Abstract
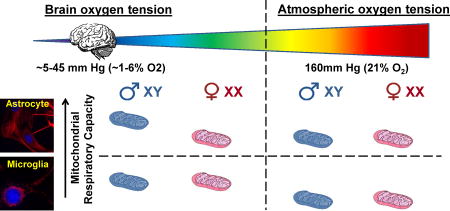
Biological sex is thought to influence mitochondrial bioenergetic function. Previous respiration measurements examining brain mitochondrial sex differences were made at atmospheric oxygen using isolated brain mitochondria. Oxygen is 160 mm Hg (21%) in the atmosphere, while the oxygen tension in the brain generally ranges from ~5–45 mm Hg (~1–6% O2). This study tested the hypothesis that sex and/or brain physiological oxygen tension influence the mitochondrial bioenergetic properties of primary rat cortical astrocytes and microglia. Oxygen consumption was measured with a Seahorse XF24 cell respirometer in an oxygen-controlled environmental chamber. Strikingly, male astrocytes had a higher maximal respiration than female astrocytes when cultured and assayed at 3% O2. Three percent O2 yielded a low physiological dissolved O2 level of ~1.2% (9.1 mm Hg) at the cell monolayer during culture and 1.2–3.0% O2 during assays. No differences in bioenergetic parameters were observed between male and female astrocytes at 21% O2 (dissolved O2 of ~19.7%, 150 mm Hg during culture) or between either of these cell populations and female astrocytes at 3% O2. In contrast to astrocytes, microglia showed no sex differences in mitochondrial bioenergetic parameters at either oxygen level, regardless of whether they were non-stimulated or activated to a proinflammatory state. There were also no O2- or sex-dependent differences in proinflammatory TNF-α or IL-1β cytokine secretion measured at 18 hours activation. Overall, results reveal an intriguing sex variance in astrocytic maximal respiration that requires additional investigation. Findings also demonstrate that sex differences can be masked by conducting experiments at nonphysiological O2.
Keywords: respiration, gender, hyperoxia, hypoxia, lipopolysaccharide, cytokine
Graphical abstract
1. Introduction
Mitochondria are organelles responsible for ATP production, consuming oxygen in the process. Mitochondrial ATP production is especially important in the brain, as the central nervous system (CNS) consumes ~20% of the total oxygen inspired, while only accounting for ~2% of the total body weight (Silver and Erecinska, 1998). Mitochondrial dysfunction is a nearly ubiquitous occurrence in neurodegenerative diseases (Lin and Beal, 2006, Fiskum et al., 1999) and acute CNS injuries (Fiskum, 2000, Demarest and McCarthy, 2015, Demarest et al., 2016, Robertson et al., 2006). While mitochondrial respiratory impairment is associated with numerous pathologies, evidence for association comes primarily from studies using isolated brain mitochondria, which are derived from both neurons and glia, or from primary neuronal cultures. Roles for mitochondrial respiratory function/dysfunction in neuroglia such as astrocytes and microglia are comparatively understudied, which is surprising since neuroglia are thought to comprise ~50% of the brain, with variation by species (Azevedo et al., 2009, Herculano-Houzel, 2014).
Astrocytes were initially regarded as “glue” or “housekeeping” cells of the brain. However, they are now recognized to have many pivotal functions such as regulation of ion homeostasis (Olsen et al., 2015), neurotransmitter recycling (Schousboe et al., 1993), and control of cerebral blood flow (Cabezas et al., 2014). Astrocytes also carry out important bioenergetic roles in the brain, including regulation of brain glucose uptake, production and storage of brain glycogen, and provision of metabolic and antioxidant support for neurons (Belanger et al., 2011).
Microglia are the resident immune cells of the brain (Kreutzberg, 1996) that play diverse roles in physiology, including surveying the surrounding environment to clear cellular debris, synaptic pruning, and promotion of synaptogenesis (Tremblay et al., 2011, Wu et al., 2015). In response to brain pathology, microglia become “activated” and secrete cytokines, an initially protective process that becomes maladaptive when failing to resolve in a timely fashion (Brown and Bal-Price, 2003, Block et al., 2007). A shift in cellular bioenergetics from oxidative phosphorylation to glycolysis occurs during microglial activation (Voloboueva et al., 2013, Orihuela et al., 2015).
There is increasing evidence of sex differences in mitochondrial function in both health and disease. A recent study found that isolated brain mitochondria from female mice have higher Complex I-linked respiration than male mitochondria at 3 months, a difference that no longer exists by 20 months or following ovariectomy (Gaignard et al., 2015). Multiple studies demonstrated that the glutathione antioxidant defense system, which protects mitochondrial bioenergetic function, is elevated in females compared to males (Gaignard et al., 2015, Demarest et al., 2016). In adults, this effect is abolished by ovariectomy (Gaignard et al., 2015). While these studies suggest that sex hormones may directly regulate mitochondrial function, fewer studies have examined whether there are intrinsic sex differences in mitochondrial function at the cellular level. A sex difference in respiration was not apparent in brain mitochondria isolated from postnatal day 7 rats (Demarest et al., 2016). However, isolated forebrain mitochondria are derived from multiple cell types which may mask cell type-specific sex differences.
Sexual dimorphism of astrocyte morphology is already evident by the day of birth (Mong and McCarthy, 2002), suggesting that there can be early sex differences in glial cell properties. Dimorphism is thought to occur via an organizational effect of gonadal steroids in males (Mong and McCarthy, 2002). Thus, neonatally prepared glial cells from males will have had hormone exposure in utero, allowing for organizational effects (Mong et al., 1996, Mong and McCarthy, 2002), while being devoid of continued gonadal hormone exposure during in vitro development. In addition to neonatal astrocytes, sex is also thought to influence neonatal microglial cell properties, including proinflammatory gene expression (Loram et al., 2012). The precedence for intrinsic sex differences in XX vs. XY glial cells makes neonatal primary cultures an excellent system to investigate fundamental mitochondrial sex differences in defined cell populations.
The primary goal of this study was to test the hypothesis that there are sex differences in the mitochondrial respiratory properties of relatively pure populations of rat cortical astrocytes or microglia. An additional goal was to determine whether experimental oxygen tension influences the astrocytic or microglial bioenergetic function of either sex. The vast majority of in vitro work on mitochondrial function has been performed at atmospheric oxygen (160 mm Hg, 21% O2). However, atmospheric O2 tension is far higher than the pO2 that reaches cells within the brain (typically 5–45 mm Hg or ~1–6% O2) (Grote et al., 1996). Oxygen tension regulates many biochemical processes with the potential to impact mitochondrial respiration, including superoxide production (Hoffman et al., 2007), nitric oxide formation (Rengasamy and Johns, 1996) and the stability of oxygen-sensitive transcription factors such as HIF-1α (Semenza, 2012). Therefore, we hypothesized that conducting experiments at a physiologically relevant O2 level may reveal sex differences in mitochondrial bioenergetics that would not otherwise be observed. The effects of 3% O2, specifically, were investigated because early studies suggested a reduction in oxidative stress-induced changes in cultured cells at this O2 level compared to supraphysiological O2 (Parrinello et al., 2003, Busuttil et al., 2003). We refer to 3% O2 as low physiological O2 because several studies have considered in vitro 5% O2 as brain physiological O2 (Tiede et al., 2011, Zhu et al., 2012, Sun et al., 2015, Dussmann et al., 2017).
We found that male astrocytes exhibit a higher respiratory capacity than female astrocytes when cultured and assayed at 3% O2 (dissolved O2 of ~1.2–3.0%), but not when experiments are conducted at atmospheric 21% O2. However, we did not find sex differences in bioenergetic parameters or release of two key proinflammatory cytokines in non-stimulated or activated microglia, irrespective of experimental O2.
2. Materials and Methods
2.1. Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
2.2. Preparation of primary rat cortical astrocytes
All procedures were approved by the University of Maryland Institutional Animal Care and Use Committee and were compliant with the NIH Guide for the Care and Use of Laboratory Animals. Primary rat cortical astrocytes were prepared from postnatal day 1 Sprague Dawley rat pups as described (McKenna, 2012). Briefly, rats were sexed visually by comparing the distance between the genitals and anus. Only rats that were easily identifiable as male or female by at least two parties were used in this study. Rat pups were euthanized by decapitation. Cortices were then removed, homogenized by trituration, and vortexed for one minute. Brain homogenate from two pups of the same sex were then passed through a 70 µm filter before plating into two separate tissue culture-treated flasks in Eagle’s Minimal Essential Medium (EMEM, Quality Biological, Gaithersburg, MD) containing 10% fetal bovine serum (FBS), 1% non-essential amino acids (NEAA, Lonza, Walkerville, MD) and 50 µg/mL gentamicin. One flask was placed in a standard 37°C incubator at 95% air/5% CO2 (20% O2, which is 95% of atmospheric 21% O2). The other flask was placed in a 37°C 92% N2/5% CO2/3% O2 incubator (referred to as 3% O2). After two days, cell culture medium was changed to gentamycin-free medium and cells were thereafter maintained on medium without gentamicin. At 18 days in vitro, cells were trypsinized using TrypLE Express (Thermo Fisher, Waltham, MA), and sub-cultured for at least 24 hours prior to assays. Data represents preparations across 3–4 litters.
2.3. Preparation of primary rat cortical microglia
Primary rat cortical microglia were prepared from cortices of one day old Sprague-Dawley rats as previously described (Wu et al., 2010). Each primary culture preparation combined pups from 2 separate litters, with 3–4 separate preparations being utilized for each study. Briefly, cerebral cortices were dissected, homogenized by serial trituration with progressively narrower serological pipets, and plated in poly-D-lysine-coated culture flasks. Cells were maintained at 20% or 3% O2, as described above, in culture medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with penicillin (100 IU/mL) plus streptomycin (100 µg/mL) and 10% FBS. Seven days after preparation, flasks were shaken for one hour at 100 rpm using an Orbi-Shaker™ (Benchmark Scientific, Edison, NJ), after which medium was collected and centrifuged at 1,000g for 10 min to isolate microglia. To induce proinflammatory microglial activation, cells were treated with a combination of 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich, cat# L2654) and 10 ng/mL recombinant interferon-γ (IFN-γ; R&D Systems, Minneapolis, MN, cat# 485-MI-100) for 18 hours prior to bioenergetic measurements or cytokine quantification.
2.4. Dissolved Oxygen Measurements
Dissolved oxygen at the cell monolayer surface was measured using a commercially available sensor dish reader (SDR; PreSens, Regensburg, Germany) in specialized 24-well O2-sensing plates (Oxo-Dish OD-24; PreSens). These plates contain an immobilized O2-sensitive fluorescent patch within the plating surface of each well for measuring dissolved oxygen and come sterilized and calibrated by the manufacturer. Measurements were made at 15 second intervals over a 16 hour period in either a conventional 37°C, 95% air/5% CO2 humidified incubator or in the 37°C 3% O2 humidified incubator described above. Astrocytes were plated at 3.6 × 105 cells/well for measurements in the 20% O2 incubator and at 2.4 × 105 cells/well for measurements at 3% O2, yielding a comparable cell density at time of analysis (16 hours). A plating volume of 1.2 mL was used for both O2 levels. Data were acquired using SDR v4.0.0 software (PreSens), and then exported into Microsoft Excel for analysis. Dissolved O2 at the cell monolayer of microglial cultures was calculated based on the relative O2 consumption rate of the microglial cultures using Fick’s first law:
where J is the flux of O2 into the system; D is the diffusion coefficient of O2 into the media; ∇C is the change in O2 concentration in the x, y, and z directions in the culture media; and R is the cellular O2 consumption rate (Lewis et al., 2017).
2.5. XF24 microplate-based respirometry
Oxygen consumption rate (OCR) measurements from primary rat cortical astrocytes or microglia were performed using a Seahorse XF24 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA) as previously described (Gerencser et al., 2009, Clerc and Polster, 2012). Astrocytes were plated in XF24 V7 plates (Agilent Technologies) at 0.6 × 105 cells per well at 20% O2 and at 0.4 × 105 cells per well at 3% O2. Microglia were plated in V7 plates at 1.0 × 105 cells per well. Cells were allowed to attach and culture overnight. Microglia were either untreated or activated for 18 hours prior to assay, as described above. Prior to OCR assays, cells in three of the 24 wells were lysed with RIPA buffer (50 mM Tris, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate, pH 8.0). Lysates from these wells were used for protein quantification by the Pierce Micro bicinchoninic acid (BCA) Assay (Thermo Fisher), allowing subsequent normalization of O2 consumption rates to average protein per well. Artificial cerebrospinal fluid (aCSF) assay medium for respiration measurements consisted of 120 mM NaCl, 3.5 mM KCl, 0.4 mM KH2PO4, 1.3 mM CaCl2, 1 mM MgCl2, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 15 mM glucose, and 4 mg/mL fatty acid-free bovine serum albumin (BSA), pH 7.4. Cells were incubated in a CO2-free incubator at 37°C for 45 minutes prior to assay to allow temperature and pH calibration. XF24 assays consisted of cycles of 3 min mix, 2 min wait, and 2 min measure for astrocytes, and 2 min mix, 1 min wait, and 2 min measure for microglia.
For experiments performed at 3% O2, the Seahorse XF24 instrument was placed into the workspace of an Xvivo System environmental chamber (Biospherix, Ltd., Parish, NY) that also contained four cell culture incubators with independent gas control. The Xvivo workspace was at room temperature (~25°C) and regulated to 3% O2 (with no CO2). A minimum of 4 hours prior to assays, aCSF assay medium and a calibration cartridge containing XF calibrant (Agilent Technologies) were placed into a partitioned 37°C, 3% O2/97% N2/0% CO2 incubator to equilibrate to temperature and O2. Cells to be assayed at 3% O2 were cultured within another partitioned incubator set to 3% O2/92% N2/5% CO2 within the Xvivo System. Forty-five minutes prior to assays, cell culture medium was exchanged for 3% O2-equilibrated aCSF assay medium within the 3% O2-regulated Xvivo workspace and cells were then transferred to the CO2-free 3% O2 partitioned incubator. Injection port drug loading of the cartridge was also conducted at 3% O2 within the environmental chamber. XF24 assays consisted of the same mix-wait-measurement cycles described above. Importantly, three empty wells of each assay plate (A6, B6, and C6) received four successive injections of 1.0 M sodium sulfite (1:10, 1:11, 1:12, and 1:13 dilutions in the assay wells, respectively) to chemically scavenge oxygen and provide a zero O2 reference. Sodium sulfite stock (1.0 M) was made fresh immediately prior to each assay by dissolving powder in aCSF assay medium in a glass vial with a tight-fitting lid. For XF assays conducted at 3% O2, OCRs generated by the XF software were recalculated using the XF Hypoxia Rate Calculator Program (Agilent Technologies). The sodium sulfite zero oxygen reference specifically in wells A6, B6, and C6 is essential for the Hypoxia Rate software to calculate OCR at 3% O2.
2.6. ELISA analysis of TNF-α and IL-1β
Rat interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were quantified by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocols (R&D Systems).
2.7. Statistical analysis
Statistical analyses were performed using SigmaPlot 12.0 (Systat Software Inc, San Jose, CA). Two-way analysis of variance (ANOVA) was employed to evaluate the statistical significance of oxygen consumption measurements, while three-way ANOVA was used to evaluate the statistical significance of TNF-α and IL-1β measurements. A p value < 0.05 was considered significant. Tukey’s post-hoc analysis was used to compare individual groups.
3. Results
3.1. Estimation of dissolved oxygen at the cell monolayer surface
Cells that were assayed at 3% O2 were also cultured at 3% O2 and were not exposed to room air prior to measurements. Cells that were analyzed at 21% O2 were cultured at 20% O2 (95% of atmospheric 21% O2) in an incubator containing 95% air and 5% CO2. Due to cellular O2 consumption and limitations in cell culture medium O2 diffusion, dissolved O2 at the cell monolayer surface is less than the regulated O2 in air (Abaci et al., 2010). To get a better estimate of the O2 level at the cell surface for our two O2 setpoints, we used specialized Oxo-Dish plates containing O2 sensor patches directly embedded into the plates. For astrocytes cultured in 1.2 mL of media, a volume that produces a similar height of media to that used in Seahorse XF24 assay plates, O2 at the cell surface during culture was 1.17 ± 0.18% at a set point of 3% O2 and was 19.71 ± 0.13% O2 at a set point of 20% O2 (mean ± SD, n=3).
Next, we used the relative O2 consumption rates (OCR) of astrocytes and microglia to calculate the cell surface O2 level for primary microglia cultured at 3% O2. Because there were no sex differences in basal OCR (see below), cultures from males and females were combined for each cell type. The absolute (non-protein normalized) OCR of astrocytes was 69 ± 23 pmol O2/min (mean ± SD, n=11) while the absolute OCR of microglia was 49 ± 23 pmol O2/min (mean ± SD, n=6). Based on the slightly lower O2 consumption rate of microglia, the dissolved O2 at the microglial monolayer surface was estimated to be 1.69% during 3% O2 culture.
During the acquisition of O2 consumption rates by Seahorse respirometry, cells consume O2 and frequent media mixing is required. Therefore, during assays, astrocytes likely experience a dynamic O2 range of ~1.2–3.0% O2 when measured at 3% O2 while microglia likely experience a range of ~1.7–3.0% O2 under the same conditions. Cells measured at 21% O2 transiently drop below 21% O2 during 2 min measurements but O2 level quickly recovers after mixing.
3.2. Astrocyte bioenergetics
The basal OCR of male and female primary rat glial cells was measured at 3% or 21% O2. Following acquisition of basal OCR, maximal respiration was induced by the addition of the uncoupler FCCP and the cell permeable mitochondrial Complex I substrate pyruvate. Provision of excess substrate was done to preclude any differences due to insufficient substrate supply, enabling us to examine differences in OCR primarily due to differences in electron transport chain function. The Complex III inhibitor antimycin A was added last and any remaining oxygen consumption was regarded as non-mitochondrial respiration.
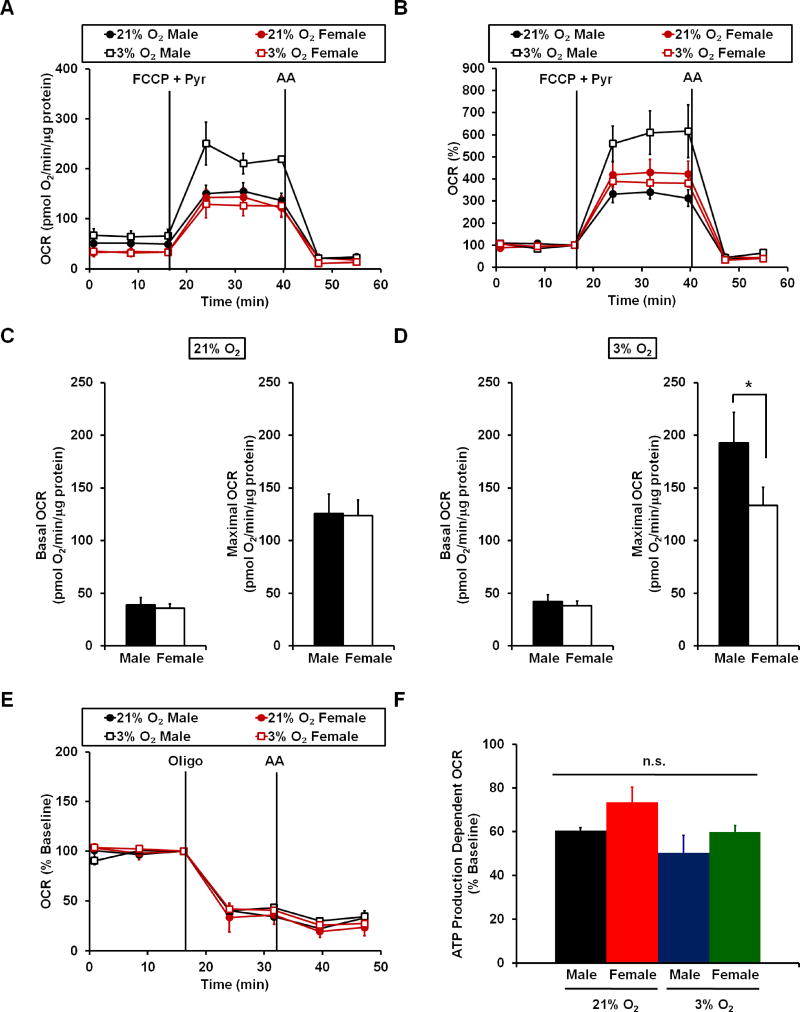
Primary rat cortical astrocytes showed no significant differences in basal OCR among male and female astrocytes at either 21% or 3% oxygen (Fig. 1A and C–D). There were also no differences among these groups in the amount of basal O2 consumption used for mitochondrial ATP synthesis, as estimated by the fraction of the basal respiration rate reduced by the ATP synthase inhibitor oligomycin (Fig. 1E and F). However, at 3% O2 male astrocytes demonstrated a significantly higher maximal respiration rate than female astrocytes (Fig. 1A–D). Male astrocytes at 3% O2 also had a higher maximal respiration rate than either male or female astrocytes at 21% O2. The difference in maximal respiration rate remained significant even after respiration was normalized to basal respiration (Fig. 1B), indicating that male astrocytes have greater spare respiratory capacity than female astrocytes at 3% O2. Spare respiratory capacity, the difference between basal and maximal respiration, is thought to reflect the capability of cells to respond to increased energy demand with an elevation in oxidative phosphorylation (Nicholls, 2009).
Figure 1. A sex difference in astrocyte respiration is observed at 3% oxygen but not at 21% oxygen.
(A) Representative traces of oxygen consumption rate (OCR) measurements from male and female primary rat cortical astrocytes cultured and tested at 21% atmospheric or 3% low physiological oxygen. FCCP (6 µM), pyruvate (Pyr, 10 mM) and antimycin A (AA, 1 µM) were added when indicated. Traces are mean ± standard deviation of three wells of cells from the same animal that were cultured and assayed at the different O2 values (i.e. one male and one female). (B) Representative traces of the data described in A after normalization to the third basal respiration measurement prior to FCCP + Pyr addition. (C–D) Bar graph representations of basal and maximal OCR at 21% O2 (C) and at 3% O2 (D). (E) Representative traces of normalized OCR measurements in male and female rat cortical astrocytes at 21% and 3% oxygen. Oligomycin (oligo, 0.3 µg/mL), and antimycin A (AA, 1 µM) were added when indicated. (F) Bar graph representation of ATP production-dependent OCR at 21% vs. 3% O2. Data are presented as mean ± standard error, n=5–8 astrocyte preparations across 3–4 separate litters derived from different dams. *p<0.05.
3.3. Microglial bioenergetics
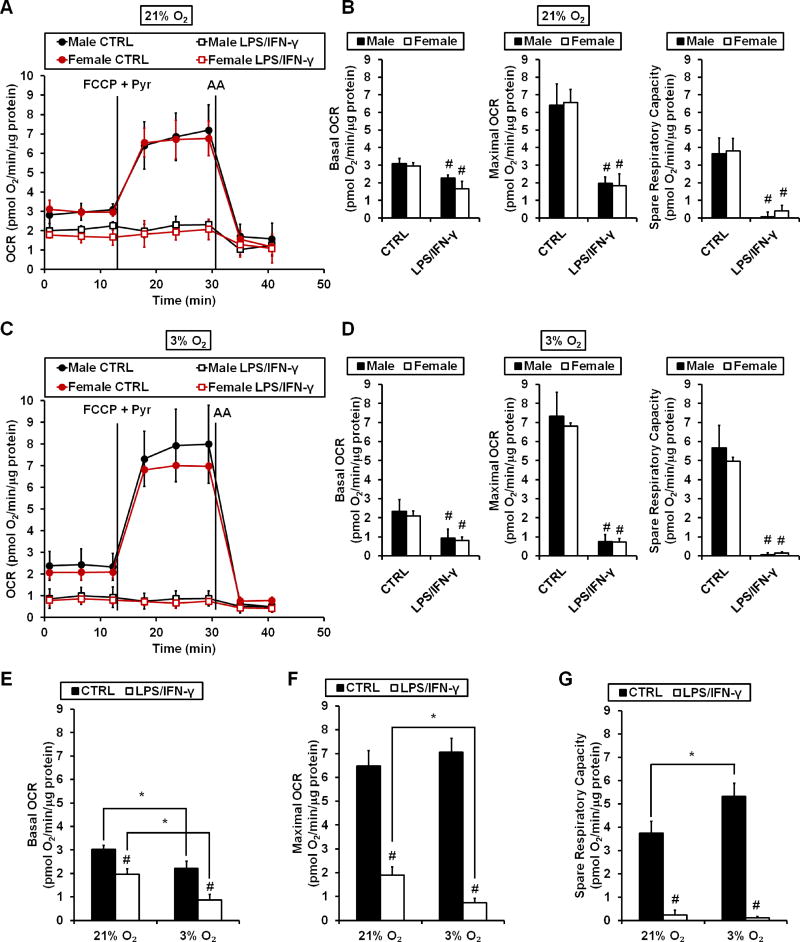
Primary rat cortical microglia were either untreated (CTRL) or stimulated with LPS plus IFN-γ (LPS/IFN-γ) for 18 hours prior to oxygen consumption measurements. There was no difference between male and female cells in basal OCR, maximal OCR, or spare respiratory capacity at either 3% or 21% O2 (Fig. 2A–D). In both male and female controls, basal OCR was lower at 3% O2 compared to 21% O2, an effect that remained consistent following LPS/IFN-γ stimulation (Fig. 2E). There was no oxygen tension-dependent difference in maximal OCR (Fig. 2F), yet spare respiratory capacity of untreated microglia (CTRL) was significantly higher at 3% O2 compared to 21% O2 (Fig. 2G). At both 21% O2 (Fig. 2A and B) and 3% O2 (Fig. 2C and D), basal and maximal OCR were significantly suppressed by LPS/IFN-γ treatment. There were no sex differences in the extent of inhibition at either oxygen tension. However, both basal and maximal OCR were impaired to a greater degree at 3% O2 compared to 21% O2 following LPS/IFN-γ treatment (Fig. 2E and F).
Figure 2. LPS/IFN-γ induces a greater respiratory impairment in microglia at 3% O2 compared to 21% O2, regardless of sex.
(A) Representative traces of oxygen consumption rate (OCR) measurements from primary rat cortical microglia at 21% O2 following 18 hours of LPS (100 ng/mL) plus IFN-γ (10 ng/mL) stimulation (LPS/IFN-γ) or control (CTRL) treatment. FCCP (4 µM), pyruvate (Pyr, 10 mM) and antimycin A (AA, 1 µM) were added when indicated. (B) Quantification of basal OCR, maximal OCR, and spare respiratory capacity from the experiments described in (A). (C) Representative traces of OCR measurements from cortical microglia at 3% O2 following 18 hours of LPS/IFN-γ stimulation or CTRL treatment. Drug additions were as in (A). (D) Quantification of basal OCR, maximal OCR, and spare respiratory capacity from the experiments described in (C). (E–G) Comparison of basal OCR (E), maximal OCR (F), and spare respiratory capacity (G) from microglia (combined data of male and female) at 21% vs. 3% O2. Traces in (A) and (C) are mean ± standard deviation of three wells of cells from the same microglial preparation and are representative of 3–4 independent experiments using different preparations. Data in (B) and (D) are mean ± standard deviation, n = 3–4 microglial preparations derived from different animals. Data in (E–G) are mean ± standard deviation, n=6–8 microglial preparations derived from different animals. Each individual preparation utilized litters stemming from separate dams. #p <0.05 compared to CTRL of the same sex or the same O2. *p<0.05 for 21% vs. 3% O2.
3.4. Microglial release of proinflammatory TNF-α and IL-1β in response to LPS/IFN-γ
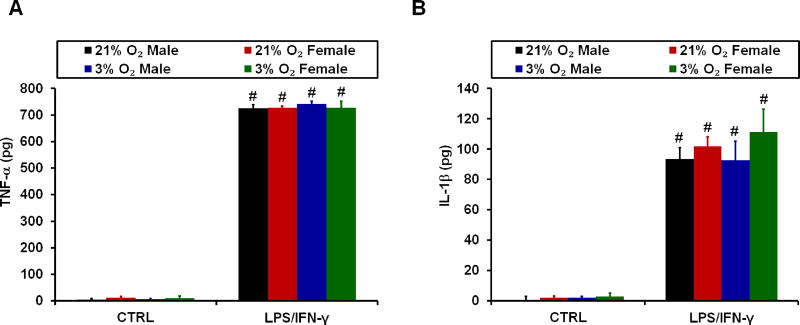
A previous study found that neonatal male cortical microglia expressed more IL-1β mRNA than female cells upon 4 hours of LPS stimulation (Loram et al., 2012). Therefore, despite finding no sex differences in the microglial mitochondrial response to LPS/IFN-γ, we decided to test whether sex influences microglial secretion of proinflammatory cytokines. We also evaluated whether physiological oxygen tension, compared to atmospheric O2, affects cytokine secretion. There were no significant sex or oxygen tension-dependent differences in the levels of TNF-α (Fig. 3A) or IL-1β (Fig. 3B) released by microglia stimulated with LPS/IFN-γ for 18 hours. There were also no differences in basal cytokine release from untreated cells (CTRL, Fig. 3A and B).
Figure 3. Lack of effect of sex or oxygen tension on microglial release of key proinflammatory cytokines.
Primary rat cortical microglia were untreated (CTRL) or treated with 100 ng/mL LPS plus 10 ng/mL IFN-γ (LPS/IFN-γ) for 18 hours, and the release of (A) TNF-α and (B) IL-1β were quantified by ELISA. Data are presented as mean ± standard deviation, n = 3–4 microglial preparations derived from different animals, with each preparation utilizing litters stemming from separate dams. #p <0.05 compared to CTRL.
4. Discussion
This study demonstrates an intrinsic sex difference in the mitochondrial bioenergetics of rat cortical astrocytes at low physiological 1.2–3.0% O2 that was not observed at atmospheric O2. Male astrocytes exhibited a higher maximal respiration rate than female astrocytes, but only when oxygen consumption rate was measured at 3% O2 following 3% O2 culture. In contrast to astrocytes, there were no sex differences in the respiratory characteristics of rat cortical microglia at either 3% or 21% O2, regardless of whether they were non-stimulated or activated by LPS/IFN-γ. Nevertheless, there were some oxygen tension-dependent differences in both nonstimulated and LPS/IFN-γ-stimulated microglia.
Non-stimulated microglia displayed slightly lower basal respiration at 3% O2 compared to atmospheric O2. Although maximal OCR was not different, the difference in basal respiration resulted in greater spare respiratory capacity when microglia were at 3% O2. As expected, LPS/IFN-γ stimulation curbed respiration at both oxygen levels, consistent with the reported metabolic shift from oxidative phosphorylation towards glycolysis upon activation (Voloboueva et al., 2013, Orihuela et al., 2015). However, LPS/IFN-γ induced a slightly greater suppression of both basal and maximal OCR at 3% O2 compared to 21% O2. Activated microglia produce nitric oxide (NO), which contributes to respiratory inhibition (Moss and Bates, 2001). One mechanism by which NO may do so is by competing with molecular oxygen at Complex IV of the electron transport chain (ETC) (Brown and Cooper, 1994, Cleeter et al., 1994). A greater inhibitory effect of NO on the ETC at 1.2–3.0% O2 compared to 21% O2 is predicted based on a competition mechanism, potentially accounting for the greater suppression of OCR that was observed when microglia were activated and assayed at 3% O2.
The Organizational/Activational Hypothesis of hormone action states that gonadallyderived steroid hormones create lasting sex differences in brain circuitry that are then activated by sex-specific hormones in adulthood (McCarthy et al., 2012). Importantly, activation of the testes during a critical window during the late stages of embryonic development leads to a surge in production of the hormone testosterone in males (Gillies and McArthur, 2010, Kight and McCarthy, 2014). This testosterone is aromatized to estradiol. In rodents, it is estradiol that plays a significant role in the masculinization or de-feminization of neural circuitry (referred to as organization) by influencing processes such as synaptogenesis and neurite outgrowth (Wilson and Davies, 2007). Because cultured cells are not exposed to the same levels of sex hormones that circulate in the brain, any sex differences observed in vitro would likely be caused by organizational modifications occurring in utero or intrinsic differences in gene expression between XX and XY chromosome-containing cells.
One concern involving in utero hormone exposure involves the role of alpha-fetoprotein, a plasma glycoprotein produced during fetal life (Andrews et al., 1982). Alpha-fetoprotein is thought to protect the developing female brain against masculinization through the binding of circulating estradiol (Bakker et al., 2006). As the level of alpha-fetoprotein may vary from mother to mother, we used litters from multiple dams to minimize differences due to variability in in utero hormone exposure. However, to determine the robustness and generalizability of the astrocyte sex difference identified in Sprague Dawley cortical astrocytes, a future goal is to test whether our findings extend to astrocytes derived from other rat strains, or from other species.
The sex difference in mitochondrial respiration found in astrocytes but not microglia at 3% O2 is consistent with the possibility of a lasting organizational modification occurring in utero specifically in astrocytes. Lasting in vitro sex differences have also been found in neurons, for example, in gamma-aminobutryic acid (GABA) responses in hippocampal neuronal cultures (Nunez and McCarthy, 2009). Intrinsic neuronal sex differences in mitochondrial properties have also been reported, including XX-XY differences in mitochondrial biogenesis (Sharma et al., 2014), morphology (Sharma et al., 2014), and recruitment of cell death signaling (Du et al., 2004, Sharma et al., 2014). The observation of mitochondrial sex differences in vitro in neurons and astrocytes but not microglia may be explained by the developmental origin of the brain cells. Both neurons and astrocytes are thought to originate from radial glial cells (Malatesta et al., 2003, Malatesta et al., 2000), whereas microglia are thought to arise from precursor cells originating in the yolk sac (Alliot et al., 1999). It may be that lasting sex differences observed in neurons and astrocytes develop from alterations occurring in precursor radial glial cells, adaptations that would not be found in yolk sac-derived microglia. Nevertheless, literature supports sex differences in microglial cytokine production during early development (Loram et al., 2012, Crain et al., 2013) or following injury (Mirza et al., 2015), indicating that the topic requires further study.
The mechanism underlying the higher respiratory capacity observed in male astrocytes compared to female astrocytes at 3% O2 remains to be elucidated. It may be that male astrocytes cultured and assayed at 3% O2 express rate-limiting enzymes involved in aerobic energy metabolism at higher levels than female cells or male cells at 21% O2. A transcriptome study identified increased expression of the gene encoding Complex I subunit Ndufa5 in astrocytes cultured at 4% O2 relative to 20% O2 (Chadwick et al., 2011). However, cells were pooled from both sexes, making the contribution of male astrocytes to this increase unclear. It is also possible that protein degradation/turnover of electron transfer chain subunits is differentially regulated, for example, by damage due to oxidative stress. Such alterations would not necessarily lead to a sex difference in basal respiration, which is regulated by energy demand. However, a relative increase in the levels of ETC proteins is predicted to provide males with a greater respiratory capacity, as indicated by maximal respiration measured in the presence of an uncoupler. Respiration in the presence of uncoupler is independent of energy demand because the protonmotive force required for ATP synthesis is dissipated. Therefore, uncoupled OCR measured with excess substrate is a good measure of ETC capacity.
Perhaps the best-characterized protein that is a candidate for mediating differences in mitochondrial protein expression is hypoxia-inducible factor-1α (HIF-1α), the oxygen-sensitive subunit of the transcription factor HIF-1 (Semenza, 2012). HIF-1α is constitutively degraded at atmospheric O2, with the prolyl hydroxylase enzymes necessary for this degradation using oxygen as a substrate (Epstein et al., 2001). Because prolyl hydroxylase activity is O2-dependent, degradation is limited under low-oxygen conditions, including within the physiological oxygen range (Epstein et al., 2001). Stabilization of HIF-1α is necessary for a wide variety of transcriptional processes, including regulation of cellular metabolism (Semenza, 2012) and, specifically, ETC Complex IV subunit composition (Fukuda et al., 2007). Interestingly, sex effects on Complex IV subunit transcription have also been reported (Roemgens et al., 2011). Alternatively, other factors could be responsible for modifying the function of the electron transport chain. For instance, if there is greater basal production of nitric oxide in female astrocytes compared to male cells, the higher NO concentration may result in inhibition of maximal respiration in female cells at 3% O2. This would lead to a higher respiratory capacity in male cells. The absence of a sex difference at 21% O2 might then be explained by an inability of NO to effectively compete with O2 when oxygen is more abundant. Additionally, it is possible that the ETC is modified post-translationally in a sex-dependent manner, such as by S-nitrosylation, phosphorylation, or acetylation. Proteomic studies are needed to help resolve whether there are male-female differences in the composition or post-translational modification of the ETC in cultured astrocytes at 3% O2.
A limitation of our study is that although the brain experiences a range of oxygen tensions, our experiments were performed at a single physiological oxygen tension. Notably, we were able to estimate O2 level at the cell monolayer surface at the 3% O2 set point, showing that dissolved O2 is actually ~1.2% during culture of astrocytes and ~1.7% during culture of microglia. These O2 levels are closer to the bottom of the physiological range rather than the 3.5% midpoint. It is possible that the sex difference in astrocyte mitochondrial bioenergetics observed at 1.2–3.0% O2 may decrease, increase, or disappear at the upper or lower O2 limits of the physiological range. Similarly, it is possible that a sex difference in microglial bioenergetics would be revealed elsewhere within the physiological O2 range.
The most significant contribution of our study is demonstration of the importance of controlling oxygen tension when studying in vitro sex differences of glial cells. Additional studies are required to determine the mechanisms behind the dichotomous bioenergetic profiles of male and female astrocytes at low physiological oxygen. It also remains to be seen whether the slight quantitative but not qualitative difference in microglial respiratory impairment at 3% O2 compared to atmospheric O2 during proinflammatory activation is functionally significant.
Male astrocytes have greater respiratory capacity than female at a physiological O2.
No sex differences in astrocyte respiration are seen at atmospheric O2.
No sex differences in microglial respiration are observed at either O2.
Respiration is more inhibited when microglia are activated at a physiological O2.
Acknowledgments
The authors thank Dr. Jessica Mong for critically reading the manuscript and expert comments. This work was supported by the National Institutes of Health [grant numbers R01NS085165, R01NS091099, and P01HD016596] and by the M. Jane Matjasko Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abaci HE, Truitt R, Luong E, Drazer G, Gerecht S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am. J. Physiol Cell Physiol. 2010;298:C1527–C1537. doi: 10.1152/ajpcell.00484.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Andrews GK, Dziadek M, Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J. Biol. Chem. 1982;257:5148–5153. [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob FW, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Bakker J, De MC, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- Busuttil RA, Rubio M, Dolle ME, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–294. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Cabezas R, Avila M, Gonzalez J, El-Bacha RS, Baez E, Garcia-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick W, Boyle JP, Zhou Y, Wang L, Park SS, Martin B, Wang R, Becker KG, Wood WH, III, Zhang Y, Peers C, Maudsley S. Multiple oxygen tension environments reveal diverse patterns of transcriptional regulation in primary astrocytes. PLoS. ONE. 2011;6:e21638. doi: 10.1371/journal.pone.0021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Clerc P, Polster BM. Investigation of mitochondrial dysfunction by sequential microplate-based respiration measurements from intact and permeabilized neurons. PLoS. ONE. 2012;7:e34465. doi: 10.1371/journal.pone.0034465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg. Biomembr. 2015;47:173–188. doi: 10.1007/s10863-014-9583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G. Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem. 2016;137:714–729. doi: 10.1111/jnc.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Dussmann H, Perez-Alvarez S, Anilkumar U, Papkovsky DB, Prehn JH. Single-cell time-lapse imaging of intracellular O2 in response to metabolic inhibition and mitochondrial cytochrome-c release. Cell Death. Dis. 2017;8:e2853. doi: 10.1038/cddis.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Gaignard P, Savouroux S, Liere P, Pianos A, Therond P, Schumacher M, Slama A, Guennoun R. Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice. Endocrinology. 2015;156:2893–2904. doi: 10.1210/en.2014-1913. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote J, Laue O, Eiring P, Wehler M. Evaluation of brain tissue O2 supply based on results of PO2 measurements with needle and surface microelectrodes. J. Auton. Nerv. Syst. 1996;57:168–172. doi: 10.1016/0165-1838(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am. J. Physiol Heart Circ. Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- Kight KE, McCarthy MM. Using sex differences in the developing brain to identify nodes of influence for seizure susceptibility and epileptogenesis. Neurobiol Dis. 2014;72(Pt B):136–143. doi: 10.1016/j.nbd.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lewis DM, Tang V, Jain N, Isser A, Xia Z, Gerecht S. Collagen Fiber Architecture Regulates Hypoxic Sarcoma Cell Migration. ACS Biomater. Sci. Eng. 2017 doi: 10.1021/acsbiomaterials.7b00056. (in press) [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res. 2012;37:2613–2626. doi: 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm. Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res. Dev. Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Moss DW, Bates TE. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci. 2001;13:529–538. doi: 10.1046/j.1460-9568.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neurosci. 2009;158:623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling. J Neurosci. 2015;35:13827–13835. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2015 doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy A, Johns RA. Determination of Km for oxygen of nitric oxide synthase isoforms. J. Pharmacol. Exp. Ther. 1996;276:30–33. [PubMed] [Google Scholar]
- Robertson CL, Soane L, Siegel ZT, Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Dev. Neurosci. 2006;28:432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- Roemgens A, Singh S, Beyer C, Arnold S. Inducers of chemical hypoxia act in a gender- and brain region-specific manner on primary astrocyte viability and cytochrome C oxidase. Neurotox. Res. 2011;20:1–14. doi: 10.1007/s12640-010-9213-z. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L. Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci. 1993;15:359–366. doi: 10.1159/000111356. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Johnston MV, Hossain MA. Sex differences in mitochondrial biogenesis determine neuronal death and survival in response to oxygen glucose deprivation and reoxygenation. BMC. Neurosci. 2014;15:9. doi: 10.1186/1471-2202-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I, Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv. Exp Med Biol. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- Sun X, Voloboueva LA, Stary CM, Giffard RG. Physiologically normal 5% O2 supports neuronal differentiation and resistance to inflammatory injury in neural stem cell cultures. J. Neurosci. Res. 2015;93:1703–1712. doi: 10.1002/jnr.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede LM, Cook EA, Morsey B, Fox HS. Oxygen matters: tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death. Dis. 2011;2:e246. doi: 10.1038/cddis.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Emery JF, Sun X, Giffard RG. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013;587:756–762. doi: 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331–359. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- Wu J, Wrathall JR, Schachner M. Phosphatidylinositol 3-kinase/protein kinase Cdelta activation induces close homolog of adhesion molecule L1 (CHL1) expression in cultured astrocytes. Glia. 2010;58:315–328. doi: 10.1002/glia.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Aja S, Kim EK, Park MJ, Ramamurthy S, Jia J, Hu X, Geng P, Ronnett GV. Physiological oxygen level is critical for modeling neuronal metabolism in vitro. J. Neurosci. Res. 2012;90:422–434. doi: 10.1002/jnr.22765. [DOI] [PubMed] [Google Scholar]