Abstract
Mechanosensitve pathways in chondrocytes are essential for maintaining articular cartilage homeostasis. Traumatic loading increases cartilage oxidation and causes cell death and osteoarthritis. However, sub-lethal doses of the pro-oxidant molecule tert-Butyl hydroperoxide (tBHP) protects against loading-induced chondrocyte death. We hypothesized that compressive cyclic loading at moderate strains (<20%) causes sub-lethal cartilage oxidation that induces an adaptive increase in the endogenous antioxidant defense network. We tested this hypothesis by subjecting healthy bovine articular cartilage explants to in vitro static or cyclic (1 Hz) compressive loading at 50kPa (15% strain, “physiologic”) versus 300kPa (40% strain, “hyper-physiologic”) for 12 hours per day for 2 days. We also treated unloaded explants with 100μM tBHP for 12 hours per day for 2 days to differentiate between biomechanical and chemical pro-oxidant stimulation. All loading conditions induced glutathione oxidation relative to unloaded controls, but only the 50kPa cyclic loading condition increased total glutathione content (2-fold). This increase was associated with a greater expression of glutamate-cysteine ligase, the rate-limiting step in glutathione synthesis, compared to 300kPa cyclic loading. 50kPa cyclic loading also increased the expression of superoxide dismutase-1 and peroxiredoxin-3. Like 50kPa loading, tBHP treatment also increased total glutathione content. However, tBHP treatment and 50kPa cyclic loading differed in their effect on the expression of genes regulating antioxidant defense and cartilage matrix synthesis and degradation. These findings suggest that glutathione metabolism is a mechanosensitive antioxidant defense pathway in chondrocytes and that intermittent pro-oxidant treatment alone is insufficient to account for all changes in mediators of cartilage homeostasis associated with cyclic loading.
Keywords: Oxidative stress, Mechanobiology, Glutathione, Articular Cartilage, Superoxide Dismutase
INTRODUCTION
During joint loading, articular cartilage compresses and distributes joint stress while serving as a low-friction surface that minimizes joint wear. Cartilage withstands a range of biomechanical stresses by balancing the synthesis and degradation of the cartilage extracellular matrix1,2. Chondrocytes regulate this balance by sensing and responding to an interaction of genetic and environmental factors. The environmental factors include biochemical signals (e.g. cytokines and growth factors), local matrix composition, and biomechanical stresses such as tension, compression, shear, or fluid pressure acting on the cells. These signals, if excessive, promote catabolism and impair the structural integrity of the cartilage. Thus, with limited capacity for self-repair, adaptive mechanosensitive feedback pathways that promote chondrocyte resilience in response to changing biomechanical stress are critical for maintaining cartilage function throughout life3.
One potential mechanosensitive feedback system involves enzymes and signaling mechanisms that balance the reducing and oxidizing (i.e., “redox”) conditions of the cell. Although oxidative stress is commonly associated with age-related cellular pathologies, pro-oxidants, such as hydrogen peroxide, regulate many fundamental cellular processes, including proliferation, differentiation, growth, and cell survival4. One of the first examples of the generation of reactive pro-oxidant molecules (i.e., reactive oxygen species, or ROS) under physiologic conditions was in contracting skeletal muscle5. Subsequent studies have elucidated multiple mechanisms by which cellular strain generates ROS6,7. In chondrocytes, physiologic and hyper-physiologic cellular strains increase ROS production8–11. How this occurs in chondrocytes is not well understood; however, injurious loading has been shown to increase mitochondrial-derived ROS11. Moreover, suppression of mitochondrial ROS following traumatic loading reduces cell death12, suggesting that mitochondria are a source of damaging ROS and may be susceptible to oxidative stress following a traumatic load. Under non-injurious conditions, however, mitochondrial ROS production is critical for maintaining cellular metabolism13,14. Thus, in chondrocytes, as in other cells types, regulating the cellular redox environment is critical for maintaining cellular homeostasis15. A fundamental way that cells tune this redox balance is through an intricate and adaptive endogenous antioxidant system.
Our goal was to gain further insight into the effect of physiologic and hyperphysiologic compressive stresses under static and dynamic conditions on the endogenous cartilage antioxidant system. We hypothesized that physiologic cyclic loading increases chondrocyte resilience by upregulating the expression and activity of endogenous antioxidants. Previous studies have shown that cyclic compressive loading at physiologic strains (≤20%) reduces cytokine-mediated extracellular matrix degradation16–20. This protection is attributed to an inhibition of NF-κB signaling, an oxidant-sensitive signaling pathway18,21. Understanding the effect of different types of biomechanical stresses on the chondrocyte antioxidant system may explain how the magnitude and frequency of biomechanical loading either worsens or protects against cartilage catabolism. Moreover, it may also provide insight into how treating cartilage with non-lethal doses of the pro-oxidant tert-butyl hydroperoxide (tBHP) prior to applying a damaging loading cycle reduces cell death and extracellular matrix degradation22. Sub-lethal tBHP preconditioning may provide protection against damaging ROS by upregulating antioxidant pathways to improve redox regulation. Thus, we also compared the effect of tBHP versus cyclic compressive loading on antioxidant defense networks. We conducted these studies using healthy bovine cartilage explants and an in vitro compressive loading system.
MATERIALS AND METHODS
Cartilage Explant Culture
Young adult (8–30 mo) bovine metacarpophalangeal (fetlock) joints were purchased from a local abattoir within 4 hours of death. Macroscopically healthy cartilage explants were excised from load-bearing regions using a scalpel and 4-mm biopsy punch. The average explant thickness was 0.75 mm ± 0.07 mm (mean ± SD). Tissue explants were cultured in 48-well plates with 1mL of culture media containing low glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% heat inactivated fetal bovine serum, 100U/ml penicillin, 100μg/ml streptomycin, 10mM HEPES, and 0.1mM non-essential amino acids (Invitrogen) at 37°C and 5% CO2.
Explant Mechanical Loading
After 3 days in culture, site-matched explants were transferred to either free-swell control plates or 6-well loading plates modified for use with a Flexcell FX5000 system (Flexcell International) as described previously2. Flexcell pressure-force conversions were independently verified at multiple loading-well positions by monitoring pressure-induced forces using a 22-N load cell (0–22 Newtons with range +/− 0.50%) and Electro-Force 3100 Mechanical Testing System (Bose). Cartilage explants were subject to a 10-gram (g) tare pre-load prior to initiating compressive loading, which is equivalent to 8kPa. Additional compressive forces were then applied dynamically using a sine waveform at a frequency of 1 Hz for 12 hours per day for 2 days. Cartilage explants were loaded during the first 12 hours of each 24-hour cycle; consequently, explants were harvested on day 2 following 12-hrs of being subjected to the tare loading condition only. In a subset of experiments, explants were continuously compressed statically for 2 days.
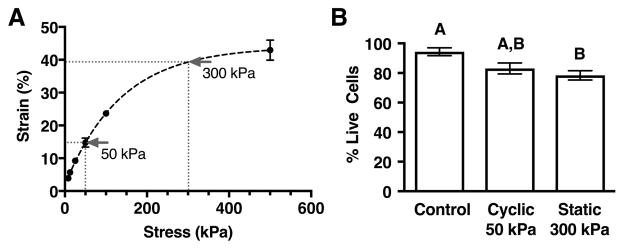
Experimental stress levels were selected based on generating strains that straddle a transition from anti-catabolic (≤20% strain) to pro-catabolic (≥30% strain)20. Explants were axially loaded under unconfined compression using a Bose Electro-Force Mechanical Testing System. We applied forces ranging between 10g and 640g, corresponding to stress values between 8kPa and 500kPa, respectively. Cartilage explants from 2 – 4 animals per force level were maintained at a given load for up to 2 hours to reach steady-state, at which time the deformation was recorded. An equilibrium stress-strain curve was then determined from the force and deformation data (Figure 1A). We selected compressive loads of 50 and 300kPa for our testing because they corresponded to equilibrium strains of approximately 15% and 40%, respectively. For all experiments, loaded explants were cultured under the same conditions as the unloaded controls (supplemented DMEM culture media at 37°C and 5% CO2). At the completion of loading treatments, explants were weighed, placed in cryotubes, snap frozen in liquid nitrogen, and stored at −80°C for later analysis. Cell viability following loading was evaluated by confocal microscopy as described below.
Figure 1.
Stress-strain characteristics and cell viability for compressive loading parameters. A. Equilibrium strains of cartilage explants were measured using an Electro-Force 3100 Mechanical Testing System operated in load-control. Loads ranging from 8 – 500kPa stresses were applied to explants in unconfined compression (N=2–4 per stress level). Stress levels of 50kPa and 300kPa were selected to generate strains of approximately 15% and 40% in subsequent experiments. B. Chondrocyte viability decreased with loading from 94% to ~80%, but it did not differ between a physiologic loading condition (cyclic 50kPa loading) and a hyper-physiologic loading condition (static 300kPa loading). Viability was assessed by confocal microscopy in 3 separate regions per explant per animal (N=4). Bars not sharing a letter are significantly different from each other (p<0.05). Values are mean ± SEM.
Live-Dead Cell Imaging
Following an initial subset of loading experiments, we assessed chondrocyte viability by confocal microscopy using a LIVE/DEAD viability/cytotoxicity kit (Life Technologies). Explants were bisected, rinsed, and stained with calcein AM and ethidium homodimer-1 following the manufacturer’s protocol. Explants were coated with citi-fluor and imaged at 3 separate locations covering the surface to deep zones. Samples were placed in a coverglass chamber and imaged at 20× magnification using a Zeiss LSM510 confocal microscope and 500mW argon laser. Images were analyzed using Image-J software with automated thresholding and cell counting. Results were obtained from explants from 4 animals for each loading condition and were reported as %live cells (Figure 1B).
Explant Pro-Oxidant Treatment
Bovine cartilage explants were harvested from 4 animals and cultured for 3 days in 48-well plates to equilibrate. Explants were then treated with 100μM tert-Butyl hydroperoxide (tBHP; Sigma) diluted in culture medium for 12 hrs/day for 2 days. Untreated site-matched and animal-matched control explants were maintained in standard culture medium. Media were changed every 12 hours for all groups, with tBHP-supplemented medium applied during the first 12 hours of each 24-hour cycle. Explants were not loaded during these experiments. Following 2 days, explants were weighed, snap frozen in cryotubes with liquid nitrogen, and stored at −80°C for later analysis.
Glutathione Content and Oxidation Ratio
We used a DTNB based Glutathione Assay Kit (Cayman Chemical) to measure total glutathione (tGSH) and oxidized glutathione (GSSG) levels following manufacturer instructions. Briefly, two explants per animal were cryopulvarized and suspended in 265uL 10mM MOPS, 1mM EDTA, and 10μL protease inhibitor cocktail (Sigma). Homogenate was pulse sonicated 3×10 sec, and cleared at 13,000 RCF for 15 min. Supernatant was incubated with 4mM 2-vinylpyridine (Sigma) or water to quantify GSSG or tGSH, respectively. Reduced glutathione (GSH) was calculated as the difference of tGSH and GSSG. Samples were tested in duplicate using a Sunrise microplate reader to monitor changes in absorbance at 405nm (Tecan). Net glutathione oxidation was calculated as the ratio of GSH:GSSG.
RNA and Protein Extraction
Frozen explants were cryopulverized and homogenized in TRIzol® Reagent (Ambion) on ice. Cartilage mRNA and protein were isolated following the manufacturer’s protocol, including the addition of high salt precipitation solution (Molecular Research Center, Inc.) to minimize glycosaminoglycan co-purification. mRNA was further purified using an RNeasy Plant Kit (Qiagen) following the manufacturer’s protocol. Protein samples were dissolved in 1% SDS for Mass Spectrometry analyses.
Quantitative RT-PCR
mRNA from 2 explants per animal was pooled and synthesized into cDNA using Quantitect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. Quantitative RT-PCR was performed in duplicate using RT2 primers and RT2 SYBR green (Qiagen) on a CFX96 thermocycler (Bio-Rad). Target genes were normalized to the geometric mean of 6 reference genes (GAPDH, B2M, RPLP01, ACTB, HPRT1, and TBP) following the comparative Ct method. Gene expression changes due to loading (N=3) and tBHP (N=4) treatments were evaluated by the ΔΔCt method, with animal-matched unloaded and untreated samples, respectively, serving as the referent control condition.
Quantitative Mass Spectrometry Analysis
We utilized Selected Reaction Monitoring (SRM) Mass Spectrometry to quantify protein abundance from 2 pooled explants per animal as previously described23. We determined cartilage protein concentration by the BCA Protein Assay (Thermo Scientific) following manufacturer instructions for small volumes. 3pmol equine serum albumin (ESA) (Rockland Immunochemicals) was added to each 20μg protein sample as an internal standard. Samples were separated by short-gel electrophoresis, trypsin digested, and analyzed using a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific) operated in the selected reaction monitoring mode with a splitless nanoflow HPLC system (Eksigent). Data were processed using Pinpoint to find and integrate the correct peptide chromatographic peaks. To quantify protein abundance, the relative quantity of each protein was first normalized to the ESA internal standard and then to the geometric mean of six stable cellular reference proteins: (ATP5B, GAPDH, PPIA, RPS27A, VDAC, VIM). Loaded and tBHP treated samples were analyzed relative to the average of the site-matched unloaded control samples. The following proteins were not consistently detected in a sufficient number of samples to include in the final analysis: AKR1B1, CALR, GPX1, GPX3, GPX4, GSTA3, GSTM1, GSTP1, MSRA, NNT, PHB, PHB2.
Superoxide Dismutase Activity
We measured cartilage superoxide dismutase (SOD) activity spectrophotometrically in triplicate using a Superoxide Dismutase Activity Assay (Cayman Chemical) and a microplate reader (Tecan) following the manufacturer’s protocol. Two frozen explants per animal were cryopulverized and suspended in 400μL 50mm KPO4 pH 7.2. Sample homogenate was then pulse-sonicated 3×10 seconds on ice and cleared at 12,000 RCF. The supernatant was separated and incubated with water or 10mM KCN for 45 minutes. KCN selectively inhibits SOD1 and SOD3 allowing for the separate quantification of SOD2. The combined activity of SOD 1 and 3 was calculated as total SOD activity minus SOD2 activity.
Statistical Analyses
Significant differences between loaded and unloaded samples or tBHP treated and untreated samples were determined by paired Students’ t-tests. The effects of loading magnitude and frequency were analyzed by 2-Factor Analysis of Variance. Between group differences were determined by Holm-Sidak’s multiple comparisons post-hoc test. Differences in gene expression due to loading and tBHP treatments were evaluated by Kruskal-Wallis test with Dunn’s multiple comparisons post-hoc analysis for determining between-group differences. All tests were conducted using the statistical software program Prism 6.0c or 7 for Mac OSX. Significance was determined as p<0.05. Tests were conducted on site-matched treatment and free-swell control samples obtained from 3 – 7 cows as specified by “N” in the figure legends. Technical and/or biological replicates, as indicated previously in the methods, were averaged to generate one value per animal, and degrees of freedom for statistical purposes were based on the number of independent animals from which samples were obtained.
RESULTS
Glutathione Oxidation and Content
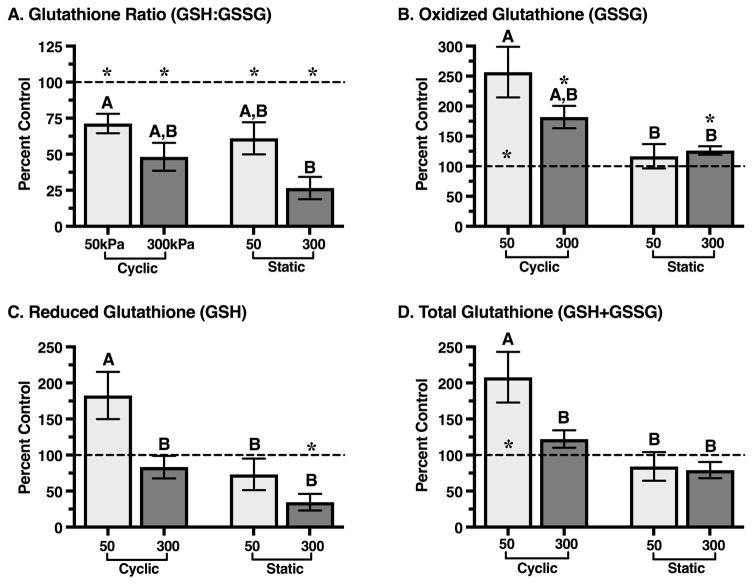
We measured cartilage glutathione oxidation and content as markers of tissue oxidation and antioxidant capacity. Reduced (GSH) and oxidized (GSSG) glutathione levels were normalized to explant wet weight and expressed as a percentage of the unloaded site-matched control explant from the same animal. In unloaded control explants from all loading experiments, the ratio of reduced to oxidized glutathione (GSH:GSSG) was 3.29 ± 1.15 (mean ± SD), and the total glutathione content (GSH+GSSG) was 298 ± 72 μM/g wet weight. Explants from two animals that were cyclically loaded at 50kPa were excluded from analysis because the glutathione content exceeded the standard curve; however, the values followed the same trends and their exclusion does not alter the interpretation of the results.
Compared to unloaded control samples, all loading conditions stimulated glutathione oxidation, as indicated by the reduced GSH:GSSG ratio (p<0.05, Figure 2A). The reduction in GSH:GSSG was greater in samples loaded at 300kPa versus 50kPa (p=0.0086) and was not altered by the type of loading (cyclic vs. static). The reduction in GSH:GSSG was primarily due to an increase in the level of oxidized glutathione, GSSG (Figure 2B). GSSG was elevated the most in 50kPa cyclic loaded samples, and overall it was elevated more with cyclic versus static loading (p=0.0023). Both the type (cyclic vs. static) and magnitude (50 vs. 300kPa) of loading altered the content of reduced glutathione in cartilage (Figure 2C). Specifically, 50kPa of cyclic loading increased GSH (p=0.06) whereas 300kPa of static loading decreased GSH (p=0.011). Consequently, only the 50kPa cyclic loading condition significantly altered total glutathione content (Figure 2D). Total glutathione content increased approximately 2-fold with 50kPa of cyclic loading (p=0.037); in other loading conditions, however, it remained unchanged.
Figure 2.
Effect of intermittent cyclic versus continuous static compressive loading at 50 and 300 kPa on cartilage glutathione content and oxidation. Compared to unloaded controls, all loading conditions decreased the ratio of reduced to oxidized glutathione; whereas, only 50 kPa of cyclic loading increased total glutathione content. A. Ratio of reduced to oxidized glutathione (GSH:GSSG), expressed as a percent of GSH:GSSG measured from explant harvest site and animal-matched free-swelling control samples. B. Oxidized glutathione content (GSSG) was increased with loading in all loading conditions except 50 kPa static loading. C. Reduced glutathione content (GSH) was significantly greater in 50 kPa cyclically-loaded samples. D. Total glutathione content (GSH+GSSG) was increased with 50 kPa cyclic loading and did not change with other loading conditions. Sample sizes: 50 kPa cyclic (N=5); 300 kPa cyclic (N=7); 50 kPa static (N=4); 300 kPa static (N=4). Bars not sharing a letter are significantly different from each other (p<0.05; Holm-Sidak’s multiple comparisons test); *p<0.05 from control. Values are mean ± SEM.
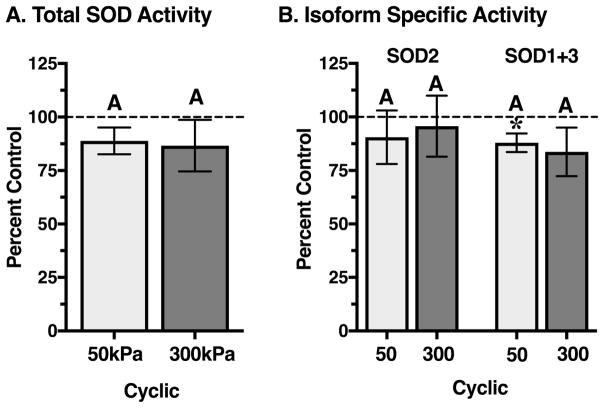
Superoxide Dismutase Activity
Based on the significant differences in total glutathione content caused by 50 versus 300 kPa cyclic loading, we focused our subsequent analyses on these two loading conditions. Total superoxide dismutase activity incorporates the actions of three isoforms: SOD1 (cytosolic, Cu-Zn-SOD), SOD2 (mitochondrial, Mn-SOD), and SOD3 (extracellular, Cu-Zn-SOD). In unloaded cultured bovine cartilage, the total SOD (tSOD) activity was 6.0 ± 1.7 U/g wet weight (mean ± SD). The isoform-specific activity of SOD2 was 28.3 ± 7.7% of tSOD activity (mean ± SD). Neither 50kPa nor 300kPa cyclic loading significantly altered tSOD activity, although the values tended to be lower than controls (Figure 3A). Similarly, 50kPa and 300kPa cyclic loading did not substantially alter the isoform-specific activities of SOD2 or combined SOD1+3 (Figure 3B). Nevertheless, 50kPa loading did significantly reduce SOD1+3 activity (p=0.03) by ~10%, which was similar in magnitude to 300kPa.
Figure 3.
Effect of 50 and 300kPa intermittent cyclic compressive loading on cartilage superoxide dismutase activity. Superoxide dismutase (SOD) converts superoxide to hydrogen peroxide. Three SOD isoforms exist: SOD1 (cytosolic), SOD2 (mitochondrial), and SOD3 (extracellular). A. Total SOD activity was not altered by either loading magnitude, but it tended to be lower compared to site and animal-matched unloaded control samples. B. The isoform-specific activity of SOD2 was unaltered by loading magnitude, similar to the combined activity of SOD1 and 3. Isoform-specific rates tended to be reduced relative to controls. Sample sizes: 50 kPa cyclic (N=7); 300 kPa cyclic (N=3). Bars not sharing a letter are significantly different from each other (p<0.05; Holm-Sidak’s multiple comparisons test); *p<0.05 from site-matched control. Values are mean ± SEM.
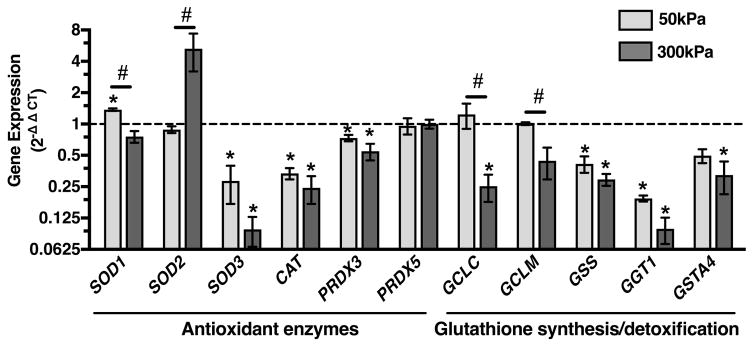
Antioxidant and Glutathione Synthesis Gene Expression
To gain further insight into mechano-responsive antioxidant regulatory pathways, we examined the effect of 50 and 300kPa cyclic loading on several major antioxidant genes and genes associated with the regulation of glutathione synthesis and cellular detoxification. The two magnitudes of compressive loading differentially regulated the expression of all three SOD isoforms (Figure 4). 50kPa of cyclic loading upregulated SOD1 (1.4-fold, p=0.01), downregulated SOD3 (3.5-fold, p=0.02), and did not alter SOD2. In contrast, 300kPa of loading upregulated SOD2 (5-fold, p=0.18), downregulated SOD3 (10-fold, p=0.001), and did not alter the expression of SOD1. These differential effects of loading magnitude on gene expression were not observed for the downstream hydrogen peroxide antioxidants catalase (CAT) or peroxiredoxin 3 or 5 (PRDX3, PRDX5). For these genes, both 50 and 300kPa of cyclic loading downregulated the expression of CAT and PRDX3 by an average of 3.5 and 1.6-fold, respectively, and did not alter the expression of PRDX5. These results indicate specific mechano-sensitive changes in antioxidant expression associated with different cellular compartments, such as the mitochondria (e.g., SOD2 and PRDX3), the cytosol (e.g., SOD1 and PRDX5), and the extracellular matrix (e.g., SOD3).
Figure 4.
Effect of 50 and 300 kPa intermittent cyclic compressive loading on the expression of cartilage antioxidant and glutathione synthesis and cellular detoxification genes. Data are expressed as a fold-change relative to average unloaded control samples. Both loading magnitudes down-regulated the expression of numerous genes, including superoxide dismutase 3 (SOD3), catalase (CAT), peroxiredoxin 3 (PRDX3), glutathione synthetase (GSS), and gamma-glutamyltransferase-1 (GTT1). Some genes, however, were differentialy regulated, including superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM). Peroxiredoxin 5 (PRDX5) was the only gene whose expression was unaltered by loading. Y-axis is log-2 scale; N=3 per loading group. *p<0.05 from unloaded controls; #p<0.05 between loading magnitudes. Values are mean ± SEM.
Among the glutathione regulatory genes we measured, 50kPa cyclic loading maintained gene expression levels of both the glutamate-cysteine ligase catalytic (GCLC) and modifier (GCLM) subunits similar to those of unloaded samples and significantly higher than samples loaded at 300kPa (p=0.013 and 0.044, respectively; Figure 4). Glutamate-cysteine ligase is the first and rate-limiting step in glutathione synthesis. However, cyclic loading at both 50kPa and 300kPa downregulated the expression of all other genes tested involved in glutathione synthesis and cellular detoxification. These genes included glutathione synthetase (GSS), gamma-glutamyltransferase 1 (GGT1), and glutathione s-transferase (GSTA4). This effect was greatest for GGT1, which was downregulated 10-fold (p=0.001) by 300kPa of loading. GGT1 is a plasma membrane enzyme that removes the γ-glutamyl group from extracellular GSH to facilitate cellular reuptake of cysteine for de novo synthesis of GSH.
Antioxidant Protein Networks
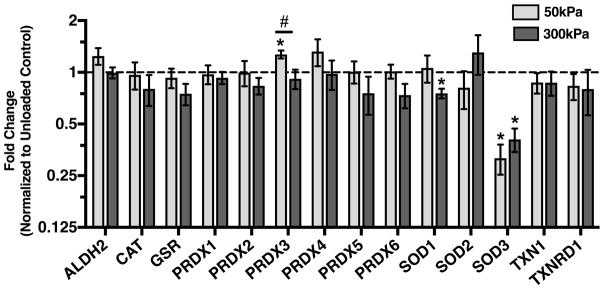
We utilized Selected Reaction Monitoring (SRM) Mass Spectrometry to quantify protein abundance in cartilage explants following 50 or 300kPa of cyclic loading as well as in site-matched unloaded controls. Cyclic loading had a minimal impact on antioxidant protein levels (Figure 5). The most substantial effect was on SOD3, which decreased by approximately 3-fold following either 50kPa or 300kPa of cyclic loading (p<0.0001 and p=0.011, respectively). Additionally, 300kPa of loading decreased SOD1 content by 25% (p=0.037). In contrast, 50kPa of loading increased peroxiredoxin 3 content by 27% (p=0.007). Thus, in comparison to the changes in gene expression, cyclic loading induced fewer changes in antioxidant protein content relative to unloaded controls.
Figure 5.
Effect of 50 and 300kPa intermittent cyclic compressive loading on the content of cartilage antioxidant proteins. Data are expressed as a fold-change relative to average unloaded control samples. Both loading magnitudes had minimal impact on antioxidant protein content. 50kPa loading increased peroxiredoxin 3 (PRDX3); whereas, 300kPa of loading decreased superoxide dismutase 1 (SOD1). Both loading magnitudes down-regulated the extracellular SOD isoform, SOD3. Abbreviations: ALDH2 (aldehyde dehydrogenase 2, mitochondrial), CAT (catalase), GSR (glutathione reductase), PRDX1-6 (peroxiredoxins 1–6), SOD1-3 (superoxide dismutase 1–3), TXN1 (thioredoxin 1), and TXNRD1 (thioredoxin reductase 1). Y-axis is log-2 scale; 50kPa: N=7; 300kPa: N=3. *p<0.05 versus unloaded control; #p<0.05 between loading magnitudes. Values are mean ± SEM.
Comparison to Pro-oxidant Treatment Without Loading
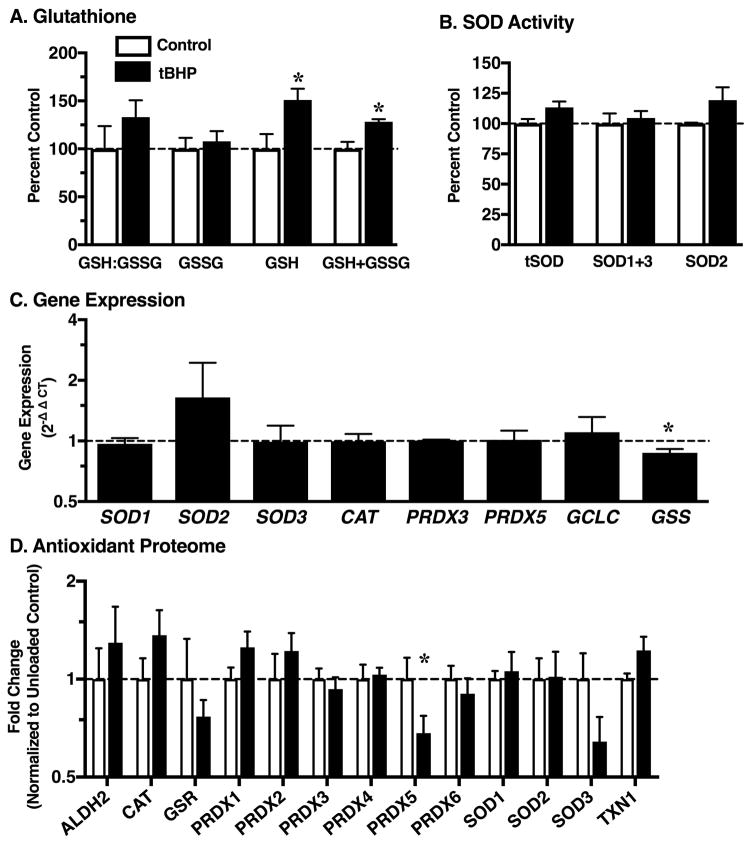
We treated explants ±100μM tert-Butyl hydroperoxide (tBHP) to model increased cellular oxidation and hydrogen peroxide-mediated signaling. The rationale for this experiment was based on two observations. First, cyclic loading induced cartilage oxidation as indicated by a decrease in the GSH:GSSG ratio. Second, previous work has shown that pre-conditioning cartilage with 100μM tBHP reduced the acute effects of mechanical stress on cell death and proteoglycan catabolism as well as increased the expression of catalase22. We found that treatment with tBHP for 12-hr periods over 2 days increased the amount of reduced and total glutathione content in cartilage (Figure 6A), but it did not alter the GSH:GSSG ratio or the amount of oxidized glutathione. Shorter periods of tBHP treatment (1 and 6-hrs) did not induce glutathione oxidation either (data not shown). Interestingly, though, the increase in total glutathione content was observed at the 6-hr time point. tBHP treatment did not alter SOD activity (total or isoform specific; Figure 6B), and it had a minimal effect on antioxidant gene expression (Figure 6C). There was a trend for increased SOD2 expression and a 15% reduction in glutathione synthetase expression (p=0.047). At the protein level, tBHP treatment induced minimal changes as well. PRDX5 was reduced by approximately 30% with tBHP (p=0.038), and there was a trend for reduced SOD3 (p=0.06) (Figure 6D). tBHP treatment also caused a trend for increased thioredoxin 1 (p=0.052) and catalase (p=0.07).
Figure 6.
Effect of 100μM tert-Butyl hydroperoxide (tBHP) treatment on cartilage mediators of oxidative homeostasis. tBHP generates hydrogen peroxide, which is used to model the effects of loading-independent increases in cellular oxidation and hydrogen peroxide-mediated signaling. Data are expressed as a fold-change relative to average site- and animal-matched untreated control samples. A. Reduced (GSH) and oxidied (GSSG) glutathione levels. B. Total and isoform-specific SOD activity assay. C. tBHP-induced changes in gene expression normalized to untreated control samples. D. tBHP-induced changes in antioxidant protein content. Data are expressed as fold-change relative to the average untreated control samples. *p<0.05 versus untreated control. N=4. Values are mean ± SEM.
Effect of Cyclic Loading versus tBHP Treatment on Cartilage Gene Expression
Our initial results showed that both static and cyclic loading at two different stress magnitudes induced net glutathione oxidation in cartilage as defined by a reduction in the GSH:GSSG ratio. In contrast, only the 50kPa cyclic loading condition increased total glutathione content in cartilage. Treating cartilage with tBHP did not reduce the ratio of GSH:GSSG, but it did increase total glutathione content like 50kPa of cyclic loading. Additional similarities included a reduction in the expression of glutathione synthetase (GSS) and SOD3. To further understand potential differences in the effects of cyclic compressive loading versus intermittent pro-oxidant treatment we compared the changes in expression of a panel of genes involved in cartilage homeostasis (Figure 7). mRNA was isolated from cartilage explants cyclically loaded at 50 or 300kPa for 12-hr increments over 2 days and compared to mRNA isolated from explants treated ±100μM tert-Butyl hydroperoxide (tBHP) using the same 12-hr on/off block design for 2 days.
Figure 7.
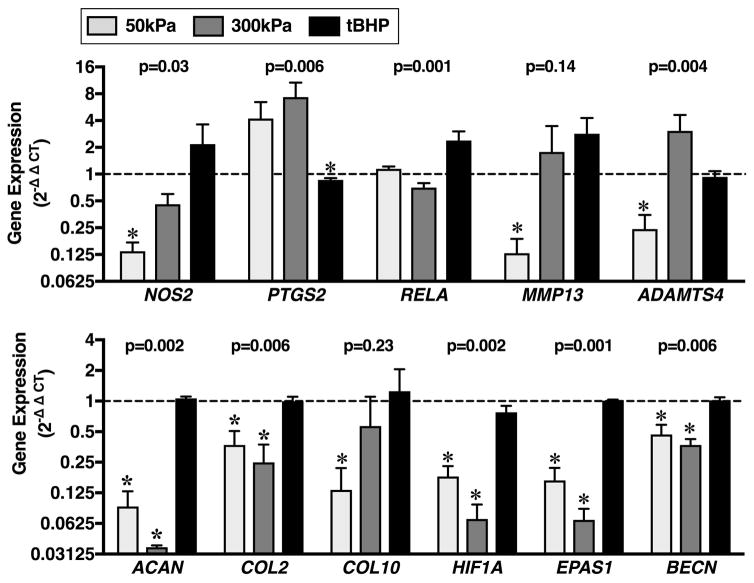
Effect of 50 and 300 kPa cyclic compressive loading and 100μM tert-Butyl hydroperoxide (tBHP) treatment on the expression of genes involved in cartilage homeostasis. Cartilage explants were loaded or treated with tBHP for 12-hrs followed by 12-hrs of tare loading or standard culture medium, respectively, repeated for 2 days. Data are expressed as a fold-change relative to site- and animal-matched unloaded or untreated control samples. There were significant differences between loading versus pro-oxidant treatment effects on gene expression as indicated by p-values above bar graphs (Kruskal-Wallis). *p<0.05 versus unloaded or untreated control. N=3 for loading treatments, and N=4 for tBHP treatment. Y-axis is log-2 scale. Values are mean ± SEM.
The effect of loading and tBHP treatment differed for the majority of genes tested, including inflammatory mediators (NOS2, PTGS2, RELA), the aggrecanase ADAMTS4, extracellular matrix components (ACAN, COL2), and cellular metabolic response mediators (HIF1A, EPAS1, BECN). In particular, 50kPa of cyclic loading downregulated the expression of nitric oxide synthase 2 (NOS2), matrix metalloproteinase-13 (MMP13), a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4), aggrecan (ACAN), type II collagen (COL2), type X collagen (COL10), hypoxia-inducible factor 1-alpha (HIF1A), hypoxia-inducible factor 2-alpha (EPAS1), and beclin 1 (BECN) (Figure 7). 300kPa of cyclic loading downregulated the expression of the same extracellular matrix and cellular metabolic response genes, but it did not reduce the expression of pro-inflammatory or catabolic-associated genes. In contrast, tBHP treatment did not alter the expression of any of the genes tested except for a slight but significant reduction in PTGS2 (Figure 7). Thus, pro-oxidant treatment with the hydrogen peroxide generating substrate tBHP did not induce changes in cartilage gene transcription that mirror the anti- or pro-catabolic effects of 50 or 300kPa of compressive loading, respectively.
DISCUSSION
We hypothesized that physiologic cyclic loading would increase chondrocyte resilience by upregulating the expression and activity of endogenous antioxidants. Our experiments tested this hypothesis using several approaches: 1) quantifying the total content and ratio of reduced:oxidized glutathione, 2) measuring the total and isoform-specific activity of superoxide dismutase, 3) measuring the change in gene expression of multiple cellular antioxidant enzymes and glutathione synthesis and detoxification enzymes, and 4) quantifying changes in protein abundance across a network of cellular antioxidants. By focusing on a comparison of 50kPa versus 300kPa of cyclic compressive stress, we sought to relate these findings to the differences associated with anti- versus pro-catabolic levels of cartilage mechanical loading.
Our results generated several primary findings in relation to our hypothesis. First, in support of the hypothesis, 50kPa of cyclic loading was the only loading condition that increased the total tissue content of glutathione, which is the primary thiol-based intracellular ROS defense peptide. Second, although 50 and 300kPa stresses upregulated SOD1 and SOD2, respectively, neither substantially altered the enzymatic activity of these key cellular antioxidants. Thus, these data provide conflicting results in support of our hypothesis. Finally, the gene transcript and protein levels of most antioxidants that we evaluated did not change with either 50 or 300kPa cyclic loading. Furthermore, of those that did change, more antioxidants decreased rather than increased with loading, especially for gene transcripts. Considering these findings as a whole, they suggest that glutathione synthesis, more than antioxidant enzyme upregulation, is a central mechanosensitive antioxidant response in chondrocytes.
Glutathione has previously been shown to be a central mediator of chondrocyte redox homeostasis. For example, the GSH:GSSG ratio is significantly reduced in non-osteoarthritic cartilage from aged donors due to an increase in GSSG24. In addition, inhibition of glutathione synthetase or glutathione reductase, which converts GSSG back to GSH, reduced chondrocyte glutathione content and sensitized cells to nitric oxide and hydrogen peroxide-induced cell death24,25. Furthermore, the protective effect of N-acetylcysteine treatment against loading-induced chondrocyte cell death has been attributed to the ability of N-acetylcysteine to provide an intracellular supply of cysteine for GSH synthesis11. GSH is synthesized in a two-step enzymatic process. First, glutamate-cysteine ligase (GCLC and GCLM) catalyze the rate-limiting formation of γ-glutamylcysteine from glutamate and cysteine. Second, glutathione synthetase (GSS) catalyzes the formation of GSH from γ-glutamylcysteine and glycine. In the current study, 50kPa cyclic loading maintained the expression of GCLC and GCLM; whereas, 300kPa loading reduced the expression of these rate-limiting enzymes relative to unloaded samples. Both levels of loading reduced the expression of gamma-glutamyltransferase 1, which mediates the extracellular catabolism of GSH to facilitate GSH re-synthesis via the gamma-glutamyl cycle26. Thus, the increased levels of total glutathione (GSH+GSSG) with 50kPa cyclic loading may reflect an overall increase in substrate flux through the de novo GSH synthesis pathway. Upregulation of GSH biosynthesis does not require an increase in the expression of GCLC, the rate-limiting enzyme, because its activity is subject to multiple levels of regulation. For example, GCLC activity is upregulated by pro-oxidants, such as hydrogen peroxide, as well as by oxidized glutathione itself, which is increased following cyclic loading. Understanding how mechanical loading induces changes in chondrocyte metabolic flux27 may provide new insight into the mechanobiology of glutathione synthesis and redox homeostasis.
Many studies suggest that biomechanically-induced oxidative stress regulates mechanosensitive signaling mechanisms in chondrocytes14,15,28,29. One potential example is by glutathione-mediated modulation of NF-κB signaling30. Cyclic loading at a 10% strain level suppresses cytokine-induced NF-κB signaling; whereas, loading at a 30% strain level enhances it18. This transition is largely mediated through changes in IκB phosphorylation and protein turnover18. Glutathione has been shown to modulate NF-κB signaling, both positively and negatively, through multiple mechanisms, including changes in the quantity and redox ratio of glutathione, activation of glutathione peroxidase, and by glutathionylation of IκB kinases (IKKs), IκB, or NF-κB subunits31–33. Our finding that 50kPa cyclic loading increased GSH while also lowering the ratio of GSH:GSSG raises questions about how glutathione may contribute to altered NF-κB signaling. Interestingly, the finding that 100μM of tBHP pre-treatment reduced the acute effects of mechanical stress on cell death and proteoglycan catabolism22 may be mediated in part through altered NF-κB signaling30,34. In our study, a common finding between tBHP treatment and 50kPa of cyclic loading was a significant increase in glutathione content. Future studies are needed to understand how changes in glutathione content and redox status alter NF-κB signaling in chondrocytes.
Efforts to enhance chondrocyte antioxidant defense systems via chemical, pharmacological, or biomechanical means may help improve the clinical success of musculoskeletal tissue engineering. Many applications involve aging and injury-related diseased conditions, which are pro-inflammatory and pro-oxidative. Increasing cell survival and function in these conditions could improve the engraftment and maintenance of tissue-engineered constructs. Chemical and pharmacological mimetics of chondrocyte mechanical loading can accelerate functional tissue engineering of cartilage without the need for mechanical loading bioreactors35–37. Perhaps mimetics of chondrocyte mechanical loading could also be used to improve resistance to oxidative stress.
The conclusions of this study are limited by several factors. For example, despite our attempt to characterize multiple cellular antioxidant networks, several potentially important omissions remain. These include the free radical scavenging properties of micronutrients, such as tocopherols (vitamin E), ascorbic acid (vitamin C), and carotenoids (vitamin A), whose metabolism, bioavailability, and function may be altered by mechanical loading under in vivo conditions. We also did not evaluate the activation or levels of oxidative stress response factors, such as NRF2/Keap1, FoxOs, heat shock factors, or sirtuins15. Furthermore, we did not quantify changes in the rate or source of ROS production under the different loading conditions. This information could help to identify which antioxidants are most proximal to the source of ROS production, such as the antioxidants SOD2 and PRDX3 for mitochondrial ROS10,38,39. New methods to assess organelle-specific measures of ROS production and oxidative stress in vivo are becoming more widely available and offer new opportunities for future investigations40,41. Finally, additional evidence is needed to define the mechanism by which cyclic loading upregulates GSH synthesis in a stress-dependent manner.
In summary, we found that cyclic compressive loading of healthy bovine cartilage alters the endogenous cartilage antioxidant network. Higher compressive stress (40% strain) increased glutathione oxidation and caused a reduction in the expression of the rate-limiting enzymes for glutathione synthesis. Higher stress also increased the expression of the mitochondrial antioxidant SOD2 while decreasing the cytosolic isoform SOD1, consistent with mitochondrial-derived ROS production at higher loads. Moderate compressive stress (15% strain) caused glutathione oxidation as well, but it also increased total glutathione content and upregulated SOD1 gene expression. Pro-oxidant conditioning of cartilage using tBHP increased glutathione content without altering any other loading-associated changes. These findings suggest that glutathione synthesis, more than antioxidant enzyme upregulation, is a central pro-oxidant and mechanosensitive mediator of antioxidant defense in chondrocytes.
Acknowledgments
We thank Dr. Yao Fu, Dr. Rachel Lane, Caroline Kinter, Joanna Hudson, Erin Hutchison, Grahm Roach, and Dr. Rachel Chang for technical assistance and Aaron Simmons for critical feedback in the preparation of the manuscript. We also thank Drs. Luke Szweda, Ken Humphries, Mary Beth Humphrey, Scott Plafker, and Christopher West for advice, technical assistance, and intellectual input. This work was supported by grants from the NIH (F32 AR060155 to Dr. Issa) and the Arthritis National Research Foundation, Arthritis Foundation, and Oklahoma Center for the Advancement of Science and Technology (to Dr. Griffin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. RI, MB, MK, and TMG have no conflicts to disclose.
Footnotes
Author Contributions: All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be published.
References
- 1.Sah RL, Kim YJ, Doong JY, et al. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 2.Piscoya J, Fermor B, Kraus V, et al. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthritis Cartilage. 2005;13(12):1092–1099. doi: 10.1016/j.joca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Adams J, Leddy HA, McNulty AL, et al. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014;16(10):451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biology. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 6.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11(7):1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333(6048):1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki K, Fukuda K, Matsukawa M, et al. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: involvement of reactive oxygen species. Arthritis Rheum. 2003;48(11):3151–3158. doi: 10.1002/art.11305. [DOI] [PubMed] [Google Scholar]
- 9.Brouillette MJ, Ramakrishnan PS, Wagner VM, et al. Strain-dependent oxidant release in articular cartilage originates from mitochondria. Biomech Model Mechanobiol. 2014;13(3):565–572. doi: 10.1007/s10237-013-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike M, Nojiri H, Ozawa Y, et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci Rep. 2015;5:11722. doi: 10.1038/srep11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman MC, Ramakrishnan PS, Brouillette MJ, Martin JA. Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function. Arthritis & Rheumatology. 2016;68(3):662–671. doi: 10.1002/art.39460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin W, McCabe D, Sauter E, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057–1063. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JA, Martini A, Molinari A, et al. Mitochondrial electron transport and glycolysis are coupled in articular cartilage. Osteoarthritis Cartilage. 2012;20(4):323–329. doi: 10.1016/j.joca.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff KJ, Ramakrishnan PS, Brouillette MJ, et al. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. J Orthop Res. 2013;31(2):191–196. doi: 10.1002/jor.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gassner R, Buckley MJ, Georgescu H, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163(4):2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1 beta-induced release of nitric oxide and PGE2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthritis Cartilage. 2003;11(9):688–696. doi: 10.1016/s1063-4584(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS ONE. 2009;4(4):e5262. doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torzilli PA, Bhargava M, Park S, Chen CTC. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2010;18(1):97–105. doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Frank EH, Wang Y, et al. Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-α and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthritis Cartilage. 2013;21(12):1933–1941. doi: 10.1016/j.joca.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dossumbekova A, Anghelina M, Madhavan S, et al. Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum. 2007;56(10):3284–3296. doi: 10.1002/art.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan P, Hecht BA, Pedersen DR, et al. Oxidant conditioning protects cartilage from mechanically induced damage. J Orthop Res. 2010;28(7):914–920. doi: 10.1002/jor.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rindler PM, Plafker SM, Szweda LI, Kinter M. High Dietary Fat Selectively Increases Catalase Expression within Cardiac Mitochondria. J Biol Chem. 2013;288(3):1979–1990. doi: 10.1074/jbc.M112.412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Carlo M, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 25.Baker MS, Feigan J, Lowther DA. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J Rheumatol. 1989;16(1):7–14. [PubMed] [Google Scholar]
- 26.Karp DR, Shimooku K, Lipsky PE. Expression of gamma-glutamyl transpeptidase protects ramos B cells from oxidation-induced cell death. J Biol Chem. 2001;276(6):3798–3804. doi: 10.1074/jbc.M008484200. [DOI] [PubMed] [Google Scholar]
- 27.Salinas D, Minor CA, Carlson RP, et al. Combining Targeted Metabolomic Data with a Model of Glucose Metabolism: Toward Progress in Chondrocyte Mechanotransduction. PLoS ONE. 2017;12(1):e0168326–16. doi: 10.1371/journal.pone.0168326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckwalter JA, Martin JA, Brown TD. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology. 2006;43(3–4):603–609. [PubMed] [Google Scholar]
- 29.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiology of Aging & Age-related Diseases. 2012;2(1):17470. doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan MJ, Liu Z-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275(28):21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 32.Lou H, Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282(40):29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 33.Jones JT, Qian X, van der Velden JLJ, et al. Glutathione S-transferase pi modulates NF-κB activation and pro-inflammatory responses in lung epithelial cells. Redox Biology. 2016;8(C):375–382. doi: 10.1016/j.redox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1(Suppl 1):e000061. doi: 10.1136/rmdopen-2015-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampat SR, Dermksian MV, Oungoulian SR, et al. Applied osmotic loading for promoting development of engineered cartilage. J Biomech. 2013;46(15):2674–2681. doi: 10.1016/j.jbiomech.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleswarapu SV, Athanasiou KA. TRPV4 channel activation improves the tensile properties of self-assembled articular cartilage constructs. Acta Biomater. 2013;9(3):5554–5561. doi: 10.1016/j.actbio.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNulty AL, Leddy HA, Liedtke W, Guilak F. TRPV4 as a therapeutic target for joint diseases. Naunyn-Schmiedeberg’s Arch Pharmacol. 2014;388(4):437–450. doi: 10.1007/s00210-014-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott JL, Gabrielides C, Davidson RK, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69(8):1502–1510. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins JA, Wood ST, Nelson KJ, et al. Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes. J Biol Chem. 2016;291(13):6641–6654. doi: 10.1074/jbc.M115.693523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klomsiri C, Nelson KJ, Bechtold E, et al. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Meth Enzymol. 2010;473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan A, Cochemé HM, Pun PBL, et al. Using exomarkers to assess mitochondrial reactive species in vivo. BBA - General Subjects. 2014;1840(2):923–930. doi: 10.1016/j.bbagen.2013.05.026. [DOI] [PubMed] [Google Scholar]