Abstract
Classic studies in bone mechanobiology have established the importance of loading parameters on the anabolic response. Most of these early studies were done using loading methods not currently in favor, and using non-murine species. Our objective was to re-examine the effects of several loading parameters on the response of cortical bone using the contemporary murine axial tibial compression model. We subjected tibias of 5-month old, female C57Bl/6 mice to cyclic (4 Hz) mechanical loading and examined bone formation responses using dynamic and static histomorphometry. First, using a reference protocol of 1200 cycles/day, 5 days/week for 2 weeks, we confirmed the significant influence of peak strain magnitude on periosteal mineralizing surface (Ps.MS/BS) and bone formation rate (Ps.BFR/BS) (p<0.05, ANOVA). There was a significant induction of periosteal lamellar bone at a lower threshold of approx. -1000 με and a transition from lamellar-woven bone near −2000 με. In contrast, on the endocortical surface, bone formation indices did not exhibit a load magnitude-dependent response and no incidence of woven bone. Next, we found that reducing daily cycle number from 1200 to 300 to 60 did not diminish the bone formation response (p>0.05). On the other hand, reducing the daily frequency of loading from 5 consecutive days/week to 3 alternate days/week significantly diminished the periosteal response, from a loading-induced increase in Ps.MS/BS of 38% (loaded vs. control) for 5 days/week to only 15% for 3 days/week (p<0.05). Finally, we determined that reducing the study duration from 2 to 1 weeks of loading did not affect bone formation outcomes. In conclusion, cyclic loading to −1800 με peak strain, at 4 Hz and 60 cycles/day for 5 consecutive days (1 week) induces an increase in periosteal lamellar bone formation with minimal incidence of woven bone in 5-month old C57Bl/6 female mice. Our results provide a basis for reduction of loading duration (daily cycles and study length) without loss of anabolic effect as measured by dynamic histomorphometry.
Keywords: bone mechanobiology, bone formation, mouse tibial loading, in vivo mechanical loading, mouse model
Introduction
In vivo studies of altered skeletal loading have contributed greatly to our understanding of the mechanisms that govern bone adaptation [1, 2]. While a number of species and loading models have been used historically, most recent studies have been conducted in mice using non-invasive axial loading of either the forelimb (ulna) [3] or the lower leg (tibia) [4, 5]. Our focus here is the murine model of axial tibial compression, which has been widely used since its original descriptions in 2005 [6–13].
Numerous parameters can influence bone’s anabolic response to mechanical loading [1, 2, 14]. These are predicated on the use of a dynamic stimulus [15], referred to as cyclic loading, typically applied in daily “bouts” [16]. Relevant parameters include: peak load (or strain) magnitude, strain rate, cyclic frequency (cycles/sec), shape of loading waveform, duration of daily bouts (time or cycle number), rest insertion (between cycles or between bouts), and duration of study (days or weeks of loading). The importance of these parameters has been established through studies (reviewed elsewhere [1, 2]) that pre-date the use of murine models. In particular, foundational results have been obtained using the isolated avian ulnar model [15, 17–19], the rat four-point tibial bending model [16, 20–22], and the rat ulnar axial loading model [23–25]. Although there is little reason to doubt the generalizability of these earlier results, few studies have examined the effects of loading parameters on bone outcomes using the mouse axial tibial loading model. Because parameter values used for murine axial tibial loading can differ widely between research groups, additional data specific to this model will facilitate comparisons between studies as well as the rational design of protocols for new studies.
There are some data on the effect of loading parameters on bone response in the mouse axial tibial compression model. The importance of peak force (or strain) magnitude has been confirmed in several studies. Lynch et al. [6] reported that adult C57Bl/6 mice did not respond to a loading protocol that applied +1200 με peak strain, but exhibited an anabolic response when loaded to +2100 με. We reported a strain magnitude-dependent cortical response in studies of BALB/c and C57Bl/6 inbred mice at different ages [7, 26], and others have reported a linear strain-dependent response in young-adult C57Bl/6 mice [8, 9]. An inconsistent finding of these studies is the force at which woven bone is observed. In 4–5 month old C57Bl/6 female mice, we observed some woven bone in 25% of tibias loaded to 8 N [26], whereas others reported that woven bone does not appear until forces exceed 10 or 12 N [8, 9]. The reason for this discrepancy is unclear. Considering factors other than magnitude, we previously compared two protocols that differed in several parameters (cyclic frequency, strain rate, cycles/day, rest intervals) and found differences in the rate of bone accrual [27], although we did not dissect which parameters contributed to the differences. Recently, Yang et al. [28] evaluated the effects of daily loading duration and rest insertion in a 2-week study in C57Bl/6 mice. They reported that cortical bone accrual increased with cycle number, although there were diminishing gains with increasing cycles from 36 to 216 to 1200. They also found that insertion of a 10-s rest between cycles did not affect bone accrual.
The choice of loading parameters has practical implications for animal handling, anesthesia exposure, and ease of implementation. Protocols that use shorter daily loading bouts, fewer days/week, and shorter study length may be advantageous provided that they elicit a sufficient anabolic response. For example, the findings of Yang et al. [28] provide rationale for briefer daily loading (fewer cycles, no rest insertion). Regarding study length, initial studies of mouse axial loading used 2-week (or longer) loading durations [3–5], yet more recent studies have reported significant loading effects in mice using only 3 or 5 days of loading [7, 16, 29, 30]. Direct assessment of the effect of study duration on bone formation outcomes has not been reported.
Our objective was to examine the effects of several loading parameters on the anabolic response of cortical bone in the mouse axial tibial compression model. Although this model is also useful for studying cancellous bone adaptation, we focused on cortical bone to limit the scope of the study and because the foundational studies of loading parameters from other species have examined cortical responses. We focused on issues that have been relatively underexplored with the mouse tibial model: 1) the incidence of woven bone as a function of force (strain) magnitude; and 2) the effect of parameters related to duration and number of daily loading bouts. We started with a “reference” protocol based on recent studies, and then varied parameters individually to determine their effect on cortical bone formation assessed by dynamic histomorphometry. We concluded by comparing the “reference” protocol with a strain-matched “revised” protocol that produces similar increases in bone formation with fewer daily cycles and a shorter study duration.
Methods
Animals
This study was approved by the Washington University Institutional Animal Care and Use Committee (IACUC). Female C57BL/6 mice were purchased at 12–13 weeks age from the -Charles River Laboratory (Wilmington, MA, USA) or Jackson Laboratory (Bar Harbor, ME, USA) and were housed until use at 5 months age, when they had completed the rapid phase of skeletal growth [31, 32] and are considered young-adult [33]. A total of 83 mice were used. Tibias from four pairs were damaged during histological processing and not useable, leaving 79 pairs of tibiae for analysis. Mice were housed in group cages (up to five per cage) in a central facility with ad libitum access to water and regular chow (Purina 5053; Purina, Saint Louis, MO, USA).
In vivo tibial compression
Prior to each daily loading session (bout), mice were transported from the housing facility to the lab where loading was performed. They were anesthetized (3% isoflurane) and their right lower leg was placed in a vertical orientation in a materials testing system (ElectroPuls E1000; Instron, Norwood, MA, USA). Loading fixtures and animal positioning were similar to our recent studies [26, 27]. We have reported that animal transport, handling and daily anesthesia did not affect bone structure in similarly aged mice [27]. A small pre-load was applied (−0.5 N) and then the tibia was loaded cyclically through the knee (above) and foot (below) in axial compression. The temporal parameters of the triangle waveform were as described [4, 28], with the input command for each cycle consisting of a linear ramp to peak in 0.075 s, return to pre-load in 0.075 s, followed by 0.1 s dwell. Frequency was fixed at 4 Hz. The left tibiae were not loaded and served as contralateral controls. Mice received buprenorphine (0.1 mg/kg s.c.) immediately after each loading bout for analgesia, and were returned to their cages for unrestricted activity. By routine observation we did not observe signs of tissue damage or lameness.
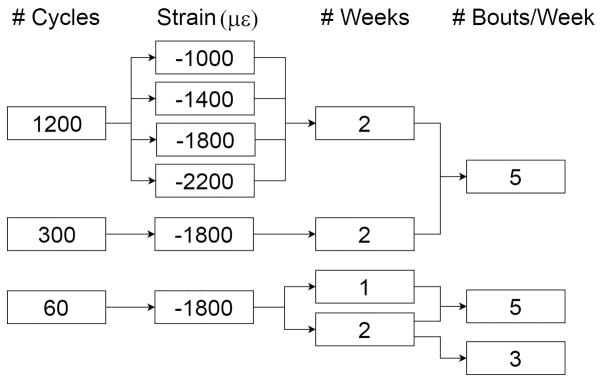
The “reference” loading protocol was one we have used in recent studies and is known to be anabolic for cortical bone in young-adult C57Bl/6 mice [6, 26, 27] (Table 1). The parameters that were varied from the reference values were, in order: a) peak compressive strain, b) number of load cycles/day, c) number of loading days (bouts)/week, and d) number of weeks of loading. (We also varied the input command waveform, from triangular [reference] to haversine, with the intent of comparing waveform shape. Post hoc analysis revealed that the actuator output for the two waveforms was more similar than different, and thus this part of the study proved inconclusive and is presented in the Supplement.) Animals were distributed across 10 groups (n = 4–10) with each group assigned to a unique set of loading parameters (Fig. 1). The study design was not fixed a priori and was not intended to examine all possible combinations of parameters. We proceeded incrementally to consider each of the four variables one at a time in a way that avoided redundancy and reduced the parameter combinations to the ones we deemed of most practical relevance. (See Tables 2, 3 for Groups.)
Table 1.
Parameters of the “reference” loading protocol, based on recent studies [26, 27]. Variations in each of these parameters were evaluated, except for waveform frequency.
| Peak strain | −2200 με |
| Cycles/day | 1200 |
| Days/week | 5 |
| Weeks | 2 |
| Waveform shape (input command) | Triangle |
| Waveform frequency | 4 Hz |
Figure 1.
Experimental design showing the four loading parameters that were varied. Each “path” corresponds to one of the eight experimental groups.
Table 2.
Periosteal (Ps) bone formation indices. Values are mean ± SD.
| Group | n | Cycles | Strain (με) | Weeks | Days/Week | Side | sLS/BS (%) | dLS/BS (%) | MS/BS (%) | MAR (um/day) | BFR/BS (um/day) | Incidence Wo.B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1200 | 1000 | 2 | 5 | Control | 11.2 ± 6.2 | 1.4 ± 1.8 | 7 ± 4 | 1.0 ± 0.5 | 0.1 ± 0.1 | 0 |
| Loaded | 19.1 ± 12.2* | 12.8 ± 13 | 22.4 ± 13.1 | 1.4 ± 0.5 | 0.4 ± 0.2* | 0 | ||||||
| 2 | 6 | 1200 | 1400 | 2 | 5 | Control | 20.6 ± 10.6 | 0.5 ± 1.3 | 11 ± 6.3 | 0.8 ± 0.2 | 0.1 ± 0 | 0 |
| Loaded | 32.7 ± 7.5* | 21.3 ± 21.7* | 37.7 ± 21.1* | 1.4 ± 0.3 | 0.6 ± 0.4 | 0 | ||||||
| 3 | 8 | 1200 | 1800 | 2 | 5 | Control | 15.3 ± 9.4 | 0.9 ± 0.8 | 8.6 ± 4.7 | 0.8 ± 0.3 | 0.1 ± 0 | 0 |
| Loaded | 28.5 ± 9.5* | 38.5 ± 17.9* | 53.7 ± 15.3* | 1.5 ± 0.4* | 0.8 ± 0.3* | 1 | ||||||
| 4 | 7 | 1200 | 2200 | 2 | 5 | Control | 15.7 ± 7.7 | 1.0 ± 1.1 | 8.8± 4.3 | 1.0 ± 0.2 | 0.1 ± 0 | 0 |
| Loaded | 23.5 ± 13.2* | 48 ± 11.5* | 63.5 ± 10.4* | 1.7 ± 0.5 | 1.0 ± 0.3* | 3 | ||||||
| 5 | 8 | 300 | 1800 | 2 | 5 | Control | 18.2 ± 7.9 | 1.1 ± 0.8 | 10.2 ± 4.4 | 0.7 ± 0.2 | 0.1 ± 0 | 0 |
| Loaded | 23.7 ± 10.2 | 48.3 ± 29* | 62.2 ± 26.9* | 1.8 ± 0.7* | 1.2 ± 0.8* | 2 | ||||||
| 6 | 9 | 60 | 1800 | 2 | 5 | Control | 28.5 ± 21.8 | 2.9 ± 1.9 | 17.1 ± 12.4 | 1.0 ± 0.4 | 0.2 ± 0.2 | 0 |
| Loaded | 24.5 ± 5.7 | 42.9 ± 22* | 55.2 ± 20.2* | 1.4 ± 0.5* | 0.8 ± 0.5* | 0 | ||||||
| 7 | 9 | 60 | 1800 | 1 | 5 | Control | 16.0 ± 9.1 | 3.2 ± 5.9 | 11.1 ± 9.3 | 1.1 ± 0.3 | 0.1 ± 0.1 | 0 |
| Loaded | 34.4 ± 12.6* | 33.8 ± 7.5* | 53.3 ± 8.7* | 1.7 ± 0.3* | 0.9 ± 0.3* | 1 | ||||||
| 8 | 9 | 60 | 1800 | 2 | 3 | Control | 19.2± 9.9 | 3.1 ± 2.5 | 12.7 ± 6.8 | 0.9 ± 0.1 | 0.1 ± 0.1 | 0 |
| Loaded | 35.0 ± 13.8* | 10.2 ± 7.9* | 27.7 ± 10.2* | 1.0 ± 0.2 | 0.3 ± 0.2* | 0 |
Loaded different from Control, p < 0.05
Table 3.
Endocortical (Ec) bone formation indices. Values are mean ± SD.
| Group | n | Cycles | Strain (με) | Weeks | Days/Week | Side | sLS/BS (%) | dLS/BS (%) | MS/BS (%) | MAR (um/day) | BFR/BS (um/day) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1200 | 1000 | 2 | 5 | Control | 70.9 ± 15.3 | 7.3 ± 6.1 | 54.5 ± 19 | 1.0 ± 0.3 | 0.5 ± 0.1 |
| Loaded | 77.3 ± 2.5 | 4.8 ± 5.3 | 54.7 ± 20.5 | 1.5 ± 0.8 | 0.8 ± 0.3 | ||||||
| 2 | 6 | 1200 | 1400 | 2 | 5 | Control | 72.6 ± 10.4 | 6.6 ± 13.1 | 42.9 ± 11.4 | 0.9 ± 0.2 | 0.4 ± 0.2 |
| Loaded | 66.7 ± 7.8 | 16.7 ± 11.1 | 50.0 ± 12.2 | 1.8 ± 0.5* | 1.0 ± 0.2* | ||||||
| 3 | 8 | 1200 | 1800 | 2 | 5 | Control | 58.5 ± 24 | 7.0 ± 8.1 | 36.3 ± 13.7 | 1.1 ± 0.3 | 0.4 ± 0.2 |
| Loaded | 47.7 ± 15.2 | 13.7 ± 7.9 | 37.5 ± 8.3 | 1.3 ± 0.3 | 0.5 ± 0.1 | ||||||
| 4 | 7 | 1200 | 2200 | 2 | 5 | Control | 66.0 ± 15.1 | 6.6 ± 11.1 | 39.6 ± 11.9 | 1.1 ± 0.4 | 0.5 ± 0.3 |
| Loaded | 55.7 ± 8.0 | 12.8 ± 6.1 | 40.7 ± 7.6 | 1.9 ± 0.4* | 0.8 ± 0.2 | ||||||
| 5 | 8 | 300 | 1800 | 2 | 5 | Control | 69.3 ± 26.4 | 7.6 ± 9.3 | 42.2 ± 17.2 | 0.7 ± 0.2 | 0.3 ± 0.2 |
| Loaded | 60.1 ± 36.8 | 25.2 ± 12.8* | 55.2 ± 21.1* | 1.7 ± 0.7* | 1.0 ± 0.5* | ||||||
| 6 | 9 | 60 | 1800 | 2 | 5 | Control | 71.8 ± 11.2 | 8.5 ± 7.7 | 49.3 ± 15.1 | 1.0 ± 0.3 | 0.5 ± 0.2 |
| Loaded | 65.6 ± 36.6 | 27.5 ± 11.5* | 60.3 ± 14.9 | 1.4 ± 0.3 | 0.8 ± 0.4 | ||||||
| 7 | 9 | 60 | 1800 | 1 | 5 | Control | 41.2 ± 11.4 | 6.6 ± 10.1 | 27.2 ± 7.5 | 1.0 ± 0.3 | 0.3 ± 0.2 |
| Loaded | 44.2 ± 8.4 | 26.6 ± 13.8* | 48.7 ± 14.6* | 1.5 ± 0.3* | 0.7 ± 0.3* | ||||||
| 8 | 9 | 60 | 1800 | 2 | 3 | Control | 50.9 ± 19.9 | 20.4 ± 24.2 | 45.8 ± 18.8 | 1.0 ± 0.5 | 0.5 ± 0.3 |
| Loaded | 48.2 ± 17.5 | 33.2 ± 24.1* | 57.3 ± 18 | 1.3 ± 0.3 | 0.8 ± 0.4* |
NB: There was zero incidence of endocortical woven bone.
Loaded different from Control, p < 0.05
Based on a recent strain analysis [34], we selected peak forces of −4.2, −5.5, −7 and –8 N to generate corresponding peak periosteal strains of −1000, −1400, −1800, and −2200 με, values selected to span the lower range of the anabolic window [20, 24]. Throughout this paper, we use these strain values to describe loading magnitudes. Notably, these values are for the diaphyseal site 5 mm proximal to the distal tibiofibular junction (TFJ), and denote the estimated peak magnitude in the cross-section, which occurs at the postero-lateral apex [35]. This magnitude at this site is 1.5–2 times greater than the value at the site of peak tension located on the antero-medial tibial surface [34], a common site for strain reporting in this model. Also of note, we did not adjust the waveform temporal parameters when varying loading magnitude, and thus the loading (strain) rate varied as a direct function of force (strain) magnitude [1]. Estimated peak loading (strain) rates varied from 49–100 N/s (0.012–0.027 s−1).
The number of daily loading cycles was varied from 60 to 300 to 1200, similar to the range evaluated by Yang et al. [28]. The number of loading bouts per week was varied from 5 consecutive days to 3 alternate days, representing two commonly used approaches [3, 9, 24, 26, 36]. In the 5 bouts/week loading scheme, mice were loaded on days 1–5 and 8–12. In the 3 bouts/week loading scheme, mice were loaded on days 1, 3, 5, 8, 10, and 12. The number of weeks of loading was varied from 2 to 1, representing two reported approaches [3, 7, 16, 20, 24, 26]. In the 1-week loading schemes, mice were loaded on days 1–5.
Histomorphometry
Double fluorochrome labels were administered to allow assessment of dynamic indices of bone formation. The timing of the labels was chosen to approximate prior study designs that administered the first label several days after the initial loading bout, and the second label 5–9 days later, several days before death [3, 7, 16, 20, 24, 26]. With this approach, the inter label interval should capture the estimated time of peak rate of bone formation, which has been reported to occur 5–8 days after a loading bout [16, 19, 21]. In the 1-week loading scheme, mice were injected with calcein green (10 mg/kg IP; Sigma, Saint Louis, MO, USA) and alizarin complexone (30 mg/kg IP; Sigma) on days 5 and 10, respectively, and were euthanized on day 12. In the 2-week loading scheme, mice received calcein and alizarin on days 5 and 12, respectively, and were euthanized on day 15. Both tibiae were harvested and embedded in plastic. Diaphyseal transverse sections were cut at the site of strain gauge analysis (5 mm proximal to the distal TFJ), which corresponds approximately to a site 37% of the tibial length from the proximal end as described by others [9, 28]. (Unpublished data from our lab show that loading induces a similar bone formation response across the 25–50% tibial region.) Initial sections were cut at 100 μm thickness on a saw-microtome (SP 1600; Leica, Wetzlar, Germany) then ground manually to 30 μm thickness and mounted on glass slides. Images were obtained using a fluorescent microscope (Axio Observer D1; Carl Zeiss, Oberkochen, Germany) and analyzed using commercial software (Osteo II; BIOQUANT, Nashville, TN, USA). We determined standard measures of bone formation [37], including lamellar indices: single- and double-labeled surface per bone surface (sLS/BS [green or red label], dLS/BS [green and red label separated by interlabel gap]), and mineral apposition rate (MAR); woven indices: woven surface (Wo.S); and combined lamellar and woven indices: total mineralizing surface (MS/BS) and total bone-formation rate (BFR/BS), on the periosteal (Ps) and endocortical (Ec) surfaces.
Static histomorphometry measures were also computed to determine the effect of loading on cortical bone structure. We report: cortical area (Ct.Ar), total area (Tt.Ar), and cortical width (Ct.Wi).
Statistical analysis
Paired Student’s t-tests were used to compare the bone formation indices between the right (loaded) and left (control) tibiae, as test of a local loading effect. One-way analysis of variance (ANOVA) was used to study the effects of loading parameters on bone formation, for loaded (right) and non-loaded (left) limbs separately, with Tukey post-hoc tests for multiple comparisons. Statistical significance was considered as p < 0.05, with trends noted for 0.05 < p < 0.10. Analysis was performed with Prism 7.0 (GraphPad, La Jolla, CA, USA).
Results
Control tibias
ANOVA/Tukey analysis of control (non-loaded) samples revealed few effects of loading parameters. The only significant differences were that Ec.MS/BS and Ec.BFR/BS were lower for 1 week than 2 weeks of loading (p < 0.05, Group 7 vs. 6, Table 3). Overall, these findings indicate that values from control samples were similar across groups.
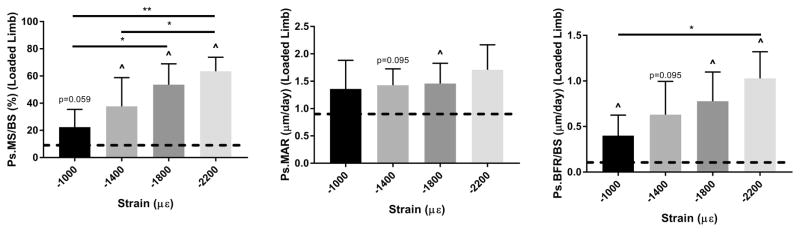
Periosteal bone formation exhibits dose response with increasing strain magnitude
Ten daily bouts of tibial loading induced a strain magnitude dependent anabolic response, primarily evident as increased periosteal mineralizing surface in the loaded limbs compared to contralateral controls (Figs. 2, 3; Groups 1–4, Table 2). Control tibias had an average Ps.MS/BS of 9.0%, independent of group (p > 0.05), indicating a mostly quiescent surface. Loading stimulated an increase in Ps.MS/BS (%) ranging from +15.4 at −1000 με (loaded vs. control, p = 0.06) to +54.7 at −2200 με (p < 0.001). Ps.MAR was less strongly enhanced by loading, with only the −1800 με group reaching significance (loaded vs. control, +0.7 μm/day, p < 0.05). Ps.BFR/BS was increased significantly in loaded vs. control bones in the −1000, −1800 and −2200 με groups, but the loading effect did not reach significance in the −1400 με group (p = 0.095). Increasing peak strain also induced higher incidence of woven bone formation, with no woven bone detected in the −1000 and the −1400 με groups, but 1 of 8 and 3 of 7 samples showing some modest woven bone in the −1800 and −2200 με groups, respectively.
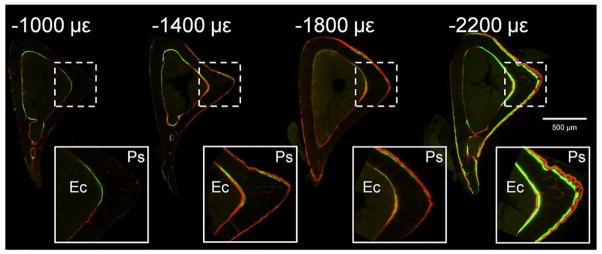
Figure 2.
Representative transverse sections of fluorochrome-labeled, loaded tibias illustrating increasing bone formation on periosteal (Ps) and endocortical (Ec) surfaces with increasing peak strain magnitude. Tibias in these groups were loaded 1200 cycles/day, 5 days/wk for 2 weeks. Mice were loaded days 1–5 and 8–12, injected with calcein on day 5 and alizarin on day 12, and euthanized on day 15. The sample in the −2200 με group has a small amount of periosteal woven bone.
Figure 3.
Periosteal bone formation increased with increasing peak strain magnitude. A significant dose-response was observed for Ps.MS/BS and Ps.BFR/BS (p < 0.05, ANOVA), but not for Ps.MAR (p = 0.50). Tibias in these groups (Groups 1–4) were loaded 1200 cycles/day, 5 days/wk for 2 weeks. Bars depict mean ± SD for the loaded (right) tibia; dashed line indicates mean value for control tibias of these groups.
^ p < 0.05, Right vs. Left; * p< 0.05, ** p< 0.01, Between groups
In contrast to the periosteal surface, loading at these force levels did not consistently enhance bone formation on the endocortical surface and there was no significant effect of loading magnitude (Table 3, Fig. S1A). Static histomorphometric indices were modestly greater in bones loaded to strains of −1400 με and higher compared to control bones, albeit without significant difference between strain groups (Table S1, Fig. S1B). The most consistent effect was seen in Ct.Wi, which was increased by 9.4% (loaded vs. control, p<0.05), 5.6% (p< 0.05) and 7.8% (p=0.089) in the −1400, −1800 and −2200 με groups, respectively.
In summary, a periosteal dose response was observed with loading of increasing magnitude, whereas the endocortical response was not magnitude dependent. There was strong induction of periosteal lamellar bone (and minimal woven bone) at a strain magnitude of −1800 με. This strain level was used for all subsequent parameter studies.
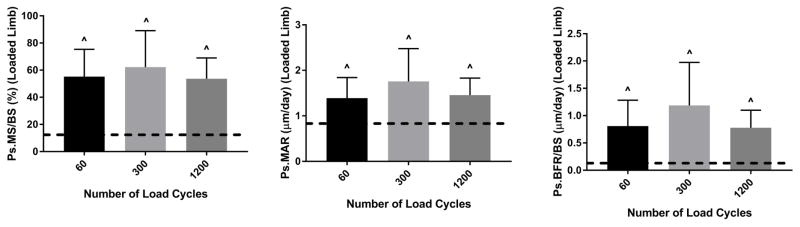
Loading-induced periosteal bone formation is independent of cycle number
Ten days of loading at −1800 με peak strain induced a significant periosteal bone formation response independent of cycle number (Fig. 4; Groups 3, 5 and 6, Table 2). Ps.MS/BS, Ps.MAR and Ps.BFR/BS were significantly increased in loaded tibias vs. control in the 60, 300 and 1200 cycle number groups, but with no significant differences between groups. For example, the increases in Ps.MS/BS were +38.1, +52.0 and +45.1%, respectively. No woven bone was found in the 60 cycle group, whereas two and one instances were found in the 300 and 1200 cycle groups, respectively. Paradoxically, there was some evidence of a negative dose response on the endocortical surface, with 60 cycles/day having a higher Ec.MS/BS and 300 cycles/day a higher Ec.BRF/BS compared to 1200 cycles/day (Fig. S2A, Table 3). But consistent with the periosteal results, Ct.Wi was increased in the loaded bones by a similar amount (5–6%, loaded vs. control, p < 0.05) for the three groups (Table S1, Fig. S2B). In summary, because loading at 60 cycles/day was sufficient for periosteal lamellar bone induction and higher cycles numbers did not enhance this response, subsequent parameter studies were conducted with 60 cycles.
Figure 4.
Periosteal bone formation was not affected by cycle number (p > 0.05, ANOVA). Each of these three groups had a significant increase in bone formation in loaded vs. control tibias. Tibias in these groups (Groups 6, 5 and 3) were loaded to −1800 με, 5 days/wk for 2 weeks. Bars depict mean ± SD for the loaded (right) tibia; dashed line indicates mean value for control tibias of these groups.
^ p < 0.05, Right vs. Left
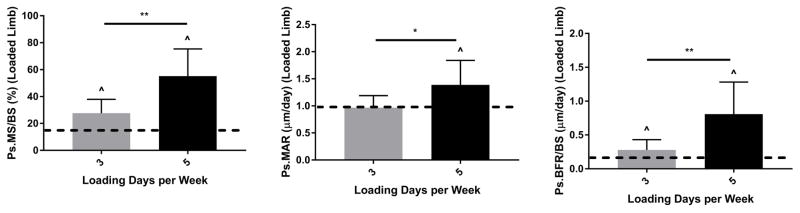
Loading 5 days/week is more anabolic than loading 3 days/week
Reducing the number of loading bouts per week from 5 to 3 led to a significantly diminished anabolic response (Fig. 5). As noted above, the protocol of loading 5 consecutive days (Mon-Fri) at −1800 με peak strain, 60 cycles/day for 2 weeks increased Ps.MS/BS (+38.1%), Ps.MAR (+0.7 μm/day) and Ps.BFR/BS (+0.7 μm/day) in loaded versus control tibias (Group 6, Table 2). In contrast, loading on alternate days (Mon, Wed, Fri) produced smaller increases in Ps.MS/BS (+15%) and Ps.BFR.BS (+0.2 μm/day) and did not stimulate a significant increase in Ps.MAR (Group 8, Table 2). Endocortical formation was likewise only modestly induced by 3 days/week of loading, although this was not significantly different from the response for 5 days/week (Fig. S3A, Table 3). On the other hand, Ct.Wi was not significantly increased with 3 days/week of loading, compared to a 5.4% increase with 5 days/week (Fig. S3B, Table S1). In summary, reducing the number of loading sessions to 3 days/week resulted in a significant decline in the anabolic response to loading at the periosteal surface, and 5 days/week was used for subsequent parameter studies.
Figure 5.
Periosteal bone formation was significantly greater with daily loading (5 days/wk) than alternate day loading (3 days/wk). Tibias in these groups (Groups 8 and 6) were loaded to −1800 με, 60 cycles/day for 2 weeks. Bars depict mean ± SD for the loaded (right) tibia; dashed line indicates mean value for control tibias of these groups.
^ p < 0.05, Right vs. Left; * p< 0.05, ** p< 0.01, Between groups
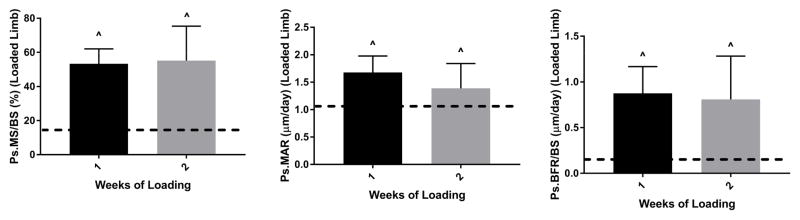
Bone formation responses were similar for 1 and 2 weeks of loading
Finally, we evaluated the effects of reducing the number of weeks of loading from 2 to 1. Despite 5 fewer loading days, the 1-week protocol stimulated similar periosteal bone formation responses as the 2-week protocol (Fig. 6). For example, the loading-induced increases in Ps.MS/BS were +38.1% and +42.2% for 2- and 1-weeks, respectively (Groups 6 and 7, Table 2). One instance of periosteal woven bone was detected in the 1-week group, while no woven bone was detected in the 2-week group. At the endocortical surface, the responses to 2 and 1 week of loading were similar (Fig. S4A, Table 3). Static histomorphometric measures were likewise not affected by study duration (Table S1, Fig. S4B). Ct.Wi was increased by 5.4 and 6.5% (loaded vs. control, p < 0.05) for the 2- and 1-week protocols, respectively. In summary, a reduction in weeks of loading did not diminish the anabolic response to loading for a protocol of −1800 με peak strain, 60 cycles/day, and 5 days/week.
Figure 6.
Periosteal bone formation was not enhanced by use of a longer study duration. Both of these groups had a significant increase in bone formation in loaded vs. control tibias. Tibias in these groups (Groups 7 and 6) were loaded to −1800 με, 60 cycles/day, 5 days/wk. Bars depict mean ± SD for the loaded (right) tibia; dashed line indicates mean value for control tibias of these groups.
^ p < 0.05, Right vs. Left
Discussion
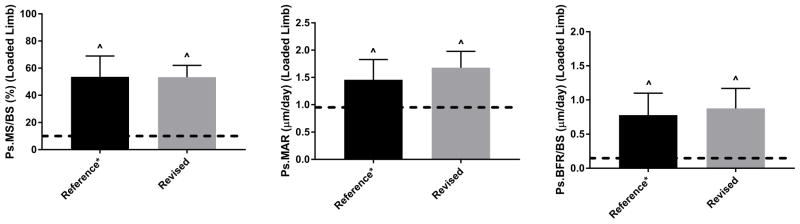
Non-invasive axial loading of mouse long bones is commonly used for in vivo study of the mechanisms that govern bone adaptation to mechanical loading, and the selection of loading parameters is an important consideration for study design. Because most studies on this topic have been conducted using loading models not currently in favor, and using non-murine species, our objective was to re-examine the effects of several loading parameters on cortical bone formation in the contemporary mouse axial tibial compression model. We focused on: 1) the lamellar-woven threshold, and 2) parameters related to the duration and number of daily loading bouts. Our findings indicate that −1800 με peak strain (−7 N peak force), applied as a 4 Hz waveform for 60 cycles/day, for 5 consecutive days (“1 week”) induces a significant increase in periosteal lamellar bone formation with minimal incidence of woven bone in 5-month old C57Bl/6 female mice. The increase in bone formation induced by this protocol did not differ from a strain-matched reference protocol, despite using fewer daily loading cycles and 5 fewer loading days (Fig. 7).
Figure 7.
Comparison of “Reference*” vs. “Revised” protocols showing equivalent induction of bone formation for a peak strain of −1800 με. The “Reference*” protocol (Group 3) was 1200 cycles/day, 5 days/wk for 2 weeks. (* denotes that this is equivalent to the “reference” parameters in Table 1 except for strain magnitude.) The “Revised” protocol (Group 7) was 60 cycles/day, 5 days/wk for 1 week. The input command waveform for both protocols was a Triangle. Bars depict mean ± SD for the loaded (right) tibia; dashed line indicates mean value for control tibias of these groups.
^ p < 0.05, Right vs. Left
A rule of bone adaptation that was established in non-murine models is that, for values of strain above a threshold, the anabolic response increases with strain magnitude [17, 20 ]. Our findings, along with other recent reports [7–9, 26], confirm this rule in the mouse axial tibial compression model. We observed a linear dose-response relationship between peak strain and periosteal bone formation rate (Ps.BFR/BS), driven by a linear increase in mineralizing surface (Ps.MS/BS) (Figs. 2, 3). The strain magnitude threshold for inducing a significant lamellar response was approximately 1000 με, consistent with the approximate threshold based on other loading models [17, 20]. In addition to this lower threshold, we were also interested in the lamellar-woven threshold. Turner et al. [20] reported periosteal woven bone in the rat tibia for peak strains of 1900 με and greater, although this result may have been complicated by the periosteal pressure inherent with their four-point bending model. Here, in 53 tibias from six groups of mice loaded 5 days/week to −1800 με peak strain (−7 N force), we observed a small amount of periosteal woven bone in 13% of cases (7/53); endocortical woven bone was not observed. In the 7 cases with woven bone, the average percent woven bone surface (Ps.Wo.S/BS) was only 14%. The occurrence of woven bone was seemingly random among these samples, e.g., it was more common in mice loaded for 2 weeks than 1 week. In similarly aged (4-month) C57Bl/6 female mice, Weatherholt et al. [8] reported no woven bone for a peak force of −9 N (2 Hz Hsine, 60 cycles/day, 3 days/week, 4 weeks). Interestingly, in the one group in our study loaded for 3 rather than 5 days/week, we did not observe woven bone, but with the trade-off of a lesser increase in lamellar bone formation. In summary, a peak compressive strain of −1800 με (−7 N) applied at 4 Hz for 60–1200 cycles/day, 5 days/week results in a potent induction of lamellar bone formation, with some periosteal woven bone in a small fraction of samples.
An important consideration when interpreting our results is the effect of strain rate. It is well established that loading at a faster rate induces a greater bone formation response [22, 25, 38]. This has implications for the strain magnitude effects reported here, because for the waveforms we used, strain rate is directly proportional to strain magnitude and frequency [1]. Since we did not adjust waveform frequency between groups of different magnitudes, the tibias loaded to a higher magnitude experienced both higher peak strains and proportionally higher strain rates; the periosteal dose-response we observed is likely tied to both of these factors. Also of note is that we used a cyclic frequency of 4 Hz, which matches the rate first used by Fritton et al. [4] and used in many subsequent murine studies [3, 6, 10, 13, 26, 39]. A rate of 2 Hz is also commonly reported [5, 8, 40–42]. We expect that the magnitude of the bone formation indices reported here is specific to 4 Hz, and would be diminished if we used a lower frequency. In particular, we hypothesize that the difference in cyclic frequency in our study versus Weatherholt et al. [8] (4 vs. 2 Hz) explains the difference in the force at the lamellar-woven transition (~7 N vs. ~10 N). Additional studies comparing cyclic frequencies would be required to test this hypothesis. Nonetheless, we believe that the relative effects of the other parameters we examined (e.g., cycle number, days/week) are generalizable to frequencies different from 4 Hz.
We were motivated in part to evaluate parameters that might reduce in vivo study time, either the time needed each day or the number of days. First, we found no effect on bone formation when the number of cycles/day was reduced from 1200 to 300 to 60 (Fig. 3). This result is mostly consistent with the report of Yang et al. [28], who reported a significant increase in tibial bone mass with as few as 36 cycles/day applied daily for 2 weeks. In slight contrast, these authors did find a small additive benefit when they increased to 216 and 1200 cycles/day. Collectively, the results of these two studies in the mouse tibial compression model are consistent with the classic rule that with increasing daily loading cycles beyond ~50 cycle/day there is limited additive enhancement of bone formation [18, 43].
A second temporal parameter that had no effect in the current experiment was duration of loading period (1 versus 2 weeks). The timeline of the 2-week protocol we used represents a common approach for bone loading studies with dynamic histomorphometry as an outcome [3, 20, 24, 26]: loading on days 1–5 and 8–12, with fluorochrome labeling on days 5 and 12, and euthanasia on day 15. Our results indicate that, despite 5 fewer loading days, equivalent bone formation indices can be induced using a 1-week loading protocol with labeling on days 5 and 10. Thus, our finding supports a 1-week loading period as not only being adequate to induce bone formation, which is well documented [7, 16, 29, 30], but as being comparable to a 2-week loading period. This provides further rationale for the use of a shorter protocol, with the dual benefits of reduced animal handling and anesthesia exposure, and reduced investigator workload. Importantly, this result is specific to a study where dynamic histomorphometric indices are the main outcomes[16]. For studies where areal or volumetric measures are a primary outcome (e.g., bone area added between labels, or Ct.Ar or Ct.Bv assessed by microCT), a longer study duration (2–6 weeks) is beneficial to allow increases in bone mass to accrue [6, 8, 28, 35, 44].
One temporal factor that affected the magnitude of bone formation was days per week, with 3 days/week (2 weeks) resulting in a significantly lower anabolic response than 5 days/week (either 1 or 2 weeks). The increased anabolic effect of 5 vs. 3 days/week is consistent with the concept that each loading bout produces a discrete amount (a “quantum”) of bone formation [16, 19], and thus more bouts for the same experimental duration will induce a larger additive response. Interestingly, this phenomenon can also be seen in assays of mRNA expression of genes related to bone formation (e.g., Col1a1), whereby incremental bouts produce additive increases in expression [45, 46]. Although more loading days/week is more anabolic, a number of investigations have successfully used a 3 day/week schedule to induce a loading response and enable comparisons between experimental groups [9, 29, 36, 40]. In our case, the 3 day/week protocol stimulated only a small increase in periosteal bone formation indices,, although this could have been enhanced by use of a higher peak strain.
The effects we observed for the periosteal surface were not necessarily the same at the endocortical surface. Most notable was the lack of a magnitude-dependent dose response at the endocortex (Fig. S1A). While the peak strain magnitude is less at the endocortex, it should still increase in proportion to force magnitude. Interestingly, the basal (control) rates of bone formation were much greater on the endocortex vs. the periosteum. For example, the average Ec.MS/BS for controls was ~40% versus the average Ps.MS/BS of ~10%. Thus, the parameters we examined may not have been adequate to stimulate a robust loading response above the relatively high baseline on the endocortical surface.
Several additional limitations should be noted. Our primary outcomes were cortical bone formation indices assessed by dynamic histomorphometry at a single diaphyseal section. We did not perform microCT (either in vivo or post mortem) to analyze 3D morphological changes, which reflect structural adaptation. Analysis of cortical changes at additional proximal or distal sites would provide a more complete description of the loading response, although loading effects are reported to be relatively consistent across sites within the 25–50% range of the tibia length [9, 28]. Likewise, although our study did not examine effects of loading on cancellous bone, other studies have shown that loading parameters have similar relative effects on cortical and cancellous adaptation [9, 28]. Finally, a focus of our study was reducing the loading duration during the in vivo part of a study, motivated in part to enhance animal welfare and to reduce investigator workload. We did not consider the time expended in sample processing and analysis, which may influence the decision to perform microCT versus histomorphometry.
In summary, we assessed the effects of several loading parameters on dynamic bone formation indices using the axial tibial loading model in 5-month, female C57Bl/6 mice. We confirmed the strong influence of peak strain magnitude, with a lower threshold of approx. −1000 με and occasional induction of small amounts of woven bone at approximately −2000 με. We found no effects of varying the daily cycle number from 60 to 1200, or the experimental length from 1 to 2 weeks. On the other hand, daily loading (5 consecutive days for 1 or 2 weeks) was more potent than alternate day loading. In conclusion, a loading protocol that engenders −1800 με peak strain (−7 N peak force), applied at 4 Hz for 60 cycles/day, for 5 consecutive days (“1 week”) induces a significant increase in periosteal lamellar bone formation with minimal incidence of woven bone in 5-month old C57Bl/6 female mice. Our results provide a basis for reduction of loading duration (daily cycles and study length) without loss of anabolic effect as measured by dynamic histomorphometry.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under Award Number R01 AR047867, P30 AR057235, T32 AR060719, and F32 AR064667. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We state that we have no conflicts to disclose.
Footnotes
Author Contributions Statement:
Study Design: DS, MDB, HMZ, NH, MJS. Data Generation: DS, MDB, HMZ, NH. Data Analysis: DS, MDB, MJS. Manuscript Draft: DS, MJS. Manuscript Editing: DS, MDB, HMZ, NH, MJS. All authors have read and approved the final submitted manuscript.
References
- 1.Robling AG, Fuchs RK, Burr DB. Basic and Applied Bone Biology. San Diego: Academic Press; 2014. Chapter 9 - Mechanical Adaptation; pp. 175–204. [Google Scholar]
- 2.Meakin LB, Price JS, Lanyon LE. The Contribution of Experimental in vivo Models to Understanding the Mechanisms of Adaptation to Mechanical Loading in Bone. Frontiers in endocrinology. 2014;5:154. doi: 10.3389/fendo.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31(3):407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- 4.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36(6):1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.De Souza RL, Matsuura M, Eckstein F, et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37(6):810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Lynch ME, Main RP, Xu Q, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49(3):439–46. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared to young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25(9):2006–15. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52(1):372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T, Meakin LB, Browne WJ, et al. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012;27(8):1784–93. doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razi H, Birkhold AI, Weinkamer R, et al. Aging Leads to a Dysregulation in Mechanically Driven Bone Formation and Resorption. J Bone Miner Res. 2015;30(10):1864–73. doi: 10.1002/jbmr.2528. [DOI] [PubMed] [Google Scholar]
- 11.Sztefek P, Vanleene M, Olsson R, et al. Using digital image correlation to determine bone surface strains during loading and after adaptation of the mouse tibia. J Biomech. 2010;43(4):599–605. doi: 10.1016/j.jbiomech.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Jing D, Baik AD, Lu XL, et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28(4):1582–92. doi: 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Butz KD, Duffy D, et al. Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis. Bone. 2014;66:131–9. doi: 10.1016/j.bone.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6(1):50–9. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. Journal of Biomechanics. 1984;17(12):897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 16.Forwood MR, Turner CH. The response of rat tibiae to incremental bouts of mechanical loading: a quantum concept for bone formation. Bone. 1994;15(6):603–9. doi: 10.1016/8756-3282(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 17.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–7. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 18.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- 19.Pead MJ, Skerry TM, Lanyon LE. Direct transformation from quiescence to bone formation in the adult periosteum following a single brief period of bone loading. J Bone Miner Res. 1988;3(6):647–56. doi: 10.1002/jbmr.5650030610. [DOI] [PubMed] [Google Scholar]
- 20.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9(1):87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 21.Forwood MR, Owan I, Takano Y, Turner CH. Increased bone formation in rat tibiae after a single short period of dynamic loading in vivo. American Journal of Physiology. 1996;270(3 Pt 1):E419–23. doi: 10.1152/ajpendo.1996.270.3.E419. [DOI] [PubMed] [Google Scholar]
- 22.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol. 1995;269(3 Pt 1):E438–42. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 23.Torrance AG, Mosley JR, Suswillo RFL, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periosteal pressure. Calcified Tissue International. 1994;54:241–7. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh Y-F, Robling AG, Ambrosius WT, et al. Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. Journal of Bone Mineral Research. 2001;16(12):2291–7. doi: 10.1359/jbmr.2001.16.12.2291. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16(5):918–24. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 26.Holguin N, Brodt MD, Sanchez ME, Silva MJ. Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone. 2014;65:83–91. doi: 10.1016/j.bone.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holguin N, Brodt MD, Sanchez ME, et al. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93(3):211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Embry RE, Main RP. Effects of Loading Duration and Short Rest Insertion on Cancellous and Cortical Bone Adaptation in the Mouse Tibia. PLoS One. 2017;12(1):e0169519. doi: 10.1371/journal.pone.0169519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxon LK, Robling AG, Castillo AB, et al. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-beta. American journal of physiology Endocrinology and metabolism. 2007;293(2):E484–91. doi: 10.1152/ajpendo.00189.2007. [DOI] [PubMed] [Google Scholar]
- 30.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–17. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14(12):2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 32.Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res. 2008;23(12):2048–59. doi: 10.1359/jbmr.080808. [DOI] [PubMed] [Google Scholar]
- 33.Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The mouse in biomedical research. 2. Burlington: Academic Press; 2007. pp. 637–72. [Google Scholar]
- 34.Patel TK, Brodt MD, Silva MJ. Experimental and finite element analysis of strains induced by axial tibial compression in young-adult and old female C57Bl/6 mice. J Biomech. 2014;47(2):451–7. doi: 10.1016/j.jbiomech.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moustafa A, Sugiyama T, Prasad J, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meakin LB, Galea GL, Sugiyama T, et al. Age-related impairment of bones’ adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res. 2014;29(8):1859–71. doi: 10.1002/jbmr.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23(4):313–8. doi: 10.1016/s8756-3282(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 39.Morse A, McDonald MM, Kelly NH, et al. Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res. 2014;29(11):2456–67. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leucht P, Temiyasathit S, Russell A, et al. CXCR4 antagonism attenuates load-induced periosteal bone formation in mice. J Orthop Res. 2013;31(11):1828–38. doi: 10.1002/jor.22440. [DOI] [PubMed] [Google Scholar]
- 41.Lara-Castillo N, Kim-Weroha NA, Kamel MA, et al. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58–66. doi: 10.1016/j.bone.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 43.Umemura Y, Ishiko T, Yamauchi T, et al. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12(9):1480–5. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- 44.Silva MJ, Brodt MD, Lynch MA, et al. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7(4):e34980. doi: 10.1371/journal.pone.0034980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holguin N, Brodt MD, Silva MJ. Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice. J Bone Miner Res. 2016;31(12):2215–26. doi: 10.1002/jbmr.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26(1):100–12. doi: 10.1002/jbmr.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.