Abstract
Background
Children, adolescents and young adults with very high-risk (VHR) B acute lymphoblastic leukemia (B-ALL) have a poor outcome and novel therapies are needed for this subgroup. AALL1131 evaluated post-Induction therapy for patients with VHR B-ALL using cyclophosphamide (CPM), etoposide (ETOP) and clofarabine (CLOF).
Methods
Patients 1–30 years of age with VHR B-ALL received modified Berlin-Frankfurt-Münster therapy post-Induction and were randomized to: (1) CPM/cytarabine/mercaptopurine/vincristine (VCR)/pegaspargase (Control Arm); (2) CPM/ETOP/VCR/pegasparagse (Experimental Arm 1); or (3) CPM/ETOP/CLOF (30mg/m2 × 5 days)/VCR/pegasparage (Experimental Arm 2) during the second half of Consolidation and Delayed Intensification.
Results
The rates of Grade 4/5 infection and Grade 3/4 pancreatitis were significantly increased in Experimental Arm 2. The dose of CLOF was therefore reduced to 20 mg/m2 × 5 days and myeloid growth factor was required following CLOF administration. Despite these changes, 4/39 (10.3%) patients developed Grade 4 infections with one of these patients developing Grade 5 acute kidney injury (AKI) attributed to CLOF versus 1/46 (2.2%) Grade 4 infections on Experimental Arm 1 and no Grade 4/5 infections on Control Arm (n=20). Four patients on Experimental Arm 2 had prolonged cytopenias >60 days versus none on the Control Arm or Experimental Arm 1. Two of these patients failed to recover counts, one with Grade 5 AKI and one removed from protocol therapy, both 92 days following the start of Consolidation Part 2.
Conclusions
CLOF as administered with CPM/ETOP on AALL1131 was associated with unacceptable toxicity.
Keywords: Toxicity, Lymphoblastic Leukemia, Acute, Childhood, Clofarabine
INTRODUCTION
The 5-year overall survival of children with newly diagnosed acute lymphoblastic leukemia (ALL) exceeds 90% with multi-agent chemotherapy1–6. However, a subset of patients remains at very high risk (VHR) of relapse and includes patients with older age, unfavorable cytogenetics, poor initial response to therapy, and/or central nervous system (CNS) leukemia. Although targeted therapy has proven effective for patients with Philadelphia chromosome positive ALL7, 8, many patients lack an actionable target9, 10. Intensification of conventional chemotherapy has remained the main therapeutic option and has proven effective but the outcome for VHR patients is still inferior11–13. Thus, the integration of new agents is an attractive option.
Clofarabine is a second generation purine nucleoside analogue designed to improve anti-cancer activity and decrease the toxicity associated with the earlier purine nucleoside analogues cladribine and fludarabine14. Clofarabine has three main mechanisms of action: (1) incorporation into DNA, (2) inhibition of ribonucleotide reductase, and (3) induction of apoptosis. In 2004, the United States Food and Drug Administration (FDA) approved the use of clofarabine for use in pediatric patients 1–21 years of age with relapsed or refractory ALL after at least two prior regimens based on a 30% response rate seen with single agent therapy at 52 mg/m2/day × 5 days15.
Two subsequent studies evaluating the combination of clofarabine, cyclophosphamide, and etoposide in relapsed or refractory ALL demonstrated response rates of 44%16 and 56%17. Given the encouraging outcome of children with relapsed or refractory B-ALL following administration of clofarabine when used in combination with cyclophosphamide and etoposide, the Children’s Oncology Group (COG) AALL1131 study randomized patients with newly diagnosed VHR B-ALL into three treatment groups: Control Arm, Experimental Arm 1 (including the combination of cyclophosphamide and etoposide), and Experimental Arm 2 (including the combination of cyclophosphamide, etoposide, and clofarabine). The safety and efficacy of the two Experimental Arms were evaluated against the Control Arm. This report summarizes the toxicities that led to the closure of Experimental Arm 2.
METHODS
Patients
AALL1131 is a Phase 3 study for children and young adults >1 to <31 years of age with newly diagnosed B-ALL. Patients eligible at diagnosis were (1) 1–9 years of age with a white blood cell count (WBC) ≥50,000/μL, (2) ≥10 to <31 years of age with any WBC, or (3) >1 to <31 years of age with testicular leukemia, CNS 3 leukemia, or steroid pretreatment in patients <10 years of age where no pre-steroid WBC was obtained. CNS3 was defined as ≥5/μL WBCs and cytospin positive for blasts in the cerebral spinal fluid (CSF) and/or clinical signs of CNS leukemia.
At the end of Induction therapy patients were eligible for randomization on the VHR stratum if any of these were found: ≥13 years of age or CNS3 at initial diagnosis, day 29 bone marrow minimal residual disease (MRD) ≥0.01% % as measure by flow cytometry at one of two central reference laboratories18, Induction failure (>25% blasts in the bone marrow (M3) on day 29), intrachromosomal amplification of chromosome 21 (iAMP21), lysine methyltransferase 2A (KMT2A) [formerly mixed-lineage leukemia (MLL)] rearrangement, or severe hypodipliody (n < 44 chromosomes and/or a DNA index < 0.81). Additionally, patients with National Cancer Institute (NCI) standard risk ALL19 enrolled on AALL0932 were eligible to be randomized post-Induction on the VHR stratum of AALL1131 if they had iAMP21, KMT2A rearrangement, severe hypodiploidy, failure to achieve remission after Induction, or were without favorable cytogenetics (no ETV6-RUNX1 or double trisomies 4+10) and had a day 29 bone marrow MRD ≥ 0.01%. Patients with Down syndrome were not eligible for randomization on the VHR stratum due to lack of available data on safety in this population known to be at risk for excess toxicity from cytotoxic agents. Toxicities were graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.
The study was approved by the NCI and by Institutional Review Boards at the individual COG member institutions prior to patient enrollment. Informed consent was obtained from parents or guardians according to Department of Health and Human Services guidelines.
Treatment
Patients enrolled on AALL1131 received a standard 4 drug Induction (Table 1). Patients identified as having VHR B-ALL were randomized 1:2:2 post-Induction to one of three arms: (1) Control Arm with COG modified augmented Berlin-Frankfurt-Münster (BFM) including cyclophosphamide, cytosine arabinoside, and mercaptopurine (during Consolidation) or thioguanine (during Delayed Intensification)11, (2) Experimental Arm 1 with cyclophosphamide and etoposide during the second half of Consolidation and Delayed Intensification, or (2) Experimental Arm 2 with cyclophosphamide, etoposide, and clofarabine during the second half of Consolidation and Delayed Intensification. The remainder of therapy was identical for patients on these 3 treatment arms. Patients with testicular leukemia at diagnosis that did not resolve by the end of Induction received testicular irradiation 2400 cGy during Consolidation. Patients with CNS3 leukemia received cranial irradiation 1800 cGy during the first 4 weeks of Maintenance. Patients with hepatic dysfunction (direct bilirubin > 1.5 × upper limit of normal (ULN) or alanine aminotransferase ≥ 3 × ULN), renal dysfunction (creatinine clearance < 70 ml/min/1.72 m2), cirrhosis, hepatitis B or C, or elevated lipase (> 2 × ULN) were excluded from participation in the VHR stratum. In addition to routine reporting, specific adverse event reporting for the VHR stratum during Consolidation Part 2 and Delayed Intensification Part 2 included: (1) Grade 2 or more infections with an absolute neutrophil count (ANC) < 500/μL and Grade 3 to 5 infections regardless of ANC after Day 29 in Consolidation and Delayed Intensification; (2) Adverse events resulting in greater than a 14 day delay in starting Interim Maintenance 1 or 2; (3) Grade 3/4 ALT, AST, Grade 4 amylase and lipase or Grade 3/4 bilirubin elevations that did not return to Grade 2 or less by the time Day 43 vincristine and asparaginase were scheduled to be administered during Consolidation or Delayed Intensification; (4) Other non-hematologic Grade 3/4 toxicities that did not return to Grade 2 or less by the time Day 43 vincristine and asparaginase were scheduled to be administered during Consolidation or Delayed Intensification; (5) Grade 3/4 pancreatitis; (6) Grade 3/4 capillary leak syndrome; (7) Grade 3/4 acute kidney injury; (8) Other Grade 3 or 4 adverse events attributable to clofarabine; and (9) Sinusoidal obstruction syndrome.
Table 1.
AALL1131 Very High Risk Treatment Regimen
| Induction | ||
|---|---|---|
| IT ARAC* Day 1 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 1, 8, 15, 22 | ||
| DEX 10 mg/m2/day Days 1–14 (< 10 year old) | ||
| PDN 60 mg/m2/day Days 1–28 (≥ 10 years old) | ||
| DAUN 25 mg/m2 Days 1, 8, 15, 22 | ||
| PEG-ASP 2,500 units/m2 Day 4 | ||
| IT MTX** Days 8, 29 (CNS3 +15, 22) | ||
| Consolidation Part 1 | ||
| CPM 1000 mg/m2 Day 1 | ||
| ARAC 75 mg/m2 Days 1–4, 8–11 | ||
| MP 60 mg/m2 Days 1–14 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 15, 22 | ||
| PEG-ASP 2,500 units/m2 Day 15 | ||
| IT MTX Days 1, 8, 15, 22 | ||
|
| ||
| Consolidation Part 2 | ||
| Control Arm | Experimental Arm 1 | Experimental Arm 2 |
| CPM 1000 mg/m2 Day 29 | CPM 440 mg/m2 Days 29–33 | CPM 440 mg/m2 Days 29–33 |
| ARAC 75 mg/m2 Days 29–32, 36–39 | ETOP 100 mg/m2 Days 29–33 | ETOP 100 mg/m2 Days 29–33 |
| MP 60 mg/m2 Days 29–42 | CLOF 30 mg/m2 Days 29–33 (20 mg/m2 post amendment) | |
| VCR 1.5 mg/m2 (2 mg max) Days 43, 50 | VCR 1.5 mg/m2 (2 mg max) Days 43, 50 | VCR 1.5 mg/m2 (2 mg max) Days 43, 50 |
| PEG-ASP 2,500 units/m2 Day 43 | PEG-ASP 2,500 units/m2 Day 43 | PEG-ASP 2,500 units/m2 Day 43 |
|
| ||
| Interim Maintenance 1 | ||
| MTX 5000 mg/m2 IV followed by leucovorin rescue Days 1, 15, 29, 43 | ||
| MP 25 mg/m2 Days 1–56 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 1, 15, 29, 43 | ||
| IT MTX Days 1, 29 | ||
| Delayed Intensification Part 1 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 1, 8, 15 | ||
| DEX 10 mg/m2/day Days 1–7, 15–21 | ||
| DOX 25 mg/m2 Days 1, 8, 15 | ||
| PEG-ASP 2,500 units/m2 Day 4 | ||
| IT MTX Day 1 | ||
|
| ||
| Delayed Intensification Part 2 | ||
| Control Arm | Experimental Arm 1 | Experimental Arm 2 |
| CPM 1000 mg/m2 Day 29 | CPM 440 mg/m2 Days 29–33 | CPM 440 mg/m2 Days 29–33 |
| ARAC 75 mg/m2/day Days 29–32, 36–39 | ETOP 100 mg/m2 Days 29–33 | ETOP 100 mg/m2 Days 29–33 |
| TG 60 mg/m2/day Days 29–42 | CLOF 30 mg/m2 Days 29–33 (20 mg/m2 post amendment) | |
| VCR 1.5 mg/m2 (2 mg max) Days 43, 50 | VCR 1.5 mg/m2 (2 mg max) Days 43, 50 | VCR 1.5 mg/m2 (2 mg max) Days 43, 50 |
| PEG-ASP 2,500 units/m2 Day 43 | PEG-ASP 2,500 units/m2 Day 43 | PEG-ASP 2,500 units/m2 Day 43 |
| IT MTX Days 29, 36 | IT MTX Days 29, 36 | IT MTX Days 29, 36 |
|
| ||
| Interim Maintenance 2 | ||
| MTX 100 mg/m2 IV escalating*** Days 1, 11, 21, 31, 41 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 1, 11, 21, 31, 41 | ||
| PEG-ASP 2,500 units/m2 Days 2, 22 | ||
| IT MTX Days 1, 31 | ||
| Maintenance**** (12 week cycles) | ||
| MTX 20 mg/m2 Days 8, 15, 22, 29, 36, 43, 50, 57, 64, 71, 78 | ||
| MP 75 mg/m2 Days 1–84 | ||
| VCR 1.5 mg/m2 (2 mg max) Days 1, 29, 57 | ||
| PDN 40 mg/m2/day Days 1–5, 29–33, 57–61 | ||
| IT MTX Days 1 (and 29 first 2 cycles for patients who did not receive CNS radiation) | ||
ARAC - cytosine arabinoside, VCR-vincristine, DEX – dexamethasone, PDN-prednisone, DAUN-daunorubicin, PEG-ASP - pegaspargase, MTX – methotrexate, CPM-cyclophosphamide, MP – mercaptopurine, ETOP – etoposide, CLOF – clofarabine, DOX – doxorubicin, TG – thioguanine; IT – intrathecal, BID - twice daily, IV – intravenous;
IT ARAC: 1–1.99 years, 30 mg; 2–2.99 years, 50 mg; ≥ 3 years, 70 mg;
IT MTX: 1–1.99 years, 8 mg; 2–2.99 years, 10 mg; 3–8.99 years,12 mg; ≥ 9 years, 15mg;
MTX dose escalated as tolerated 50 mg/m2 every 10 days;
Total duration of treatment: Females 2 years and males 3 years from start of Interim Maintenance 1
AALL1131 opened to accrual in February 2012. However the VHR arms were temporarily closed to accrual 7 months later (September 2012) due to excessive infectious toxicities observed on Experimental Arm 2. The study was amended to reduce the dose of clofarabine from 30 to 20 mg/m2/day × 5 and to provide enhanced supportive care recommendations. Myeloid growth factor (granulocyte colony stimulating factor (G-CSF) 5 mcg/kg/dose) was required beginning 24 hours following the last dose of clofarabine until neutrophil count recovery (absolute neutrophil count ≥ 750/μL on 2 consecutive days post nadir). Additionally, supportive care recommendations during Consolidation Part 2 and Delayed Intensification Part 2 for patients enrolled on Experimental Arm 2 included: (1) hospitalization until evidence of count recovery; (2) empiric coverage for both gram positive and gram negative organisms at the onset of fever, and; (3) antifungal prophylaxis and/or empiric antifungal therapy in patients with neutropenia on broad spectrum antibiotics following either 3–5 days of persistent fever or recurrence of fever. The VHR arms were reopened in December 2013 and then temporarily closed to accrual in May 2014 for a planned safety analysis. Experimental Arm 2 did not re-open and was permanently closed to accrual in September 2014 due to excessive toxicity.
Statistical Considerations
Patients classified as VHR on studies AALL0932 and AALL1131 were eligible for the VHR post-Induction randomizations on this study. A total of 135 eligible patients were randomized to the three arms from study activation to first temporary closure (2/27/2012 – 9/13/2012) (Figure 1). A total of 115 eligible patients were randomized to the three arms after accrual was restarted with the reduced dose of clofarabine (11/25/2013 – 5/5/2014) (Figure 2). Study data current as of 06/30/2015 are included in this report. Proportions of adverse events on the regimens were compared using Chi-square test or Fisher’s Exact test. All analyses were performed with SAS software (version 9.4; SAS Institute, Cary, NC).
Figure 1. Pre-Amendment Clofarabine 30 mg/m2/day × 5 Consort diagram.
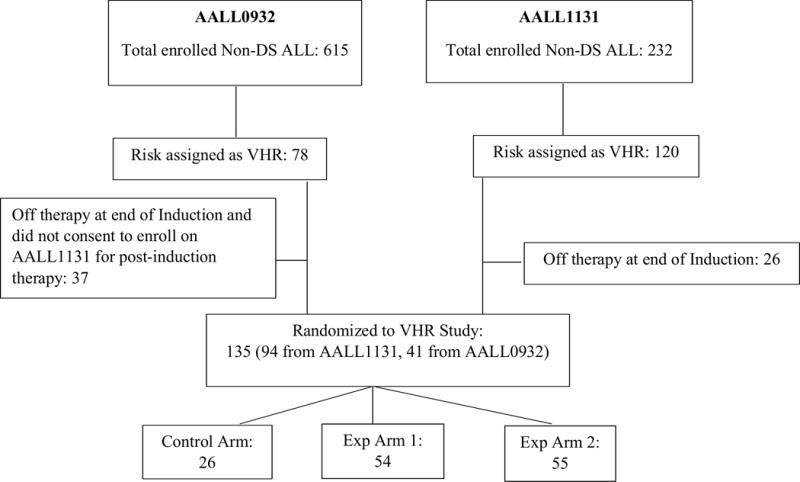
Between 2/27/2012 and 9/13/2012 (only including eligible patients)
Figure 2. Post-Amendment Clofarabine 20 mg/m2/day × 5 Consort diagram.
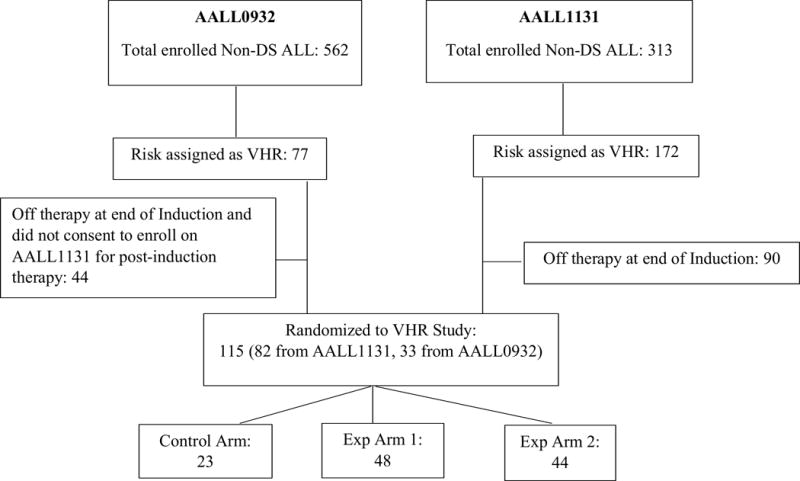
Between 11/25/2013 and 5/5/2014 (only including eligible patients)
RESULTS
VHR Strata (February 2012 – September 2012)
From February 21, 2012 until September 14, 2012, when the study was suspended due to excessive toxicities, 135 eligible patients were randomized post-Induction to the VHR arms of AALL1131. No patient had reached Delayed Intensification Part 2 at the time of study suspension. Table 2 lists protocol targeted toxicities among the 128 patients who entered Consolidation Part 2, Control Arm (n=25), Experimental Arm 1 (n=51), and Experimental Arm 2 (n=52). Significant differences among the three arms included the incidence of Grade 4/5 infections and Grade 3/4 pancreatitis. Twelve of the 52 (23.1%) patients on Experimental Arm 2 had infectious complications, including 11 Grade 4 (defined as life-threatening consequences with urgent intervention indicated) and one Grade 5 (death), compared to 1/51 (2%) and 1/25 (4%) Grade 4 infectious complications on the Experimental Arm 1 and Control Arm, respectively, p=0.0013. Infectious complications began on Experimental Arm 2 a mean of 11.1 ± 2.9 days following the start of Consolidation Part 2. Six of the 52 (11.5%) patients on Experimental Arm 2 had Grade 3/4 pancreatitis versus none of the patients on Experimental Arm 1 or Control Arm, p=0.0142.
Table 2.
Toxicities - Consolidation Part 2
| Pre-Amendment Clofarabine 30 mg/m2/day × 5 | ||||
|---|---|---|---|---|
| Targeted Toxicity | Control Arm (N=25) | Experimental Arm 1 (N=51) | Experimental Arm 2 (N=52) | p-value |
| Infection Grade (Gr) 4–5 |
1 Gr 4 |
1 Gr 4 |
12 Gr 4 (11), Gr 5 (1) |
0.0013 |
| ALT,ASTˆ,Bilirubin* Gr 3–4 |
2 | 1 | 1 | 0.3205 |
| Amylase/Lipase* Gr 4 |
0 | 1 | 4 | 0.2687 |
| Pancreatitis Gr 3–4 |
0 | 0 | 6 | 0.0142 |
| Capillary Leak Syndrome Gr 3–4 |
0 | 0 | 0 | — |
| Acute Kidney Injury Gr 3–4 |
0 | 0 | 1 | 1.0000 |
| Post-Amendment Clofarabine 20 mg/m2/day × 5 | ||||
| Targeted Toxicity | Control Arm (N=20) | Experimental Arm 1 (N=46) | Experimental Arm 2 (N=39) | p-value |
| Infection Gr 4–5 |
0 | 1 Gr 4 |
4 Gr 4 |
0.2046 |
| ALT,ASTˆ,Bilirubin* Gr 3–4 |
0 | 5 | 0 | 0.0456 |
| Amylase/Lipase* Gr 4 |
0 | 1 | 0 | 1.0000 |
| Pancreatitis Gr 3–4 |
0 | 1 | 1 | 1.0000 |
| Capillary Leak Syndrome Gr 3–4 |
0 | 0 | 1 | 0.5619 |
| Acute Kidney Injury Gr 3–5 |
0 | 0 | 1 Gr 5 |
0.5619 |
AST – aspartate aminotransferase; ALT – alanine aminotransferase
Toxicity did not return to ≤ Grade 2 by day 43 of Consolidation Part 2
Comparison between Experimental Arm 2 and the Control Arm as well as between Experimental Arm 2 and 1 were conducted for each targeted toxicity (Table 2). Grade 4 and 5 infectious complications remained increased on Experimental Arm 2 versus the Control Arm (p=0.0979) and Experimental Arm 1 (p=0.0039).
The microbiologically confirmed pathogens in each of the 3 arms of the VHR strata are shown in Table 3 and included gram positive, gram negative, and anaerobic bacterial, and fungal organisms. There were more infectious organisms identified in patients in Experimental Arm 2 (n=25), compared to the Control Arm (n=2) or Experimental Arm 1 (n=2). Notably, 10/29 (34.5%) identified organisms were fungal, with 7 of 10 identified as Aspergillus species.
Table 3.
Infectious Etiology - Consolidation Part 2
| Clofarabine 30 mg/m2/day × 5 | |||
|---|---|---|---|
| Organism Name | Control Arm (N=25) |
Experimental Arm 1 (N=51) |
Experimental Arm 2 (N=52) |
| Gram Positive | 1 | 1 | 8 |
| Streptococcus mitis | 1 | 3 | |
| Coag negative Staphylococcus | 1 | ||
| Staphylococcus aureus | 1 | ||
| Streptococcus viridans | 1 | 1 | |
| Staphylococcus epidermidis | 1 | ||
| Staphylococcus saprophyticus | 1 | ||
| Gram Negative | 0 | 1 | 7 |
| Klebsiella pneumoniae | 3 | ||
| Pseudomonas aeruginosa | 2 | ||
| Escherichia coli | 1 | 2 | |
| Anaerobic | 0 | 0 | 1 |
| Clostridium difficile | 1 | ||
| Fungus | 1 | 0 | 9 |
| Candida tropicalis | 1 | ||
| Blastoschzomyces capitatus | 1 | ||
| Aspergillus | |||
| Aspergillus nos | 1 | 3 | |
| Aspergillus terreus | 1 | ||
| Aspergillus versicolor | 1 | ||
| Aspergillus fumigatus | 1 | ||
| Geotrichum (yeast) | 1 | ||
| Total | 2 | 2 | 25 |
The mean number of days (± standard deviation) to complete the scheduled 28-day cycle of Consolidation Part 2 was 40.1 ± 8.4, 37.5 ± 6.9, and 51.3 ± 22.8 for the Control Arm, Experimental Arm 1, and Experimental Arm 2, respectively. This resulted in an increased number of days to complete Consolidation Part 2 between the Experimental Arm 2 and Control Arm (p=0.057) as well as between Experimental Arms 2 and 1 (p=0.0013).
Based on the infectious toxicities observed and treatment related delays, the study was suspended to further enrollment in September 2012 and investigators were instructed not to start any 5-day clofarabine cycle in either Consolidation Part 2 or Delayed Intensification Part 2, as well as to omit any additional clofarabine for patients that had already started a 5-day cycle. The VHR Arms were reopened to accrual in December 2013 following an amendment to reduce the dose of clofarabine and provide additional supportive care guidelines.
VHR Strata (December 2013 – May 2014)
From December 25, 2013 until May 5, 2014 when the study was temporarily closed for a planned safety analysis, an additional 115 eligible patients were randomized 1:2:2 post-Induction to the VHR arms of AALL1131. Protocol targeted toxicities among 105 patients who entered Consolidation Part 2, Control Arm (n=20), Experimental Arm 1 (n=46), and Experimental Arm 2 (n=39), are listed in Table 2. Grade 3/4 elevations of aspartate aminotransferase, alanine aminotransferase, and/or bilirubin that did not return to ≤ Grade 2 by day 43 of Consolidation Part 2 were seen in 5/46 (10.9%) patients on Experimental Arm 1 versus none of the patients on the Experimental Arm 2 or Control Arm, p=0.0456. There were no significant differences among the three arms for the remaining targeted toxicities.
During Consolidation Part 2, despite the clofarabine dose reduction and increased supportive care measures recommended for patients on Experimental Arm 2, 4/39 (10.3%) patients on Experimental Arm 2 developed Grade 4 infectious complications with one of these patients developing a Grade 5 toxicity (acute kidney injury) attributed to clofarabine. Infectious complications began on Experimental Arm 2 a mean of 9.0 ± 1.4 days following the start of Consolidation Part 2. The infections were attributed Viridans group streptococcus, Enterobacter cloacae, and Pseudomonas aeruginosa. This was in contrast to the Control Arm where no ≥ Grade 4 infections were reported and 1/46 (2.2%) patients on Experimental Arm 1. Additionally, four patients on Experimental Arm 2 developed prolonged cytopenias (>60 days), including the one patient with a Grade 5 acute kidney injury who died 92 days following the start of Consolidation Part 2 and one patient requiring removal from protocol therapy, due to pancytopenia, also 92 days following the start of Consolidation Part 2. Neither of these two patients recovered blood counts prior to day 92. No patient on either the Control Arm or Experimental Arm 1 experienced prolonged cytopenias >60 days.
The mean number of days (± standard deviation) to complete the scheduled 28-day cycle of Consolidation Part 2 was 41.3 ± 3.8, 37.5 ± 8.3, and 42.3 ± 14.4 for the Control Arm, Experimental Arm 1, and Experimental Arm 2, respectively. There was no significant difference in the number of days to complete Consolidation Part 2 between the Experimental Arm 2 and Control Arm (p=0.3515) as well as between Experimental Arms 2 and 1 (p=0.3252).
Thirteen patients reached Delayed Intensification Part 2 and received clofarabine prior to closure of Experimental Arm 2. Three of the 13 (23.1%) patients on Experimental Arm 2 developed a Grade 4 infection with Viridans group streptococcus, Streptococcus mitis, and Pseudomonas aeruginosa. None of the patients who had entered Delayed Intensification Part 2 on the Control Arm (n=8) or Experimental Arm 1 (n=25) developed a Grade 4 or 5 infection.
Due to the excessive toxicities observed and prolonged cytopenias, despite the reduction in clofarabine dose, the addition of G-CSF and other supportive care measures Experimental Arm 2 was permanently closed on September 12, 2014.
DISCUSSION
Experimental Arm 2 of AALL1131 was designed to evaluate the safety and efficacy of clofarabine, cyclophosphamide, and etoposide as part of multi-agent chemotherapy for the treatment of newly diagnosed children and young adults with VHR B-ALL. This combination and dosing scheduled had been previously tolerated with an acceptable toxicity profile in similar patients17. The starting dose of clofarabine was 30 mg/m2/day × 5, 60% of the FDA approved dose (52 mg/m2/day × 5) when given as a single agent15 and 75% of the Phase 2 dose (40 mg/m2/day × 5) given in combination with cyclophosphamide and etoposide16. Infectious toxicities associated with this combination necessitated temporary closure of the VHR strata and an amendment to reduce the dose of clofarabine to 20 mg/m2/day × 5. Despite this dose reduction, infectious toxicities continued post-amendment in addition to prolonged cytopenias and ultimately resulted in closure of Experimental Arm 2.
Clofarabine was initially studied in a Phase 1 study in 25 pediatric patients (1–19 years of age, median 12) with relapsed or refractory leukemia (ALL, n=17; AML, n=8). Most patients were heavily pretreated (1–6 prior regimens) and 9 (36%) had previously received a bone marrow transplant. The maximum tolerated dose as a single agent was 52 mg/m2/day × 5, with dose limiting toxicities of hepatotoxicity (elevated bilirubin and transaminase levels) that resolved by day 14 and skin rash; no Grade 4 or 5 infectious toxicities were reported. Of the 17 patients with ALL treated on this study, there were 4 complete responses and 1 partial response, for an overall response rate of 29%20.
Subsequently, a Phase 2 study of single agent clofarabine (52 mg/m2/day ×5) was conducted in 61 pediatric patients (1–20 years of age, median 12) with relapsed or refractory ALL. Patients again were heavily pretreated (2–6 prior regimens, median 3) with 18 (30%) having previously received a bone marrow transplant. Grade 3/4 bilirubin and alanine aminotransferase levels that resolved by day 16, occurred in 16% and 43% of patients, respectively. Infectious complications (≥ Grade 3) occurred in 69% of patients, with 15% of patients developing fungal infections. The overall response rate was 30% (18/61), with 7 complete responses, 5 complete responses without platelet recovery, and 6 partial responses15. While infection rates were high, the overall toxicities were deemed tolerable, in a heavily pre-treated population, and the overall response rate in this patient population was encouraging.
Based on the known synergy between clofarabine and DNA damaging agents as identified in pre-clinical studies21, 22, as well as clinical experience using the combination of cyclophosphamide and etoposide in the treatment of ALL23–26, a Phase 1 study was designed to combine all three agents27. The Phase 1 study of clofarabine, cyclophosphamide, and etoposide was conducted in 25 patients (2–21 years of age, median 9) with relapsed or refractory leukemia (ALL, n=20; AML, n=5). Patients received a median of 2 prior regimens and 4 (16%) had previously received a bone marrow transplant. No maximum tolerated dose was identified with the target doses reached for clofarabine (40 mg/m2/day × 5), cyclophosphamide (440 mg/m2/day × 5), and etoposide (100 mg/m2/day × 5). Infectious complications (≥ Grade 3) occurred in 72% of patients [16 Grade 3 (64%) and two Grade 4 (8%)], and hepatotoxicity (≥ Grade 3) with elevated alanine aminotransferase occurred in 38% of patients. At the final dose level one patient of six had prolonged bone marrow aplasia > 42 days. Of the 20 patients with ALL, there were 9 complete responses and 2 complete responses without platelet recovery, for response rate of 55%28. Although AALL1131 used a lower dose of clofarabine than this Phase 1 study, bone marrow aplasia was again seen in 2 of 39 patients on the AALL1131.
Based on the results of the above Phase I study, a Phase 2 study of clofarabine (40 mg/m2/day × 5), cyclophosphamide (440 mg/m2/day × 5), and etoposide (100 mg/m2/day × 5) was subsequently conducted in 25 pediatric patients (1–21 years of age, median 14) with relapsed or refractory ALL. Patients received 1–3 prior regimens (median 2), and 4 had previously received a bone marrow transplant. Of the first 8 patients enrolled, 4 developed severe hepatotoxicity suggestive of sinusoidal obstructive syndrome (SOS), with 3 resulting in multi-organ system failure and death. Of the 4 patients with SOS, 3 had concurrent infection and 3 had previously received a bone marrow transplant. At this point the study was amended to exclude patients with a prior bone marrow transplant, cirrhosis, viral hepatitis, or elevated conjugated bilirubin. Grade 3/4 bilirubin and alanine aminotransferase levels occurred in 28% and 36% of patients, respectively. Infectious complications (≥ Grade 3) occurred in 76% of patients, and acute renal failure occurred in 16% of patients. The response rate was 44% (11/25), with 7 complete responses and 4 complete responses without platelet recovery16. These results formed the basis for introducing this regimen into the AALL1131 VHR stratum. However, given the concerns of toxicities with this regimen the starting dose for clofarabine in the AALL1131 was reduced from 40mg/m2/day to 30 mg/m2/day × 5.
Despite starting at a lower dose of clofarabine than was used in the Phase 2 study (30 mg/m2/day compared to 40 mg/m2/day) and further reducing the dose to 20 mg/m2/day × 5 days, infectious complications persisted. At the dose of 30 mg/m2/day, ≥ Grade 4 infections occurred in 23.1% (12/52) of patients in Experimental Arm 2 compared to 2% (1/51) in Experimental Arm 1 and 4% (1/25) in the Control Arm. Despite lowering the dose of clofarabine further to 20 mg/m2/day, adding G-CSF and other supportive care measures, Grade 4 infections continued on Experimental Arm 2 with 10.3% (4/39) patients reported compared to none in either Experimental Arm 1 or the Control Arm. More importantly, 2 of 39 patients receiving clofarabine failed to recover counts 92 days following the start of Consolidation Part 2. One of these patients had a Grade 5 acute kidney injury and one was removed from protocol therapy. No association with age was seen among the patients with ≥ Grade 4 infectious toxicities on Experimental Arm 2, with 43.8% (7/16) occurring in patients <10 years of age (range 1–27 years of age). The addition of pegaspargase and vincristine to this course of therapy may have contributed to the increased toxicities seen on this study. Prior studies have demonstrated that pegaspargase when combined with antimetabolite therapy is associated with increased myelosuppression and infectious complications29, 30. Given the profound toxicities associated with Experimental Arm 2, it was concluded that the combination of clofarabine, cyclophosphamide, and etoposide as given on the VHR stratum of AALL1131 for children, adolescents and young adults with B-ALL was associated with unacceptable toxicity.
These results highlight the challenge of integrating new chemotherapeutic drugs and novel combinations of cytotoxic agents into the frontline setting especially in sequence with a standard but already augmented backbone. Even when doses are reduced and supportive care is enhanced, unexpected toxicities may emerge. Alternative new immunotherapeutic approaches including monoclonal antibodies and antibody conjugates, bi-specific T cell engagers and chimeric antigen receptor T cells offer great promise as adjuncts to conventional chemotherapeutic regimens in the frontline setting. However, their integration will also require carefully designed clinical trials to evaluate toxicity and efficacy.
Acknowledgments
Funding Sources:
Supported by grants U10 CA180886 and U10 CA180899 from the National Institutes of Health and by St. Baldrick’s Foundation. LG is the Ergen Chair in Pediatric Oncology. MLL is the UCSF Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia.
The opinions and assertions contained herein are the private views of the author(s) and are not to be construed as the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the US Food and Drug Administration.
Footnotes
Author Contributions:
Conceptualization: WSalzer, MBurke, MDevidas, LGore, ELarsen, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Methodology: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Validation: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Formal analysis: MDevidas, SChen
Investigation: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Resources: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Data curation: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Writing – original draft: WSalzer, MBurke, MDevidas, SChen, MLoh, ERaetz, SHunger
Writing – review and editing: WSalzer, MBurke, MDevidas, SChen, LGore, ELarsen, MBorowitz, BWood, NHeerema, ACarroll, JHilden, MLoh, ERaetz, NWinick, WCarroll, SHunger
Visualization: WSalzer, MBurke, MDevidas, SChen
Supervision: MLoh, ERaetz, NWinick, WCarroll, SHunger
Project administration: WSalzer, MBurke, MDevidas, LGore, MLoh, ERaetz, NWinick, WCarroll, SHunger
Funding acquisition: LGore, MLoh, SHunger
There are no conflicts of interest.
References
- 1.Pui CH, Yang JJ, Hunger SP, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui CH, Pei D, Campana D, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014;28:2336–2343. doi: 10.1038/leu.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 7.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slayton W, Kairalla J, Schultz K, et al. Outcomes of dasatinib plus intensive chemotherapy or stem cell transplant (SCT) for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) on Children’s Oncology Group AALL0622. J Clin Oncol. 2015;33 doi: 10.1200/JCO.2017.76.7228. abstract 10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raetz EA, Cairo MS, Borowitz MJ, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children’s Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer. 2015;62:1171–1175. doi: 10.1002/pbc.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown P, Kairalla JA, Wang C, et al. Addition of FLT3 inhibitor lestaurtinib to post-induction chemotherapy does not improve outcomes in MLL-rearranged infant acute lymphoblastic leukemia: AALL0631, a Children’s Oncology Group study. International Society of Paediatric Oncology (SIOP) Annual Meeting Abstracts. 2016:O-001. [Google Scholar]
- 11.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 14.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 15.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 16.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118:6043–6049. doi: 10.1182/blood-2011-08-374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locatelli F, Testi AM, Bernardo ME, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009;147:371–378. doi: 10.1111/j.1365-2141.2009.07882.x. [DOI] [PubMed] [Google Scholar]
- 18.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126:964–971. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Nowak BJ, Keating MJ, Plunkett W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;7:3580–3589. [PubMed] [Google Scholar]
- 22.Karp JE, Ricklis RM, Balakrishnan K, et al. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood. 2007;110:1762–1769. doi: 10.1182/blood-2007-03-081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children’s Oncology Group Study[corrected] J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biagi E, Rovelli A, Balduzzi A, De Lorenzo P, Tagliabue A, Uderzo C. TBI, etoposide and cyclophosphamide as a promising conditioning regimen for BMT in childhood ALL in second remission. Bone Marrow Transplant. 2000;26:1260–1262. doi: 10.1038/sj.bmt.1702714. [DOI] [PubMed] [Google Scholar]
- 25.Shigematsu A, Kondo T, Yamamoto S, et al. Excellent outcome of allogeneic hematopoietic stem cell transplantation using a conditioning regimen with medium-dose VP-16, cyclophosphamide and total-body irradiation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2008;14:568–575. doi: 10.1016/j.bbmt.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Johny A, Song KW, Nantel SH, et al. Early stem cell transplantation for refractory acute leukemia after salvage therapy with high-dose etoposide and cyclophosphamide. Biol Blood Marrow Transplant. 2006;12:480–489. doi: 10.1016/j.bbmt.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Hijiya N, Barry E, Arceci RJ. Clofarabine in pediatric acute leukemia: current findings and issues. Pediatr Blood Cancer. 2012;59:417–422. doi: 10.1002/pbc.24112. [DOI] [PubMed] [Google Scholar]
- 28.Hijiya N, Gaynon P, Barry E, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23:2259–2264. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- 29.Salzer WL, Devidas M, Shuster JJ, et al. Intensified PEG-L-asparaginase and antimetabolite-based therapy for treatment of higher risk precursor-B acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2007;29:369–375. doi: 10.1097/MPH.0b013e3180640d54. [DOI] [PubMed] [Google Scholar]
- 30.Harris MB, Shuster JJ, Pullen J, et al. Treatment of children with early pre-B and pre-B acute lymphocytic leukemia with antimetabolite-based intensification regimens: a Pediatric Oncology Group Study. Leukemia. 2000;14:1570–1576. doi: 10.1038/sj.leu.2401886. [DOI] [PubMed] [Google Scholar]


