Abstract
Focal chondral lesions and early osteoarthritis (OA) are responsible for progressive joint pain and disability in millions of people worldwide, yet there is currently no surgical joint preservation treatment available to fully restore the long term functionality of cartilage. Limitations of current treatments for cartilage defects have prompted the field of cartilage tissue engineering, which seeks to integrate engineering and biological principles to promote the growth of new cartilage to replace damaged tissue. Towards improving cartilage repair, hydrogel design has advanced in recent years to improve their utility. Injectable hydrogels have emerged as a promising scaffold due to their wide range of properties, the ability to encapsulate cells within the material, and their ability to provide cues for cell differentiation. Some of these advances include the development of improved control over in situ gelation (e.g. light), new techniques to process hydrogels (e.g. multi-layers) and better incorporation of biological signals (e.g. immobilization, controlled release, and tethering). This review summarises the innovative approaches to engineer injectable hydrogels towards cartilage repair.
Keywords: hydrogel, tissue engineering, orthopedics, osteoarthritis, focal chondral lesions
INTRODUCTION
Cartilage restoration surgical procedures for the treatment of chondral lesions are common in the young adult population. Still, they are not capable of regenerating functional articular hyaline cartilage, providing temporary symptomatic relief but not a cure. Tissue engineering has enabled the development of biological substitutes that restore, maintain or improve tissue functions for therapeutic purposes. Recently, injectable hydrogels acting as three-dimensional scaffolds have received increased attention for articular cartilage tissue engineering.
Injectable hydrogels generally retain a large amount of water, exhibit excellent permeability for nutrients and metabolites, and show good biocompatibility.(1) They can be administrated via a minimally invasive procedure, and are able to appropriately fill irregularly-shaped defects. Meanwhile, cells and bioactive molecules can be homogeneously incorporated into the hydrogels. Due to their physical properties that resemble the native extracellular matrix (ECM), injectable hydrogels may be suitable platforms for supporting the survival, proliferation and differentiation of incorporated cells, and promoting the regeneration of articular cartilage tissue. The purpose of this review is to summarise the innovative approaches to engineer injectable hydrogels towards cartilage repair and present an update on the use of injectable hydrogels for the treatment of focal chondral lesions in preclinical animal models and clinical trials.
TISSUE ENGINEERING
The standard concept of tissue engineering is to combine cells with a three-dimensional (3D) biomaterial scaffold to help regenerate damaged tissue. The scaffold is designed to create a 3D microenvironment that resembles specific tissues and stimulates native tissue regeneration by promoting cell-matrix interactions and cell-cell interactions, which can lead to cell differentiation and tissue growth.
Biodegradable in situ forming hydrogels have been suggested as a promising scaffold for articular cartilage tissue engineering. A main advantage of using hydrogels is the ability to inject the hydrogel as a prepolymer solution and then polymerize it in vivo. This polymerization allows the hydrogel to form into the defect anatomically. In addition, recent advances in bioprinting have granted tissue engineers the ability to assemble hydrogels ex vivo into physically relevant 3D structures. Moreover, in situ forming hydrogels have also been used as drug delivery systems, allowing for a controlled and sustained release of drugs intra-articularly over several weeks or months for the treatment of joint disease such as OA or rheumatoid arthritis. Lastly, lesions on articular cartilage normally affect cartilage and underlying subchondral bone making these lesions problematic because they extend across two distinctly different tissues, a highly compliant hyaline cartilage and a stiff subchondral bone. Contemporary hydrogels have been designed to include bi-layers or multi-layers that mimick aspects of both bone and cartilage tissues.(2)
The body of literature concerning articular cartilage tissue engineering in animal models is rapidly expanding. However, it has been reported that 90% of the new approaches that are successful in animal studies subsequently fail clinical trials.(3) Therefore, meticulous analysis of the existing short-term clinical outcomes is advocated. Such a rigorous approach is needed to guide the development of biomimetic and bioactive approaches in tissue engineering, which will lead to more successful and reliable clinical outcomes.
Articular Cartilage Properties
Articular cartilage is a fiber-reinforced composite material composed of chondrocytes surrounded by specialized ECM consisting of structural and functional proteins, glycoproteins, and glycosaminoglycans assembled in unique tissue-specific 3D microenvironment architectures. It presents a lubricated surface with low friction stress. Mechanically, human articular cartilage is a composite of materials with widely differing properties, that resist high compressive loads, ranging from 240–1000 kPa.(4) The composition and structure of cartilage tissue are always depth-dependent and can be divided into four different zones based on collagen fiber alignment and proteoglycan composition.
Approximately 70 to 85% of the whole tissue weight is water, and less than 5% accounts for chondrocytes. The remainder of the tissue is composed primarily of proteoglycans and collagen. In an aqueous environment, proteoglycans are polyanionic. In solution, the mutual repulsion of these negative charges causes an aggregated proteoglycan molecule to spread out and occupy a large volume. In the cartilage matrix, the volume occupied by proteoglycan aggregates is limited by the entangling collagen framework. The swelling of the aggregated molecule against the collagen framework is an essential element in the mechanical response of cartilage. When cartilage is compressed, the negatively charged sites on aggrecan are pushed closer together, which increases their mutual repulsive force and adds to the compressive stiffness of the cartilage. The mechanical response of cartilage is also strongly tied to the flow of fluid through the tissue. When deformed, fluid flows through the cartilage and across the articular surface. If a pressure difference is applied across a section of cartilage, fluid also flows through the tissue. These observations suggest that cartilage behaves like a sponge, albeit one that does not allow fluid to flow through it easily. On the other hand, chondrocytes have a repertoire of integrins that perform unique and crucial crosstalk with the ECM, growth factors, cytokines and mechanical stimuli. This crosstalk allows chondrocytes to respond to microenvironmental cues which will regulate their functions (proliferation, differentiation, migration, morphogenesis and survival), and will help maintain the cartilage architecture through a balanced molecular degradation/synthesis activity. For example, fibronectin has binding sites for integrins α3β1, α4β1, α5β1 and αVβ1, while the collagen binding integrins include α1β1, α2β1, α10β1 and α11β1. The intracellular MAP kinases mediate cell signaling after integrin activation, and lead to production of catabolic factors such as matrix metalloproteinases (MMP), reactive oxygen species (ROS), and nitric oxide (NO), among others.(5) Integrins sense intact ECM structures or fragments. Intact molecules do not upregulate MMP, but fragments do, to clear the damaged matrix before synthesizing new matrix. Particularly, α5β1 is activated by fibronectin fragments and arginine-glycine-aspartic (RGD, a sequence found in collagen).(5)
Hydrogel Properties
Hydrogels are crosslinked polymers that are insoluble, but swell in aqueous environments. The high water content of hydrogels can be tuned, reaching values that are similar to native cartilage at ~80% water and even higher (i.e., >90% water). This helps facilitate rapid exchange of nutrients towards and waste away from the embedded cells.(6) Hydrogels can be broadly divided into natural or synthetic crosslinked polymers, or a combination of both; and degradable and non-degradable. Natural polymers are polysaccharide (e.g., alginate, chitosan, cellulose, amylose, dextran, glycosaminoglycans, agarose, chitin), protein (e.g., collagen, gelatin, fibrin, elastin, silk, actin, myosin, soy), or proteonucleotide (e.g., RNA, DNA) based. They are favored by their general biocompatibility, and in some cases their biochemical similarity to native cartilage and feasibility to be degraded by cell-secreted enzymes. Those degradable by enzymes include glycosaminoglycans (e.g. HA), collagen, chitosan, gelatin, fibrin, elastin, actin, myosin, RNA, and DNA; while non-degradable polymers include alginate, cellulose, amylose, dextran, agarose, chitin, silk and soy. Synthetic polymers are favored by their high tunability, enabling greater control over their macroscopic properties and degradation behavior. Some examples of synthetic polymers that have been used in cartilage tissue engineering include poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), poly(lactic acid) (PLA) and polydioxanone (PDS), among others. Notably, hydrogels formed from synthetic polymers have been designed to have similar mechanical properties (compressive modulus) and frictional behavior as articular cartilage. Furthermore, a number of studies has demonstrated the ability to easily embed cells and growth factors in synthetic hydrogels. The combination of synthetic and natural polymers has emerged as a promising approach to create biomimetic hydrogels, combining the potential chondrogenic tunable characteristics of both. These biomimetic hydrogels can be designed to mimic key aspects of the native environment, while precisely adjusting the hydrogel’s mechanical, chemical and degradation properties.(7)
Hydrogel degradation is critical for ECM synthesis during cartilage tissue growth. The hydrogel degradation may occur through two predominant mechanisms: bulk degradation (e.g., hydrolysis), which results in uniform degradation of the crosslinks; and/or local degradation (e.g., enzymatic). Synthetic polymers can be designed with crosslinks that degrade by either hydrolysis or enzymes.(8) An ideal hydrogel will support joint loads, gradually degrade and transfer the joint load stimulus to the new forming tissue. Thus, it is important to tune hydrogel degradation with new tissue growth. The rate of hydrogel degradation depends on the degree of crosslinking (i.e., the more crosslinked the slower the degradation) and the choice of degradable linker, which influences the kinetics (i.e., speed) of degradation and solute diffusion coefficient.(9) The challenge is that a high crosslink density is required to support joint loads, but will slow degradation rate and negatively affect diffusion of large molecules including growth factors and newly synthesized ECM molecules. Notably, the ECM molecules of cartilage, specifically aggrecan and collagen, are too large to be transported through the crosslinks of the hydrogel and as a result degradation must occur before a macroscopic tissue can form.(10) By carefully tuning the initial properties and formulation of the hydrogel, it is possible to match degradation with new cartilage tissue growth.
Clinically, most symptomatic cartilage lesions are osteochondral with involvement of both the cartilage and bone layers. To overcome these complex lesions, multi-layer hydrogels have been created. Bi-layer hydrogels are the simplest approach, whereby the same scaffold chemistry in both layers has been used and the properties varied in each one [(e.g., pore structure and/or biochemical cues (e.g., tissue-specific ECM-analogs)(11) or incorporation of growth factors].
Moreover, incorporation of cells into the hydrogel is critical for regeneration. Cells can either infiltrate into the scaffold or exogenous cells can be delivered within the scaffold upon implantation. (11) One potential cell source is mesenchymal stem cells (MSCs) since they can be differentiated towards the chondrogenic or osteogenic lineage and inoculated in each specific layer to better reproduce the articular cartilage and subchondral bone. Endogenous cells can migrate into the hydrogel by designing hydrogels that contain chemotactic factors that will promote the migration of cells from surrounding bone marrow and synovial tissue. This is facilitated by the fact that MSCs from the marrow and synovium express multiple chemokine receptors such as CXCR1, CXCR2, CXCR4 and CCR2 which allow them to home to chemokines.(12) Examples of known chemokines involved in MSC recruitment are stromal cell-derived factor 1 (SDF-1), interleukin-8 (IL-8), platelet derived growth factors (PDGFs), and TGF-β isoforms.(12) It has been shown that addition of SDF-1 into a scaffold leads to improved articular cartilage regeneration following injury due to an increased number of MSCs at the injury site.(13) Thus, it is possible to develop acellular hydrogel constructs that provide chemokine factors to recruit endogenous MSCs from nearby marrow compartments. Further designing the hydrogels to also provide factors that promote cartilage differentiation would encourage endogenous MSCs to form articular cartilage tissue.
Another critical feature for successful cartilage healing is achieving optimal integration to the surrounding tissue. In order to accomplish this, the hydrogel needs to adhere to the surrounding tissue, including the adjacent articular surface and the subjacent subchondral bone. If integration to the surrounding smooth cartilage fails, the implanted hydrogel will become loose or fracture even under gentle daily movement. Integration or self-adhesiveness to host cartilage can be obtained from entanglements, which can form between the crosslinked tissue molecules in cartilage and the polymer chains or by chemical reactions that allow chemical bonds to local cartilage during in situ gelation. Depending on the chemistry of the monomers that form the hydrogel, non-specific reactions can occur between the polymerizing monomers and the tissue molecules (e.g., chain transfer in radical-mediated polymers).(14) Moreover, chemistries can be introduced to enhance adhesion. Among these, aldehyde groups found in self-adhesive hydrogels allow a Schiff-base reaction between the hydrogel and local cartilage tissue amines giving an adhesive property to the hydrogel.(13) Bonding to host tissue through aldehyde groups depend on multiple environmental conditions including pH, oxygen content and enzymatic challenges. Differently, bonding to the subchondral bone takes place in a “flow like” manner, migrating from the neighboring native bone in a gradual centripetal way towards the scaffold. After adhesion of the scaffold to the subchondral bone, which may be enhanced by marrow stimulation, mechanical stimulus follows. Subchondral bone is induced to migrate from the surrounding tissue, and “anchors” the scaffold/new forming tissue to the defect area. As the bond matures, it enhances the new cartilage formation and lateral interface integration.(15)
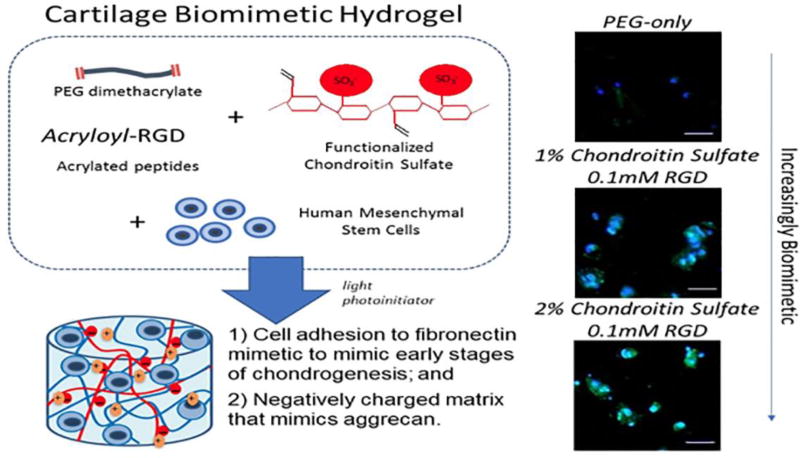
One example of a promising hydrogel system is based on crosslinked PEG into which cartilage ECM analogs including chondroitin sulfate (ChS) and RGD have been introduced to create a cartilage-like biomimetic hydrogel.(16) ChS is the main glycosaminoglycan in cartilage and creates a unique environment that is hyperosmotic and promotes tissue synthesis, especially under dynamic compression.(17) RGD, a cell adhesion peptide, provides a mechanism for cells to sense substrate stiffness and acts as a mechanosensor to cells activating integrin α5β1. RGD has been demonstrated to support chondrogenesis, with lower concentrations improving differentiation.(18)
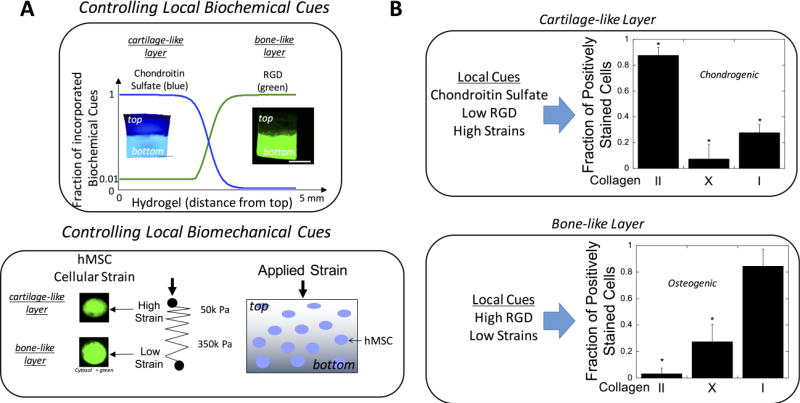
This system was developed to be photopolymerizable whereby the different components (i.e., PEG, ChS, and RGD) are modified with crosslinkable groups and, upon exposure to light, react to form a crosslinked polymer network (Figure 1). This hydrogel permits cells to be encapsulated during the hydrogel formation process, with the final combination to be formed in situ within a defect site in the body. Advantages of this photopolymerizable hydrogel include spatial and temporal control during hydrogel formation, the ability to polymerize at physiological pH and temperature, and rapid polymerization (seconds to minutes) (Figure 2).(19) In addition, this hydrogel system can be expanded by creating an injectable multilayer construct via sequential photopolymerization of layers in situ. Thus, each layer can be designed to reproduce the different layers that need to be regenerated for combined osteochondral lesions. For example, the type and concentration(s) of ECM molecules and the local stiffness can be varied within each layer of a PEG hydrogel.(11) Notably, under a compressive load, the variation in hydrogel stiffness within each layer produced high strains in the softer cartilage-like layer, low strains in the stiffer bone-like layer, and moderate strains in the interfacial layer. This led to the ability to direct differentiation fate of embedded MSCs (Figure 3).
Figure 1.
An example of a cartilage biomimetic hydrogel. The hydrogel is composed of poly(ethylene glycol) (PEG), chondroitin sulfate (ChS) and a cell adhesion peptide, RGD. Under a light source, polymerization is initiated in an aqueous precursor solution with embedded stem cells. This allows hydrogel formation and in situ delivery of cells. The incorporation of ECM molecules (e.g., chondroitin sulfate and RGD) enhances chondrogenesis of the encapsulated stem cells after two weeks in culture as shown by the presence of collagen type II (green), which was not present in the synthetic-only hydrogel. Data are reproduced with permission from Aisenbrey et al.(16)
Figure 2.
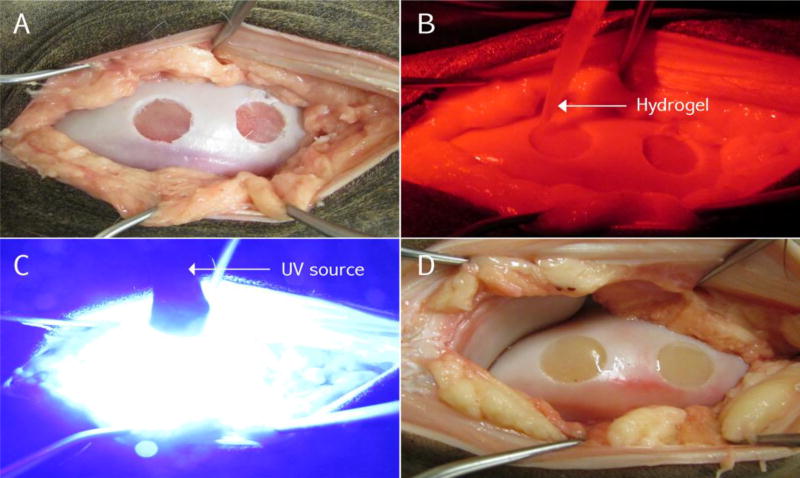
In situ hydrogel photopolymerization in a critical chondral defect in the medial femoral condyle of the knee in a cadaveric horse model, A) Osteochondral defects (10mm wide × 3 mm deep) in the medial femoral condyle; B) Injection of prepolymer into the chondral defect under red light to prevent early photopolymerization C) Exposure to 405-nm blue light to induce polymerization; D) Polymerized hydrogel, anatomically filling the chondral defects.
Figure 3.
A multi-layer PEG-based hydrogel whereby different ECM analog type(s) and/or concentration(s) were varied in each layer along with the local stiffness, resulting in spatially controlled biochemical and biomechanical cues for osteochondral tissue engineering (A). Here, the top cartilage-like layer was comprised of chondroitin sulfate (1%), and 0.1 mM RGD with a compressive modulus of 50 kPa, while the bottom layer was comprised of 10 mM RGD with a compressive modulus of 350 kPa. Under dynamic compressive loading at 2.5% strain 1Hz for 1 hr/day, differentiation of human MSCs was semi-quantified by collagen staining revealed high collagen II expression in the top layer and high collagen I expression along with mineral (not shown) in the bottom layer. These data show the ability to direct human MSCs under dynamic loading. Data are reproduced with permission from Steinmetz et al.(11)
Cell Source and Cell Encapsulation
Incorporating cells into hydrogels can be performed by: 1) seeding the cells onto prefabricated porous scaffolds, or 2) the cells are encapsulated during scaffold formation. Multiple cell lines have been investigated for cartilage repair including: chondrocytes, MSCs, adipose-derived MSCs and induced pluripotent stem cells (iPSCs) (Table 1). Yet, most therapies require a two-step approach: first, harvesting the cells; and secondly, expanding the cell population. From a FDA regulation point of view, this manipulation of tissue and cells is considered beyond minimally manipulated and requires greater regularoty oversight which could delay its translation to clinic.(20)
Table 1.
Chondrogenic polymers and cells
| Polymer | Precursor(s)/ initiator | Cells encapsulated | Degradation mechanism |
|---|---|---|---|
| Chitosan | Chitosan, lactic acid, and methacrylate / APS/TEMDA | Chondrocytes(39) | Lysozyme, hydrolytic |
| Styrenated gelatin | Styrenated gelatin / camphorquinone | Chondrocytes(40) | Enzymatic |
| HA | Methacrylated HA / Irgacure 2959 | Chondrocytes(41) | Hyaluronidase |
| Acrylated HA and PEG-(SH)4 | Human MSCs(42) | Hyaluronidase | |
| Thiol-modified HA and PEG diacrylate | Adipocyte MSCs(43) | Hyaluronidase | |
| ChS | Methacrylated chondroitin sulfate / Irgacure 2959 | Chondrocytes(7) | Chondroitinase |
| Synthetic ECM analogs | Thiol-modified HA, or Thiol-modified chondroitin sulfate, or Thiol-modified gelatin, and PEG diacrylate | Bone marrow derived MSCs(44) | Enzymatic |
| PEGylated fibrinogen | Fibrinogen-g-PEG acryloyl and PEG diacrylate / Irgacure 2959 | Bone marrow stromal cells(45) | Plasmin, MMPs |
| ELP | Genetically engineered ELP | Chondrocytes(46) | Enzymatic |
| Poly(ethylene) based | PCL-b-PEG-b-PCL dimethacrylate / Irgacure 2959 | Chondrocytes(47) | Lipase, hydrolytic |
| PEG- [poly (glycerol succinic acidmethacrylate)]/Eosin-Y, NVP, triethanolamine | Chondrocytes(48) | Hydrolytic | |
| PEG-norbornene-caprolactone, PEG-dithiol (crosslink) / photoinitiator I2959 | Chondrocytes(1) | Hydrolytic | |
| OPF and NVP/Irgacure 2959 | Chondrocytes(49) | Hydrolytic | |
| Polyfumarate based | Poly (lactide-co-ethylene oxide-co-fumarate), and MMP-diacrylate APS/TEMDA | Bone marrow stromal cells(50) | MMPs, hydrolytic |
Note. APS: ammonium persulfate; TEMDA: N,N,N0,N0-tetramethylethylenediamine; PEG: poly(ethylene glycol); HA: hyaluronic acid; ChS: chondroitin sulfate; Irgacure 2959: 2- hydroxy-1-[4-(hydroxyethoxy) phenol]-2-methyl-1-propanone; ECM: extracellular matrix; MSCs: mesenchymal stem cells; MMPs: metalloproteinases; ELP: Elastin-like polypeptides; PLA: poly(lactic acid); PCL: poly(8-caprolactone); NVP: N-vinylpyrrolidone; OPF: oligo(polyethylene glycol) fumarate.
Chondrocytes (fully differentiated cells)
Autologous chondrocyte implantation (ACI) has been used for decades in the treatment of focal chondral lesions, with good clinical outcomes. Yet, chondrocytes tend to dedifferentiate into a fibroblast-like phenotype.(21) Newer, third generation ACI, or matrix-induced autologous chondrocyte implantation (MACI) techniques have incorporated scaffolds to prevent the dedifferentiation of chondrocytes during culture and further enhance the technique. Most MACI scaffolds consist of collagen type I or III, or hyaluronic acid. Newly published data suggests good long-term clinical outcomes with the MACI technique for the treatment of focal chondral lesions in the knee. Despite these positive outcomes, limitations of this procedure include donor-site morbidity, being a staged procedure (chondrocyte harvest, expansion, and reimplantaton) and low chances of regeneration in patients over 50 years of age due to a decrease in cell proliferation and extracellular matrix secretion capacity with increased donor age. However, hydrogels have been designed to maintain the chondrogenic phenotype of chondrocytes. For example, Buschmann et al.(22) studied an in-situ gelating chitosan based hydrogel that not only adhered to the defect area, but also retained chondrocytes phenotype and potential; while Schneider et al. (23) proved that in vitro bovine chondrocytes encapsulated in photo-polymerizable PEG hydrogel maintain their phenotype and synthesize a broad repertory of cartilage-specific ECM proteins (e.g., collagens II, VI, IX, XI, aggrecan and biglycan), which increases over time. Moreover, chondrocytes embedded in 3D charged hydrogels (i.e., ChS and PEG), behave differently whether there is dynamic loading or not. With no loading, cell proliferation and proteoglycan synthesis are greatly reduced, while collagen does not seem to be affected. However, when loading is present, cell proliferation is less affected, while proteoglycan and collagen synthesis are exponentially increased. If the loading stimulus is removed, the enhanced synthesis is not maintained.(24) Thus, proving that mechanotransduction, and different extracellular cues can tune cellular behavior and hydrogels fate.
Stem Cells
Several stem cell sources have shown the ability to undergo chondrogenesis in vitro when seeded in hydrogels. These include embryonic stem cells (ESCs), MSCs and the more recently discovered iPSCs.
ESCs display unlimited self-renewal capacity while maintaining a pluripotent differentiation potential. Combining these cells with biomimetic hydrogels and growth factors (i.e., transforming growth factor beta-1, bone morphogenetic protein) has proven to be a synergistic environment for their chondrogenesis.(25) When allogenic ESCs seeded in fibrin glue were applied for treating induced knee focal osteochondral defects in sheeps, these were efficiently repaired with better outcomes (higher collagen II and proteoglycans) than the untreated control joints.(26) While promising, ESCs are associated with ethical concerns surrounding their derivation, limiting their use.(27) This has promoted significant interest in adult-derived stem cells such as MSCs, a multipotent cell population and precursor of cartilage.
MSCs can be obtained from adult tissues such as bone marrow, synovium, adipose tissue, periosteum, as well as umbilical cord and peripheral blood. In vitro studies seeding MSCs in chondrogenic 3D hydrogels such as agarose, hyaluronan, PEG or alginate have reported chondrogenic differentiation of the cells. It should also be noted that in vitro studies have demonstrated that MSC proliferation and differentiation capacity decreases with ageing and ageing-related diseases,(28) potentially hindering their clinical use in older individuals. Therefore, methods to improve the chondrogenesis of MSCs are still warranted and could potentially be achieved using hydrogels which would provide them with strong chondrogenic cues and prevent their differentiation towards unwanted tissues.
Additional cell sources continue to be explored. One such source is the iPSC, which is obtained from the reprogramming of adult cells to an early state of differentiation, resembling that of ESCs.(29) This technology could potentially be used to “rejuvenate” cells from older patients into iPSCs and might improve their progenitor cell healing and functional capacity. Xu at al.(30), in a rabbit model found that human iPSCs maintained their pluripotency in a poly-lactic based scaffold, and enhanced cartilage repair of an osteochondral defect in a 6 week period. With the advancement of cellular reprogramming techniques, it is now possible to generate iPSCs using an integration-free approach, which is safer and more amenable from a regulatory perspective for their eventual clinical use.(31) Despite this decreased risk of unwanted tissues, further studies are needed to better assess the long-term benefit of using human iPSCs for articular cartilage tissue engineering.
Preclinical Animal Models
Numerous preclinical animal models have been used to evaluate cartilage repair with different cell lines delivered in hydrogels. Animal models include: rabbit, canine, mini-pig, ovine, caprine and equine. Most of these studies suggest promising results with improved cartilage regeneration between 3 months and 2 years.(26,32–36) Significant superior histologic evidence of cartilage regeneration when using hydrogels are reported when compared to empty defects. The cell sources, collection techniques, cell processing, qualitative and quantitative characterizations, and delivery methods vary widely between studies. Cell types include autologous bone marrow mesenchymal stem cells (BMSCs) and allogeneic umbilical cord blood mesenchymal stem cells (UCB-MSCs). All lesions were located in the knee joint (Table 2-A and 2-B).
Table 2.
| -A. Small animal studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study, year |
Animal, maturity and age |
N, study type | Diagnosis | Hydrogel or scaffold |
Cell type seeded and density |
Procedure | FU period and method |
Outcomes | Adverse events |
| Ha et al.(34) 2015 | Minipig, adult, 1.5 years’ old | 6, case-control. Case: HA hydrogel/hUCB-MSCs. Control: contralateral joint, empty defect. | Trochlear groove osteochondral defect 5 mm wide × 10 mm deep | 4 % HA | CE xenogenous, hUCB-MSCs; 0.5×107 cells/ml | 1. The defect was generated and reinspected at 3 weeks for treatment. 2. An osteochondral defect 5 mm wide × 10 mm deep was generated in the injured area. 3. 1.5 ml of seeded HA hydrogel was transplanted. | 12 weeks. Clinical, macroscopic and histological with ICRS. IHC for collagen II. | The case group resulted in superior hyaline cartilage regeneration. Collagen II was higher in the case group. | Not mentioned. |
| Zhao et al.(33) 2015 | Rabbit, not specified | 4, case-control, 3 groups: (1) N 18, chitosan hydrogel/chondrocytes; (2) N 18, chitosan hydrogel; (3) N 18, control, empty. | MFC full-thickness defect 4 mm wide × 3 mm deep. | Chitosan hydrogel: 1,6-diisocyanatohexane and PEG | CE autologous costal chondrocytes; 1×107 cells/ml | 1. Chitosan hydrogel scaffold discs 1 mm wide seeded with cells for a week. 2. Generation of the defect, followed by scaffold ± chondrocytes implantation | 4. 8 and 12 weeks. Macroscopically and histologically with ICRS. IHC for collagen II. | (1) had better macroscopic and histological results. Hyaline-like cartilage, with high proteoglycan and collagen II content. | Only two had wound infection, synovitis and limited ROM. |
| Liu et al.(36) 2017 | Rabbit, not specified | Pilot study, 4 groups: (A) untreated; (B) cartilage ECM-derived scaffolds; (C) scaffolds with undifferentiated hWJMSCs; and (D) scaffolds with chondrogenically di erentiated hWJMSC-Cs group. | Femoral patellar groove osteochondral defect 4 mm wide × 1.5 mm deep | ECM of swine cartilage 4 mm wide×1.5 mm high | CE xenogenous, hWJMSCs; 3×107 cells/ml | 1. Scaffolds were injected with stem cells and cultured in normal culture medium and chondrogenic culture medium for 14 days. 2. Generation of the defect, and scaffold/hWJMSCs or hWJMSC-Cs implantation. | 3, 6, 12 and 16. Macroscopic with ICRS, histological with ICRS-I, and GAG content. | Hyaline-cartilage like layer with good surface, integration, and regenerated subchondral bone. The group C had, better appearance, histological results, and higher GAGs content. | Not mentioned. No immune rejection was detected. |
| -B. Large animal studies. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study, year |
Animal model, maturity and age |
N, study type |
Diagnosis | Hydrogel or scaffold |
Cell type seeded and density |
Procedure | FU period and method |
Outcomes | Adverse events |
| Pilichi et al.(26) 2014 | Ovine, adult, 5.5 years’ old | 22 ewes, case-control. Case: ES-like cells. Control: contralateral empty defect; a total of 44 defects | Left MFC osteochondral defect 6 mm wide × 2 mm deep | Fibrin glue | CE allogenic male sheep ES-like cells; 500,000 cells | 1. ES-like cells harvested from male embryos. 2. After generation of the defect, followed fibrin glue/ES-like cells implantation (cases). | 2 years. Clinical, macroscopic with ICRS, histological with a Caplan derived scoring system, IHC (collagen II) and FISH assays. | Cases had better histologic regeneration, with higher proteoglycan and collagen II content. FISH was positive in the treated group. | Five were excluded, because accidentally died from toxemic gastroenteritis. No immune rejection or teratoma formation. |
| Kon et al.(32) 2015 | Caprine, skeletally mature, age not specified | 20, case-control. Group (A): N 6, blood clot; Group (B): N 14 Ar-HA implant. | MFC osteochondral defect 6 mm wide × 10 mm deep. | Ar-HA | NA | Generation of defect, followed positioning of Ar-HA in a press-fit manner below the articular surface. | 6 and 12 months. Clinical, x-rays, micro-CT, ultrasound, histology and MRI. | (A, control): most were not reconstructed; defects were either empty or contained fibrous tissue. (B, case): mostly reconstructed; filling was compatible with hyaline cartilage and normal bone. | Minor adverse events: weight loss, high temperature, sneezing in both groups. |
| Goodrich et al.(35) 2016 | Equine, 2–5 years’ old | 12, case-control. Case: APEF + BMDMSCs. Control: Contralateral joint, APEF alone. | Lateral trochlear ridge osteochondral defect 15 mm wide | APEF | CE autologous BMDMSCs; 1×107cells/ml | 1. Generation of defect. 2. Positioning of APEF ± BMDMSCs in the defect. | 12 months. Macroscopically with ICRS, histologically with O' Driscoll, MRI, micro-CT, and biomechanical testing. | No macroscopic difference. Histologically, 23/24 had fair to good fill and integration. Bone formation in the case group. Micro-CT, thinner cartilage in the case group. MRI, no difference. | Not mentioned. |
Note. N: number; FU: follow-up; MFC: medial femoral condyle; NA: not applicable; ICRS: International Cartilage Repair Society; IHC: immunohistochemistry; ROM: range of motion; HA: hyaluronic acid; hUCB-MSCs: human umbilical cord blood derived mesenchymal stem cells; GAGs: glycosaminoglycans; hWJMSCs: human umbilical cord Warthon's Jelly mesenchymal stem cells; hWJMSC-Cs: chondrogenically differentiated human umbilical cord Warthon's Jelly mesenchymal stem cells.
Note. N: number; FU: follow-up; MFC: medial femoral condyle; CE: culture expanded; NA: not applicable; ES: embryonic stem; ICRS: International Cartilage Repair Society; FISH: fluorescent in situ hybridization; Ar-HA: Aragonite-hyaluronate; IHC: immunohistochemistry; CT: computed tomography; MRI: magnetic resonance imaging; APEF: autologous platelet-enriched fibrin; BMDMSCs: bone marrow derived mesenchymal stem cells.
Clinical Human Studies
Few clinical studies have been reported evaluating the use of hydrogels for the treatment of focal chondral lesions in human patients. Most of the literature includes case reports or case series. Two studies included case-control treatments. Elisseff et al.(37) in a pilot study, compared microfracture with hydrogel to microfracture alone in a focal chondral defect in the knee with a minimum follow up of 6 months suggesting higher filling and decreased water content as well as better tissue organization in the study group. Similarly, Restrepo et al.(38) in a randomized study, reported on clinical outcomes in patients treated with BST-CarGel® (Piramal Life Sciences, Bio-Orthopaedics Division) and concomitant microfracture (study group) compared to microfracture alone (control group) for the treatment of focal chondral lesions in the knee with a median follow-up of 5 years. Patients in the study group showed statitiscally better patients reported outcomes measures (PROs) and better cartilage regeneration on MRI. No major adverse events were reported in either study (Table 3). There are no ongoing clinical trials in the USA evaluating the use of hydrogels for the treatment of focal chondral lesions.
Table 3.
Clinical studies
| Study, year |
N, study type |
Age | Diagnosis | Hydrogel or Scaffold |
Cell type seeded and density |
Procedure | FU period and method |
Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Elisseeff et al.(37) 2013 | 18, pilot case-control. Case (N 15): hydrogel + MFX; vs controls (N 3): MFX. | Not mentioned | MFC focal cartilage defects of 2–4 cm2. | PEG diacrylate (PEGDA-HA) | NA | 1. CS layer on defect, 2. MFX; 3. liquid PEGDA-HA; 4. Photo-polymerization for 4 min. | 6 months. Clinical, IKDC and MRI | Pain decreased in the case group, and IKDC increased in both groups. The case group showed higher filling, decreased water content and tissue organization on MRI. | No major adverse events. 1 case of mild hemarthrosis. |
| Restrepo et al.(38) 2015 | 80, randomized case-control. Case: BST-CarGel® + MFX; vs control: MFX; 1:1 ratio. | Case: 34.3 ± 9.7; Control: 40.1 ± 10.1 | Femoral condyle focal chondral defects (ICRS grade III, IV). | Chitosan scaffold (BST-CarGel®; Piramal Life Sciences, MA, USA) | NA | 1. MFX; 2. BST-CarGel®/blood mixtures solidify in situ within approximately 10 minutes. | 5 years. Clinical, WOMAC and MRI. | Pain decreased in both groups, and WOMAC was higher in the case group. The case group had higher filling on MRI. | Most mild to moderate, less adverse events in the case group. Most frequent: knee pain. |
Note. N: number; FU: follow-up; MFC: medial femoral condyle; NA: not applicable; MRI: magnetic resonance imaging; PEG: poly(ethylene glycol); MFX: microfracture; IKDC: International Knee Documentation Committee; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Overall, when compared to microfracture alone, both animal and clinical studies suggest superior cartilage regeneration when treating chondral defects with hydrogels. In the animal studies, best results were seen when the hydrogel was combined with cell lines or with a concomitant microfracture. They prove that different sources can be used, such as allogenic, autologous or xenogenic for enhancing cartilage repair. Both clinical trials, despite not encapsulating cells in the scaffolds used, used microfracture technique which provides endogenous stem cells and growth factors. Histologically, hyaline cartilage-like formation was seen in the animal studies, yet this was not proved in either clinical trial. However, pain/function assessments and MRI are suitable indirect measures of good recovery and prognosis. Both ways of assessing results are valid and complementary.
CONCLUSION
Biodegradable cartilage biomimetic hydrogels are a promising therapeutic tool to deliver cells in vivo for the treatment of cartilage lesions and tissue engineering. Several advantages over other cartilage restoration techniques include: delivery as injectable systems, controlled in situ polymerization, mechanical support and the option of incorporating chondrogenic cells. Multiple preclinical animal models evaluating cartilage repair with hydrogels and different cell lines have shown excellent results in osteochondral defects, however there is limited experience in human patients. One randomized controlled study has been performed suggesting a clinical benefit of the use of hydrogel compared to control. Further studies, including blinded randomized control trials, will determine the true clinical effectiveness of hydrogels in cartilage repair.
Acknowledgments
SJB and EAA acknowledge support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1R01AR065441. The authors also acknowledge the Department of Education’s Graduate Assistanship in Areas of National Need to EAA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Pascual-Garrido receives research support from Biomet-Zimmer, JIT Core Using Funding Program, Washington Universtiy in St Louis and AOSSM-SANOFI. Dr. Goodrich is a shareholder for Advanced Regenerative Therapies and a consultant for Allosource and Calimmune.
Footnotes
AUTHORS CONTRIBUTION STATEMENT:
-Cecilia Pascual-Garrido: Design, drafting, editing and images.
-Francisco Rodriguez-Fontan: Drafting, editing, images and tables.
-Elizabeth A. Aisenbrey: Drafting, editing and images.
-Karin A. Payne: Drafting and editing.
-Jorge Chahla: Drafting, editing and images.
-Stephanie J. Bryant: Drafting, editing and images.
-Laurie R. Goodrich: Drafting and editing.
All authors were actively involved during the process of the manuscript development. They all read and approved the final version for submission.
The other authors report no actual or potential conflict of interest in relation to this article.
References
- 1.Neumann AJ, Quinn T, Bryant SJ. Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering. Acta Biomater. Acta Materialia Inc. 2016;39:1–11. doi: 10.1016/j.actbio.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5(November 2016):17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotech. 2014;32(1):40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 4.Beck EC, Barragan M, Tadros MH, Gehrke SH, Detamore M. Approaching the Compressive Modulus of Articular Cartilage With a Decellularized Cartilage-Based Hydrogel. Acta Biomater. 2016;38:94–105. doi: 10.1016/j.actbio.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeser RF, Hill C, States U. I ntegrins and chondrocyte–matrix interactions in articular cartilage Richard. 2014:11–6. doi: 10.1016/j.matbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicodemus GD, Bryant SJ. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng Part B Rev. 2008;14(2):149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005;1:243–52. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Jeuken RM, Roth AK, Peters RJRW, van Donkelaar CC, Thies JC, van Rhijn LW, et al. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers (Basel) 2016;8(6) doi: 10.3390/polym8060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadjiev NA, Amsden BG. J Control Release. Vol. 199. Elsevier B.V; 2015. An assessment of the ability of the obstruction-scaling model to estimate solute diffusion coefficients in hydrogels; pp. 10–6. [DOI] [PubMed] [Google Scholar]
- 10.Nicodemus GD, Skaalure SC, Bryant SJ. Gel structure impacts pericellular and extracellular matrix deposition which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels. 2011;7(2):492–504. doi: 10.1016/j.actbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmetz NJ, Aisenbrey EA, Westbrook KK, Qi HJ, Bryant SJ. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater. Acta Materialia Inc. 2015;21:142–53. doi: 10.1016/j.actbio.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101(1):135–46. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Brouillette MJ, Seol D, Zheng H, Buckwalter JAMJ. Use of recombinant human stromal cell-derived factor 1alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol (Hoboken, NJ) 2015;67(5):1274–85. doi: 10.1002/art.39049. [DOI] [PubMed] [Google Scholar]
- 14.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33(2):167–79. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Lian Q, Li D, Wang K, Hao D, Bian W, et al. Cartilage repair and subchondral bone migration using 3d printing osteochondral composites: A one-year-period study in rabbit trochlea. Biomed Res Int. 2014;2014 doi: 10.1155/2014/746138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aisenbrey EA, Bryant SJ. Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel. J Mater Chem B Mater Biol Med. 2016;4(20):3562–74. doi: 10.1039/c6tb00006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilic J, Izpisua Belmonte JC. Concise review: Induced pluripotent stem cells versus embryonic stem cells: Close enough or yet too far apart? Stem Cells. 2012;30(1):33–41. doi: 10.1002/stem.700. [DOI] [PubMed] [Google Scholar]
- 18.Liu SQ, Tian Q, Wang L, Hedrick JL, Hui JH, Yang YY, et al. Injectable Biodegradable Poly(ethylene glycol)/RGD Peptide Hybrid Hydrogels for in vitro Chondrogenesis of Human Mesenchymal Stem Cells. Macromol Rapid Commun. 2010;31(13):1148–54. doi: 10.1002/marc.200900818. [DOI] [PubMed] [Google Scholar]
- 19.Hoyle CEBC. Thiol-ene click chemistry. Angew Chemie (International ed English) 2010;49(9):1540–73. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 20.Chirba MA, Sweetapple B, Hannon CP, Anderson JA. FDA regulation of adult stem cell therapies as used in sports medicine. J Knee Surg. 2015;28(1):55–62. doi: 10.1055/s-0034-1398470. [DOI] [PubMed] [Google Scholar]
- 21.Duan L, Ma B, Liang Y, Chen J, Zhu W, Li M, et al. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am J Transl Res. 2015;7(2):194–208. [PMC free article] [PubMed] [Google Scholar]
- 22.Hoemann C, Sun J, Légaré A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr Cartil. 2005;13(4):318–29. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Schneider MC, Barnes CA, Bryant SJ. Characterization of the chondrocyte secretome in photoclickable poly(ethylene glycol) hydrogels. Biotechnol Bioeng. 2017;114(9):9–10. doi: 10.1002/bit.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villanueva I, Gladem SK, Kessler J, Bryant SJ. Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethylene glycol) and chondroitin sulfate. Matrix Biol. 2011;29(1):51–62. doi: 10.1016/j.matbio.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang NS, Varghese S, Zhang ZEJ. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12(9):2695–706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 26.Pilichi S, Rocca S, Pool RR, Dattena M, Masala G, Mara L, et al. Treatment with embryonic stem-like cells into osteochondral defects in sheep femoral condyles. 2014:1–14. doi: 10.1186/s12917-014-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson JA, Itskovitz-eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science (80-) 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Payne KA, Didiano DMCC. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18(5):705–13. doi: 10.1016/j.joca.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lietman SA. Induced pluripotent stem cells in cartilage repair. World J Orthop. 2016;7(3):149–55. doi: 10.5312/wjo.v7.i3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Shi D, Liu Y, Yao Y, Dai J, Xu Z, et al. In vivo repair of full-thickness cartilage defect with human iPSC-derived mesenchymal progenitor cells in a rabbit model. Exp Ther Med. 2017:239–45. doi: 10.3892/etm.2017.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HY, Lee TJ, Yang GM, Oh J, Won J, Han J, et al. Efficient mRNA delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. J Control Release. 2016;235:222–35. doi: 10.1016/j.jconrel.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Kon E, Filardo G, Shani J, Altschuler N, Levy A, Zaslav K, et al. Osteochondral regeneration with a novel aragonite-hyaluronate biphasic scaffold : up to 12-month follow-up study in a goat model. J Orthop Surg Res. Journal of Orthopaedic Surgery and Research. 2015;10(1):81. doi: 10.1186/s13018-015-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Chen Z, Liu K, Wan Y, Li X, Luo X, et al. Repair of articular cartilage defects in rabbits through tissue-engineered cartilage constructed with chitosan hydrogel and chondrocytes. J Zhejiang Univ Sci B. 2015;16(11):914–23. doi: 10.1631/jzus.B1500036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha C-W, Park Y-B, Chung J-Y, Park Y-G. Cartilage Repair Using Composites of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogel in a Minipig Model. Stem Cells Transl Med. 2015;4(9):1044–51. doi: 10.5966/sctm.2014-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodrich LR, Chen AC, Werpy NM, Williams AA, Kisiday JD, Su AW, et al. Addition of Mesenchymal Stem Cells to Autologous Platelet-Enhanced Fibrin Scaffolds in Chondral Defects: Does It Enhance Repair? J Bone Joint Surg Am. 2016;98(1):23–34. doi: 10.2106/JBJS.O.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Jia Y, Yuan M, Guo W, Huang J, Zhao B, et al. Repair of Osteochondral Defects Using Human Umbilical Cord Wharton ’ s Jelly-Derived Mesenchymal Stem Cells in a Rabbit Model. 2017;2017 doi: 10.1155/2017/8760383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shive MS, Stanish WD, Mccormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel ® Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. 2015 doi: 10.1177/1947603514562064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong Y, Song HQ, Gong YH, Mao ZW, Gao CYSJ. Covalently crosslinked chitosan hydrogel: properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007;3(23) doi: 10.1016/j.actbio.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Hoshikawa A, Nakayama Y, Matsuda T, Oda H, Na- kamura KMK. Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Eng. 2006;12(2333) doi: 10.1089/ten.2006.12.2333. [DOI] [PubMed] [Google Scholar]
- 41.Chung C, Mesa J, Randolph MA, Yaremchuk MBJ. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater. 2006;Res A(77A):518. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 43.Flynn L, Prestwich GD, Semple JLWK. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Liu YC, Shu XZPG. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 45.Almany LSD. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 46.McHale MK, Setton LACA. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 47.Rice MAAK. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Eng. 2007;13:683. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- 48.Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J Sport Med. 2016;4(1):2325967115625481. doi: 10.1177/2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dadsetan M, Szatkowski JP, Yaszemski MJLL. Characterization of photo-cross-linked oligo[poly (ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules. 2007;8:1702. doi: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]
- 50.He XZJE. Material properties and cyto-compatibility of injectable MMP degradable poly(lactide ethylene oxide fumarate) hydrogel as a carrier for marrow stromal cells. Biomacromolecules. 2007;8:780. doi: 10.1021/bm060671a. [DOI] [PubMed] [Google Scholar]