Abstract
Obesity is associated with an elevated risk of osteoarthritis (OA). We examined here whether high fat diet administered in young mice, compromised the attainment of articular cartilage thickness. Further, we sought to determine if low intensity vibration (LIV) could protect the retention of articular cartilage in a mouse model of diet induced obesity. Five-week-old, male, C57BL/6 mice were separated into 3 groups (n=10): Regular diet (RD), High fat diet (HF), and HF+LIV (HFv; 90Hz, 0.2g, 30 min/d, 5 d/w) administered for 6 weeks. Additionally, an extended HF diet study was run for 6 months (LIV at 15m/d). Articular cartilage and subchondral bone morphology, and sulfated GAG content were quantified using contrast agent enhanced μCT and histology. Gene expression within femoral condyles was quantified using real-time polymerase chain reaction. Contrary to our hypothesis, HF cartilage thickness was not statistically different from RD. However, LIV increased cartilage thickness compared to HF, and the elevated thickness was maintained when diet and LIV were extended into adulthood. RT-PCR analysis showed a reduction of aggrecan expression with high fat diet, while application of LIV reduced the expression of degradative MMP-13. Further, long term HF diet resulted in subchondral bone thickening, compared to RD, providing early evidence of OA pathology—LIV suppressed the thickening, such that levels were not significantly different from RD. These data suggest that dynamic loading, via LIV, protected the retention of cartilage thickness, potentially resulting in joint surfaces better suited to endure the risks of elevated loading that parallel obesity.
Keywords: osteoarthritis, chondrocytes, exercise, biomechanics, adaptation, stem cell
Introduction
Obesity is a recognized risk factor for osteoarthritis 1, a disease characterized by a thinning of articular cartilage and subchondral bone sclerosis 2. Osteoarthritis is often paralleled by debilitating joint pain, decreased mobility, and ultimately, increased likelihood of total joint replacement 3. To a large extent, obesity related cartilage ablation is presumed to result from weight-related abuse of the articular surfaces 1. While at first glance this overuse theory is plausible, it is also counterintuitive, as the obese are typically less active 4–6, and instead may challenge the cartilage surface with lower peak loads and fewer loading cycles than a healthy cohort with lower BMI 6.
In adult animal models, diet induced obesity has been shown to promote many deleterious changes consistent with osteoarthritis, such as lower proteoglycan content within articular cartilage and subchondral bone sclerosis 7–9. Fewer studies, however have investigated the consequences of an increased adipose burden on the development of articular cartilage in young, growing animals—a model of interest considering the growing incidence of obesity in the adolescent population 10. One study demonstrated that a high fat diet employed early on can induce the formation of cartilaginous osteophytes, and joint inflammation 11.
Aside from local biomechanical factors, obesity has been associated with an increased secretion of adipokines and hormones—the establishment of a chronic state of low-grade inflammation in the obese is suggested to contribute to osteoarthritis risk12. Increased expression of proinflammatory cytokines (i.e. TNF-α, IL-1, IL-6) have been reported in the synovial fluid of OA patients 13; 14, and in vitro studies have shown that these agents can induce catabolic processes in chondrocytes, specifically the activity of matrix metalloproteinases (MMPs) that are involved with the degradation of the cartilage ECM 15; 16. Further, proinflammatory cytokine levels have been associated with increased osteoarthritis severity in vivo; Griffin et al., for example, showed a significant association between serum IL-1α, leptin, and adiponectin and osteoarthritis severity 9, and in a separate study, showed that KC, leptin and IL-1Ra were positively correlated with worse knee OA scores 7. Osteoarthritis risk in obese individuals, therefore, likely includes contribution from both metabolic and biomechanical factors.
Mimicking some of the dietary consequences on cartilage morphology, extreme loading demands on the joint are known to have detrimental consequences on cartilage health. Strenuous treadmill running in mature animals has been shown to induce osteoarthritic changes in the joint, including reduced chondroitin sulfate and hyaluronic acid content 17. Defined high peak loads applied cyclically in both young and older mice resulted in decreased articular cartilage thickness, with incidences of osteophyte formation 18. In humans, extreme demands on joints, as might result from long distance running 19, weight lifting 20, and physically demanding occupational tasks 21 has been associated with a higher incidence and earlier onset of osteoarthritis.
Similar to the deterioration associated with excessive loading, the absence of loading to the joint can also be detrimental to cartilage tissue. Joint immobilization in canines, for example, resulted in altered articular cartilage mechanical properties and reduced proteoglycan content, consistent with mild cartilage degeneration22. In humans, extended bedrest has led to decreased cartilage thickness within the knee joint, an early harbinger of osteoarthritis 23.
In contrast, moderate exercise, in the form of cycling, rope skipping, and light jogging, has been shown to improve GAG content in knee cartilage of patients at high risk of developing knee OA 24, providing evidence that some mechanical signals are better than none. Daily running in high fat diet fed mice mitigated the cartilage GAG loss and subchondral bone thickening 7, achieved without any changes in body weight compared to the sedentary group, suggesting that dynamic mechanical signals may promote cartilage health while the absence of them are permissive to OA progression. The critical nature of dynamic mechanical signals is evident even at the cellular level, with time-varying compression increasing total synthesis of GAGs in chondrocytes in vitro, as compared to those cultured under static compression 25. While these data suggest that, excessive or insufficient mechanical stimulation may be deleterious to cartilage, there appears to be a ‘window’ where some degree of loading is beneficial to the tissue. The challenge when translated to the human, however, is that compliance with a daily exercise regimen is typically very poor, particularly in the obese 6.
As a surrogate for exercise, low intensity vibration (LIV) delivers a relatively high frequency (10–100Hz), low magnitude (<1.0g, where 1g is Earth’s gravitational field), mechanical signal, resulting in a challenge to the musculoskeletal system that is several orders of magnitude below that which arises during strenuous activity 26, but has been shown to be anabolic to bone 27 while simultaneously suppressing adiposity 28. Using rodent models of diet-induced obesity, recent evidence has shown that LIV biases mesenchymal stem cell (MSC) lineage selection towards the formation of higher order connective tissues such as bone, and away from adipogenesis 29. In the study reported here, we hypothesized that high fat feeding would lead to thinner articular cartilage, and that LIV would protect the retention of articular cartilage thickness otherwise challenged by the obese phenotype.
Materials and Methods
Animals and Experimental Design
The short and long-term studies were reviewed and approved by the Institutional Animal Care and Use Committee of Stony Brook University. To investigate the short-term impact of high fat diet and low intensity vibration on knee joint changes in growing mice, 30 five-week old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were weight-matched, and assigned to 3 groups (n=10/group): regular diet (RD), high fat diet (HF), and high fat diet +LIV (HFv), and single housed at the Stony Brook University Division of Laboratory Animal Resources facility. At baseline, mice were fed either a regular or high fat diet (45% kcal from fat; van Heek Series 58V8; TestDiet, Richmond, IN, USA) ad libitum. Food consumption was monitored and animals were weighed once a week. At the 2w time-point, the HFv group was subjected to LIV (0.2g, 90 Hz sine wave, 30 min/d, 5 d/w; where g is Earth’s gravitational field, or 9.8m/s2). Each day, all mice in RD and HF groups were placed on an inactive LIV platform for the same duration as HFv. Each group continued their respective diets for the following 6 weeks. At 8w, the end of the short-term protocol, all animals were euthanized with isoflurane and cervical dislocation, and thus data for the short term protocol represent mice at 13w of age.
To investigate the long-term effects of diet and low intensity vibration on cartilage in the knee joint, seven-week old male C57BL/6J mice were separated into RD (n=7), HF (n=7), and HFv (n=10) groups. At baseline, each group received their respective regular or fat diets, as described previously, and at this point, the LIV group received vibration treatment (0.2g, 90 Hz sine wave, 15 min/d, 5 d/w), while the other two groups were handled in an identical fashion but placed on an inactive device. While 30 minutes of LIV was used for the short-term study, previous research in our lab has demonstrated that 15 mins of LIV is sufficient to induce anabolic effects in the musculoskeletal system 29, and was used for the long term study. Each group continued their diet and treatment for 6 months, and at the end of the protocol, all animals were euthanized, as described previously, and thus data for the long-term protocol represent mice at 31 weeks of age.
Contrast agent enhanced microcomputed tomography assessment of articular cartilage thickness, and sGAG content
At tissue harvest, right tibias from each animal were extracted, bisected at the midshaft, and the proximal end immersed in phosphate buffered saline supplemented with proteinase inhibitor (PBS/PI), for the short-term study, and temporarily stored at 4°C. For the long-term study, proximal tibias were initially placed in 10% NBF, and switched to 70% ethanol at 4°C, until time of analysis. To enhance image intensity of the articular cartilage, a technique described by Kotwal et. al 30, known as the Equilibrium Partitioning of an Ionic Contrast agent (EPIC-μCT) was used, in which the proximal region of each tibia was incubated in a solution of 15% Ioxaglate (Hexabrix, Mallinckrodt Inc., St Louis, MO) and 85% PBS/PI at 37°C for 30 min. Hexabrix is a negatively charged hexaiodinated dimer, which has x-ray attenuation properties that are distinct from the underlying bone, allowing for segmentation of the articular cartilage. Due to the electrochemical interactions between both negatively charged Hexabrix and the sulfated glycosaminoglycans (sGAG) within the cartilage, the equilibrated concentration of Hexabrix is inversely related to the sGAG concentration within the cartilage 31. Samples were patted dry to remove adherent fluid droplets, and scanned in air using high resolution micro computed tomography (ex vivo μCT 40; Scanco Medical, Bassersdorf, Switzerland), using 6-μm isotropic voxels at 45kVp, 177 μA, and 300ms integration time for the young mice (short-term study), and 55kVp, 177 μA, and 300ms integration time for the older mice (long-term study).
The resulting 3D grayscale image of each sample was rotated to obtain sagittal sections of the tibial plateau, which were contoured every 5–10 slices, and contained the subchondral bone, cartilage, and surrounding air, while excluding the marrow space. The cartilage was segmented from the subchondral bone using global lower and upper thresholds. For the short-term study, the lower threshold was 1278 Hounsfield units (HU), and the upper was 4706 HU. For the older animals, the thresholds were 1559 and 4682 HU.
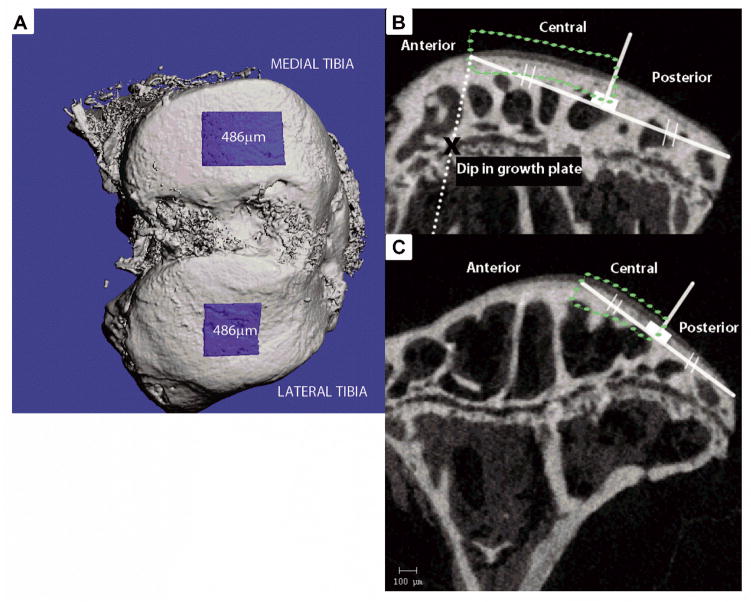
Cartilage thickness was measured in rectangular regions we defined as the central medial and lateral compartments, containing the thickest cartilage in the joint, which were postulated to be high weight bearing regions within the mouse knee. The ROIs were centered at the midline of the medial and lateral tibial plateau, the width of the ROIs spanning a distance of 480μm in the medial condyle, and 480μm in the lateral condyle (Fig 1A). In the sagittal plane, the medial central region was defined using a dip in the tibial growth plate as a landmark—a line parallel to the cortical metaphysis was drawn from the growth plate dip to the plateau surface, the point of intersection defined the start of the central region. A secant line was drawn from the start of the central region, to the posterior edge of the tibial plateau, the midpoint of the secant line serving as the endpoint of the central region (Fig 1B). For the lateral tibia ROI, the central region was defined by drawing a secant line along the relative flat posterior side of the plateau, the midpoint of the secant separating the central from the posterior region (Fig 1C). Direct distance transformation algorithms, within the Scanco Medical μCT software, were used to calculate average cartilage thickness 32. Within each cartilage ROI, relative sGAG content was measured as the average x-ray attenuation (Hounsfield Units) of the articular cartilage pixels.
Figure 1.
A) Bird’s eye view of articular cartilage and subchondral bone region of interest B) Central region of articular cartilage was isolated within the medial tibia for thickness analysis. An x indicates the landmark (dip) in the tibial growth plate, through which a line (dotted) parallel to the tibial metaphyseal cortical bone was drawn. The intersection of this line with the tibial plateau surface was used to draw a secant line to the posterior edge of the plateau, which was bisected to isolate the central region C) Central region of articular cartilage within the lateral tibia isolated for thickness analysis. A secant line was drawn over the relatively flat portion of the posterior end of the plateau, and bisected to isolate the central region.
μCT quantification of subchondral bone plate thickness
Using the EPIC-μCT scans discussed previously, manual contours were drawn to isolate the subchondral bone plate from the epiphyseal trabecular bone, within the same medial and lateral ROIs used for cartilage thickness analysis (Fig 1B and C). Global thresholds were used to segment the subchondral bone from the articular cartilage. For the short-term study, a lower threshold of 4815 HU and upper threshold of 10,000 HU was used. For the long-term study, the thresholds used were 4566 and 10,000 HU. Direct distance transformations, within the Scanco Medical μCT software, were used to calculate subchondral bone plate thickness.
Histology
Histological staining was also used to assess proteoglycan content in the short term mouse study. Intact left knee joints were decalcified in 14% EDTA, dehydrated and embedded in paraffin. Single sagittal 6-micron sections were collected at the approximate midpoint of the medial and lateral knee, stained with Toluidine blue, and counter stained with fast green. Proteoglycan content of the uncalcified cartilage was assessed in the central region of the medial and lateral tibial plateau, corresponding to the regions analyzed with the μCT. Using IMAGE J, the cartilage images were first converted to an 8-bit grey scale image in the blue channel. The average optical density within the blue channel was measured in the articular cartilage of the medial and lateral condyle of each sample. Optical density within the articular cartilage was normalized to the optical density of the growth plate within the same section, to serve as a staining control, and the ratio was assessed as a relative measure of proteoglycan content.
Gene expression within the mouse knee
Total RNA was obtained from the distal femur of the short-term diet/LIV mice, which contains cartilage, subchondral bone, and epiphyseal marrow. To extract RNA, the distal femur was cleaned to eliminate muscle tissue, crushed using a homogenizer and steel beads in the Bullet Blender, in Qiazol reagent, to lyse cells. Chloroform separation was used to isolate RNA in an aqueous phase, and was then purified using a Qiagen RNeasy kit, including DNase digestion of contaminating DNA. Gene expression levels were evaluated using a real time PCR system (StepOnePlus, Applied Biosystems). Using TaqMan gene expression assays (Applied Biosystems), genes involved with cartilage anabolism: Aggrecan (Acan) and Collagen II (Col2A), cartilage catabolism: Matrix Metalloproteinases 3 and 13 (MMP-3,13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), and joint inflammation: TNF-α, and IL-1α, were measured, with eukaryotic 18S used as a housekeeping gene, with all diet and LIV groups compared to RD.
Statistics
All data are shown as means ± SD. GraphPad Prism 6 (GraphPad Software Inc, CA, USA) was used for all statistical analyses. To determine differences between groups, a 1-way analysis of variance (ANOVA) with a Tukey post hoc analysis test was used. Differences were considered statistically significant if p ≤ 0.05. Grubb’s test, with an alpha=0.05, was used to determine if a data point was considered an outlier.
Results
High fat diet changes in body weight
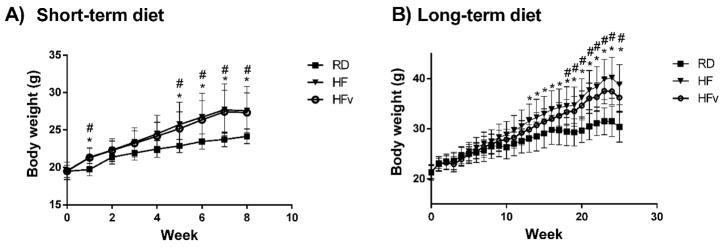
Following 8w of high fat diet, the young HF and HFv groups exhibited a 14.1% and 13.1% increase in total body weight, respectively, as compared to that realized by the regular diet group over the same time period (Fig. 2A, p ≤ 0.01). The total abdominal fat pad mass of the short-term HF and HFv groups were 168% and 159% greater, respectively, than the regular diet group (p ≤ 0.003). There were no significant differences in body weight or abdominal fat pad mass between the young HF and HFv groups.
Figure 2.
A) Average body weight and SD of the young mice in the regular diet group (RD), High-fat diet (HF), or High fat diet with LIV treatment (HFv), at each week of the short-term study. Both HF and HFv groups become significantly heavier than RD over the duration of the study, with HF body weight not being significantly different from HFv (n=10 mice per group) B) Average body weight and SD of the older mice in the RD, HF, and HFv groups at each week of the 6 month protocol. Again, HF and HFv are significantly heavier than RD, for a large duration of the study with HF body weight not being statistically different from HFv (RD, n=7; HF, n=7; HFv, n=10).
Following 24w of high fat diet, both the long-term HF and HFv groups were significantly heavier than the age-matched regular diet controls (+27.3% and +17.3%, respectively, p<0.004), while again, there were no significant differences in body weight between the older HF and HFv groups (Fig 2B).
High fat diet did not induce changes in articular cartilage thickness
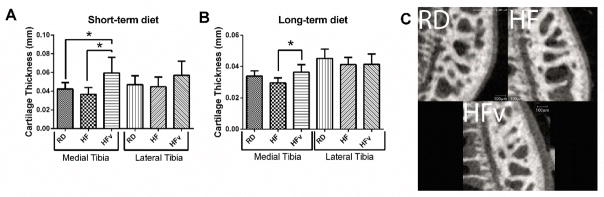
There were no significant differences in medial cartilage thickness between short-term RD and HF mice (HF 12.5% lower than RD, p=0.54), despite the 14% difference in body mass. Further, there were no significant differences in cartilage thickness between the long-term HF and RD mice (HF 8.6% lower than RD, p=0.46) (Fig 3A and 3B).
Figure 3.
A) Cartilage thickness in mice receiving 6 weeks diet/LIV (RD, n=10; HF, n=9; HFv, n=10). (B) Cartilage thickness in mice receiving the 6 months diet/LIV protocol (RD, n=7; HF, n=6; HFv, n=10) C) EPIC-μCT images of medial tibia from representative samples of RD, HF, and HFv from the 6 week diet/LIV protocol.
Likewise, in the lateral tibia, there were no differences in articular cartilage thickness between the RD and HF groups, regardless of age (HF 4.6% higher than RD, p= 0.92 for short-term; −8.0%, p= 0.46 for long-term) (Fig 3A and 3B).
Articular cartilage matrix adaptation with LIV
LIV stimulated an increase in cartilage thickness within the medial condyle, in both young and older HF mice, as compared to HF (+62.0%, p<0.001 in short-term protocol; +23.0%, p<0.010 in long-term protocol) (Fig 3A). Cartilage thickness in young HFv exceeded that measured in age-matched RD mice (+40.4%, p<0.01); while in the older mice, HFv cartilage thickness was not significantly different from RD (+7.6%, p=0.41) (Fig 3B).
Within the lateral tibial plateau, young HFv exhibited increased cartilage thickness compared to young HF, although not significantly different (+27.5%, p=0.097). In older animals, no significant difference in lateral tibial cartilage thickness was exhibited (HFv +0.40%, p= 0.998).
Neither diet nor LIV perturb sGAG content in cartilage
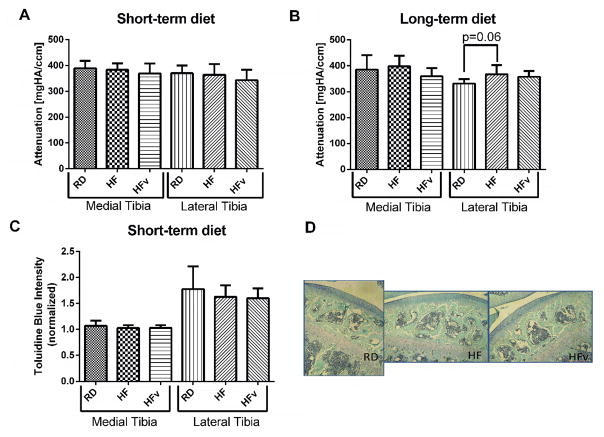
Young RD, HF, and HFv groups exhibited no significant differences in Hexabrix staining intensity (Fig 4A and 4B) nor Toluidine Blue staining intensity (Fig 4C) in the medial or lateral tibial compartments, therefore no detectable difference in sGAG content between the three groups was observed. The older HF group exhibited a higher Hexabrix staining intensity, although not significantly compared to the older RD group, in the lateral compartment (+10.8%, p= 0.06), indicating a potential trend of decreased sGAG content, whereas older HFv Hexabrix staining intensity was not significantly different from older RD controls (8.0% lower, p = 0.14) (Fig 4B).
Figure 4.
A) Average attenuation value of articular cartilage from hexabrix enhanced microCT images in animals undergoing the 6 week diet/LIV protocol (n=10 for all groups) and in (B) animals undergoing the 6 month diet/LIV protocol (RD, n=7; HF, n=7, HFv, n=10). (C) Toluidine blue intensity measured in histological sections of articular cartilage, normalized to the staining intensity of the growth plate. (RD, n=7, HF, n=8, HFv, n=8) (D) Representative toluidine blue/fast green stained histological sections from RD, HF, and HFv groups. No difference in staining intensity was detected between groups.
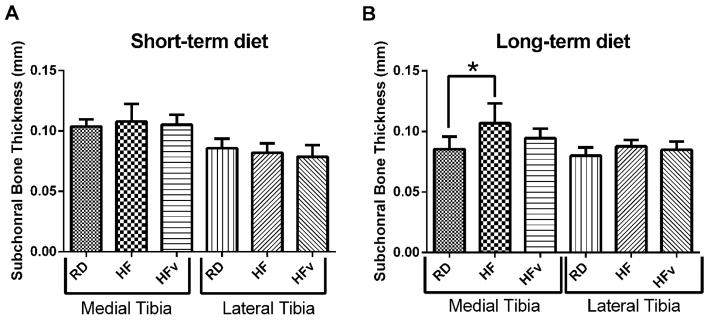
Long term high fat diet promoted subchondral bone thickening, but was mitigated with LIV
Neither short term HF nor HFv resulted in any significant changes in subchondral bone thickness as compared to young RD mice (Fig 5A). In contrast, twenty four weeks of HF resulted in a significant thickening of subchondral bone within the medial tibial plateau as compared to RD controls (+25.0%, p<0.01). However, subchondral bone in HFv was 11.6% thinner than HF (p=0.10), such that the thickness was not significantly different from older RD (+10.5%, p=0.27) (Fig 5B). In the lateral compartment however, long term high fat diet did not result in any changes in subchondral bone thickness (HF 9.8% thicker than RD, p =0.09); likewise, HFv was not statistically different from RD (HFv 6.2% thicker than RD, p=0.28).
Figure 5.
A) Subchondral bone thickness, measured via microCT in the mice receiving the 6 week diet/LIV protocol (n=10 for all groups) and in (B) the mice receiving the 6 month diet/LIV protocol (RD, n=7, HF, n=7, HFv, n=10). No differences in subchondral bone were present at the end of 6 weeks, however, after 6 months of high fat diet, subchondral bone thickening was observed in the HF group, while LIV mitigated changes in subchondral bone thickness.
Influence of short term high fat diet and LIV on anabolic, catabolic, and inflammatory gene expression in the mouse knee
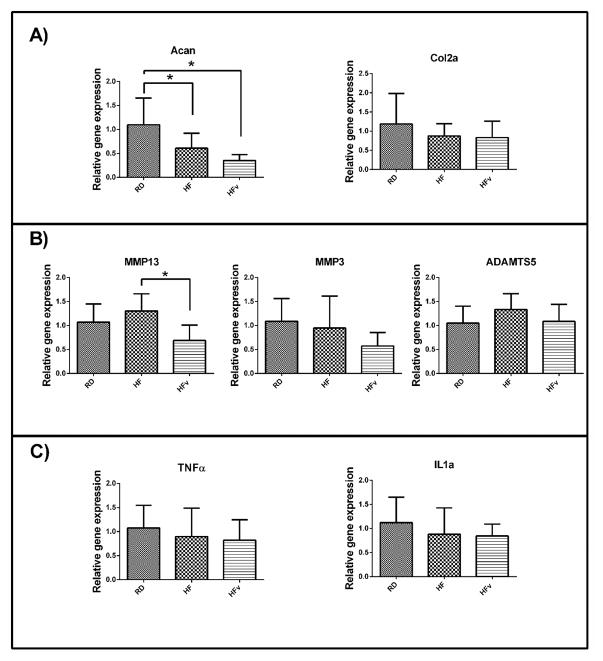
HF and HFv groups both exhibited a significant decrease in aggrecan gene expression compared to RD (−44.6% for HF, p=0.02; −68.0% for HFv, p<0.001). However, LIV also induced a significant reduction in MMP-13 expression compared to HF (−47.2%, p<0.01) to levels trending lower than RD (−35.0%, p=0.06) (Fig 6B). No differences in Col2a, MMP-3, TNF-α or IL-1α gene expression were detected between RD, HF, and HFv groups.
Figure 6.
Relative expression of (A) anabolic genes (B) catabolic genes and (C) inflammatory cytokines, within the femoral condyles of the mice undergoing the 6 week diet/LIV protocol (n=10 for all groups). * indicates p<0.05. HF and HFv groups exhibited a significant decrease in aggrecan gene expression within the knee. HFv also exhibited a significant decrease in MMP-13 gene expression compared to HF.
Discussion
Contrary to our hypothesis of high fat diet resulting in cartilage thinning, eight weeks of high fat diet in young, growing mice did not reduce cartilage thickness within the knee joint, as compared to age-matched controls fed a regular diet. These data suggest that excess adiposity accumulated over this period of musculoskeletal system development – in and of itself – was not a contributory factor in cartilage thinning. Further, a long-term challenge (24w) of excess adiposity in adult mice presented with a cartilage phenotype indistinguishable from the much lighter, age-matched controls fed a regular diet.
While cartilage thickness remained unchanged from obesity, brief, daily exposure to LIV induced a significant increase in cartilage thickness in young obese mice, which was exhibited even when diet and LIV were extended into adulthood. The elevated cartilage thickness due to LIV was more evident in the medial, rather than the lateral compartment of the knee. The medial region is presumed to have a greater weight bearing responsibility 33; this local response to a focal stimulus suggests a mechanically-mediated mechanism of perception and response that is regional, rather than a systemic adaptation to these mechanical signals.
Sulfated GAG content was not significantly different between young RD, HF, and HFv mice in either the medial and lateral compartments, as determined by Hexabrix staining intensity, although previous studies have reported decreased sGAG content with high fat diet 7; 8, and increased sGAG production with moderate exercise 7. Although sGAG content did not vary between groups, RT-PCR analysis showed that aggrecan gene expression within the knee was reduced in both HF and HFv groups (short-term study), suggesting that high fat diet suppressed anabolic processes in joint tissue, and LIV was unable to mitigate this effect. However, LIV significantly reduced MMP-13 expression, compared to HF. MMP-13 is a major type II collagen-degrading collagenase34, and the suppression of its expression via LIV may help in preserving the mechanical integrity of the tissue, mitigating the degenerative risk.
The mechanism in which LIV increased thickness of cartilage, however, is still unclear. Considering that anabolic aggrecan gene expression was reduced in both HF and HFv groups at 13 weeks of age, and no significant change in ADAMTS5 expression, a major aggrecanse, was observed, there is some ambiguity in how articular thickness was increased. It is possible that LIV inhibited the activity of aggrecanases through other pathways not explored in this study. Also, future studies investigating the early effects of LIV on cartilage tissue (i.e. less than 8 weeks of treatment), may provide more insight into the processes leading to thicker cartilage.
Consistent with early symptoms of joint degeneration, long term obese mice demonstrated a thickening of the subchondral bone. Subchondral bone thickening has been previously shown to be associated with the appearance of cartilage lesions and chondrocyte apoptosis, and suggested to exacerbate the deterioration of the bearing surface 35. In concert with LIV’s capacity to increase articular cartilage thickness, these low magnitude mechanical signals also mitigated changes in subchondral bone thickness. There was, however, no significant correlation between articular cartilage thickness and subchondral bone plate thickness (data not shown), regardless of whether all samples were pooled, or the RD, HF, and HFv groups were analyzed individually. This suggests that the changes observed in cartilage, and the mitigation of subchondral bone thickness associated with LIV, occurred via independent processes. Also, while previous literature has reported an association between subchondral bone changes and articular cartilage degeneration, we observed changes in subchondral bone without cartilage thinning or reduced sGAG content. Perhaps the high fat diet would need to be extended, beyond our six month protocol, to observe these degenerative changes.
LIV was introduced here as a surrogate, not a replacement for exercise. These data suggest that brief, daily exposure to mild mechanical signals have the capacity to increase articular cartilage thickness, while also mitigating the subchondral bone morphology changes exhibited in obese animals. In the context of the juvenile obesity crisis, these data suggest that excess adiposity–while not necessarily catabolic to cartilage thickness – may result in the suppression of anabolic processes in the joint, along with degenerative changes in the subchondral bone, a consequence that is alleviated by introducing some form of mechanical signal, thus protecting and preserving the joint for a longer period. At the very least, exercise, salutary to so many physiologic systems, should be introduced early as a preventative and treatment modality for obesity 6, with both direct and indirect benefits being realized 36; 37.
It is important to highlight that recent research has reported that repeated exposure to low intensity vibration induced marked degeneration of murine knee joints, including meniscal tears and focal damage to the cartilage surface within the medial joint compartment 38. From previous studies in our laboratory 27; 39–41, we have not observed damage to connective tissues, whether bone, cartilage, tendon, IVD, muscle or ligament. Further, in clinical trials evaluating the capacity of LIV to preserve bone under conditions of disease or age 42–47, no adverse event was reported relative to knee, hip or back pain. It is important to note that this research group used CD-1 mice in their study, however, when repeating the study in C57BL/6 mice, the same strain used in our study, joint degeneration due to LIV was not detected 48. Nevertheless, the susceptibility of the CD-1 mice to joint degeneration in response to LIV will be important to investigate further. Additionally, in rats, studies have indicated that low intensity vibration accelerates cartilage degeneration after ovariectomy21 and anterior cruciate ligament transection49. However, in our current investigation of LIV administration, we do not report any signs of detrimental LIV effects on the obese mouse knee. While it is important to recognize that vibration, especially at high magnitudes, is capable of causing significant damage to a number of physiologic systems, the LIV signal used here is considered safe by ISO for exposures up to four hours each day 50. However, the negative effects of LIV reported in the literature will be important to consider in future studies.
Our study is limited in that we do not investigate the causes of OA, but whether high fat diet can influence cartilage thickness during development. Also, while we found that LIV increased cartilage thickness, there is a lack of mechanical testing data to indicate whether these changes had a beneficial impact on the mechanical properties of the tissue. Additionally, our attempt to quantify sulfated GAG content is limited in that the measurements are indirect, based on the electrochemical interactions between the contrast agent Hexabrix and the sGAG chains within cartilage tissue. More robust assays for quantification of sulfated proteoglycans exist, such as the dimethylmethylene blue assay, however, isolating sufficient cartilage tissue for analysis is a challenge in the mouse model. Further, the integrity of the collagen II network, a key component of the cartilage extracellular matrix, was not investigated in this study. In addition, joint morphology was investigated in limited joint surfaces–OA is characterized by localized cartilage lesions, and a more in-depth characterization of both hindlimbs would ensure that all cartilage abnormalities were accounted for.
This current study provides evidence for negative consequences of high fat diet on articular cartilage development in young animals; while the diet did not induce changes in cartilage thickness, our short term diet study indicated a reduction in anabolic processes in the knee joint. Reaching adulthood, a thickening of the underlying subchondral bone was measured, representing an early sign of joint degeneration. In contrast, administration of low intensity vibration appears to mitigate catabolic processes within the knee, with an overall increase in cartilage thickness persisting into adulthood, achieved without a thickening of subchondral bone. Time varying mechanical signals, even if exceedingly small, can potentially contribute to establishing a more robust load-bearing system to withstand the challenges of obesity.
Future studies are needed to investigate the biological mechanism and signaling pathways that may be responsible for mechanically mediated anabolic signals to cartilage. Further, in vitro studies need to be combined with in vivo models of joint degeneration to specifically determine how low intensity vibration affects chondrocyte proliferation, differentiation, and matrix secretion and degradation. Perhaps, beyond the systemic benefits of exercise that are widely recognized, there are direct, focal benefits of dynamic loading of cartilage. These studies ultimately may provide the bases for a non-drug approach to slow the onset of osteoarthritis, a disease that is likely to increase with obesity.
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants AR43498 and EB 14351 and the Center for Biotechnology, Stony Brook University. The funding agencies have no role in the study design, data acquisition and analysis, decision to publish, or the preparation of this manuscript.
We thank Patryk Krzesaj, Tenzin Samphel, and Kimberly DeCarr for help with image analysis, and Alyssa Tuthill for experimental assistance. C. T. Rubin is a founder of Marodyne Medical, Inc. and has several USPTO applications under review for the ability of mechanical signals to control musculoskeletal and metabolic disorders. The other authors declare no competing interests.
Footnotes
Conflict of Interest:
C. T. Rubin is a founder of Marodyne Medical, Inc. and has several USPTO applications under review for the ability of mechanical signals to control musculoskeletal and metabolic disorders. The other authors declare no competing interests.
Author Contributions:
Research study design, data acquisition and interpretation, review of paper—TP,BA,MC,CR. Data acquisition and analysis, review of paper—VB
References
- 1.Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis: the Framingham Study. Annals of internal medicine. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter J, Mankin H. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional course lectures. 1997;47:487–504. [PubMed] [Google Scholar]
- 3.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. The American journal of nursing. 2012;112:S13–19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 4.Olds TS, Ferrar KE, Schranz NK, et al. Obese adolescents are less active than their normal-weight peers, but wherein lies the difference? Journal of Adolescent Health. 2011;48:189–195. doi: 10.1016/j.jadohealth.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Bullen BA, Reed RB, Mayer J. Physical activity of obese and nonobese adolescent girls appraised by motion picture sampling. The American journal of clinical nutrition. 1964;14:211–223. doi: 10.1093/ajcn/14.4.211. [DOI] [PubMed] [Google Scholar]
- 6.Page A, Cooper AR, Stamatakis E, et al. Physical activity patterns in nonobese and obese children assessed using minute-by-minute accelerometry. International journal of obesity. 2005;29:1070–1076. doi: 10.1038/sj.ijo.0802993. [DOI] [PubMed] [Google Scholar]
- 7.Griffin TM, Huebner JL, Kraus VB, et al. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis and rheumatism. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner AM, Henn CM, Drewniak EI, et al. High dietary fat and the development of osteoarthritis in a rabbit model. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:584–592. doi: 10.1016/j.joca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis research & therapy. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata M, Ochi H, Hara Y, et al. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PloS one. 2013;8:e60706. doi: 10.1371/journal.pone.0060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45:387–398. [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MD, Triantafillou S, Parker A, et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. The Journal of rheumatology. 1997;24:365–371. [PubMed] [Google Scholar]
- 14.Orita S, Koshi T, Mitsuka T, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC musculoskeletal disorders. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis & Rheumatology. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-jun N-terminal kinase, and nuclear factor κB: Differential regulation of collagenase 1 and collagenase 3. Arthritis & Rheumatology. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Franciozi CE, Tarini VA, Reginato RD, et al. Gradual strenuous running regimen predisposes to osteoarthritis due to cartilage cell death and altered levels of glycosaminoglycans. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:965–972. doi: 10.1016/j.joca.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Ko FC, Dragomir C, Plumb DA, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis and rheumatism. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis and rheumatism. 1996;39:988–995. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 20.Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis and rheumatism. 1995;38:539–546. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 21.Cameron KL, Hsiao MS, Owens BD, et al. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis and rheumatism. 2011;63:2974–2982. doi: 10.1002/art.30498. [DOI] [PubMed] [Google Scholar]
- 22.Leroux M, Cheung HS, Bau J, et al. Altered mechanics and histomorphometry of canine tibial cartilage following joint immobilization. Osteoarthritis and cartilage. 2001;9:633–640. doi: 10.1053/joca.2001.0432. [DOI] [PubMed] [Google Scholar]
- 23.Liphardt AM, Mundermann A, Koo S, et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:1598–1603. doi: 10.1016/j.joca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis and rheumatism. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 25.Davisson T, Kunig S, Chen A, et al. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20:842–848. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 26.Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nature reviews Rheumatology. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. Journal of Bone and Mineral Research. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin C, Capilla E, Luu Y, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proceedings of the National Academy of Sciences. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotwal N, Li J, Sandy J, et al. Initial application of EPIC-muCT to assess mouse articular cartilage morphology and composition: effects of aging and treadmill running. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:887–895. doi: 10.1016/j.joca.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L, Lin AS, Levenston ME, et al. Quantitative assessment of articular cartilage morphology via EPIC-microCT. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:313–320. doi: 10.1016/j.joca.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurwitz DE, Sumner DR, Andriacchi TP, et al. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31:423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 34.Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. European cells & materials. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamli Z, Robson Brown K, Tarlton JF, et al. Subchondral bone plate thickening precedes chondrocyte apoptosis and cartilage degradation in spontaneous animal models of osteoarthritis. BioMed research international. 2014;2014 doi: 10.1155/2014/606870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. NutrJ. 2005;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbeau P, Johnson MH, Howe CA, et al. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity (Silver Spring) 2007;15:2077–2085. doi: 10.1038/oby.2007.247. [DOI] [PubMed] [Google Scholar]
- 38.McCann MR, Patel P, Pest MA, et al. Repeated Exposure to High-Frequency Low-Amplitude Vibration Induces Degeneration of Murine Intervertebral Discs and Knee Joints. Arthritis & Rheumatology. 2015;67:2164–2175. doi: 10.1002/art.39154. [DOI] [PubMed] [Google Scholar]
- 39.Pagnotti GM, Chan ME, Adler BJ, et al. Low Intensity Vibration Mitigates Tumor Progression and Protect Bone Quantity and Quality in a Murine Model of Myeloma. Bone. 2016 doi: 10.1016/j.bone.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frechette DM, Krishnamoorthy D, Adler BJ, et al. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. Journal of applied physiology. 2015;119:27–36. doi: 10.1152/japplphysiol.01020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnamoorthy D, Frechette DM, Adler BJ, et al. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporosis International. 2016;27:747–756. doi: 10.1007/s00198-015-3289-5. [DOI] [PubMed] [Google Scholar]
- 42.Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. JBone MinerRes. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 43.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. JBone MinerRes. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 44.Gilsanz V, Wren TAL, Sanchez M, et al. Low-Level, High-Frequency Mechanical Signals Enhance Musculoskeletal Development of Young Women With Low BMD. Journal of Bone and Mineral Research. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 45.Kiel DP, Hannan MT, Barton BA, et al. Low-Magnitude Mechanical Stimulation to Improve Bone Density in Persons of Advanced Age: A Randomized, Placebo-Controlled Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:1319–1328. doi: 10.1002/jbmr.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leonard MB, Shults J, Long J, et al. Effect of Low-Magnitude Mechanical Stimuli on Bone Density and Structure in Pediatric Crohn’s Disease: A Randomized Placebo-Controlled Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:1177–1188. doi: 10.1002/jbmr.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mogil RJ, Kaste SC, Ferry RJ, Jr, et al. Effect of Low-Magnitude, High-Frequency Mechanical Stimulation on BMD Among Young Childhood Cancer Survivors: A Randomized Clinical Trial. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2015.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerr GJ, McCann MR, Branch JK, et al. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2017;25:421–425. doi: 10.1016/j.joca.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Qin J, Chow S-H, Guo A, et al. Low magnitude high frequency vibration accelerated cartilage degeneration but improved epiphyseal bone formation in anterior cruciate ligament transect induced osteoarthritis rat model. Osteoarthritis and Cartilage. 2014;22:1061–1067. doi: 10.1016/j.joca.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Muir J, Kiel DP, Rubin CT. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. Journal of Science and Medicine in Sport. 2013;16:526–531. doi: 10.1016/j.jsams.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]