement is upstream of ROS production and is necessary for Siglec-8-mediated eosinophil ROS production and apoptosis. Therefore, it seems likely that active Akt negatively regulates eosinophil ROS scavengers, and that this is a key molecular event that triggers accumulation of ROS leading to eosinophil apoptosis following Siglec-8 engagement.
MAPKs are an evolutionarily conserved family of serine/threonine kinases that include ERK1/2, JNK and p38 that have been shown to control a number of intracellular processes, including cell transcription, proliferation, survival and apoptosis.33, 53 The present work strongly suggests that Siglec-8-mediated eosinophil apoptosis requires the activation of JNK1 and p38, and activation of JNK1 is downstream of Siglec-8-mediated ROS production. This is in line with multiple studies reporting that ROS can activate JNK and promote cell apoptosis in various cell types.34 Additionally, in eosinophils, it has been reported that ROS can activate JNK and promote apoptosis.54 Unexpected was our observation that pharmacological inhibition of p38 inhibited Siglec-8-mediated ROS production, suggesting that p38 acts upstream of NADPH oxidase. Although it is traditionally thought that oxidative stress activates p38 to promote cell death,34 other lines of evidence support the concept that p38 regulates NADPH oxidase activity in granulocytes. In fact, activation of eosinophils and neutrophils with a variety of stimuli (formyl-peptides, endotoxin, phorbol esters) leads to activation of p38, which is necessary for NADPH oxidase activation and ROS generation,55–59 further supporting our observation that p38 mediates NADPH oxidase activity following Siglec-8 engagement. Unlike Siglec-E engagement on mouse neutrophils, which negatively regulates p38 phosphorylation, current findings are in agreement with other data showing Siglec-5/Siglec-14 engagement promotes endotoxin or Group B streptococcus (GBS) dependent phosphorylation of p38.48, 60
Although speculative, the current findings may have implications for our understanding of the function of Siglec-8 on eosinophils in health and disease, as well as the safety and suitability of Siglec-8 as a therapeutic target. Regarding the former, one possible scenario suggested by the finding that Siglec-8 engagement activates adhesion, ROS generation and death could be that when eosinophils encounter a natural ligand, such as an endogenous sialoside-containing structure in the airways,61 the interaction selectively facilitates attachment of the eosinophil, leading to removal of the eosinophil via apoptosis. Regarding potential safety concerns that might arise when Siglec-8 is “activated” on a cytokine-primed eosinophil in vivo by a therapeutic agent such as an antibody, one might see a rapid drop in circulating eosinophils via margination if β2-integrins are activated, but at the same time Siglec-8 engagement, even of IL-5-primed cells, does not result in detectable degranulation or necrosis, an important concern that has been rightfully raised when trying to eliminate eosinophils for therapeutic benefit.62 Clinical trials with humanized Siglec-8 mAbs have already begun, with at least one phase I study completed and phase II studies underway (https://clinicaltrials.gov/ct2/results?cond=&term=allakos&cntry1=&state1=&recrs=), so more information regarding safety and efficacy should be forthcoming.
In conclusion, the work reported here has uncovered a novel mechanism and pathway by which IL-5-primed eosinophils undergo cell death. Following engagement of Siglec-8, downstream activation of β2-integrins promotes enhanced eosinophil adhesion, which is necessary for NADPH oxidase activation, ROS generation, activation of a subset of tyrosine kinases and ultimately eosinophil apoptosis (Figure 7). Studies employing F(ab′)2 versions of Siglec-8 mAb and polymeric Siglec-8 glycan ligands confirmed that specific engagement of Siglec-8, independent of any possible role for FcɣR on eosinophils, was responsible for the observed biological activities. Our current results challenge the established dogma implicating CD33-related Siglecs as inhibitory receptors and provides a novel mechanism for regulation of Siglec-8 function in cytokine-primed human eosinophils.
Figure 7. Schematic diagram of the proposed Siglec-8 signaling mechanism in IL-5 primed eosinophils.
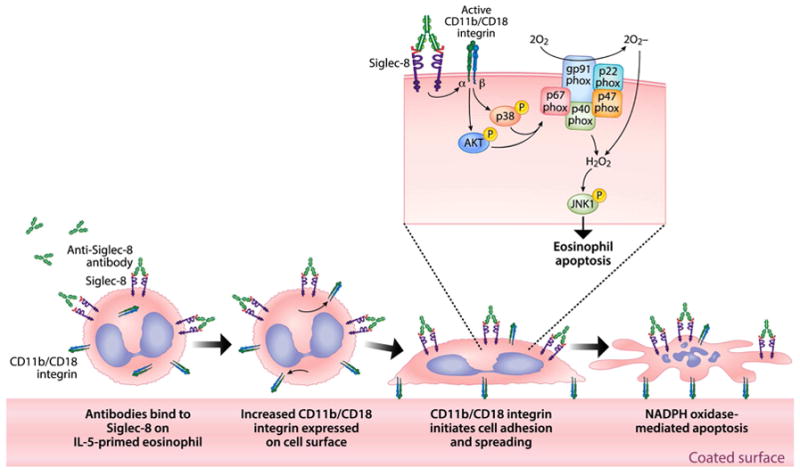
Siglec-8 engagement on IL-5 primed eosinophils (shown here with a Siglec-8 mAb but likely true of other Siglec-8-specific ligands) promotes CD11b/CD18 expression, activation and β2 integrin-dependent eosinophil adhesion, which is necessary for NADPH oxidase activation, ROS generation, activation of Akt, p38 and JNK1, and ultimately eosinophil apoptosis. Artwork by Jacqueline Schaffer
Supplementary Material
Key messages.
This is the first detailed description of how a CD33-related Siglec, namely Siglec-8, has the ability to activate, rather than inhibit, cellular responses.
Siglec-8 engagement on IL-5 primed cells promoted β2-integrin dependent eosinophil adhesion, which was absolutely required for subsequent ROS generation and cell death.
These Siglec-8 functions on IL-5 primed eosinophils require the downstream intracellular activation of NADPH oxidase, Akt, p38 and JNK1.
Acknowledgments
This work was supported by grants AI72265 and HL107151 from the National Institutes of Health and P41 GM103490 from the National Institute of General Medical Sciences.
We wish to thank Nick Lu and Paul Bryce for helpful discussions and Corwin Nycholat, Nenad Tomasevic and John Leung for providing crucial reagents.
Abbreviations
- 6′-O-Sulfo-3′SLN-PAA
6′-sulfo-sialyl-N-acetyl-D-lactosamine-polyacrylamide
- Akt
Protein Kinase B
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- c2E2
chimeric 2E2 mAb in which the mouse IgG1 Fc from clone 2E2 has been replaced with human IgG4 Fc
- ECP
Eosinophil cationic protein
- GO
Gene ontology
- HSP27
Heat shock protein 27
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- ITSM
Immunoreceptor tyrosine-based switch motif
- JNK
c-Jun N-terminal kinase
- LC-MS
Liquid chromatography- mass spectrometry
- MAPK/ERK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- PAA
polyacrylamide
- p38
p38 mitogen-activated protein kinase
- PARP
Poly ADP ribose polymerase
- PI3K
Phosphatidylinositide 3-kinase
- ROS
Reactive oxygen species
- SFK
Src family kinase
- SHP
Src-homology region 2 domain-containing phosphatases
- Siglec
Sialic acid-binding immunoglobulin-like lectin
Footnotes
Disclosure statement
Dr. Bochner has current or recent consulting or scientific advisory board arrangements with, or has received honoraria from, Sanofi-Aventis, Pfizer, Biogen Idec, TEVA, AstraZeneca and Allakos; and owns stock in Allakos and Glycomimetics. He receives publication-related royalty payments from Elsevier and UpToDate™, and is a co-inventor on Siglec-8-related patents licensed to Allakos, and thus may be entitled to a share of royalties received by Johns Hopkins University. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23:157–65. doi: 10.1016/j.blre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Wechsler ME, Fulkerson PC, Bochner BS, Gauvreau GM, Gleich GJ, Henkel T, et al. Novel targeted therapies for eosinophilic disorders. J Allergy Clin Immunol. 2012;130:563–71. doi: 10.1016/j.jaci.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2015;135:598–608. doi: 10.1016/j.jaci.2014.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 8.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’Alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105:1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 9.Foussias G, Yousef GM, Diamandis EP. Molecular characterization of a Siglec8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec8 gene. Biochem Biophys Res Commun. 2000;278:775–81. doi: 10.1006/bbrc.2000.3866. [DOI] [PubMed] [Google Scholar]
- 10.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 11.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–24. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 12.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437–45. doi: 10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2012;57:169–74. doi: 10.1016/j.cyto.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J Biol Chem. 1999;274:11505–12. doi: 10.1074/jbc.274.17.11505. [DOI] [PubMed] [Google Scholar]
- 16.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J Biol Chem. 2001;276:45128–36. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- 17.Kitzig F, Martinez-Barriocanal A, Lopez-Botet M, Sayos J. Cloning of two new splice variants of Siglec-10 and mapping of the interaction between Siglec-10 and SHP-1. Biochem Biophys Res Commun. 2002;296:355–62. doi: 10.1016/s0006-291x(02)00885-9. [DOI] [PubMed] [Google Scholar]
- 18.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem. 2005;280:19843–51. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 19.Kano G, Bochner BS, Zimmermann N. Regulation of Siglec-8-induced intracellular reactive oxygen species production and eosinophil cell death by Src family kinases. Immunobiology. 2017;222:343–9. doi: 10.1016/j.imbio.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansel TT, Pound JD, Pilling D, Kitas GD, Salmon M, Gentle TA, et al. Purification of human blood eosinophils by negative selection using immunomagnetic beads. J Immunol Methods. 1989;122:97–103. doi: 10.1016/0022-1759(89)90339-6. [DOI] [PubMed] [Google Scholar]
- 21.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 2009;330:608–12. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, et al. The Peripheral Blood Eosinophil Proteome. J Proteome Res. 2016;15:1524–33. doi: 10.1021/acs.jproteome.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villen J, Gygi SP. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc. 2008;3:1630–8. doi: 10.1038/nprot.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato M, Abraham RT, Okada S, Kita H. Ligation of the beta2 integrin triggers activation and degranulation of human eosinophils. Am J Respir Cell Mol Biol. 1998;18:675–86. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- 27.Simon HU. Integrin expression by eosinophils. Allergy. 2000;55:791–2. doi: 10.1034/j.1398-9995.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 28.Bochner BS. Systemic activation of basophils and eosinophils: markers and consequences. J Allergy Clin Immunol. 2000;106:S292–302. doi: 10.1067/mai.2000.110164. [DOI] [PubMed] [Google Scholar]
- 29.Dri P, Cramer R, Spessotto P, Romano M, Patriarca P. Eosinophil activation on biologic surfaces. Production of O2- in response to physiologic soluble stimuli is differentially modulated by extracellular matrix components and endothelial cells. J Immunol. 1991;147:613–20. [PubMed] [Google Scholar]
- 30.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 31.Trnka J, Blaikie FH, Smith RA, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44:1406–19. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med. 1997;22:787–94. doi: 10.1016/s0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- 33.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 34.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–89. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 35.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 36.Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–21. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 37.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao H, Crocker PR. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132:18–26. doi: 10.1111/j.1365-2567.2010.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–58. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 40.Johansson MW, Annis DS, Mosher DF. alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–10. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, Prescott A, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood. 2013;121:2084–94. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson MW, Gunderson KA, Kelly EA, Denlinger LC, Jarjour NN, Mosher DF. Anti-IL-5 attenuates activation and surface density of beta(2) -integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy. 2013;43:292–303. doi: 10.1111/j.1365-2222.2012.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han ST, Mosher DF. IL-5 induces suspended eosinophils to undergo unique global reorganization associated with priming. Am J Respir Cell Mol Biol. 2014;50:654–64. doi: 10.1165/rcmb.2013-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–67. [PubMed] [Google Scholar]
- 46.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–60. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMillan SJ, Sharma RS, Richards HE, Hegde V, Crocker PR. Siglec-E promotes beta2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung. J Biol Chem. 2014;289:20370–6. doi: 10.1074/jbc.M114.574624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen KA, Hamzeh-Cognasse H, Palle S, Anselme-Bertrand I, Arthaud CA, Chavarin P, et al. Role of Siglec-7 in apoptosis in human platelets. PLoS One. 2014;9:e106239. doi: 10.1371/journal.pone.0106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–42. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 52.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–70. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–33. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Hasala H, Zhang X, Saarelainen S, Moilanen E, Kankaanranta H. c-Jun N-terminal kinase mediates constitutive human eosinophil apoptosis. Pulm Pharmacol Ther. 2007;20:580–7. doi: 10.1016/j.pupt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto K, Kuribayashi F, Nakamura M, Takeshige K. Involvement of p38 MAP kinase in not only activation of the phagocyte NADPH oxidase induced by formyl-methionyl-leucyl-phenylalanine but also determination of the extent of the activity. J Biochem. 2006;140:739–45. doi: 10.1093/jb/mvj204. [DOI] [PubMed] [Google Scholar]
- 56.El Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 57.Detmers PA, Zhou D, Polizzi E, Thieringer R, Hanlon WA, Vaidya S, et al. Role of stress-activated mitogen-activated protein kinase (p38) in beta 2-integrindependent neutrophil adhesion and the adhesion-dependent oxidative burst. J Immunol. 1998;161:1921–9. [PubMed] [Google Scholar]
- 58.Lynch OT, Giembycz MA, Barnes PJ, Hellewell PG, Lindsay MA. ‘Outside-in’ signalling mechanisms underlying CD11b/CD18-mediated NADPH oxidase activation in human adherent blood eosinophils. Br J Pharmacol. 1999;128:1149–58. doi: 10.1038/sj.bjp.0702892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch OT, Giembycz MA, Barnes PJ, Lindsay MA. Pharmacological comparison of LTB(4)-induced NADPH oxidase activation in adherent and non-adherent guinea-pig eosinophils. Br J Pharmacol. 2001;134:797–806. doi: 10.1038/sj.bjp.0704314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211:1231–42. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, et al. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 2017 doi: 10.1093/glycob/cwx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Persson C, Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014;189:628–33. doi: 10.1164/rccm.201311-2069OE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.