Abstract
Background
Chemotherapy-induced nausea and vomiting remain common, distressing side effects of chemotherapy. Acupressure has been reported to prevent chemotherapy-induced nausea (CIN) in adults, but has not been well studied in children.
Methods
In this multi-center, prospective, randomized, single-blind, sham-controlled trial, we compared acute phase nausea severity in patients 4 to 18 years of age receiving highly emetic chemotherapy with standard antiemetic agents combined with acupressure bands versus sham bands. Patients wore acupressure or sham wrist bands continuously on each day of chemotherapy and for up to 7 days afterward. CIN severity in the delayed phase and chemotherapy-induced vomiting (CIV) control in the acute and delayed phases were also compared.
Results
Of the 187 patients randomized, 165 contributed nausea severity assessments during the acute phase. Acupressure bands did not reduce CIN severity in the acute phase (odds ratio (OR): 1.33; 95% confidence limits (CL): 0.89 to 2.00, where OR < 1 favors acupressure). Acupressure bands also did not reduce CIN severity in the delayed phase (OR: 1.23; 95% CL: 0.75 to 2.01). Furthermore, acupressure bands did not improve daily vomiting control during the acute (OR: 1.57; 95% CL: 0.95 to 2.59) or delayed phases (OR: 0.84; 95% CL: 0.45 to 1.58). No serious adverse events were reported.
Conclusion
Acupressure bands were safe but did not improve CIN or CIV in pediatric patients receiving highly emetic chemotherapy.
Keywords: acupressure, nausea, vomiting, chemotherapy, supportive care, pediatrics
Introduction
Despite advances in antiemetic prophylaxis, chemotherapy-induced nausea and vomiting are still common and are among the most distressing side effects of chemotherapy.1 Evaluation of chemotherapy-induced nausea (CIN) by pediatric patients has only recently been possible through the development and validation of pediatric, self-report measures such as the Pediatric Nausea Assessment Tool (PeNAT).2 Recent studies indicate that almost all pediatric patients receiving highly emetogenic chemotherapy (HEC) experience chemotherapy-induced nausea (CIN).3,4
Many patients and families are interested in using non-pharmacologic therapies to control symptoms.5 Acupuncture and acupressure are traditional Chinese medicine techniques that seek to improve health by regulating basal energy through acupoint stimulation.6 The P6 acupoint, located on the ventral surface of the wrist, purportedly has antiemetic action. When used as an adjunct therapy, acupuncture (sterile needle insertion) at the P6 acupoint has improved CIN control in adults.7 Acupressure is similar to acupuncture but uses pressure rather than needles. Wrist bands, the most common type of acupressure, apply pressure to the P6 acupoint. Studies of acupressure band use for chemotherapy-induced nausea and vomiting in adults report mixed results with efficacy reported predominantly in women and in patients with a high expectation of benefit.8–13
To date two feasibility studies have studied acupressure for chemotherapy-induced nausea and vomiting control in children: one evaluated wrist bands and the other auricular acupressure.14,15 Both concluded that a future pediatric trial of acupressure was feasible. Neither was designed to evaluate the efficacy of acupressure in controlling CIV or CIN. Thus, the role of acupressure in reducing CIN or CIV in children is unknown.
The primary objective of this trial was to compare CIN control in the acute phase provided by standard antiemetic agents (ondansetron or granisetron with or without dexamethasone and aprepitant) combined with acupressure bands versus sham bands in children 4 to 18 years of age receiving HEC. Secondary objectives were to compare CIN control in the delayed phase and to compare CIV control in the acute and delayed phases.
Methods
This was a multi-center, international, prospective, randomized, single-blind, sham-controlled trial (www.clinicaltrials.gov, NCT01346267). It was approved by the Institutional Review Board at each participating center. Each patient or their guardian provided informed consent. Children provided assent when able to do so.
Patients
English-speaking patients aged 4 to 18 years with non-relapsed cancer and with an English-speaking guardian were eligible. On initial trial activation, patients receiving cisplatin ≥ 50mg/m2/dose were eligible. In January 2013, the eligibility criteria were expanded to include patients who were scheduled to receive one of the following three possible regimens classified as HEC:16 (i) cisplatin ≥ 50mg/m2/dose, (ii) ifosfamide plus etoposide or doxorubicin, or (iii) cyclophosphamide plus an anthracycline. Patients may have previously received chemotherapy other than HEC.16 Patients with a prior history of acupressure use or who were planned to receive antiemetic agents other than ondansetron, granisetron, dexamethasone or aprepitant on a scheduled basis were excluded.
Definitions
A chemotherapy block was defined as a period of consecutive days when chemotherapy was administered. The acute phase began with administration of the first chemotherapy dose of a chemotherapy block and continued until 24 hours after administration of the last chemotherapy dose of the block. The delayed phase began at the end of the acute phase and continued until the first chemotherapy dose of the next chemotherapy block was given, to a maximum of 7 days. An emetic episode was defined as a vomit or retch separated from another vomit or retch by at least 1 minute.
Study Interventions
Acupressure bands (Sea-Bands®) are knitted elasticized wrist bands which apply pressure on the P6 acupoint by means of a 1 cm internal plastic stud. Sham bands differed from acupressure bands in only one feature: sham bands lacked the internal plastic stud. Both acupressure bands and sham bands had an external plastic stud which, in the acupressure bands, was situated directly above the internal plastic stud. The bands are intended to fit snugly enough to apply gentle continuous pressure to the P6 acupoint. The appropriate band size (adult or pediatric) was determined for each patient.
The randomization sequence was computer-generated and patients were randomized 1:1 to acupressure bands or sham bands prior to the first day of chemotherapy. Randomization was stratified by chemotherapy regimen and planned antiemetic regimen during the acute phase in block sizes of four. A study team member chose the band size, taught the patient or guardian how to place the bands, administered the first PeNAT assessment, taught the guardian how to administer the PeNAT and instructed the patient or guardian on diary completion. This person was not blinded to the study arm allocation of the patient. The allocation was concealed to all other healthcare providers, investigators, patients and families. At the end of the study period, patients or guardians were asked which type of band (acupressure or sham) they believed the patient had been wearing.
At least 30 minutes prior to the administration of the first chemotherapy dose, the bands (real or sham) were placed on both wrists with the external plastic stud positioned at the P6 acupoint. The patient or their guardian was asked to check the band position at least once a day. Patients were asked to wear the bands continuously throughout both the acute and delayed phases. Patients were allowed to remove the bands up to 4 times a day for up to 15 minutes at a time.
Concomitant Antiemetic Prophylaxis
On the days that chemotherapy was administered, all patients received either granisetron or ondansetron intravenously or by mouth on a scheduled basis (i.e. round-the-clock, not as needed). At trial activation, patients without brain tumors were permitted to receive dexamethasone as a scheduled antiemetic as per institutional/physician preference. The protocol was amended in January 2013 to permit all patients to receive scheduled dexamethasone and for patients who were 12 years of age and older to receive scheduled aprepitant in conjunction with dexamethasone.17 Thus, patients received one of three possible scheduled antiemetic regimens in addition to the study intervention: ondansetron/granisetron monotherapy, ondansetron/granisetron plus dexamethasone or, in patients 12 years of age and older, ondansetron/granisetron plus dexamethasone plus aprepitant. Non-scheduled (as needed, PRN) use of agents for breakthrough chemotherapy-induced nausea and vomiting was permitted and tracked.
The name, dose and time of administration of all antiemetic agents the patient received were abstracted from the health record during the inpatient portion of the study period. During the outpatient portion of the study period, this information was recorded by the patient or guardian in a patient diary.
Outcomes
CIN and CIV Assessment
Using the PeNAT, each patient self-assessed the severity of their nausea before administration of the first chemotherapy dose of the study block and at least four times a day (on awakening, mid-day, late afternoon, and bedtime) plus any time the patient felt nauseated or their guardian suspected that the patient felt nauseated. PeNAT scores range from 1 (no nausea) to 4 (worst nausea).
Each patient was given a structured diary on which to record the time of each emetic episode, the PeNAT scores and, for outpatients only, the name and time of antiemetic agents administered. The frequency and duration of band removal was also noted on the diary. Families were contacted at least once during the study period by a study team member to answer any questions and to remind them to complete the diary. Patients and guardians returned the completed diaries to the participating center in person or by mail.
Adverse Event Reporting
Each patient’s health record was reviewed for possible adverse events using the Common Terminology Criteria for Adverse Events v4.0.18 In addition, any adverse event attributed to the bands by a patient or their guardian was recorded.
Statistical Methods
Sample size
A proportional odds model with repeated measure analysis was used to estimate the treatment effect of acupressure and to calculate sample size. A sample of 200 patients, 100 in each study arm, was estimated to detect a cumulative odds ratio (OR) of 0.45 in nausea severity between study arms at 2-sided alpha level of 0.05 with power greater than 80%.
Analysis
The primary study endpoint was nausea severity during the acute phase of chemotherapy, defined as the maximum PeNAT score observed during each 24 hour period. The primary analysis took a modified intent-to-treat approach since patients were required to have contributed at least 1 PeNAT score during the acute phase to be included.
The duration of the acute phase varied with the chemotherapy regimen, as per study design. Due to diminishing numbers of patients who received chemotherapy for longer than 3 days, data from days 4 to 7 of the acute phase were collapsed and considered to be from a single day. A Generalized Estimating Equations (GEE) for proportional odds cumulative logit model was used to test treatment efficacy (acupressure bands vs. sham bands) on repeated measures of daily maximum PeNAT score during the acute phase. The chemotherapy regimen, antiemetic drugs actually taken, treatment (acupressure bands vs. sham bands), day within the acute phase and the day by treatment interaction were included in the model.
In addition, we determined the maximum PeNAT score for the entire acute phase for each patient. Generalized linear regression (a proportional odds cumulative logit model) was applied to compare the maximum nausea severity during the entire acute phase between study groups, adjusting for the effects of chemotherapy and antiemetic regimens. As sensitivity analyses, the primary analysis was repeated in two subsets of patients: 1) those who provided at least 2 PeNAT scores each day of the acute phase and 2) those who reported being compliant with application of the bands. The secondary analysis of CIN during the delayed phase was conducted using the same methods.
The daily number of emetic episodes was classified as no episodes, 1–2 episodes, or greater than 2 episodes. A GEE model with proportional odds cumulative logit for ordinal data was used to test treatment efficacy (acupressure bands vs. sham bands) on repeated measures during each entire phase. The ordinal levels of emetic episodes during the entire phase between study groups was compared using a generalized linear regression model. In addition, CIV control during the entire acute or delayed phase was classified as complete (no emetic episodes and no use of breakthrough antiemetic agents), partial (one or two emetic episodes in any 24 hour period and/or use of breakthrough antiemetic agents) or failed (more than 2 emetic episodes in any 24 hour period). The proportions of patients in each of the CIV control categories were compared between study groups using a proportional odds cumulative logit model for ordinal data. This analysis accounted for the duration of the acute phase, chemotherapy agents and antiemetic agents administered.
To explore the similarities or differences of the acupressure effect in patient populations based on sex and age, an interaction with treatment (acupressure bands vs. sham bands) was included in the GEE models along with the individual terms of sex or age.
Statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC). All tests of significance were two tailed. P <0.05 was considered statistically significant. An odds ratio (OR) < 1 indicates that the acupressure group had a better outcome than the sham group while an OR > 1 indicates that the acupressure group had a worse outcome than the sham group. Reasons for early withdrawal, compliance and perception of study group allocation were compared between two treatment groups using a Pearson Chi-square test. The length of follow-up was compared between groups using a Wilcoxon Rank Sum test.
Results
Patients
From May 9, 2011 to May 18, 2012 and December 12, 2012 to May 16, 2016, 187 patients receiving care in 27 institutions (Supplemental Table I) were randomized. During the gap in enrollment the sponsoring organization changed. At initial activation, the study was sponsored by the Children’s Oncology Group with financial support through the Division of Cancer Prevention, National Cancer Institute. In 2012, the SunCoast CCOP Research Base assumed sponsorship of the study. The National Cancer Institute provided financial support throughout.
Of the 187 randomized patients, 165 entered the acute phase and contributed at least 1 PeNAT score during the acute phase, 137 participated in both the acute and delayed phases, 124 patients provided at least 2 PeNAT scores on each day of the acute phase and 125 reported compliance with band application (Figure 1). Characteristics of the patients and their treatments are presented in Table 1. Study groups did not differ with respect to the duration of the acute phase (p=0.63).
Figure 1.
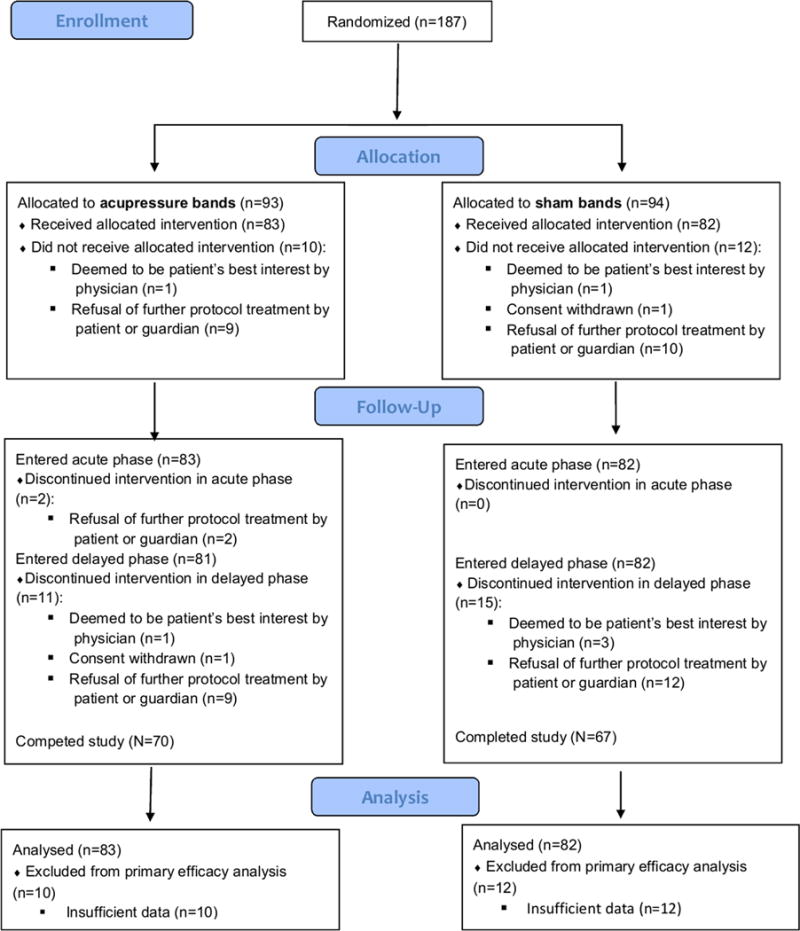
Consort Diagram.
Table 1.
Characteristics of enrolled patients who entered the acute phase and their treatments
| Acupressure (N=83) |
Sham (N=82) |
All patients (N=165) |
|
|---|---|---|---|
|
| |||
| Patient Characteristics at Enrollment | |||
|
| |||
| Sex (Male:Female; N) | 46:37 | 50:32 | 96:69 |
|
| |||
| Mean Age (yrs; SD) | 12.5(4.3) | 12.9(4.2) | 12.7(4.2) |
|
| |||
| Race (White:Other; N) | 53:30 | 56:26 | 109:56 |
|
| |||
| Diagnosis (N): | |||
| Osteosarcoma: | 16 (19%) | 17 (21%) | 33 |
| Hodgkin Lymphoma: | 20 (24%) | 29 (35%) | 49 |
| Ewings sarcoma: | 9 (11%) | 6 (7%) | 15 |
| Other: | 38 (46%) | 30 (37%) | 68 |
|
| |||
| Chemotherapy-Naive (N) | 81 (98%) | 78 (95%) | 159 |
|
| |||
| Treatment Characteristics | |||
|
| |||
| Median Duration of Acute Phase (days; interquartile range) | 4 (3–5) | 4 (3–4) | 4 (3–4) |
| Duration of Acute Phase (N) | |||
| 1-day | 2 | 3 | 5 |
| 2-day | 10 | 15 | 25 |
| 3-day | 21 | 21 | 42 |
| 4-day | 27 | 30 | 57 |
| 5-day | 11 | 5 | 16 |
| 6-day | 7 | 4 | 11 |
| 7-day | 5 | 4 | 9 |
|
| |||
| Chemotherapy Agents Received (N) | |||
| cisplatin ≥ 50 mg/m2/dose: | 33 (40%) | 31 (38%) | 64 |
| cyclophosphamide + anthracycline: | 38 (46%) | 40 (39%) | 78 |
| ifosfamide + anthracycline: | 12 (14%) | 11 (13%) | 23 |
|
| |||
| Antiemetics Received for Prophylaxis (N) | |||
| ondansetron/granisetron: | 39 (47%) | 32 (39%) | 71 |
| ondansetron/granisetron + dexamethasone: | 22 (27%) | 25 (30%) | 47 |
| ondansetron/granisetron, dexamethasone + aprepitant: | 22 (27%) | 25 (30%) | 47 |
N: number of patients; SD: standard deviation. Percentages may not total 100% because of rounding.
CIN Severity: Acute Phase
Acute phase CIN severity did not differ between study groups (acupressure bands vs. sham bands: OR: 1.33; 95% confidence limits (CL): 0.89 to 2.00) (Supplemental Table 2). When the analysis was repeated in the subset of patients who contributed 2 PeNAT scores each day of the acute phase, no difference in nausea severity was again observed (acupressure bands vs. sham bands: OR: 1.21; 95% CL: 0.76 to 1.92). A post-hoc analysis was undertaken to determine if the time of day (morning, mid-day, afternoon or bedtime) that the maximum PeNAT score was recorded influenced the treatment effect in patients who reported two or more PeNAT scores on each day of the acute phase. The findings of this analysis (OR: 1.24; 95% CL: 0.78 to 1.97) are similar to the findings without adjusting for the time of day of PeNAT assessment (Supplemental Table 3). Most (92%) patients had nausea. Complete CIN control during the entire acute phase was uncommon (acupressure bands vs. sham bands: 5% vs. 11%) and not different between study groups (OR: 2.13; 95% CL: 0.61 to 7.69). This analysis was adjusted for the duration of the acute phase.
Analysis of interactions of the primary efficacy variable across sex, chemotherapy and antiemetic subgroups did not identify any subgroup of subjects for which acupressure provided a benefit over sham (data not shown).
CIN Severity: Delayed Phase
In the delayed phase, 144 patients (acupressure bands: 75; sham bands: 69) reported at least one PeNAT score (Supplemental Table 2). Delayed phase CIN severity did not differ between study groups (acupressure bands vs. sham bands: OR: 1.23; 95% CL: 0.75 to 2.01).
Complete CIN control during the delayed phase was observed in 17% and 26% of patients in the acupressure bands and sham bands groups, respectively. The cumulative OR of having complete CIN control during the entire delayed phase was 1.56 (95% CL: 0.68 to 3.57) in the acupressure bands vs. sham bands groups.
CIV Control: Acute Phase
Most patients (78%) vomited. No difference in daily CIV control during the acute phase was observed between study groups (acupressure bands vs. sham bands: OR: 1.57; 95% CL: 0.95 to 2.59) (Figure 2). There was also no difference in the proportion of patients who did not experience an emetic episode during the entire acute phase between study groups (acupressure bands vs. sham bands: 41% vs. 42%; OR: 0.96; 95% CL: 0.50 to 1.84). Similarly, there was no difference in the proportions of patients who experienced complete, partial or failed CIV control between study groups (P=0.33) (Table 2).
Figure 2. Frequency of Emetic Episodes on Each Day of the Acute Phase.
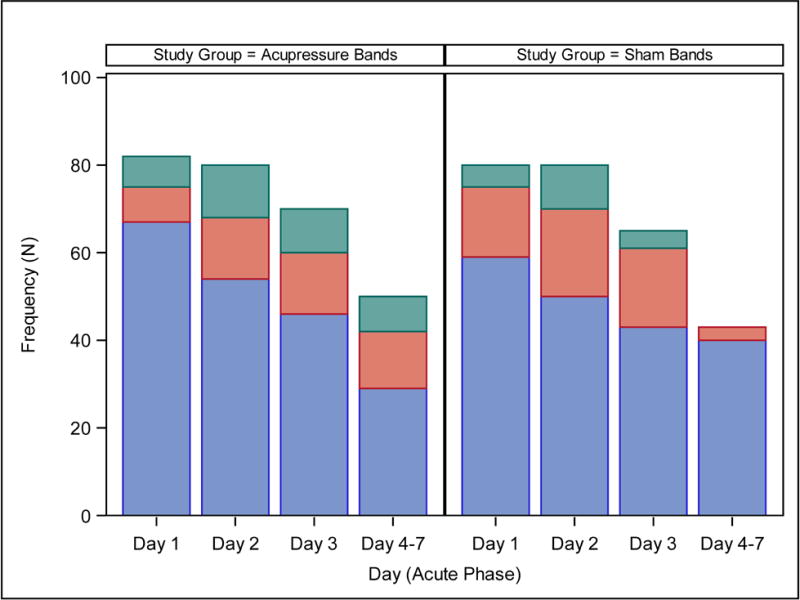
Blue: No emetic episode; Orange: 1–2 emetic episodes; Green: >2 emetic episodes.
Table 2.
Chemotherapy-induced vomiting control in children receiving standard antiemetic agents plus acupressure bands or sham bands.
| Complete CIV Control (Number of Children; %) |
Partial CIV Control (Number of Children; %) |
Failed CIV Control (Number of Children; %) |
|
|---|---|---|---|
| Acute Phase* | |||
| Acupressure | 17 (21%) | 43 (52%) | 23 (28%) |
| Sham | 19 (23%) | 48 (59%) | 15 (18%) |
| Delayed Phase¥ | |||
| Acupressure | 35 (47%) | 29 (39%) | 11 (15%) |
| Sham | 31 (45%) | 28 (41%) | 10 (14%) |
N= 165 (acupressure: 83; sham: 82);
N=144 (acupressure: 75; sham: 69). Percentages may not total 100% because of rounding.
CIV Control: Delayed Phase
Daily CIV control during the delayed phase did not differ between study groups (acupressure bands vs. sham bands: OR: 0.84; 95% CL: 0.45 to 1.58). There was also no difference in the proportion of patients who never experienced an emetic episode during the entire delayed phase between study groups (acupressure bands vs. sham bands: 60% vs. 51%; OR: 0.53; 95% CL: 0.24 to 1.15) or in the proportion of patients who experienced complete, partial or failed CIV control during the entire delayed phase (P=0.32) (Table 2).
Compliance with Treatment
During the acute phase, no differences were observed between study groups with respect to the number of patients who reported taking off the bands more than was permitted (acupressure bands vs. sham bands: 29% (24/83) vs. 20% (16/80); P=0.16) or in the number of patients who reported that band placement was not verified (acupressure bands vs. sham bands: 8% (7/83) vs. 4% (3/80); P=0.32). Similarly, no differences were observed in these proportions in the delayed phase (data not shown).
Perception of Study Group Allocation
Approximately half of patients or their guardians made a judgment regarding their study group allocation; approximately one third were correct. The proportion of patient and guardians who made a correct judgment regarding study group allocation was not different between study groups (P=0.97) (Supplemental Table 4).
Adverse events
Six adverse events (acupressure bands: 4; sham bands: 2) were described as possibly or definitely related to the study intervention. Adverse events mostly commonly arose due to complaints that the bands were too tight (3/6). No serious adverse events possibly or definitely attributed to the bands were reported and all adverse events resolved.
Discussion
In this large, randomized, single-blinded, pediatric trial, acupressure bands did not improve CIN or CIV control in children 4 to 18 years of age receiving HEC and ondansetron/granisetron-based antiemetic prophylaxis. Our findings remained the same whether control was defined on a daily basis or on the basis of the entire acute or delayed phase. No serious adverse effects were attributed to the use of acupressure bands or sham bands.
The findings of other randomized trials evaluating acupressure bands for CIN and CIV control have been mixed. Those that have observed a positive effect of acupressure bands tended to include mostly female patients receiving chemotherapy of variable emetogenicity and compared acupressure bands to electro-stimulation bands or usual care rather than sham bands.8,10,12,19 One of these studies noted that patients who expected acupressure bands to convey benefit reported less severe nausea than did those patients who expected no benefit from the acupressure bands.8 Thus, the benefit of acupressure bands was attributed, at least in part, to a placebo effect. We did not assess the pre-existing beliefs regarding acupressure held by our patients or their guardians. Similarly, Molassiotis et al13 speculated that patients may benefit from wearing sham bands via a placebo effect or via a biological effect of wrist pressure without P6 acupoint stimulation. Acupressure trials that compared acupressure bands vs. sham bands, enrolled both male and female patients and adjusted for chemotherapy emetogenicity have observed no benefit with respect to vomiting or nausea control.11,13
Patient enrollment into our study was ended abruptly due to a change in the funding model supporting our research base. It is important to consider the impact of discontinuation of patient accrual prior to reaching the planned sample size of 200. Given that the ORs for CIN control all favored sham and considering the magnitude of the ORs and their CLs in this study, it is unlikely that a positive treatment effect of acupressure would have been observed in a larger sample size.
The strengths of this study are its size, randomized and single-blinded design, the use of sham bands, the use of patient self-report of nausea severity using a validated tool, and confirmation of the primary analysis through sensitivity analyses. Nevertheless, its findings are limited to the population studied: children aged 4 to 18 years receiving multiple or single day HEC with antiemetic prophylaxis that includes ondansetron or granisetron. The results of this study cannot be generalized to children receiving chemotherapy of lower emetogenicity, to acupressure at acupoints other than P6 or to other modes of acupoint stimulation such as electric acustimulation.
It is possible that patients who reported compliance did not apply the acupressure bands properly or wear them continuously as stipulated in the protocol. However, it is unlikely that acupressure bands would be worn more optimally in clinical practice. It is also possible that simply wearing wrist bands without an internal stud applying pressure, as in the sham group, provided an antiemetic or sham effect. In response to this concern investigators have designed 3-arm trials: acupressure, sham acupressure and no bands.8,13 We made the pragmatic decision when designing this trial not to include a third arm due to concerns that patients and families would decline to participate in a trial with a low probability of receiving active treatment. The fact that 92% of patients in our trial experienced nausea argues against the feasibility of including a third study arm.
Patients and guardians did not adhere completely to the desired schedule of nausea severity assessment and, thus, some nausea assessments were unavailable. While we do not believe that this missing data would have influenced our estimate of the treatment effect, more frequent nausea assessment would have improved our understanding of the extent of nausea severity and its variability within and between patients.
Our study is the first pediatric trial of acupressure bands. Though acupressure bands were well-tolerated, we observed no improvement in CIN or CIV control in pediatric patients receiving HEC and ondansetron/granisetron-based antiemetic prophylaxis. Acupressure delivered using different modalities or at sites other than P6 may merit study. Almost all of our patients had nausea and most vomited during the acute phase. Rather than the double or triple agent prophylaxis recommended by the clinical practice guideline current at the time of this trial,17 most patients received ondansetron or granisetron monotherapy and, therefore, did not receive guideline-consistent antiemetic care. It is clear that CIN and CIV control in pediatric patients is suboptimal. Provision of clinical practice guideline consistent antiemetic prophylaxis and rigorous evaluation of novel approaches to antiemetic prophylaxis are required to provide effective supportive care.
Supplementary Material
Précis.
In this multi-center, prospective, randomized, single-blind, sham-controlled trial, we compared the control of acute phase nausea in 165 patients 4 to 18 years of age receiving highly emetic chemotherapy with standard antiemetic agents combined with acupressure bands versus sham acupressure bands. Acupressure bands were safe but did not improve nausea control in pediatric patients receiving highly emetic chemotherapy.
Acknowledgments
We are grateful to the patients and families who participated in this trial. We thank the research staff of the participating sites who recruited patients and ensured compliance with the study protocol. We also thank Viki Huegel, Angelina Fink, Lauren Bello-Matricaria, Julie Garcia and Ashley Kattlus for their assistance and support and Nusheen Rostayee for administrative assistance.
Funding support:
This study was sponsored by the National Cancer Institute, the Children’s Oncology Group and the SunCoast CCOP Research Base (UG1-CA189955 and U10-CA095861). Sea-Bands and sham bands were provided free of charge by Sea-Band International, Newport, RI, USA.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Contributors’ Statement:
L Lee Dupuis: conceptualization, methodology, validation, investigation, data curation, writing–original draft, writing–review and editing, supervision, project administration, visualization, and funding acquisition
Kara M Kelly: conceptualization, methodology, investigation, writing–review and editing
Jeffrey P Krischer: supervision, project administration, writing–review and editing, and funding acquisition
Anne-Marie Langevin: investigation, data curation, writing–review and editing
Roy N Tamura: methodology, validation, formal analysis, data curation, writing–review and editing
Ping Xu: methodology, validation, formal analysis, data curation, writing–review and editing
Lu Chen: methodology, writing–review and editing
E Anders Kolb: investigation, writing–review and editing
Nicole J Ullrich: methodology, investigation, writing–review and editing
Olle Jane Z. Sahler: methodology, investigation, writing–review and editing
Ann Stratton: methodology, writing–review and editing
Eleanor Hendershot: methodology, writing–review and editing
Lillian Sung, MD: conceptualization, methodology, supervision, visualization and writing–review and editing, and funding acquisition
Thomas W McLean: conceptualization, methodology, validation, investigation, resources, data curation, writing–original draft, writing–review and editing, supervision, project administration, visualization and funding acquisition
References
- 1.Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: A systematic review. Critical Reviews in Oncology/Hematology. 2016;99:13–36. doi: 10.1016/j.critrevonc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis LL, Taddio A, Kerr EN, Kelly A, MacKeigan L. Development and validation of a pediatric nausea assessment tool (PeNAT) for use by children receiving antineoplastic agents. Pharmacotherapy. 2006;26:1221–1231. doi: 10.1592/phco.26.9.1221. [DOI] [PubMed] [Google Scholar]
- 3.Flank J, Sparavalo J, Vol H, et al. The burden of chemotherapy-induced nausea and vomiting in children receiving hematopoietic stem cell transplantation conditioning: a prospective study. Bone Marrow Transplantation. 2017;52(9):1294–1299. doi: 10.1038/bmt.2017.112. [DOI] [PubMed] [Google Scholar]
- 4.Vol H, Flank J, Lavoratore S, et al. Poor chemotherapy-induced nausea and vomiting control in children receiving intermediate or high-dose methotrexate. Support Care Cancer. 2016;24(3):1365–1371. doi: 10.1007/s00520-015-2924-1. [DOI] [PubMed] [Google Scholar]
- 5.Bishop F, Prescott P, Chan Y, Saville J, von Elm E, Lewith G. Prevalence of complementary medicine use in pediatric cancer: a systematic review. Pediatrics. 2010;125(4):768–776. doi: 10.1542/peds.2009-1775. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Dodd M, Dibble S, Abrams D. Review of acupressure studies for chemotherapy-induced nausea and vomiting control. J Pain Symptom Manage. 2008;2008(5):529–544. doi: 10.1016/j.jpainsymman.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952–960. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roscoe JA, Morrow GR, Hickok JT, et al. The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting. A University of Rochester Cancer Center Community Clinical Oncology Program multicenter study. J Pain Symptom Manage. 2003;26(2):731–742. doi: 10.1016/s0885-3924(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 9.Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complementary Therapies in Medicine. 2007;15(1):3–12. doi: 10.1016/j.ctim.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Avci H, Ovayolu N, Ovayolu O. Effect of acupressure on nausea-vomiting in patients with acute myeoloblastic leukemia. Holistic Nursing Practice. 2016;30(5):257–262. doi: 10.1097/HNP.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 11.Genc A, Can G, Aydiner A. The efficiency of the acupressure in prevention of the chemotherapy-induced nausea and vomiting. Support Care Cancer. 2013;21:253–261. doi: 10.1007/s00520-012-1519-3. [DOI] [PubMed] [Google Scholar]
- 12.Genc F, Tan M. The effect of acupressure application on chemotherapy-induced nausea, vomiting, and anxiety in patients with breast cancer. Palliative and Supportive Care. 2015;13:275–284. doi: 10.1017/S1478951514000248. [DOI] [PubMed] [Google Scholar]
- 13.Molassiotis A, Russel W, Hughes J, et al. The effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: a randomized controlled trial. J Pain Symptom Manage. 2014;47:12–25. doi: 10.1016/j.jpainsymman.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Jones E, Isom S, Kemper KJ, McLean TW. Acupressure for chemotherapy-associated nausea and vomiting in children. Journal of the Society for Integrative Oncology. 2008;6(4):141–145. [PubMed] [Google Scholar]
- 15.Yeh C, Chien L-C, Chiang Y, Lin S, Huang C, Ren D. Reduction in nausea and vomiting in children undergoing cancer chemotherapy by either appropriate or sham auricular acupuncture points with standard care. Journal of Alternative and Complementary Medicine. 2012;18(4):334–340. doi: 10.1089/acm.2011.0102. [DOI] [PubMed] [Google Scholar]
- 16.Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncology. 2016;27(suppl 5):v119–v133. doi: 10.1093/annonc/mdw270. [DOI] [PubMed] [Google Scholar]
- 17.Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2013;60:1073–1082. doi: 10.1002/pbc.24508. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010 [Google Scholar]
- 19.Roscoe JA, Matteson SE, Morrow GR, et al. Acustimulation wrist bands are not effective for the control of chemotherapy-induced nausea in women with breast cancer. J Pain Symptom Manage. 2005;29(4):376–384. doi: 10.1016/j.jpainsymman.2004.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


