Abstract
Mesenchymal stem cells (MSCs) hold great promise for regenerative therapies and tissue engineering applications given their multipotential differentiation capacity. However, MSC isolation and expansion are typically performed on super-physiologically stiff tissue culture plastic (TCP), which may alter their behavior and lead to unintended consequences upon implantation. In contrast, electrospun nanofibrous scaffolds possess physical and mechanical properties that are similar to that of native tissue. In this study, we investigated whether isolation and expansion of juvenile bovine MSCs directly onto electrospun nanofibrous scaffolds better preserves MSC phenotype and stemness compared to TCP. Our data show that culture of MSCs on electrospun scaffolds reduces proliferation, decreases cellular senescence, and better maintains stemness compared to cells isolated and expanded on TCP, likely due to a reduction in cell contractiility Furthermore, in contrast to electrospun scaffolds, TCP biased MSCs towards a fibrotic phenotype that persisted even after the cells were reseeded onto a different substrate. Cells pre-cultured on electrospun scaffolds exhibited a heightened response to mechanical stimuli and greater chondrogenesis in methacrylated hyaluronic acid hydrogels. These data suggest that alternative substrates that better approximate the native cell environment should be used to preserve endogenous MSC behavior and may improve their success in tissue engineering applications.
Keywords: Mesenchymal stem cells, Nanofibrous scaffolds, Cell culture, Mechanobiology
INTRODUCTION
Mesenchymal stem cells (MSCs) can be isolated from a variety of tissues or organs including bone marrow, trabecular bone, adipose tissue, and skeletal muscle1,2. Given that they are clonogenic, self-renewing, and can differentiate toward a variety musculoskeletal lineages1, they are a promising cell source for regenerative medicine and tissue engineering applications. However, since MSCs represent only a small fraction of the cells harvested from any given tissue, they must be expanded in vitro through multiple population doublings to achieve sufficient numbers for therapeutic application.
The stem cell ‘niche’ refers the microenvironment in which cells reside in native tissues. This native environment may play an important role in regulating their proliferation and differentiation and mobilization after injury. For instance, stem cells within in dense fibrous connective tissues, including tendon, ligament and meniscus reside in collagen rich milieu while those in bone marrow reside in a softer less fibrous environment. Neither of these environments is replicated in the process of tissue culture expansion on a stiff substrate, such as tissue culture plastic (TCP). Indeed, several reports have noted limited MSC expansion potential in vitro, and decreased differentiation capacity with increased passage number3–5. Moreover, it is also well known that substrate stiffness can modulate MSC behavior and differentiation6–9, and that substrate stiffness effects can persist even after cells are placed onto a new substrate10. Therefore, it is critical to understand the effects of isolating and expanding MSCs on supra-physiologically stiff TCP prior to their use in a given tissue engineering application. It may be that mimicking the in vivo stem cell niche in vitro would improve MSC stemness, proliferation, and differentiation capacity.
Existing data suggest that the act of isolation itself may impact lineage specification, and that prolonged exposure to mechanical stimuli may instill a ‘mechanical memory’ in MSCs10–12. For instance, Engler et al. showed that substrates having the elastic moduli of brain (0.1~ 1 kPa), muscle (~17 kPa), or bone (~40 kPa) direct MSC differentiation into neural-like, myoblast-like, and osteoblast-like lineages, respectively6. Recent studies reported that culturing MSCs on tissue culture plastic (E = ~ 3GPa) increased nuclear translocation of the Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding domain (TAZ), which remained nuclear even after these cells were transferred to soft poly(ethylene glycol) (PEG) hydrogels (E = ~ 2 kPa)10. This persistent nuclear localization of YAP, or ‘priming’ of their mechanobiologic response had a functional consequence, with osteogenic differentiation potential enhanced by prior culture on stiff substrates10. Additionally, MSC expansion on stiff culture substrates can activate a fibrotic cell program and/or promote replicative senescence, influencing self-renewal and pluripotency10,11. As such, routine isolation and passaging on TCP may inadvertently and permanently alter cell phenotype and stem cell potentiality. In the context of orthopaedic tissue engineering, passaging on stiff TCP may constrain differentiation potential of MSCs and/or predispose them to a particular (e.g., myofibroblastic or osteogenic) phenotype10,11, which would limit their utility and potentially induce unintended consequences upon implantation.
Nanofibrous scaffolds fabricated by electrospinning have been widely used for tissue engineering applications as they possess physical and mechanical properties that are similar to the native extracellular matrix (ECM)13,14. Cell morphology and organization on these scaffolds can be modulated by changes in fiber alignment and size13,15,16. For instance, culture of MSCs on aligned electrospun nanofibrous scaffolds results in an elongated cell morphology and organized ECM deposition compared to cells on non-aligned nanofibrous scaffolds13. Similarly, MSC nuclear morphology is sensitive to changes in this fiber organization15,17. Early reports of cells on nanofibrous scaffolds also showed that signaling pathways were altered compared to culture of these same cells on TCP18–21. Along similar lines, we recently showed that plating TCP-expanded MSCs onto aligned nanofibrous scaffolds rapidly decreased their nuclear localization of YAP/TAZ22. This is an interesting finding, given that the nanofibrous material itself [poly (ε-caprolactone), PCL] has a high modulus, but the cells appear to sense this material substrate as soft when presented in a fiber form. This is potentially due to the low fiber volume fraction of these scaffolds (generally <10%) or the manner in which cells probe the networks, interrogating for example the bending stiffness of the fibers. Indeed, work by Baker and colleagues recently showed that proliferation on fibrous networks was slower than when cells were plated on solid gels formed from the same material until such time as the cells had contracted the fiber network, at which point proliferation increased23. These data suggest that replicating the stiffness and fibrous topography of native tissue with electrospun nanofibrous scaffolds during MSC isolation and expansion may help to preserve their inherent properties.
Given these findings, the objective of this study was to compare the effect of isolating and passaging MSCs on TCP versus nanofibrous scaffolds, with a focus on their mechanosensitivity and differentiation capacity. Specifically, we investigated whether isolation and expansion of MSCs on TCP altered the MSC phenotype compared to culture and expansion on nanofibrous scaffolds. In addition to time-invariant cues from the micro-environment, dynamic mechanical cues also regulate lineage specification. For instance, dynamic tensile loading of MSC-laden aligned nanofibrous constructs enhanced YAP nuclear localization22 in the short term and collagen production and mechanical properties of the construct over longer term24. Thus, to determine whether different expansion conditions influenced the mechanical response of MSCs, we evaluated their short term response to dynamic tensile deformation. Finally, to query the long term effects of different expansion conditions, we examined whether these changes persist after encapsulating the passaged cells into photo-crosslinked hyaluronic acid (HA) constructs. Our findings suggest that pre-culture and expansion of MSCs on TCP alters cell phenotype and may predispose the cells to a particular lineage prior to their use in tissue engineering applications. Alternative substrates that better approximate the cell’s native environment should therefore be used to preserve endogenous MSC behavior and prevent unintended consequences upon implantation.
MATERIALS AND METHODS
Preparation of nanofibrous scaffolds
Poly(ε-caprolactone) (PCL) nanofibrous scaffolds (PCL, mol. wt. 80 kDa, Shenzhen Bright China Industrial Co., Ltd., China; 60 cm2 for MSC isolation, 4.67 cm2 for MSC expansion) were fabricated via electrospinning as in 15. Briefly, a total of 13 mL of PCL solution (14.3% wt/vol in 1:1 tetrahydrofuran and N,N-dimethylformamide) was loaded into a syringe (20 mL). The PCL solution was extruded at a rate of 2.5 mL/hour through a stainless-steel needle (18G, charged to +13 kV) and fibers were collected on a stationary, grounded collecting surface to form non-aligned scaffolds (NAL). To produce aligned scaffolds (AL), fibers were collected on a mandrel rotating with a surface velocity of ~10m/s13,15. The scaffolds were hydrated and disinfected in ethanol (100, 70, 50, 30%; 30 minutes/step), and incubated in a fibronectin (20 μg/ml) solution overnight to enhance cell attachment.
MSC isolation and expansion
Mesenchymal stem cells were isolated from juvenile bovine bone marrow as in 25 and immediately plated onto three different substrates: aligned nanofibrous scaffolds (AL, 60 cm2), non-aligned (NAL) nanofibrous scaffolds (60 cm2), or tissue culture plastic (TCP, 60 cm2). MSCs were expanded in serum-containing growth media (high glucose DMEM with 1% penicillin/streptomycin/fungizone and 10% fetal bovine serum). Actin was visualized by staining with AlexaFluor 488 phalloidin (A12379, Invitrogen, Life Technologies, Carlsbad, CA, USA) and ImageJ was used to measure cell spread area and cell aspect ratio (CAR).
Analysis of gene expression
To isolate total RNA, 1mL of Trizol reagent was added to MSC-seeded scaffolds. MSCs on TCP were collected using a cell scraper in 1mL of Trizol. mRNA amount was quantified using a Nanodrop spectrometer (260/280 ratio: 1.7~2.1, 260/230 ratio: 2.0~2.2; ND-1000, Nanodrop Technologies, Wilmington, DE, USA). Using the SuperScript First Strand Synthesis kit (Invitrogen, Life Technologies, Carlsbad, CA, USA), cDNA was synthesized. Equal amounts of mRNA and then cDNA were loaded into the reactions. Amplification was carried out using an Applied Biosystems Step One Plus real-time PCR system with the Fast SYBR Green Reaction Mix (#4385617, Applied Biosystems, Foster City, CA, USA). Expression levels of Nanog, β-galactosidase (β-gala), type-I collagen (COL-I) and type-II collagen (COL-II) were determined using the standard curve method, and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Assessment of MSC proliferation
MSC proliferation on three different substrates during expansion was investigated using the 5-bromo-2′-deoxy-uridine (BrdU) labeling kit (Cat. #: 11 296 736 001, Roche) according to the manufacturer’s instructions. Briefly, cell culture media was aspirated and the cells were incubated with BrdU labeling solution at 37°C, 5% CO2 for 60 minutes. Cells were then fixed with ethanol fixative for 20 minutes at −20°C, and incubated with the Anti-BrdU working solution for 30 minutes. Anti-mouse-Ig-fluorescein working solution was added for 30 minutes at 37°C. DAPI was used to visualize cell nuclei. Fluorescent signal was detected using an inverted microscope (Nikon T30, Nikon Instruments, Melville, NY).
Traction force microscopy (TFM)
For traction force experiments, MSCs from each of the three substrates were trypsinized and re-seeded onto fluorescent microsphere (0.2-μm-diameter, F8810, Invitrogen, Carlsbad, CA)-embedded polyacrylamide (PA) hydrogels (Young’s Modulus, E = 10 kPa, 3000 cells/cm2, prepared on glass slides). Cells were allowed to attach to the hydrogels for ~18 hrs. Cells and embedded beads were imaged using a Deltavision Deconvolution Microscope in an environmental chamber (37°C, 5% CO2). TFM data analysis (stack alignment, PIV, and FTTC) was carried out using an FTTC plug in in Image J and a custom MATLAB script as in 26,27.
Assessment of YAP nuclear localization
To evaluate YAP nuclear localization, MSCs at each time point were fixed in 4% paraformaldehyde for 30 minutes at 37°C and then permeabilized with 0.05% Triton X-100 in PBS supplemented with 320mM sucrose. Samples were then incubated with anti-YAP antibody in 1% BSA in PBS (1:200, sc-101199, Santa Cruz Bio, Dallas, Texas) overnight at 4°C, followed by incubation with Alexa-Fluor 546 goat anti-mouse secondary (1:200, A-11030, Molecular Probes, Grand Island, NY) at RT for 1 hour. To visualize cells, actin was stained with Alexa-Fluor 488 (1:1000, A12379, Molecular Probes, Grand Island, NY) for 30 minutes and 4′, 6-diamidino-2-phenylindole (DAPI, ProLong ® Gold antifade reagent with DAPI, P36935, Molecular Probes, Grand Island, NY) to stain nuclei. Using a fluorescence microscope with a 100× objective (Zeiss Axioplan-2 fluorescent microscope, Jena, Germany), images were taken and the average YAP staining intensity and localization were quantified using ImageJ (with nuclear staining intensity normalized to cytoplasmic staining) as in 22,28. To investigate the effect of acto-myosin contractility on YAP nuclear localization, MSCs cultured on TCP through P2 were treated with Y27632 (Y27, 10 μM, for 1 h, EMD Millipore, Bedford, MA) for 1 hour prior to fixation and imaging.
Assessment of mechanosensitivity and persistence of altered phenotype
MSCs from each of the three different substrates (AL, NAL or TCP) were re-seeded on fresh aligned-PCL nanofibrous scaffolds (AL, 60 × 5 mm2) followed by 2 days of pre-culture in basal growth media (BM). MSC-seeded scaffolds were placed into to a custom tensile loading bioreactor24 and dynamic tensile loading (DL, 1Hz, 3%, 6 hours) was applied in basal growth media. Expression of aggrecan was evaluated for each condition using RT-PCR, as described above. In addition, persistence of an altered cell phenotype was examined. To do so, MSCs isolated and expanded on the three different substrates (AL, NAL or TCP) through P4 were re-plated onto TCP in serum-containing BM. After 3 days, cells were imaged to measure differences in cell morphology using image J, as described above.
Chondrogenic culture
To evaluate the effect of different isolation/expansion procedures on matrix production in chondrogenic culture, MSCs (2 ×10^7 cells/ml) isolated and expanded on either NAL scaffold or TCP for three passages were encapsulated in methacrylated hyaluronic acid (MeHA) hydrogels. MeHA was produced by reacting 65 kDa HA (Lifecore; Chaska, MN, USA) with methacrylic anhydride (Sigma Aldrich; St Louis, MO, USA) as previously described29. Formed constructs were cultured in TGF-β3 containing chondrogenic media for 20 days after which samples were harvested and sulfated glycosaminoglycan (s-GAG) content was assessed30. Briefly, s-GAG content was measured using the 1,9-dimethylmethylene blue (DMMB) dye binding assay with chondroitin-6 sulfate as a standard and s-GAG content is reported normalized to the construct wet weight (as in 31).
Statistical analyses
Normality tests were performed using IBM SPSS Statistics (v.24, Armonk, New York). For data sets that were not normally distributed, a log-transformation was performed before statistical analysis. Statistical analysis was performed using ANOVA with post hoc testing (SYSTAT v.10.2, Point Richmond, CA). Results are expressed as mean ± the standard error of the mean (S.E.M.) or standard deviation (S.D.), as indicated in the figure legends. Differences were considered statistically significant at p<0.05.
RESULTS
Effect of Substrate on Cell Behavior
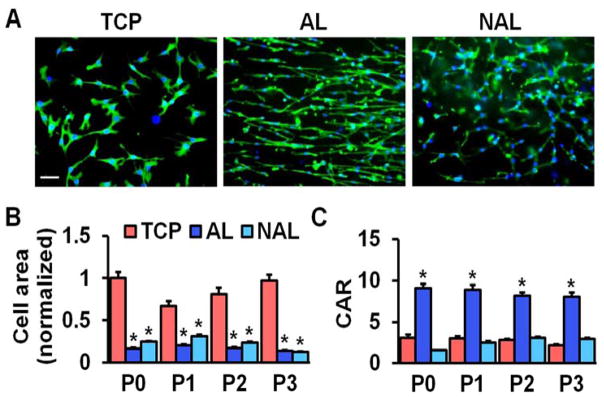
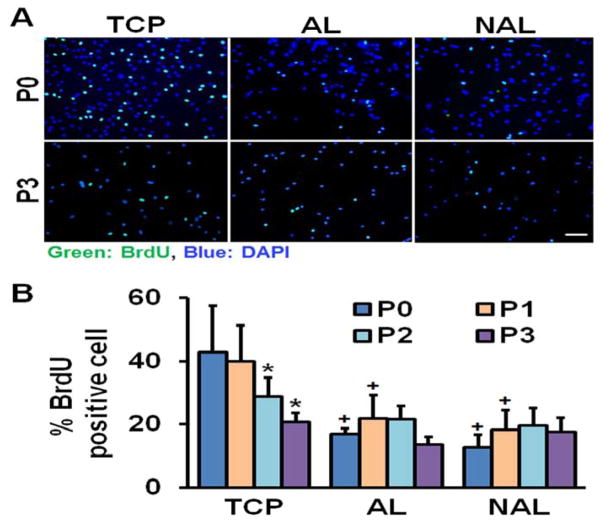
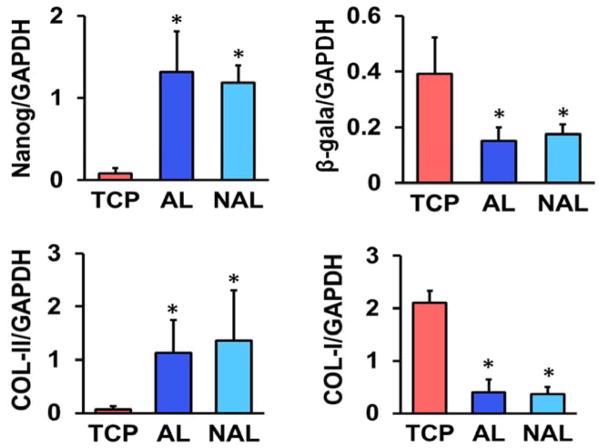
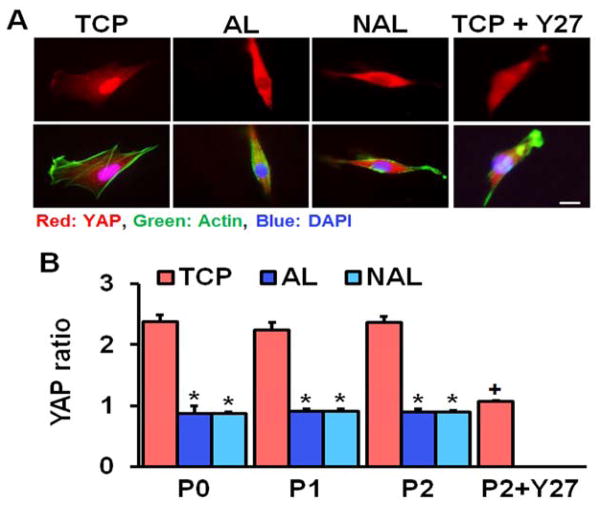
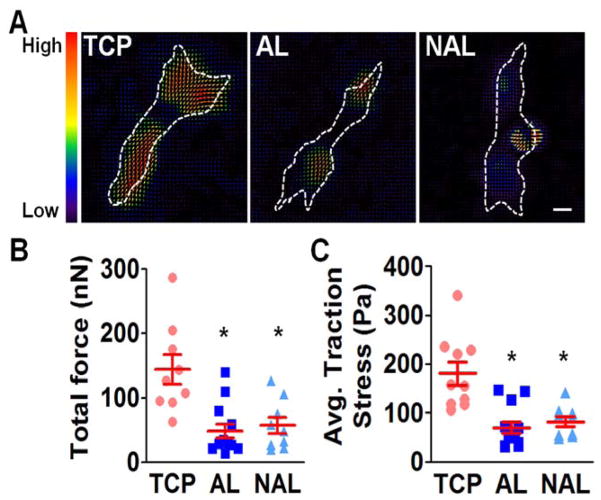
The use of tissue culture plastic (TCP) or electrospun scaffolds for isolation and expansion of MSCs dramatically altered cell phenotype. MSCs had a significantly greater spread area on TCP versus either scaffold type, and this increased cell area persisted with passaging (Fig. 1). Cells seeded on TCP were also rounder, with a lower cellular aspect ratio (CAR), which was comparable to cells cultured on non-aligned scaffolds. In contrast, the aligned electrospun scaffolds produced cells that were oriented in the fiber direction with a high CAR. Additionally, as indicated by the BrdU incorporation rate, MSCs proliferated slowly on both scaffold types across all passages, while proliferation was high during early passages on TCP and dropped with further expansion (Fig. 2). This change in proliferation rate suggests that MSCs may be activated by TCP during early culture, leading to potential loss of stemness and increased likelihood for early senescence. To test this, we next evaluated gene expressions of MSC stemness and senescence. Interestingly, after only one passage on TCP, MSCs exhibit significantly lower expression of the stemness marker Nanog and significantly greater expression of the senescence marker β-galactosidase, compared to either scaffold type (Fig. 3A–B). Additionally, MSCs cultured on TCP expressed lower amounts of collagen II and greater amounts of collagen I (Fig. 3C–D), suggesting that TCP may prime MSCs towards a pro-fibrotic phenotype. This agrees with the finding that YAP nuclear localization (and hence activation) is significantly increased on TCP compared to either scaffold type (Fig. 4A–B). Interestingly, treatment with the Rho kinase inhibitor Y27632 significantly decreased YAP nuclear localization in MSCs on TCP, suggesting that the effect of expansion substrate on MSC phenotype may be mediated by differences in cell contractility (Fig. 4A–B). This finding of a role for altered contractility with expansion on TCP was further supported by the results of traction force measurements after transfer to polyacrylamide gels. This assay showed that MSCs pre-cultured on TCP produced greater total force and stresses on the gel surface than cells that had been pre-cultured on electrospun scaffolds (Fig. 5).
Figure 1.
MSC morphology when isolated and expanded on TCP, AL, or NAR scaffolds. (A) F-actin staining (green) and nuclear staining (blue), scale bar = 100 μm, normalized to mean cell area on TCP at passage 0 (P0), error bars indicate S.D. (B) Quantification of cell area and (C) cellular aspect ratio (CAR) as a function of passage number (n = ~55 cells, *: p<0.05 vs. TCP, two-way ANOVA with Fisher’s LSD, mean ± S.E.M).
Figure 2.
MSC proliferation on TCP, AL, or NAR scaffolds. (A) BrdU staining of MSCs on different substrates at passage 0 (P0) and passage 3 (P3), scale bar = 100 μm. (B) Quantification of % BrdU positive MSCs on different substrates as a function of passage number (n = 5 image fields, *: p<0.05 vs. P0, +: p<0.05 vs. TCP, two-way ANOVA with Fisher’s LSD, mean ± S.D.).
Figure 3.
Expression of Nanog, β-galactosidase (β-gala), type-I collagen (COL-I), and type-II collagen (COL-II), normalized to GAPDH (n = 3, *: p<0.05 vs. TCP, One-way ANOVA with Fisher’s LSD) in passage 1 cells on different substrates (mean ± S.D.).
Figure 4.
Substrate type and cellular contractility influence YAP nuclear localization in MSCs. (A) YAP staining (red), F-actin staining (green), and nuclear staining (blue), scale bar = 20 μm. (B) Quantification of nuclear localization at different passages and with inhibition of contractility (n = ~20, *: p<0.05 vs. TCP using two-way ANOVA across groups and passage number only with Fisher’s LSD, +: p<0.05 vs. P0 TCP from one-way ANOVA across only TCP groups, Fisher’s LSD, Y27: Y27632, Rho kinase inhibitor, mean ± S.E.M.).
Figure 5.
Traction force in MSCs isolated on TCP, AL, or NAL scaffolds. (A) Traction force maps for MSCS on 10 kPa polyacrylamide gels after isolation of on various substrates (dashed line: cell outline, scale bar = 10 μm). Quantification of total force (B) and average traction stress (C) after isolation on various substrates (n= ~10 cells per group, * p<0.05 vs. TCP, One-way ANOVA with Fisher’s LSD, mean ± S.E.M.).
Persistence of Altered Cell Phenotype
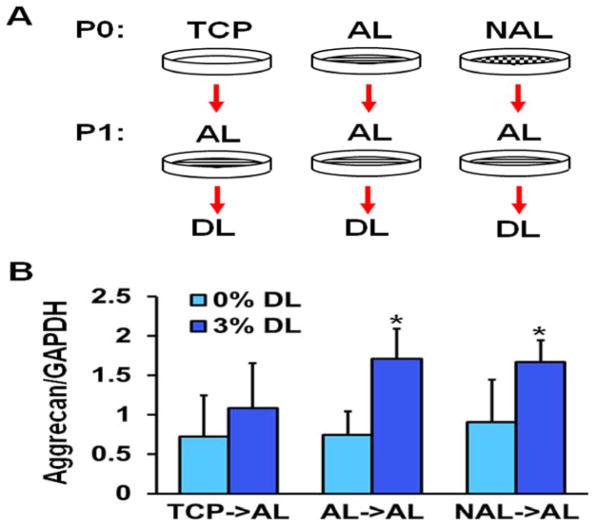
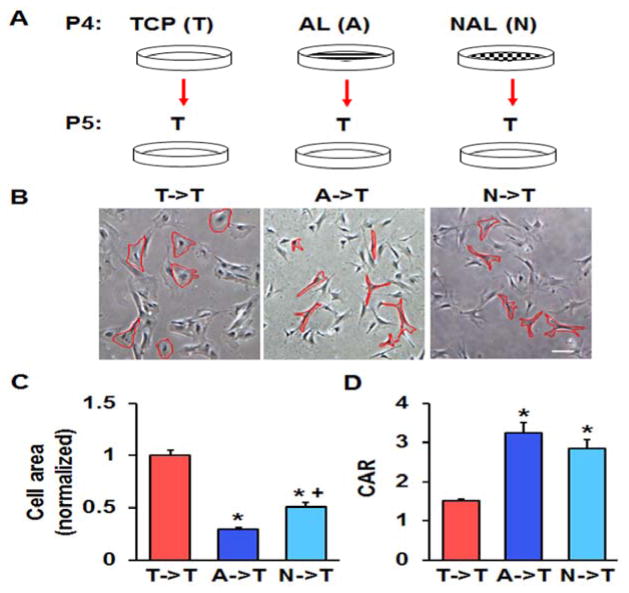
The traction force microscopy results suggested that the effects of culturing MSCs on stiff TCP persists even after switching to a more compliant substrate. To investigate this further, we cultured MSCs on either TCP or electrospun scaffolds for one passage and then switched them to aligned scaffolds, which were dynamically loaded. Interestingly, we found that MSCs pre-cultured on either aligned or non-aligned scaffolds responded to dynamic loading by increasing the expression of the chondrogenic marker aggrecan, whereas pre-culture of MSCs on TCP eliminated this effect (Fig. 6). We also performed the opposite experiment, taking MSCs pre-cultured on TCP or electrospun scaffolds and reseeding them onto TCP. Here, we found that the MSCs pre-cultured on either electrospun scaffold type, when placed onto TCP, had lower spread areas and greater cellular aspect ratios (Fig. 7). Finally, expanding MSCs for three passages on TCP or non-aligned scaffolds and seeding them into HA hydrogels demonstrated that TCP pre-culture reduced GAG accumulation within the constructs (Fig. 8). Together, this suggests that not only does TCP alter MSC phenotype compared to electrospun scaffolds, but also that these effects persist after reseeding the cells onto different substrates or into tissue engineered constructs.
Figure 6.
Response of MSCs initially isolated onto TCP, AL, or NAL scaffolds after transfer to aligned scaffold and with mechanical perturbation. (A) Schematic of study design and (B) expression of aggrecan with the application of dynamic tensile loading (DL, 3% stretch for 6 hours, *: p<0.05 vs. 0% DL, n = 6 from 2 replicates, two-way ANOVA with Fisher’s LSD, mean ± S.D.).
Figure 7.
Persistence of altered MSC phenotype after transfer to TCP. (A) Schematic of study design. (B) Bright-field images of P5 MSCs pre-cultured on TCP (T->T), AL (A->T), or NAL (N->T) scaffolds that were re-plated onto TCP (bar = 100 μm). (C) Quantification of cell area (normalized to area on TCP) and (C) cell aspect ratio (CAR) (n = ~50 cells, *: p<0.05 vs. T->T, +: p<0.05 vs A->T, one-way ANOVA with Fisher’s LSD, mean ± S.E.M.).
Figure 8.
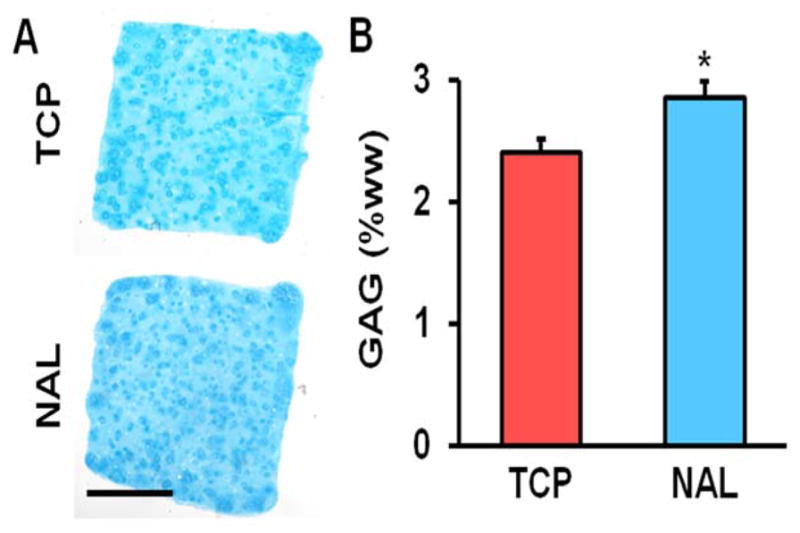
Matrix deposition in MSC-seeded HA constructs as a function of expansion conditions. (A) Alcian blue staining for proteoglycans (scale bar = 1 mm) and (B) GAG content per wet weight on day 20 (n = 4, *: p<0.05 vs. TCP, t-test, mean ± S.D.).
DISCUSSION
In this study, we investigated whether electrospun scaffolds serve as a better substrate for the isolation and expansion of MSCs compared to standard culture on tissue culture plastic. We found significant differences in cell phenotype between TCP and both aligned and non-aligned scaffolds. Importantly, it appears that the electrospun scaffolds maintain MSC stemness and prevent early cell senescence as compared to cells seeded on TCP. Our findings suggest that TCP activates MSCs, causing them to proliferate and become biased towards a fibrotic differentiation pathway, mediated by an increase in MSC contractility and YAP activation. These effects of TCP on MSC behavior is consistent with existing data demonstrating a clear link between increased substrate stiffness and cellular proliferation, contractility, YAP activity, and osteogenesis10. These effects did not appear to be dependent on fiber alignment or cell morphology, since MSCs exhibit very similar behaviors on either aligned or non-aligned scaffolds despite their different aspect ratios (Fig. 1). These data suggest that isolation and expansion of MSCs on supra-physiologically stiff TCP alters their phenotype, which may be undesirable depending on the ultimate intended use for the cells.
We also investigated whether the changes induced in MSC behavior by TCP pre-culture persist after moving to a new substrate. We found that TCP pre-culture reduced the mechanically-induced expression of aggrecan in MSCs on aligned electrospun scaffolds, which is consistent with the notion that TCP culture primes MSCs towards non-chondrogenic lineages. Additionally, TCP pre-culture reduced the expression of the stemness marker, Nanog, which otherwise persisted through three passages on nanofibrous scaffolds (Fig. S1). Finally, TCP-expanded MSCs encapsulated in an HA gel produced less proteoglycan than MSCs expanded on electrospun scaffolds for three passages.
Finally, we found that MSCs cultured on electrospun scaffolds prior to seeding on TCP had lower spread areas and greater aspect ratios (i.e., more spindle shaped morphology) compared to cells cultured continuously on TCP. This finding is consistent with the notion that TCP culture accelerates cellular senescence, which is characterized by larger and rounder cell shapes32–34. Several recent studies have sought to manipulate cell spreadingto control stemness during expansion. For instance, Nakashima et al. recently reported that MSC cultured under hypoxic conditions remained smaller in size and increased their expression of stem cell markers and had an improved differentiation capacity35. In addition, Wong et al. recently reported that MSCs cultured on hyaluronan (HA) coated TCP were smaller in size, remained largely quiescent, and maintained their differentiation potential with passage compared to MSCs cultured on untreated TCP36. Other methods, including micro-contact printing have been used to restrict cell spread area. Work from Picollo et al. showed that restricting cell area limited YAP/TAZ nuclear localization, independent of the substrate stiffness37. The link between cell spread area and differentiation outcomes were originally demonstrated by McBeath et al., who showed that MSCs cultured on large micropattered-islands underwent osteogenesis, while MSCs on small micropattered-islands became adipocytes38 when they were cultured in media that equally favored both outcomes. Further, several other recent studies have also documented that the effects of substrate stiffness on MSC behavior and commitment persist long after reseeding the cells to a new substrate or changing the substrate stiffness10,11,39. These studies suggest that MSCs retain a so-called “mechanical memory” of the substrates to which they are exposed.
In this work we chose to compare MSC behavior on TCP versus electrospun scaffolds since these materials are often used in tissue engineering applications. By using the same materials for the isolation and expansion of MSCs as for the ultimate intended tissue engineering application (e.g., meniscus replacement), we hoped to minimize any unintended alterations in MSC phenotype due to the mechanical memory induced by expansion on TCP. For that same reason, we also compared the effects of aligned versus non-aligned scaffolds. Overall, we found that MSC phenotypes were similar between aligned and electrospun scaffolds. Importantly, YAP nuclear localization and cell contractility were similar across scaffold types, suggesting that the stiffness of the fiber networks that was presented to the cells was similar in both scaffold formulations. The similarity in MSC behavior on the scaffolds and dramatic difference with cells seeded on TCP suggests that fiber alignment does not affect MSC behavior to the same degree as substrate stiffness. This finding is further supported by a recent study demonstrating similar effects of TCP on MSC behavior in comparison to soft substrates with no fibrous topography11,40. Therefore, the important consideration in choosing a substrate for MSC expansion may not be the particular substrate type (i.e., electrospun scaffold versus gel) but rather the substrate stiffness.
In conclusion, this study demonstrated that culture of MSCs on TCP has an immediate and persistent effect of cell phenotype that may be detrimental to their use in tissue engineering applications. Specifically, MSC expansion on TCP appears to activate cells, causing an increase in proliferation and senescence as well as early commitment towards a fibrotic phenotype. Increased contractility and YAP nuclear localization in cells on TCP compared to electrospun scaffolds suggests that the altered phenotypes are due to differences in substrate stiffness and MSC mechanotransduction. Importantly, these effects persist even after reseeding the cells to a new substrate or encapsulating them in hydrogels. Our findings suggest that supra-physiologically stiff TCP may not be an appropriate substrate for MSC isolation and expansion for tissue engineering purposes. Instead, it is important to carefully select a substrate with an appropriate stiffness to best maintain MSC stemness and a differentiation capacity that is relevant to the ultimate tissue engineering application.
Supplementary Material
Expression of Nanog in MSCs isolated and expanded on TCP, AL, or NAL scaffolds at passage 0 (P0) and passage 3 (P3), n = 3, *: p<0.05 vs. TCP, two-way ANOVA with Fisher’s LSD, mean ± S.D.).
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB02425, R01 EB008722, R01 AR056624, and F32 AR070562) and the Penn Center for Musculoskeletal Disorders (P30 AR069619).
Footnotes
Author Contributions:
SJH, SES, DHK and RLM designed the studies. SJH, SES, DHK and KSS performed the experiments. SJH, SES, DHK, KSS and RLM analyzed and interpreted the data. SJH, SES, and RLM drafted the manuscript, and all authors have read and approved the final submitted manuscript.
References
- 1.Pittenger MF. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science (80) 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15(2):109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 3.Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS One. 2008;3(5):e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajpai VK, Mistriotis P, Andreadis ST. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012;8(1):74–84. doi: 10.1016/j.scr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y-K, Chen CS. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J Cell Mol Med. 2013;17(7):823–32. doi: 10.1111/jcmm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Abdeen AA, Kilian KA. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci Rep. 2014;4:5188. doi: 10.1038/srep05188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13(6):547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Chen Xi, Talele Nilesh P, Boo Stellar, et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater. 2016;13(6):379–389. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 12.Heo S-J, Thorpe SD, Driscoll TP, et al. Biophysical Regulation of Chromatin Architecture Instills a Mechanical Memory in Mesenchymal Stem Cells. Sci Rep. 2015;5(5):16895. doi: 10.1038/srep16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker BM, Gee AO, Metter RB, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29(15):2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li WJ, Laurencin CT, Caterson EJ, et al. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 15.Heo SJ, Nerurkar NL, Baker BM, et al. Fiber stretch and reorientation modulates mesenchymal stem cell morphology and fibrous gene expression on oriented nanofibrous microenvironments. Ann Biomed Eng. 2011;39(11):2780–2790. doi: 10.1007/s10439-011-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W-J, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 17.Nathan AS, Baker BM, Nerurkar NL, Mauck RL. Mechano-topographic modulation of stem cell nuclear shape on nanofibrous scaffolds. Acta Biomater. 2011;7(1):57–66. doi: 10.1016/j.actbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Zeng D, Ding L, et al. Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol. 2015;16:22. doi: 10.1186/s12860-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Wei Y, Zhang X, et al. Lower extent but similar rhythm of osteogenic behavior in hBMSCs cultured on nanofibrous scaffolds versus induced with osteogenic supplement. ACS Nano. 2013;7(8):6928–6938. doi: 10.1021/nn402118s. [DOI] [PubMed] [Google Scholar]
- 20.Smith LA, Liu X, Hu J, Ma PX. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30(13):2516–2522. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Dean D, Wallace J, et al. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2011;30(15):2516–2522. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driscoll TP, Cosgrove BD, Heo S-J, et al. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys J. 2015;108(12):2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker BM, Trappmann B, Wang WY, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14(12):1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011;17(9–10):1445–1455. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthr Cartil. 2008;16(9):1074–1082. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng Q, Duchemin-Pelletier E, Deshiere A, et al. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc Natl Acad Sci U S A. 2012;109(5):1506–1511. doi: 10.1073/pnas.1106377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo SJ, Han WM, Szczesny SE, et al. Mechanically Induced Chromatin Condensation Requires Cellular Contractility in Mesenchymal Stem Cells. Biophys J. 2016;111(4):864–874. doi: 10.1016/j.bpj.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosgrove BD, Mui KL, Driscoll TP, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15(12):1297–1306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson IE, Kestle SR, Zellars KH, et al. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed Mater. 2012;7(2):24110. doi: 10.1088/1748-6041/7/2/024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Martin JT, Elliott DM, et al. Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomater. 2015;12(1):21–29. doi: 10.1016/j.actbio.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. BBA - Gen Subj. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 32.Grzesiak J, Marycz K, Czogala J, et al. Comparison of Behavior, Morphology and Morphometry of Equine and Canine Adipose Derived Mesenchymal Stem Cells in Culture. Int J Morphol. 2011;29(3):1012–1017. [Google Scholar]
- 33.Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Li Y, Tollefsbol TO. Cell senescence culturing methods. Methods Mol Biol. 2013;1048:1–10. doi: 10.1007/978-1-62703-556-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed NE-MB, Murakami M, Kaneko S, Nakashima M. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs) Sci Rep. 2016;6:35476. doi: 10.1038/srep35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong TY, Chang CH, Yu CH, Huang LLH. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell. 2017;16(3):451–460. doi: 10.1111/acel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 38.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 39.Balestrini JL, Chaudhry S, Sarrazy V, et al. The mechanical memory of lung myofibroblasts. Integr Biol. 2012;4(4):410. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 40.Talele NP, Fradette J, Davies JE, et al. Expression of alpha-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Reports. 2015;4(6):1016–1030. doi: 10.1016/j.stemcr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of Nanog in MSCs isolated and expanded on TCP, AL, or NAL scaffolds at passage 0 (P0) and passage 3 (P3), n = 3, *: p<0.05 vs. TCP, two-way ANOVA with Fisher’s LSD, mean ± S.D.).