Abstract
Mutations in the transcription factor genes FOXE3, HSF4, MAF, and PITX3 cause congenital lens defects including cataracts that may be accompanied by defects in other components of the eye or in non-ocular tissues. We comprehensively describe here all the variants in FOXE3, HSF4, MAF, and PITX3 genes linked to human developmental defects. A total of 52 variants for FOXE3, 18 variants for HSF4, 20 variants for MAF, and 19 variants for PITX3 identified so far in isolated cases or within families are documented. This effort reveals FOXE3, HSF4, MAF and PITX3 to have 33, 16, 18, and 7 unique causal mutations, respectively. Loss-of-function mutant animals for these genes have served to model the pathobiology of the associated human defects, and we discuss the currently known molecular function of these genes, particularly with emphasis on their role in ocular development. Finally, we make the detailed FOXE3, HSF4, MAF, and PITX3 variant information available in the Leiden Online Variation Database (LOVD) platform at http://www.LOVD.nl/FOXE3, http://www.LOVD.nl/HSF4, http://www.LOVD.nl/MAF, and http://www.LOVD.nl/PITX3. Thus, this article informs on key variants in transcription factor genes linked to cataract, aphakia, corneal opacity, glaucoma, microcornea, microphthalmia, anterior segment mesenchymal dysgenesis, and Ayme-Gripp syndrome, and facilitates their access through web-based databases.
Keywords: cataract, aphakia, anterior segment mesenchymal dysgenesis, Ayme-Gripp syndrome, microcornea, LOVD
Background
Perturbation of early eye development, specifically during the morphogenesis of the anterior segment tissues – the lens and cornea – can result in congenital cataract, aphakia, anterior segment mesenchymal dysgenesis, coloboma (defined as missing tissue in or around the eye; can affect lens, macula, optic nerve, uvea, retina, choroid, iris and eyelids), microphthalmia and microcornea, among other ocular defects. Here we focus on four transcription factor-encoding genes FOXE3 (MIM# 601094), HSF4 (MIM# 602438), MAF (MIM# 177075), PITX3 (MIM# 602669), mutations in which lead to ocular defects with a subset of shared features, but commonly exhibiting defects in the lens, including cataract. Cataract is an eye disease arising due to the loss of ocular lens transparency, and depending on the age of onset can be classified as congenital or age-related. Age-related cataract is among the leading causes of blindness worldwide (Shiels and Hejtmancik, 2017). Congenital cataract can present either as the primary phenotype (isolated or non-syndromic) or one among multiple phenotypes (syndromic), and between 25–50% of congenital cataract cases are estimated to be caused by an underlying genetic alteration (Shiels and Hejtmancik, 2015, 2017). Predominantly, congenital cataract defects are linked to mutations in genes that encode various crystallin proteins, cytoskeletal proteins, or gap junction proteins (Shiels et al., 2010). Additionally, mutations in several regulatory molecules, including transcription factor-encoding genes, are linked to anterior eye and lens defects (Sowden, 2007; Reis and Semina, 2011; Shiels and Hejtmancik, 2017). However, there is no recent article comprehensively summarizing variants in the transcription factor genes FOXE3, HSF4, MAF, and PITX3 in human, especially with regards to genotype-phenotype correlation and informing on the significance of specific variants.
We provide here a detailed mutation update of FOXE3, HSF4, MAF and PITX3. We present a graphical representation of the location of all currently known variants in these transcription factor genes on the genomic, cDNA, and protein levels, which serve to highlight their significance in context of the protein structure, especially in relation to conserved regions and functional domains. Further, we also provide a map for these genes that informs on the conservation of the mutated amino acids across several vertebrate species. We then discuss in detail the various animal resources, based on both targeted gene deletions as well as spontaneous mutations, that have been used to model the human defects and to gain insights into the molecular regulatory function of these proteins. Finally, we make the compiled variant profiles including DNA and protein changes, mutation type (germline, somatic or de novo), segregation and frequency for these genes publicly available by depositing and updating this information to the Leiden Online Variation Database (LOVD) platform. Using this freely available database, end-users can readily access variant information, as well as graphical summary through UCSC Genome Browser, Ensembl or NCBI sequence viewer.
Genotype-Phenotype Correlation
In the following sections, we focus on summarizing all the currently known variants (both mutations and polymorphisms as of December 19, 2017) in the four transcription factor genes FOXE3, HSF4, MAF and PITX3 that have key function in eye development and are linked to cataract, among other defects, in human patients. We also discuss the functional and clinical significance of the variations. Sequence variations in these genes are described by following the mutation nomenclature guidelines outlined in http://varnomen.hgvs.org (den Dunnen et al., 2016).
Disease-Causing FOXE3 Variants
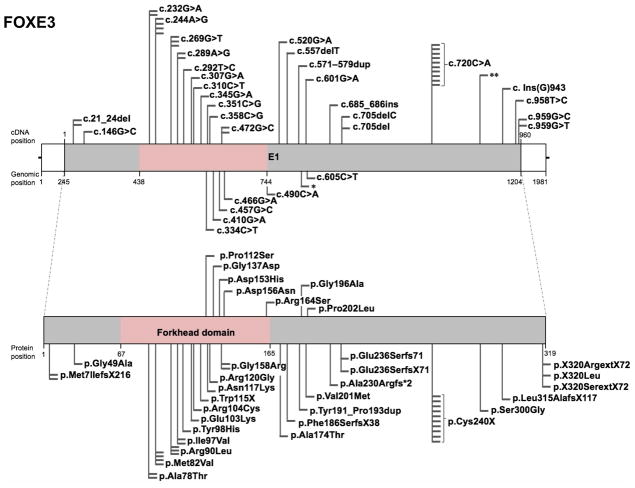
The forkhead box (FOX) gene family encodes transcription factor proteins that contain a DNA-binding region called the “forkhead” domain (Weigel et al., 1989). Mutations in the FOX family gene FOXE3 are associated with distinct ocular phenotypes, commonly involving microphthalmia, aphakia, and glaucoma, and predominantly affecting the anterior segment tissues, the lens and cornea. These are: microphthalmia (47 affected individuals), aphakia (18 affected individuals), glaucoma (17 affected individuals), corneal opacities (13 affected individuals), coloboma with or without affecting the optic disc (13 affected individuals), sclerocornea (18 affected individuals), anterior segment dysgenesis (5 affected individuals), microcornea (5 affected individuals), Peters anomaly (7 affected individuals), nystagmus (4 affected individuals), congenital and early childhood onset cataracts (3 affected individuals), anophthalmia (2 affected individuals), corneal ectasia with central scarring (2 affected individuals), absence of iris (2 affected individuals), myopia (2 affected individuals), retinal dysplasia (2 affected individuals), corneal limbal insufficiency (1 affected individual), corneal neovascularization (1 affected individual), and aniridia (1 affected individual) in reported cases (Table 1). Interestingly, in addition to ocular phenotypes, mutations in FOXE3 have recently been associated with thoracic aortic aneurysms and acute aortic dissections (TAADs) (Kuang et al., 2016). Over the past two decades, 52 studies have reported 33 unique FOXE3 mutations – classified as 6 frameshift, 23 missense, 3 nonsense and 1 nonstop mutation– in 110 affected individuals from diverse populations. Of the 33 unique FOXE3 mutations, 15 present in the forkhead domain are missense variants, predicted as damaging or experimentally validated as non-functional or low affinity proteins (Fig. 1, Table 1). Of these fifteen, 10 are associated with eye defects, which include c.232G>A (p.Ala78Thr), c.244A>G (p.Met82Val), c.269G>T (p.Arg90Leu), c.289A>G (p.Ile97Val), c.292T>C (p.Tyr98His), c.307G>A (p.Glu103Lys), c.310C>T (p.Arg104Cys), c.351C>G (p.Asn117Lys), c.358C>G (p.Arg120Gly) and c.472G>C (p.Gly158Arg), whereas 5 are associated with thoracic aortic aneurysms and acute aortic dissections (TAADs), which include c.334C>T (p.Pro112Ser), c.410G>A (p.Gly137Asp), c.457G>C (p.Asp153His), c.466G>A (p.Asp156Asn), and c.490C>A (p.Arg164Ser). Further, protein sequence alignment reveals that amino acids residues are conserved among vertebrates for all of the 15 mutations present in the forkhead domain (Fig. 2). Thus, it is interesting to note that specific mutations in the forkhead DNA-binding domain have specific outcomes with regards to either ocular or aortic defects. However, of the other 17 mutations present outside of the forkhead domain, only the amino acid encoded at position c.Ins(G)943 (p.Leu315AlafsX117) is conserved among vertebrates. Further, of these 17 mutations in non-conserved amino acids, majority were predicted to be damaging/deleterious or to encode non-functional proteins, as reported in the original publications (Table 1). One particular FOXE3 mutation c.720C>A (p.Cys240X) – located downstream of the forkhead domain reported in ten unrelated individuals to occur in the homozygous state – is frequently associated with aphakia and microphthalmia, among other eye defects. FOXE3 variants (c.146G>C (p.Gly49Ala), c.601G>A (p.Val201Met), c.898A>G (p.Ser300Gly), c.618C>G (p.Ala206Ala)) have been identified in both affected and control populations at similar frequencies and are therefore unlikely to be disease-associated (Iseri et al., 2009; Reis et al., 2010; Jimenez et al., 2011). Interestingly, the frequency of these variants are found to be different in Caucasian, Hispanic, African American and Asian population controls. Additionally, three silent (c.510T>C (p.Ala170Ala), c.618C>G (p.Ala206Ala), c.828C>G (p.Pro276Pro)) and two missense (c.587G>C (p.Gly196Ala), c.898A>G (p.Ser300Gly)) FOXE3 variants have been identified at similar frequencies in the anterior segment disorder patient population as well as in the control population (Semina et al., 2001). A complete list of FOXE3 variants, including nucleotide and predicted amino acid changes are given in Table 1 and further details on probands are given in Supp. Table S1. The location of these changes, relative to important domains, are indicated in a schematic representation of the FOXE3 gene and protein (Fig. 1). The updated FOXE3 gene variants information is deposited in the Leiden Online Variation Database (LOVD) platform at http://www.LOVD.nl/FOXE3.
Table 1.
FOXE3 human mutations
| Nuc. Pos. | AA Pos. | Vt | AD/ AR |
Prot. Func. |
A A C on . |
P at h o. |
A p |
A sd |
A no |
A n |
C | C o |
FT AA Ds |
G | C o l |
M c |
M t |
N y |
P a |
Sc | V d |
Pt. Info. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.21_24del | p.Met7IlefsX216 | fs | AR | nr | n | u | y | n | n | n | n | y | n | n | y | n | y | n | n | n | n | Proband | (Iseri et al., 2009) |
| c.21_24del | p.Met7IlefsX216 | fs | AR | nr | n | u | y | n | n | n | n | y | n | y | n | n | n | n | n | n | n | Cousin of proband | |
| c.21_24del | p.Met7IlefsX216 | fs | AR | Exp- d | n | p | y | n | n | n | n | y | n | y | n | n | n | n | n | n | n | Proband | (Islam et al., 2015) |
| c.146G>C | p.Gly49Ala | ms | AD | Pre-nd | n | p | n | n | n | n | n | n | n | n | y | y | y | n | n | n | n | Proband | (Iseri et al., 2009) |
| c.146G>C | p.Gly49Ala | ms | AD | Pre-nd | n | p | n | n | n | n | y, cr | n | n | n | n | n | n | n | n | n | n | Mother of proband | |
| c.146G>C | p.Gly49Ala | ms | AD | Pre-nd | n | p | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Maternal grandmother of proband | |
| c.232G>A | p.Ala78Thr | ms | NA | Pre-d | y | m | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Second affected case | (Plaisancié et al., 2017) |
| c.232G>A | p.Ala78Thr | ms | NA | Pre-d | y | m | n | n | n | n | n | n | n | n | n | n | y | n | y | n | n | Two affected family (2) members (siblings) | (Plaisancié et al., 2017) |
| c.244A>G | p.Met82Val | ms | NA | nr | y | u | n | n | n | n | n | n | n | n | n | n | y | n | y | y | n | First affected case | (Plaisancié et al., 2017) |
| c.244A>G | p.Met82Val | ms | NA | nr | y | u | n | n | n | n | n | n | n | n | n | n | y | n | y | n | Two affected family (1) members (siblings) | (Plaisancié et al., 2017) | |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | y | n | n | n | n | n | n | y | n | n | n | n | n | y | n | Proband | (Iseri et al., 2009) |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | n | y | n | n | n | n | n | y | n | n | n | n | n | n | n | Second affected family member (25 years age) | |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Third affected family member (24 years age) | |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | n | y | n | n | n | n | n | y | n | n | n | n | n | n | n | Fourth affected family member | |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | n | n | n | n | n | n | n | n | n | n | n | n | n | y | n | Fifth affected family member | |
| c.244A>G | p.Met82Val | ms | AR | Pre-d | y | p | n | n | n | n | n | n | n | n | n | n | n | n | n | y | n | Sixth affected family member | |
| c.244A>G | p.Met82Val | ms | AR | nr | y | p | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Proband | (Reis et al., 2010) |
| c.269G>T | p.Arg90Leu | ms | NA | Exp- d | y | m | n | n | n | n | n | y | n | y | n | n | n | n | y | n | n | One affected case | (Ormestad et al., 2002) |
| c.269G>T | p.Arg90Leu | ms | NA | nr | y | u | n | n | n | n | n | n | n | n | n | n | y | n | y | y | n | First affected case | (Plaisancié et al., 2017) |
| c.269G>T | p.Arg90Leu | ms | AR | Exp- nd | y | m | y | n | n | n | n | y | n | y | n | n | n | n | n | n | n | Proband | (Islam et al., 2015) |
| c.289A>G | p.Ile97Val | ms | NA | Pre-d | y | m | n | n | n | n | y, cg | n | n | n | n | n | n | n | n | n | y | Proband | (Gillespie et al., 2014) |
| c.289A>G | p.Ile97Val | ms | AR | Pre-d | y | u | n | y | n | n | n | y | n | n | n | n | y | n | n | n | n | Proband | (Ullah et al., 2016) |
| c.289A>G | p.Ile97Val | ms | AR | Pre-d | y | u | n | y | n | n | n | y | n | n | n | n | y | n | n | n | n | Five affected family member | |
| c.292T>C | p.Tyr98His | ms | AR | Pre-d | y | p | y | n | n | n | n | y | n | n | y | n | y | n | n | n | n | Proband | (Ali et al., 2010) |
| c.292T>C | p.Tyr98His | ms | AR | Pre-d | y | p | y | n | n | n | n | y | n | n | y | n | y | n | n | n | n | Eight affected family member | |
| c.307G>A | p.Glu103Lys | ms | AR | nr | y | m | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Proband | (Khan et al., 2016) |
| c.307G>A | p.Glu103Lys | ms | AR | Pre-d | y | m | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Five affected family member | (Chen et al., 2017) |
| c.310C>T | p.Arg104Cys | ms | NA | Pre-d | y | m | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Second affected case | (Plaisancié et al., 2017) |
| c.334C>T | p.Pro112Ser | ms | NA | Pre-nd | n | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.345G>A | p.Trp115X | ns | NA | nr | y | u | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Third affected case | (Plaisancié et al., 2017) |
| c.351C>G | p.Asn117Lys | ms | AR | nr | y | m | n | n | n | n | n | y | n | n | n | y | n | y | n | n | n | Proband | (Khan et al., 2016) |
| c.358C>G | p.Arg120Gly | ms | AR | Exp- d | y | p | y | n | n | n | n | y | n | y | n | n | n | n | n | n | n | Proband | (Islam et al., 2015) |
| c.410G>A | p.Gly137Asp | ms | NA | Pre-d | y | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.457G>C | p.Asp153His | ms | AD | Pre-d | y | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Eight affected family member | (Kuang et al., 2016) |
| c.466G>A | p.Asp156Asn | ms | NA | Pre-d | y | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.472G>C | p.Gly158Arg | ms | NA | nr | y | u | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Third affected case | (Plaisancié et al., 2017) |
| c.472G>C | p.Gly158Arg | ms | AR | nr | y | m | y | n | n | n | n | n | n | n | n | n | y | n | n | y | n | First affected family member (10 years age) | (Saboo et al., 2017) |
| c.472G>C | p.Gly158Arg | ms | AR | nr | y | m | y | n | n | n | n | y | n | n | n | n | n | n | n | y | n | Second affected family member (9years age) | |
| c.472G>C | p.Gly158Arg | ms | AR | nr | y | m | y | n | n | n | n | n | n | n | n | n | n | n | n | y | n | Third affected family member (55 years age) | |
| c.490C>A | p.Arg164Ser | ms | NA | Pre-d | y | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.520G>A | p.Ala174Thr | ms | NA | Pre-nd | n | u | n | n | y | n | n | n | n | n | n | n | n | n | n | n | n | Proband | (Jimenez et al., 2011) |
| NA | p.Gly196Ala | ms | NA | Pre-nd | n | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.557delT | p.Phe186SerfsX38 | fs | AR | nr | n | p | y | y | n | n | n | n | n | n | n | n | n | n | n | y | n | Proband | (Reis et al., 2010) |
| c.557delT | p.Phe186SerfsX38 | fs | AR | nr | n | p | n | n | n | n | n | y | n | n | n | n | n | n | n | n | n | Mother of proband | |
| c.571_579dup | p.Tyr191_Pro193dup | fs | NA | nr | n | n | n | n | n | y | y | y | n | n | n | n | n | y | n | n | n | Proband | (Brémond-Gignac et al., 2010) |
| c.685_686insTCCGGAGC | p.Ala230Argfs*2 | fs | NA | nr | n | n | n | n | n | n | n | n | n | n | n | n | n | n | n | y | n | Proband | (Chassaing et al., 2014) |
| c.601G>A | p.Val201Met | ms | NA | nr | n | n | n | n | y | n | y | n | n | n | n | y | y | n | n | n | n | Proband | (Jimenez et al., 2011) |
| c.605C>T | p.Pro202Leu | ms | NA | Pre-nd | n | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
| c.705delC | p.Glu236SerfsX71 | ms | AR | nr | n | p | n | n | n | n | n | n | n | n | n | n | y | n | n | y | n | Proband | (Reis et al., 2010) |
| c.705del | p.Glu236Serfs71 | fs | AR | Exp- d | n | p | y | n | n | n | n | y | n | y | n | n | n | n | n | n | n | Proband | (Islam et al., 2015) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | m | y | n | n | n | n | y | n | n | n | n | y | n | n | y | n | Seven affected family member | (Ali et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | m | y | n | n | n | n | n | n | n | n | n | n | n | n | n | n | Seven affected family member | (Anjum et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | m | y | n | n | n | n | n | n | n | n | n | n | n | n | n | n | Proband | (Anjum et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | n | y | n | n | n | n | y | n | y | n | n | y | n | n | n | n | Proband | (Reis et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | n | n | n | n | n | n | y | n | y | y | n | y | y | n | n | n | Proband | (Reis et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | n | n | n | n | n | n | y | n | y | n | n | y | y | n | n | n | Proband | (Reis et al., 2010) |
| c.720C>A | p.Cys240X | ns | AR | nr | n | n | y | y | n | n | n | n | n | n | n | n | y | n | n | n | n | Three affected family member | (Valleix et al., 2006) |
| c.720C>A | p.Cys240X | ns | NA | nr | n | n | n | y | n | n | n | y | n | y | y | n | n | n | y | n | n | Proband | (Khan et al., 2016) |
| c.720C>A | p.Cys240X | ns | NA | nr | n | n | n | n | n | n | n | n | n | n | n | n | y | n | n | n | n | Proband | (Jimenez et al., 2011) |
| c.720C>A | p.Cys240X | ns | NA | nr | n | n | n | n | n | n | n | n | n | n | n | n | n | n | n | y | n | Proband | (Chassaing et al., 2014) |
| c.Ins(G)943 | p.Leu315AlafsX117 | fs | NA | nr | y | m | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Proband | (Semina et al., 2001) |
| c.Ins(G)943 | p.Leu315AlafsX117 | fs | NA | nr | y | m | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Mother of proband | |
| c.958T>C | p.X320ArgextX72 | ms | AD | nr | NA | p | y | n | n | n | y | n | n | n | y | n | y | n | y | y | n | Proband | (Iseri et al., 2009) |
| c.958T>C | p.X320ArgextX72 | ms | AD | nr | NA | p | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Mother of proband | |
| c.958T>C | p.X320ArgextX72 | ms | AD | nr | NA | p | n | n | n | n | y | n | n | n | y | n | n | n | n | n | n | Maternal uncle of proband | |
| c.958T>C | p.X320ArgextX72 | ms | AD | nr | NA | p | n | n | n | n | y | n | n | n | n | n | n | n | n | n | n | Maternal auntof proband | |
| c.958T>C | p.X320ArgextX72 | ms | AD | nr | NA | p | y | n | n | n | n | n | n | n | n | n | n | n | n | n | n | Maternal grandmother of proband | |
| c.959G>T | p.X320L | nt | AD | nr | NA | m | n | n | n | n | n | y | n | n | n | n | n | n | y | n | n | Proband | (Doucette et al., 2011) |
| c.959G>T | p.X320L | nt | AD | nr | NA | m | n | n | n | n | y | y | n | n | n | y | n | n | n | n | n | Father of proband | |
| c.959G>T | p.X320L | nt | AD | nr | NA | m | n | n | n | n | y | n | n | n | n | y | n | n | n | n | n | Three paternal aunts of proband | |
| c.959G>T | p.X320L | nt | AD | nr | NA | m | n | n | n | n | y, cg | n | n | n | n | n | n | n | n | n | n | Three paternal cousins of proband | |
| c.959G>T | p.X320L | nt | AD | nr | NA | m | n | y | n | n | y | y | n | n | n | n | n | n | n | n | n | Two affected family members of proband | |
| c.959G>C | p.X320SerextX72 | ns | AD | nr | NA | n | n | n | n | n | y, cg | n | n | n | n | n | n | n | n | n | n | Proband | (Brémond-Gignac et al., 2010) |
| NA | p.Ser300Gly | ms | NA | Pre-nd | NA | m | n | n | n | n | n | n | y | n | n | n | n | n | n | n | n | Proband | (Kuang et al., 2016) |
Notes: NA, not reported in original article; Nuc. Pos., nucleotide position in CDS; AA Pos., amino acid position; Vt, variant type; fs, frameshift; ms, missense; ns, nonsense; nt, nonstop; AD, autosomal dominant; AR, autosomal recessive; NA, not available; Prot. Func., protein function; nr, not reported; Exp-d, experimental-damaging; Exp-nd, experimental-not damaging; Pre-d, predicted-damaging; Pre-nd, predicted-not damaging; n, no; y, yes; AA con., amino acid conservation; Patho., pathogenicity; Ap, Aphakia; Asd, Anterior segment dysgenesis; Ano, Anophthalmia; An, Aniridia; C, Cataract; cr, cerulean; cg, congenital; Co, Corneal opacity/abnormal cornea; Fc, Facial characteristics; FTAADs, Familial thoracic aortic aneurysms and acute aortic dissections; G, Glaucoma; Col, Iris coloboma/coloboma/abnormal iris; Mc, Microcornea; Mt, Microphthalmia; Ny, Nystagmus; Pa, Peters anomaly; Sc, Sclerocornea; Vd, vitreoretinal dysplasia; Pt. Info., patient information; Ref., reference
Figure 1. Schematic of FOXE3 gene, protein domains, and position of mutations.
(A) The human FOXE3 gene (top) spans a single exon (E1) that encodes the FOXE3 protein (bottom), which contains the forkhead DNA-binding domain (pink). Nucleotide positions for cDNA and genomic DNA in the reference sequence (hg38) and the corresponding amino acid positions in the protein sequence are given. The UCSC genome browser (https://genome.ucsc.edu) was used to obtained cDNA, genomic and protein position information from FOXE3 human GRCh38/hg38 assembly (reference sequence NM_012186, uc001crk.3 at chr1: 47,416,072–47,418,052). Total 52 mutation studies, of which 33 represent unique mutations identified in independent studies are indicated as flags. These include 6 frameshift, 23 missense, 3 nonsense and 1 nonstop mutation. Nucleotide changes in DNA and amino acid changes in protein sequence are indicated. Changes in cDNA that are not documented in original article are represented as (*) or (**). Variants linked to eye defects are shown above the gene (and below the protein) and those associated with thoracic aortic aneurysms and acute aortic dissections are shown below the gene (and above the protein).
Figure 2. Multiple sequence alignment of FOXE3 protein sequences.
Protein sequences for FOXE3 downloaded from the UCSC browser for Homo sapiens, Mus musculus, Gallus gallus, Xenopus tropicalis and Danio rerio shows amino acid conservation across different vertebrate species. Amino acid changes caused by specific human mutations are indicated by yellow arrowheads. The sequence consensus is presented for each amino-acid and the forkhead domain is indicated by green arrow.
Disease-Causing HSF4 Variants
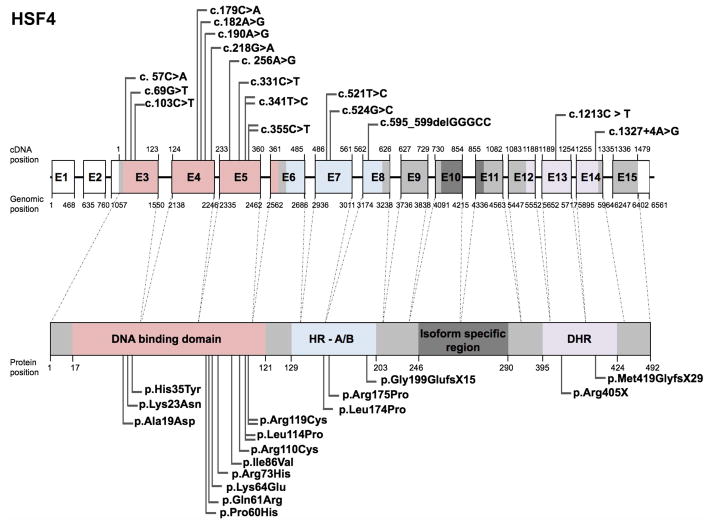
The heat shock factor (HSF) family of genes encode transcriptional regulators that have a conserved winged helix-turn-helix domain that binds DNA in a sequence-specific manner (Akerfelt et al., 2010). Mutations in the HSF family protein HSF4 are predominantly associated with congenital and early childhood onset cataract, and have been found to be inherited in both autosomal dominant and recessive modes. HSF4 mutation-linked cataracts are commonly of the lamellar sub-type, wherein the opacity is localized between the inner nuclear and outer cortical lens fiber cell layers (Table 2). In two reported cases, in addition to cataract, the eye defect nystagmus was observed (Behnam et al., 2016). Further, one HSF4 exonic sequence variant was associated with age-related cataract (Shi et al., 2008). Sixteen studies have reported a total of 14 unique HSF4 mutations in 136 affected individuals (Fig. 3, Table 2). Majority of the HSF4 mutations described to date are missense (13), but frameshift mutations in 2 cases, and a nonsense mutation in 1 case, have also been described. Of the 16 unique mutations, 11 located in the HSF4 DNA-binding domain are conserved among vertebrates: c.57C>A (p.Ala19Asp), c.69G>T (p. Lys23Asn), c.103C>T (p.His35Tyr), c.179C>A (p.Pro60His), c.182A>G (p.Gln61Arg), c.190A>G (p.Lys64Glu), c.218G>A (p.Arg73His), c.256A>G (p.Ile86Val), c.331C>T (p.Arg110Cys), c.341T>C (p.Leu114Pro), c.355C>T (p.Arg119Cys) (Table 2) (Fig. 3). Three mutations (c.521T>C (p.Leu174Pro), c.524G>C (p.Arg175Pro), c.595_599delGGCC (p.Gly199GlufsX5)) are located in hydrophobic repeat (HR- A/B) region and two mutations (c.1213C>T (p.Arg405X), c.1327+4A>G (p.Met419GlyfsX29)) are located in downstream of hydrophobic repeat (DHR) region. Protein sequence alignment shows that more than 50% of the following reported HSF4 mutations cause a change of an amino acid that is conserved across different vertebrate species: c.57C>A (p.Ala19Asp), c.69G>T (p. Lys23Asn), c.103C>T (p.His35Tyr), c.179C>A (p.Pro60His), c.190A>G (p.Lys64Glu), c.218G>A (p.Arg73His), c.256A>G (p.Ile86Val), c.341T>C (p.Leu114Pro), c.355C>T (p.Arg119Cys), c.521T>C (p.Leu174Pro) c.524G>C (p.Arg175Pro) and c.1327+4A>G (p.Met419GlyfsX29) (Fig. 4). Additionally, a missense variant (c.347G>A (p.Arg116His), originally described as c.1243G>A) was reported for HSF4 in an age-related cataract population (n=150; 3/150) as well as in a control group (n=100; 2/100) (Shi et al., 2008). Upon evaluation of all the HSF4 variants, it is interesting to note that although all mutations identified in the DNA binding domain are missense variants, they are inherited autosomal dominantly, whereas mutations identified in the HR-A/B and DHR regions are inherited autosomal recessively. Based on these correlations, it can be speculated that perturbation of the DNA binding domain affecting only one allele is sufficient to result in early onset-cataract, in turn suggesting that this domain is integral to HSF4 function. On the contrary, no autosomal dominant cases have been reported for mutations affecting other functional domains of HSF4, suggesting that in such cases both alleles have to be perturbed in order to observe a clinical phenotype. A complete list of HSF4 variants, including nucleotide and predicted amino acid changes are given in Table 2 and further details on probands are given in Supp. Table S2. The updated HSF4 gene variants information is deposited in the Leiden Online Variation Database (LOVD) platform at http://www.LOVD.nl/HSF4.
Table 2.
HSF4 human mutations
| Nuc. Pos. | AA Pos. | Vt | AD/AR | Prot. Func. | AA Con. | Patho. | C | Ny | Pt. info. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| c. 57C>A (c. 53C>A *) | p.Ala19Asp (p.Ala20Asp *) | ms | AD | nr | y | m | y, la | n | Sixty-nine sporadic cases | (Bu et al., 2002) |
| c. 57C>A (c. 53C>A *) | p.Ala19Asp (p.Ala20Asp *) | ms | AD | nr | y | m | y, la | n | One affected family member | |
| c.69G>T | p. Lys23Asn | ms | AD | Pre-d | y | p | y, cg, cr | n | Proband | (Lv et al., 2014) |
| c.69G>T | p. Lys23Asn | ms | AD | Pre-d | y | p | y, ct | n | Eight affected family members of proband | |
| c.103C>T | p.His35Tyr | ms | NA | Pre-d | y | m | y, cg | y | Proband | (Gillespie et al., 2014) |
| c.103C>T | p.His35Tyr | ms | NA | Pre-d | y | m | y, cg | y | Mother of proband | |
| c.179C>A | p.Pro60His | ms | NA | Pre-d | y | m | y, nu | n | One non-familial sporadic affected | (Li et al., 2016) |
| c.182A>G (c.1078A>G *) | p.Gln61Arg | ms | NA | nr | n | m | y, se | n | one out of one-fifty cases | (Shi et al., 2008) |
| c.190A>G | p.Lys64Glu | ms | AD | Pre-d | y | m | y, la | n | Seven affected family members | (Berry et al., 2017) |
| c.218G>A (c.221G>A *) | p.Arg73His (p.Arg74His *) | ms | AD | nr | y | p | y, cg | n | Seven affected family members | (Ke et al., 2006) |
| c. 256A>G | p.Ile86Val (p.Ile87Val *) | ms | AD | nr | y | m | y, la | n | Proband | (Bu et al., 2002) |
| c. 256A>G | p.Ile86Val (p.Ile87Val *) | ms | AD | nr | y | m | y, se | n | Father of proband | |
| c.331C>T | p.Arg110Cys (p.Arg111Cys *) | ms | AD | nr | n | m | y, cg | n | Six affected family members | (Liu et al., 2015) |
| c.341T>C (c.348T>C *) | p.Leu114Pro (p.Leu115Pro *) | ms | AD | nr | y | m | y, la | n | Proband | (Bu et al., 2002) |
| c.341T>C (c.348T>C *) | p.Leu114Pro (p.Leu115Pro *) | ms | AD | nr | y | m | y, la | n | Thirty affected family members | |
| c.341T>C | p.Leu114Pro | ms | NA | nr | y | m | y, la | n | Four affected family members | (Hansen et al., 2009) |
| c.341T>C | p.Leu114Pro | ms | NA | nr | y | m | y, po | n | One affected family member | |
| c.355C>T (c.362C>T *) | p.Arg119Cys (p.Arg120Cys *) | ms | AD | nr | y | m | y, ma | n | Proband | (Bu et al., 2002) |
| c.355C>T | p.Arg119Cys | ms | NA | nr | y | m | y, la | n | Two affected family members | (Hansen et al., 2009) |
| c.521T>C | p.Leu174Pro | ms | AR | Pre-d | y | m | y, cg | y | Proband | (Behnam et al., 2016) |
| c.521T>C | p.Leu174Pro | ms | AR | Pre-d | y | m | y, cg | y | Brother of proband | |
| c.524G>C | p.Arg175Pro | ms | AR | nr | y | m | y, cg, ct | n | Five affected family members (siblings) | (Forshew et al., 2005) |
| c.595_599delGGGCC | p.Gly199GlufsX15 | fs | AR | nr | n | m | y, cg | n | Five affected family members | (Forshew et al., 2005) |
| c.1213C > T | p.Arg405X | ns | AR | nr | n | m | y, cg, ec | n | Eight affected family members | (Sajjad et al., 2008) |
| c.1327+4A>G | p.Met419GlyfsX29 | fs | AR | nr | y | m | y, cg, to | n | Twenty-two affected family members | (Smaoui et al., 2004) |
Notes: NA, not reported in original article;
indicates nucleotide and amino acid positions as indicated in the original article; Nuc. Pos., nucleotide position in CDS; AA Pos., amino acid position; Vt, variant type; fs, frameshift; ms, missense; ns, nonsense; nt, nonstop; AD, autosomal dominant; AR, autosomal recessive; NA, not available; Prot. Func., protein function; nr, not reported; Pre-d, predicted-damaging; n, no; y, yes; AA con., amino acid conservation; Patho., pathogenicity; p, pathogenic; m, may be pathogenic; C, Cataract; cr, cerulean; cg, congenital; ct, cortical; ec, early childhood; la, lamellar; ma, marner; nu, nuclear; po, polar; se, senile; to, total; Ny, Nystagmus; Pt. Info., patient information; Ref., reference
Figure 3. Schematic of HSF4 gene, protein domains, and position of mutations.
(A) The human HSF4 gene spans fifteen exons (E1-E15) that encode the HSF4 protein (bottom). While there are multiple HSF isoforms, the specific isoform (HSF4 isoform b) that has high expression in the lens is described here because of its relevance to the cataract defect. Exons 3 through 6 encode the DNA binding domain (pink), exons 6 through 8 encode an oligomerization domain termed hydrophobic repeat (HR- A/B) (blue) and exons 12 through 14 encode a region termed as downstream of hydrophobic repeat (DHR) (purple). HSF4b is derived by alternative mRNA splicing of exons 10 and 11 (exon 8 and 9 in the originally reported study). Nucleotide positions for cDNA and genomic DNA in the reference sequence (hg38) and the corresponding amino acid positions in the protein sequence are given. The UCSC genome browser (https://genome.ucsc.edu) was used to obtained cDNA, genomic and protein position information from HSF4 human GRCh38/hg38 assembly (reference sequence NM_001040667.2 at chr16:67163385–67169945). Total 18 mutations, of which 16 represent unique mutations identified in independent studies are indicated as flags. These include 2 frameshift, 13 missense and 1 nonsense mutation. Nucleotide changes in DNA and amino acid changes in protein sequence are indicated. In Table 2 * indicates nucleotide and amino acid positions as indicated in the original article
Figure 4. Multiple sequence alignment of HSF4 protein sequences.
Protein sequences for HSF4 downloaded from UCSC browser for Homo sapiens, Mus musculus, Gallus gallus, Xenopus tropicalis and Danio rerio shows amino acid conservation across different vertebrate species. Amino acid changes caused by specific human mutations are indicated by yellow arrowheads. The sequence consensus is presented for each amino-acid. HSF-DNA binding domain and vertebrate heat shock transcription factor domains are indicated by green arrow.
Disease-Causing MAF Variants
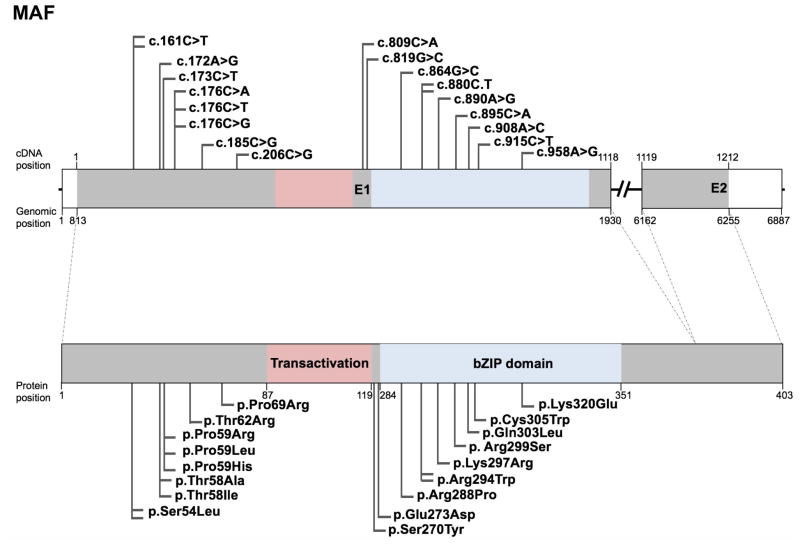
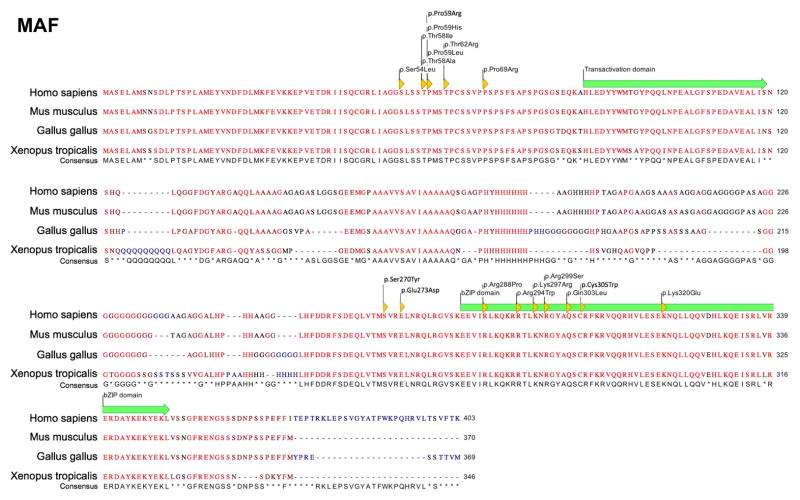
The musculoaponeurotic fibrosarcoma (MAF) gene family encodes transcription factors that contain a basic-leucine zipper (bZIP) domain, which functions in protein dimerization for binding to DNA (Kataoka, 2007). Mutations in the MAF gene are reported in eighty individuals that exhibit various ocular defects including congenital cataract. Mutations in MAF are also linked to Ayme-Gripp syndrome, which presents with congenital cataract in addition to flat facial features resembling Down syndrome, as well as brachycephaly, deafness, intellectual disability, and seizures. Besides these, MAF mutations are associated with other defects such as iris coloboma (13 affected individuals), glaucoma (3 affected individuals), microcornea (15 affected individuals), microphthalmia (3 affected individuals), myopia (3 affected individuals), nystagmus (8 affected individuals), and Peters anomaly (3 affected individuals). Twenty studies have reported eighteen unique mutations in 83 affected individuals in MAF that includes 1 translocation event t(5;16)(p15.3;q23.2) and 17 missense mutations (Table 3). Interestingly, a significant subset (seven) of the MAF mutations associated with eye defects are located within the bZIP domain-encoding region c.864G>C (p.Arg288Pro), c.880C>T (p.Arg294Trp), c.890A>G (p.Lys297Arg), c.895C>A (p. Arg299Ser), c.908A>C (p.Gln303Leu), c.915C>T (p.Cys305Trp), c.958A>G (p.Lys320Glu)), but no mutations have been identified to lie within the transactivation domain-encoding region in human (although such a mutation is reported in mouse, see the animal model section below) (Fig. 5). However, eight mutations are within the N-terminal upstream of the transactivation domain (c.161C>T (p.Ser54Leu), c.172A>G (p.Thr58Ala), c.173C>T (p.Thr58Ile), c.176C>G (p.Pro59Arg), c.176C>A (p.Pro59His), c.176C>T (p.Pro59Leu), c.185C>G (p.Thr62Arg), c.206C>G (p.Pro69Arg)) are associated with Ayme-Gripp syndrome (Fig. 5). Protein sequence alignment shows that all the amino acids affected in MAF missense mutations are conserved in vertebrates (Fig. 6). While the transactivation domain activates transcription by forming a dimer, the bZIP domain facilitates dimerization that results in recognition of the Maf recognition element (MARE) on the target genes activated by Maf dimer complexes. Based on the genotype-phenotype correlations drawn from the MAF mutation spectrum in affected individuals, we speculate that the transactivation domain is functionally critical, and thus mutations in this region lead to lethality. Furthermore, mutations in the N-terminal region upstream of the transactivation domain, may disrupt dimerization, protein stability, and/or protein function, resulting in the syndromic clinical phenotypes observed. Lastly, mutations in the bZIP domain that result in exclusively eye related-defects suggest that the MAF bZIP domain may have unique functions in the eye in addition to its known regulatory roles in other tissues. These speculations warrant further work on specifically investigating the significance of individual domains in MAF in the eye and other tissues of interest using animal models. Furthermore, while proteins in the small MAF family, MafG and MafK, have been associated with cataract in animal models (Agrawal et al., 2015), no variants have been identified in human patients yet. Interestingly, although small MAFs lack the transactivation domain present in large MAFs, they share the bZIP leucine zipper region, which may harbor potential causative variants in patients with cataract and other tissue-defects. A complete list of MAF variants, including nucleotide and predicted amino acid changes are given in Table 3 and further details on probands are given in Supp. Table S3. The updated MAF gene variants information is deposited in the Leiden Online Variation Database (LOVD) platform at http://www.LOVD.nl/MAF.
Table 3.
MAF human mutations
| Nuc. Pos. | AA Pos. | Vt | AD/ AR |
Prot. Func. |
AA Con . |
Pat ho. |
Ap | C | C o |
F c |
G | H | C o l |
M c |
M t |
N y |
P a |
Sy | Pat. Info. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.161C>T | p.Ser54Leu | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 39 years, female | (Aymé and Philip, 1996; Niceta et al., 2015) |
| c.161C>T | p.Ser54Leu | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 43 years, female) | (Niceta et al., 2015) |
| c.172A>G | p.Thr58Ala | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 27 years, female | (Gripp et al., 1996; Niceta et al., 2015) |
| c.173C>T | p.Thr58Ile | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 22 monthss, male) | (Nakane et al., 2002; Niceta et al., 2015) |
| c.176C>G | p.Pro59Arg | ms | NA | Pre-d | y | m | y | y, cg, nu | n | y | y | y | n | n | n | n | n | Asperger | Proband | (Javadiyan et al., 2017) |
| c.176C>G | p.Pro59Arg | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | n | Mother of proband | |
| c.176C>A | p.Pro59His | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 9 years, female) | (Keppler-Noreuil et al., 2007; Niceta et al., 2015) |
| c.176C>A | p.Pro59His | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 5 years, female) | (Niceta et al., 2015) |
| c.185C>G | p.Thr62Arg | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 21 months, male) | (Niceta et al., 2015) |
| c.206C>G | p.Pro69Arg | ms | NA | Pre-d | y | m | n | y | n | y | n | y | n | n | n | n | n | Ayme-Gripp | Proband (age 21 months, male) | (Gripp et al., 1996; Niceta et al., 2015) |
| c.809C>A | p.Ser270Tyr | ms | AD | Pre-d | y | m | n | y, nu | n | n | n | n | n | y | n | y | n | n | Proband | (Dudakova et al., 2017) |
| c.809C>A | p.Ser270Tyr | ms | AD | Pre-d | y | m | n | y, cg, nu | n | n | n | n | n | y | n | y | n | n | Half brother of proband | |
| c.819G>C | p.Glu273Asp | ms | AD | Pre-d | y | m | n | y, cr | n | n | n | n | n | n | n | n | n | n | Two affected family members (father and son) | (Ma et al., 2016) |
| c.864G>C | p.Arg288Pro | ms | AD | nr | y | m | n | y, ct, nu | n | n | n | n | y | y | n | n | n | n | Two affected family members | (Jamieson et al., 2002) |
| c.880C>T | p.Arg294Trp | ms | AD | Pre-d | y | m | n | y, cg | n | n | n | n | n | n | n | n | n | n | Probands from thirty families | (Ma et al., 2016) |
| c.880C>T | p.Arg294Trp | ms | AD | Pre-d | y | m | n | y, nu | n | n | n | n | n | n | n | y | n | n | Seven affected family members | (Sun et al., 2014) |
| c.890A>G | p.Lys297Arg | ms | AD | nr | y | m | n | y, cr | n | n | n | n | n | n | n | n | n | n | Proband (8 years age) | (Vanita et al., 2006) |
| c.890A>G | p.Lys297Arg | ms | AD | nr | y | m | n | y | n | n | n | n | n | n | n | n | n | n | Five affected family members | |
| c.890A>G | p.Lys297Arg | ms | AD | nr | y | m | n | y | n | n | n | n | y | y | n | n | n | n | Six affected family members | |
| c.895C>A | p. Arg299Ser | ms | NA | nr | y | m | n | y, cg, po, nu | n | n | n | n | y | n | n | n | n | n | Four affected family members | (Hansen et al., 2007, 2009) |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y, cg | n | n | n | n | n | n | n | n | n | n | Proband | (Narumi et al., 2014) |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y, cg | n | n | n | n | n | n | n | n | n | n | Mother of proband | |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y | n | n | n | n | n | n | n | n | n | n | Maternal grandmother of proband | |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y, la | n | n | n | n | y | y | n | n | n | n | First maternal cousin of proband | |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y, nu | n | n | y | n | n | n | n | n | n | n | Second maternal cousin of proband | |
| c.908A>C | p.Gln303Leu | ms | NA | Pre-d | y | m | n | y | n | n | n | n | n | y | n | n | n | n | Mother and sister of second maternal cousin of proband | |
| c.915C>T | p.Cys305Trp | ms | AD | Pre-d | y | m | n | y, cg | n | n | y | n | n | n | n | n | n | n | Proband | (Ma et al., 2016) |
| c.958A>G | p.Lys320Glu | ms | NA | nr | y | m | n | y, cg | n | n | n | n | n | n | n | n | n | n | Proband | (Hansen et al., 2009) |
| c.958A>G | p.Lys320Glu | ms | NA | nr | y | m | n | n | n | n | n | n | n | y | n | n | n | n | One affected family member | |
| t(5;16)(p15.3;q23.2) | - | - | NA | nr | y | m | n | y | n | n | n | n | n | n | n | n | n | n | Two affected family member | (Jamieson et al., 2002) |
| t(5;16)(p15.3;q23.2) | - | - | NA | nr | y | m | n | y, cg | y | n | n | n | y | n | y | n | y | n | Other affected family members |
Notes: NA, not reported in original article; Nuc. Pos., nucleotide position in CDS; AA Pos., amino acid position; Vt, variant type; fs, frameshift; ms, missense; ns, nonsense; nt, nonstop; AD, autosomal dominant; AR, autosomal recessive; NA, not available; Prot. Func, protein function; nr, not reported; Pre-d, predicted- damaging; n, no; y, yes; AA con., amino acid conservation; Patho., pathogenicity; m, maybe pathogenic; Ap, Aphakia; C, Cataract; cr, cerulean; cg, congenital; ct, cortical; la, lamellar; nu, nuclear; po, polar; Co, Corneal opacity/abnormal cornea; Fc, Facial characteristics; G, Glaucoma; H, Hearing loss; Col, Iris coloboma/coloboma/abnormal iris; Mc, Microcornea; Mt, Microphthalmia; Ny, Nystagmus; Pa, Peters anomaly; Sy, Syndrome; Pt. Info., patient information; Ref., reference
Figure 5. Schematic of MAF gene, protein domains, and position of mutations.
(A) The human MAF gene (top) spans two exons (E1-E2) that encode the HSF4 protein (bottom). Exon 1 encodes the transactivation domain (pink) and the bZIP domain (blue). Nucleotide positions for cDNA and genomic DNA in the reference sequence (hg38) and the corresponding amino acid positions in the protein sequence are given. The UCSC genome browser (https://genome.ucsc.edu) was used to obtained cDNA, genomic and protein position information from MAF human GRCh38/hg38 assembly (reference sequence NM_005360, uc002ffm.4 at chr16:79,593,838–79,600,714). Total 20 mutations, of which 18 represent unique mutations identified in independent studies are indicated as flags. These include 17 missense and 1 translocation mutation. Nucleotide changes in DNA and amino acid changes in protein sequence are indicated.
Figure 6. Multiple sequence alignment of MAF protein sequences.
Protein sequences for MAF downloaded from UCSC browser for Homo sapiens, Mus musculus, Gallus gallus, and Xenopus tropicalis shows amino acid conservation across different vertebrate species. Amino acid changes caused by specific human mutations are indicated by yellow arrowheads. The sequence consensus is presented for each amino-acid. Transactivation domain and bZIP domain are indicated by green arrow.
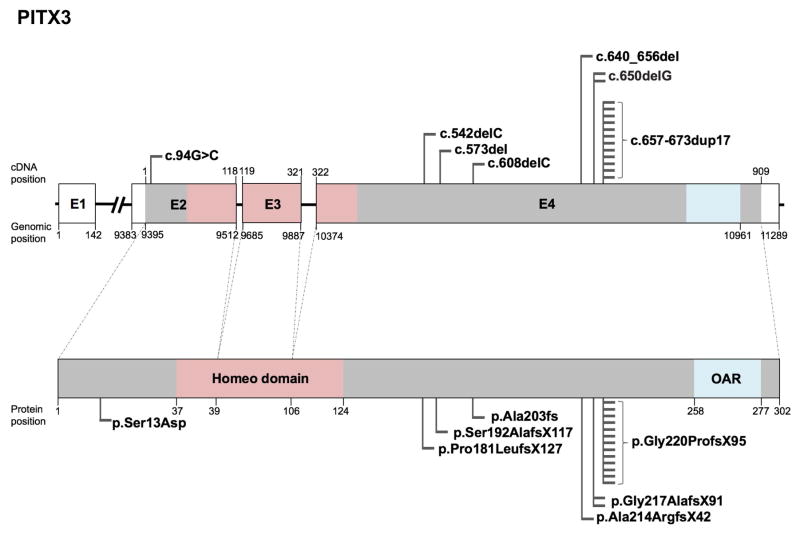
Disease-Causing PITX3 Variants
The pituitary homeobox (PITX) family of proteins are transcription factors that contain two domains, the homeobox domain and the OAR (otp, aristaless, rax) domain that function in DNA-binding and potentially transactivation, respectively. Mutations in the PITX family gene PITX3 cause congenital cataract and anterior segment mesenchymal dysgenesis (ASMD) (Semina et al., 1998). Presently, nineteen studies have reported seven unique mutations with only 1 missense, and 6 frameshift PITX3 mutations in 168 individuals and are associated with the following defects: ASMD (87 affected individuals), congenital or early childhood onset cataract (155 affected individuals), corneal opacity (9 affected individuals), microphthalmia (3 affected individuals), microcornea (4 affected individuals), nystagmus (3 affected individuals) and glaucoma (1 affected individual) (Table 4). The missense PITX3 mutation (p.Ser13Asp) reported (Semina et al., 1998) is inherited autosomal dominantly, and is the only mutation altering Exon 2 of PITX3. Exon 2 is the first coding exon, and based on the serine (polar) to aspartic acid (acidic) amino acid substitution, it can be inferred that this alteration likely affects the protein structure/function significantly, even though the mutation is in-frame and the protein is likely still expressed. Furthermore, majority of the PITX3 mutations identified thus far are in exon 4 and interestingly no mutation has yet been described in the homeodomain- or the OAR domain-encoding region (Fig. 7). Sequence alignment demonstrate that the Ser13 residue in PITX3, which is affected by the c.94G>C mutation, is conserved in vertebrates (Fig. 8). A complete list of PITX3 variants, including nucleotide and predicted amino acid changes are given in Table 4 and further details on probands are given in Supp. Table S4. The updated PITX3 gene variants information is deposited in the Leiden Online Variation Database (LOVD) platform at http://www.LOVD.nl/PITX3.
Table 4.
PITX3 human mutations
| Nuc. Pos. | AA Pos. | Vt | AD/AR | Prot. Func. | AA Con. | Patho. | Ap | Asd | C | Co | G | Mc | Mt | Ny | Sc | Pat. Info. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.94G>A | p.Ser13Asp | ms | AD | nr | y | m | n | n | y, cg | n | n | n | n | n | n | Proband | (Semina et al., 1998) |
| c.542delC | p.Pro181LeufsX127 | fs | AD | nr | y | m | n | n | y, po | n | n | n | n | n | n | Proband | (Berry et al., 2011) |
| c.542delC | p.Pro181LeufsX127 | fs | AD | nr | y | m | n | n | y, po | n | n | n | n | n | n | Eight affected family members | |
| c.573del | p.Ser192AlafsX117 | fs | AD | Exp-d | n | m | n | n | y, cg | y | n | y | n | n | n | Proband | (Verdin et al., 2014) |
| c.573del | p.Ser192AlafsX117 | fs | AD | Exp-d | n | m | n | n | y, cg | y | n | y | n | n | n | Brother of proband | |
| c.573del | p.Ser192AlafsX117 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | y | n | n | n | Father of proband | |
| c.608delC | p.Ala203fs | fs | NA | nr | n | m | n | n | y, cg | n | n | n | n | n | n | Seven affected family members | (Liu et al., 2017) |
| c.640_656del | p.Ala214ArgfsX42 | fs | AR | nr | y | m | n | y | y, cg | n | n | n | y | n | y | Proband | (Aldahmesh et al., 2011) |
| c.650delG | p.Gly217AlafsX91 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Six affected family members | (Berry et al., 2004) |
| c.650delG | p.Gly217AlafsX91 | fs | AD | nr | n | m | n | n | y, po | y | n | n | y | n | n | Twenty-eight affected family members | Bidinost et al 2006 |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | NA | nr | n | m | n | y | n | n | n | n | n | n | n | Eight affected family members | (Semina et al., 1998) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, cg, po,ec | n | n | n | n | n | n | Nine affected family members | (Berry et al., 2004) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | y | y, cg, po,ec | n | n | n | n | n | n | One affected family members | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Seven affected family members | (Berry et al., 2004) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | y | y, po | n | n | n | n | n | n | Four affected family members | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Seventeen affected family members | (Berry et al., 2004) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Eighteen affected family members | (Berry et al., 2004) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Twenty affected family members | (Finzi et al., 2005) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | y | n | y, po | n | n | n | n | y | n | One affected family member | (Burdon et al., 2006) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y, po | n | n | n | n | n | n | Twenty eightaffected family member | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | nr | n | m | n | n | y | y | n | n | n | n | n | Proband | (Summers et al., 2008) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y | y | n | n | n | n | n | Proband | (Verdin et al., 2014) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | y | y, cg | n | n | n | n | n | n | Brother of proband | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | n | n | n | n | Father of proband | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | y | y | y | n | y | n | Proband | (Verdin et al., 2014) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | n | n | n | n | Five affected family members | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | y | y, cg | y | n | n | n | n | n | Proband | (Verdin et al., 2014) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | n | n | n | n | Brother of proband | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | n | n | n | n | Mother of proband | |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | y | n | n | n | y | n | Proband | (Verdin et al., 2014) |
| c.657_673dup17 | p.Gly220ProfsX95 | fs | AD | Exp-d | n | m | n | n | y, cg | n | n | n | n | n | n | Five affected family members |
Notes: NA, not reported in original article; Nuc. Pos., nucleotide position in CDS; AA Pos., amino acid position; Vt, variant type; fs, frameshift; ms, missense; ns, nonsense; nt, nonstop; AD, autosomal dominant; AR, autosomal recessive; NA, not available; Prot. Func, protein function; nr, not reported; Exp-d, experimental-damaging; n, no; y, yes; AA con., amino acid conservation; Patho., pathogenicity; m, maybe pathogenic; Ap, Aphakia; Asd, Anterior segment dysgenesis; C, Cataract; cg, congenital; ec, early childhood; po, polar; Co, Corneal opacity/abnormal cornea; G, Glaucoma; Mc, Microcornea; Mt, Microphthalmia; Ny, Nystagmus; Sc, Sclerocornea; Pt. Info., patient information; Ref., reference
Figure 7. Schematic of PITX3 gene, protein domains, and position of mutations.
(A) The human PITX3 gene (top) consists of four exons (E1-E4), of which exons 2 through 4 encode the PITX3 protein. Exons 2 through 4 encode the homeodomain (pink) and exon 4 encodes the OAR domain (blue). Nucleotide positions for cDNA and genomic DNA in the reference sequence (hg38) and the corresponding amino acid positions in the protein sequence are given. The UCSC genome browser (https://genome.ucsc.edu) was used to obtained cDNA, genomic and protein position information from PITX3 human GRCh38/hg38 assembly (reference sequence NM_005029, uc001kuu.2 at chr10:102,230,186–102,241,474). Total 19 mutations, of which 7 represent unique mutations identified in independent studies are indicated as flags. These include 6 frameshift and 1 missense mutation.
Figure 8. Multiple sequence alignment of PITX3 protein sequences.
Protein sequences for PITX3 downloaded from UCSC browser for Homo sapiens, Mus musculus, Gallus gallus, Xenopus tropicalis and Danio rerio shows amino acid conservation across different vertebrate species. Amino acid changes caused by specific human mutations are indicated by yellow arrowheads. The sequence consensus is presented for each amino-acid. Homeodomain and OAR domain are indicated by green arrow.
Functional Insights from Animal Models and Biochemical Assays
Various animal models such as zebrafish, Xenopus, chicken, and mouse have been applied to investigate the function of Foxe3, Hsf4, Maf and Pitx3 in development and organogenesis. Notably, multiple gene-specific perturbation mouse mutants have been derived for all four genes. The characterization of these animal models has informed on the pathobiology of the underlying defects, in turn elucidating the molecular function of these transcription factors in ocular and non-ocular tissue development. These various animal models and the principle findings from their studies are discussed below for each of these genes.
FOXE3 Animal Models and Mechanistic Insights into the Pathology of Anterior Segment Defects
FOXE3 expression in the developing ocular lens is conserved in vertebrates as revealed from studies on fish (zebrafish, Danio rerio), frog (Xenopus) and mammals (mouse, human) (Kenyon et al., 1999; Blixt et al., 2000; Brownell et al., 2000; Semina et al., 2001; Shi et al., 2006; Swindell et al., 2008). Foxe3 expression in mouse lens development is initiated early at the lens placode stage and in later stages is restricted to the anterior epithelium of the lens (AEL). In addition, Foxe3 is also expressed in the forebrain, midbrain and the pharyngeal arches in mouse development (Blixt et al., 2000; Brownell et al., 2000; Kuang et al., 2016). Several distinct types of Foxe3 perturbation mouse models – exhibiting either gain-of-function or loss-of-function – have been characterized. These include mice carrying spontaneous mutations, namely dyl (dysgenetic lens) (Sanyal and Hawkins, 1979; Blixt et al., 2000; Brownell et al., 2000) and rct (Rinshoken cataract) (Wada et al., 2011), two independent mouse targeted gene knockout mouse models (Medina-Martinez et al., 2005; Blixt et al., 2007), as well as a transgenic mouse with Cryaa-promoter driven Foxe3 over-expression in lens fiber cells (Landgren et al., 2008). In the dyl mouse mutant, two closely located co-segregating missense mutations (Phe93Leu, Phe98Ser) were identified in the DNA-binding domain encoding region of the Foxe3 gene, which compromise its DNA-binding function. Mice homozygous for the dyl locus exhibit fully penetrant anterior segment defects characterized by the failed separation of the lens vesicle from the surface ectoderm (that contributes to the future corneal epithelium) (Sanyal and Hawkins, 1979; Blixt et al., 2000; Brownell et al., 2000). Interestingly, dyl heterozygous mice also exhibit lens and cornea defects with features of Peters anomaly, albeit at reduced penetrance, and therefore have been suggested as an animal model for this eye disorder (Ormestad et al., 2002). Homozygous rct mutant mice carry a 22-bp deletion in a putative Foxe3 cis-regulatory element that results in reduced Foxe3 expression in the lens, and causes cataract and microphthalmia (Wada et al., 2011).
Targeted deletion of Foxe3 in mice causes eye defects similar to those observed in homozygous dyl mice, although there are some minor differences between two independently developed gene-knockout models (Medina-Martinez et al., 2005; Blixt et al., 2007). Marker gene expression analysis of the various Foxe3 null mice has indicated that FOXE3 is required for promoting proliferation of cells of the AEL (explaining the reduced size of the mutant lens) while preventing their pre-mature differentiation into fiber cells. Ablation of FOXE3 results in a progressive loss of AEL cells, defective fiber differentiation and induction of apoptosis, which causes abnormal spaces in the posterior region of the lens (Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005; Blixt et al., 2007). Notably, FOXE3 negatively regulates an inhibitor of cyclin-dependent kinase (CDKN1C, p57KIP2) as well as a transcription factor (PROX1) that is required for proper fiber cell differentiation. In support of this, the expression of the fiber cell enriched crystallin genes, namely α- and β-Crytallins, are abnormally up-regulated in Foxe3 null mouse lens AEL (Brownell et al., 2000). In addition to the lens defects, Foxe3 deficiency in mice causes a defect in the differentiation of the periocular mesenchyme into corneal endothelium, trabecular meshwork and the different cell types of the iris and the ciliary body stroma, and therefore offers an explanation for the human ocular phenotypes involving the iris and cornea observed in patients with FOXE3 mutations (Blixt et al., 2007). Further, Foxe3 heterozygous mice exhibit a partial closure of the filtration angle by iridocorneal tissue, which may serve to explain the observed glaucoma defects in a subset of human patients (Blixt et al., 2007).
The molecular consequence of ectopic Foxe3 expression in lens fiber cells, which results in severe lens defects in mice, has been investigated by expression profiling microarrays (Landgren et al., 2008). These microarray data indicate that FOXE3 interferes with fiber cell differentiation by mis-regulating genes involved in maintenance of various cellular properties including its shape, cytoskeleton, organelle degradation and extracellular matrix. For example, genes associated with fiber cell differentiation (Capn3, Casp7, Bcl2l13) are down-regulated, while those associated with AEL (S100a1, Sdpr) are up-regulated upon FOXE3 over-expression in fiber cells. Further, expression of a Peters anomaly associated truncated FOXE3 mutant protein in human cell lines results in the down-regulation of DNAJB1 on both mRNA and protein levels, suggesting that FOXE3 may directly control autophagy factors in ocular development (Khan et al., 2016). In addition to the mouse models that compromise Foxe3 function, a mouse mutant (“vacuolated lens”, vl) – carrying a Foxe3 polymorphism that results in amino acid change Leu23Pro and a Gpr161 truncation mutation – exhibit cataract, in turn suggesting Foxe3 as a genetic modifier of the cataract phenotype (Matteson et al., 2008).
Further, various biochemical assays have been used to investigate the different human FOXE3 variants with regards to alterations in their DNA-binding properties and transactivation of reporter gene expression (Islam et al., 2015). The protein products encoded by the FOXE3 variants linked to primary aphakia in humans c.21_24del, c.358C>G, and c.705del showed loss of DNA-binding and reduced activation of luciferase reporter. Interestingly, while the protein product of the FOXE3 variant c.269G>T retained its DNA-binding property, it showed reduced activation in luciferase reporter assays. This study provides evidence that specific FOXE3 variants result in functionally defective proteins, thus suggesting altered FOXE3 function, rather than reduced dosage, as the pathogenic mechanism for FOXE3 dominant mutations (Islam et al., 2015).
Finally, the necessity of FOXE3 for proper lens development is conserved in vertebrates, although some differences in its function are observed between fish and mice. In zebrafish, foxe3 knockdown using antisense morpholinos causes a small lens phenotype that exhibits multiple layers of epithelial cells and abnormal fiber cells (Shi et al., 2006; Swindell et al., 2008). Thus, while Foxe3 mutant mice exhibit an epithelial cell proliferation defect, zebrafish foxe3 morphants harbor an undifferentiated population of epithelial cells. However, both mouse and fish Foxe3 mutants exhibit fiber cell defects and a down-regulation of the platelet-derived growth factor receptor pdgfrα, similar to that observed in Foxe3 mutant mice (Blixt et al., 2000; Medina-Martinez et al., 2005; Swindell et al., 2008). Interestingly, the zebrafish foxe3 morphant lenses exhibited mis-expression of genes (e.g. rhodopsin) normally expressed in the retina, indicating its requirement for cell-specific gene regulation in lens development (Shi et al., 2006). Together, these studies on animal models provide mechanistic insights into FOXE3 gene regulatory network in eye development and into the underlying pathology of ocular defects associated with its deficiency.
HSF4 Animal Models and Mechanistic Insights into the Pathology of Cataract
Apart from the DNA-binding domain near the amino-terminal, HSF proteins also contain leucine zipper-like heptad repeat A and B (HR-A/B) domains that are required for HSF monomer trimerization and binding to DNA. Among the HSF factors, HSF4 is unique because it does not contain an additional leucine zipper-like HR-C domain (a hydrophobic repeat at the carboxyl-terminal), which normally functions in HSF1 and HSF2 to inhibit the formation of active trimers (Nakai et al., 1997). This suggests that HSF4 may be able to bind to HSEs under non-stress conditions and therefore may participate in developmental events. Further, HSF4 is expressed as two splice forms, HSF4a and HSF4b (HSF4b has an additional 30 amino acids and is expressed in the lens), that function as a transcriptional repressor and a transcriptional activator, respectively (Tanabe et al., 1999; Smaoui et al., 2004). Finally, a motif for a unique phosphorylation-dependent sumoylation (PDSM) translational modification has been identified in the regulatory domain of HSF4, which may control its function (Hietakangas et al., 2006).
Hsf4 expression has been reported at high levels in embryonic and adult mouse lens (Fujimoto et al., 2004; Min et al., 2004; Lachke et al., 2012) and its expression in the human brain, heart, lung, pancreas and skeletal muscle is also documented (Nakai et al., 1997; Fujimoto et al., 2004). So far, HSF4 function in development has been investigated using multiple different Hsf4 mouse mutant alleles that were generated either by targeted inactivation, BAC transgenesis, or were identified as a spontaneous mutation (Fujimoto et al., 2004; Jablonski et al., 2004; Min et al., 2004; Talamas et al., 2006; Shi et al., 2009; He et al., 2010; Liang et al., 2011; Gangalum et al., 2014; Jing et al., 2014). Targeted Hsf4 deletion in mice results in lens fiber cell defects and cataract (Fujimoto et al., 2004; Min et al., 2004; Shi et al., 2009). Further, a spontaneous mutation in Hsf4 intron 9 (termed “lens opacity 11” locus (lop11)) has been identified in the RIIIS/J mouse strain (Talamas et al., 2006). Hsf4lop11 mice carry a 61-bp insertion of early transposable element (ETn) in intron 9 that functions as a pseudo-exon. This insertion generates a chimeric Hsf4 transcript, which encodes a truncated, non-functional HSF4 protein with a 132 amino acid deletion in the carboxyl terminus (Talamas et al., 2006; Liang et al., 2011). The same mutation was identified in the mouse termed “lens disrupter 1” (ldis1) that exhibits cataract (Jablonski et al., 2004). Hsf4lop11 mice exhibit severe fiber cell defects and cataract at postnatal day (P) 12 and involve fiber cell denucleation defects (Talamas et al., 2006; Liang et al., 2011). In addition to these mutant mice, BAC transgenesis-derived mice, which model the lamellar opacity observed in human childhood cataract, have been described previously (Gangalum et al., 2014). This approach has led to specifically modelling the lamellar cataract observed in human HSF4 p.Arg116His mutation (Jing et al., 2014).
Gene expression profiling studies on Hsf4 mouse mutant lenses have identified several potential target genes that may be involved in the cataract pathobiology. For example, Hsf4 targeted knockout mouse early postnatal (P2) lens exhibits downregulation of γ-crystallin genes (Cryga, Crygb, Crygc, Cryge, Crygf), all of which carry HSEs in their promoter regions (Fujimoto et al., 2004). Down-regulation of γ-crystallin genes is also exhibited by ldis1 mouse mutants (Jablonski et al., 2004). Further, the heat shock protein HSPB1 (also known as HSP25, HSP27), which is known to interact with αA-crystallins, is down-regulated in Hsf4 null mouse lens (Fujimoto et al., 2004; Min et al., 2004). In an independently generated Hsf4 germline knockout mouse mutant, the cataract-linked and fiber cell expressed genes Bfsp1, Bfsp2 and Crygs are down-regulated in the lens at age 8-weeks (Shi et al., 2009). Further, transcriptional activity assays suggest that Bfsp1 and Bfsp2 are direct targets of HSF4. A two-dimensional (2-D) electrophoretic analysis identifies a post-translational modification defect in αA-Crystallin and shows that CRYGS was also reduced at the protein-level in the Hsf4 mutant lens, as were the calcium activated proteases LP82 and CALPAIN2, which are responsible for post-translational modifications in differentiating fiber cells (Shi et al., 2009). Finally, microarray-based genome-level transcriptional profiling has been performed on lenses of two independent Hsf4 targeted knockout mouse mutants at postnatal stages P0 and P10 (Min et al., 2004; He et al., 2010). Newborn Hsf4 null lenses exhibit 1428 differentially expressed genes, a subset of which are commonly regulated by other transcription factors in the lens (He et al., 2010). Microarray analysis at P10 stage Hsf4 null lens revealed that the range of HSF4-target genes is well beyond those associated with stress-response and also involves genes functioning in lens development (He et al., 2010), which is also independently suggested by chromatin-immunoprecipitation (ChIP) assays for HSF4 in stage P2 mouse lens (Fujimoto et al., 2008). Both microarray studies identified Dnase2b – known to be required for fiber nuclear degradation (Nishimoto et al., 2003) – to be significantly reduced in Hsf4 null mutant lenses, providing an explanation for the de-nucleation defect associated with Hsf4 deficiency. Interestingly, Hsf4 has been shown to regulate transcription of Dnase2b by direct binding to its promoter (Cui et al., 2013; He et al., 2016).
Thus, these cellular, biochemical and molecular studies using animal models are beginning to define a HSF4-regulatory network operational in differentiating lens fiber cells that when perturbed causes cataract.
MAF Animal Models and Mechanistic Insights into the Pathology of Eye Defects
MAF (c-MAF) encodes a leucine zipper transcription factor that exhibits conserved expression in vertebrate eye development (Yang and Cvekl, 2007). In mice, Maf is expressed in the lens placode, and subsequently in the lens vesicle and lens fiber cells (Kawauchi et al., 1999; Kim et al., 1999; Ring et al., 2000). Outside of the eye, MAF expression is detected in the cartilage (of basioccippital bone, limb, rib), chondrocytes, cochlea, dorsal root ganglia neurons, dorsal spinal cord, forebrain, heart, intestine, kidney, liver, lung, muscle, perichondrium of the skeleton, skin, spleen, uterus, and in the T helper (Th) cells – Th2 and Th17 (Sakai et al., 1997; Ring et al., 2000; Sato et al., 2011; Wende et al., 2012; Niceta et al., 2015). In mice, targeted deletion of Maf (homozygous null) results in early postnatal mortality and ocular developmental defects including microphthalmia as well as lens defects (Kawauchi et al., 1999; Kim et al., 1999; Ring et al., 2000). In Maf−/− mutant mouse, lens fiber cells exhibit normal development until E12.5, but beyond this stage, they fail to elongate. Further, Maf−/− mutant lenses exhibit reduced expression of several Crystallin genes (e.g. Crystallins αA-, βB2-, βA3/A1-, βA4-, and γD-). In agreement with this, MAF-binding motifs (termed MAF response elements (MAREs) are identified in the cis-regulatory regions of several Crystallin genes (Cui et al., 2004; Rajaram and Kerppola, 2004; Yang et al., 2004; Yang and Cvekl, 2005; Yang et al., 2006). Together these data show indicate that MAF is an important regulator of the Crystallin gene regulatory network in the lens. Further, besides the ocular phenotypes, Maf−/− mice also show defects in terminal differentiation of chondrocytes during bone development (MacLean et al., 2003) – a phenotype which is not entirely surprising, as MAF is shown to be expressed in chondrocytes (Sakai et al., 1997).
In addition to targeted deletion, spontaneous – as well as ENU-induced – Maf mutant alleles have been identified in mice. Interestingly, the mouse spontaneous Maf missense mutation c.1803G>A (p.Arg291Gln), termed “opaque flecks in lens” (Ofl), lies near the human MAF mutations p.Arg288Pro and p.Arg294Trp, which are in the basic domain of the encoded protein (Lyon et al., 2003; Sun et al., 2014; Ma et al., 2016). While in human, the p.Arg288Pro mutation causes cortical or nuclear cataract, microcornea and iris coloboma, and the p.Arg294Trp causes nuclear cataract (Table 3), in mouse, the p.Arg291Gln mutation causes cataract accompanied by anterior segment abnormalities, which is influenced by the genetic background (Lyon et al., 2003). Interestingly, heterozygous Ofl mutant mice exhibit pulverulent cataract and homozygous Ofl mutants exhibit – in addition to ocular defects – renal tubular nephritis and early postnatal lethality (Lyon et al., 2003). Biochemical assays indicate that the mouse Olf p.Arg291Gln mutation alters the DNA-binding affinity of MAF, and thus reduces its transactivation potential at target promoters, similar to the human p.Arg288Pro mutation (Perveen et al., 2007). This finding offers a molecular explanation for the observed dominant negative effect of the Olf mutant allele that causes the cataract phenotype in heterozygous state in these mouse mutants, which is not observed in Maf targeted knockout mice. Another Maf gene mouse mutant, designated MafENU424, has been identified in an N-ethyl-N-nitrosourea (ENU) mutagenesis screen (Perveen et al., 2007). This Maf missense mutation (c.269A>T, p.Asp90Val) affects a conserved aspartate residue in the transactivation domain of the protein, which in homozygous state, results in isolated cataract. Further, biochemical studies show that the p.Asp90Val mutation causes MAF-mediated transactivation to be non-receptive to inhibition by the protein kinase A pathway, and therefore results in elevated promoter activity, which interesting includes the Pitx3 promoter (Perveen et al., 2007). These studies show that specific changes in conserved regions of the MAF protein can result in loss-of-function (e.g. mouse p.Arg291Gln, human p.Arg288Pro) or gain-of-function (p.Asp90Val) variants.
In addition to these mutants, a mouse line overexpressing Maf in has been developed (Ho et al., 1998). Using these mice, it has been demonstrated that MAF negatively regulates Th1 differentiation while positively regulating Th2 differentiation. Finally, in addition to these various loss-of-function and gain-of-function Maf mouse mutant alleles, a recent study has generated a targeted conditional Maf mouse mutant line (Wende et al., 2012). In conjunction with a Isl1Cre detetor mouse, this conditional Maf mouse line has been utilized to demonstrate an unprecedented function for MAF in mechanoreceptors (Wende et al., 2012). Together, these findings suggest that the range of phenotypes associated with MAF deficiency may be broader than previously anticipated.
PITX3 Animal Models and Mechanistic Insights into the Pathology of Eye Defects
In mice, PITX3 expression is detected in the lens placode starting at E9.75, the entire lens vesicle at E10.5, and in both the anterior epithelial cells and the posteriorly localized fiber cells at E11.5 through E12.5, before being restricted in the epithelium at E14.5 and later stages (Semina et al., 1997; Ho et al., 2009; Medina-Martinez et al., 2009). In addition to the lens, PITX3 is also strongly expressed in mouse midbrain mesodiencephalic dopaminergic neurons starting from E11.5 and this expression is conserved between mouse, rat, and human (Smidt et al., 1997; Zhao et al., 2004). Further, PITX3 is detected in tongue, incisors, sternum, vertebrae and limbs (Semina et al., 1998) and in skeletal muscle cells (Coulon et al., 2007). Both spontaneous and targeted knockout mouse mutants have been described for Pitx3 (Semina et al., 1997, 2000; Ho et al., 2009; Rosemann et al., 2010).
The spontaneous mutation aphakia (ak) is recessive and causes a small eye phenotype with the absence of lens and eyelids (Varnum and Stevens, 1968; Grimm et al., 1998). Pitx3 was mapped near the ak locus on mouse chromosome 19 and its expression was absent in ak/ak homozygous mutant lens (Semina et al., 1997, 2000). While the Pitx3 coding sequence is intact in ak mice, these animals carry a double deletion – a distant intergenic deletion plus another removing exon 1 and part of intron 1 – that segregates with the ak allele (Semina et al., 2000; Rieger et al., 2001). The deletion of the Pitx3 upstream sequence carrying potential regulatory elements (for transcription factors such as MAF) is considered the basis of the ak eye-related phenotypes (Semina et al., 2000). Subsequent analysis has shown that ak mutant lenses exhibit reduced proliferation and elevated apoptosis, as well as defects in fiber differentiation as indicated by reduced expression of Prox1, p57Kip2 and various crystallins, including the abnormal distribution of γ-crystallin transcripts (Medina-Martinez et al., 2009). While one study found no genetic interaction between Pitx3 and Foxe3 (Medina-Martinez et al., 2009), other findings suggest that PITX3 transcriptionally controls Foxe3 expression (Ahmad et al., 2013). Interestingly, pitx3 knockdown in zebrafish results lens and retina defects and in reduced foxe3 lens expression (Shi et al., 2005, 2006), suggesting that PITX3 mediated regulation of FOXE3 is conserved in vertebrates. In addition to the ocular defects, and as expected from PITX3 expression pattern, ak mice also exhibit significantly reduced numbers of dopaminergic neurons (Hwang et al., 2003).
Besides ak, another spontaneous recessive mutation, Pitx3eyl (c.416insG), has been identified in mouse, which results in a frameshift that is predicted to include 121 different amino acids downstream of the homeodomain (Rosemann et al., 2010). Homozygous Pitx3eyl/eyl mutants have ocular and neuronal defects similar to ak mutants, and additionally exhibit defects in the spleen and liver. Further, these mutants have behavioral defects resembling human Parkinson patients (Rosemann et al., 2010). Recently, yet another new spontaneous nonsense recessive Pitx3 mutation (c.444C>A; p. Tyr148>X) called miak (microphthalmia and aphakia) has been identified in the Japanese mouse strain Mus musculus molossinus, which causes aphakia and microphthalmia (Wada et al., 2014). Homozygous Pitx3miak/miak mice exhibit an over-expression of the mutant PITX3 protein that is predicted to have a C′ 155 amino acid truncation. These mutants have delayed differentiation of lens fiber cells and showed reduction in important lens proteins such as FOXE3, MIP, αβ-, β-, and γ-Crystallins. Finally, germline targeted Pitx3 deletion mouse mutants have been generated. Interestingly, while FOXE3 is reduced in the Pitx3 null mouse lenses in agreement with other Pitx3 mouse mutant models, PROX1 expression does not appear to be reduced as in ak lenses, but rather is up-regulated in the lens anterior epithelium, suggesting that PITX3 functions to repress PROX1 in these cells (Ho et al., 2009). Further, β- and γ-crystallin expression was abnormally detected in early stages of lens development in Pitx3 null mice. These findings suggest that PITX3 has distinct functions in epithelial cell maintenance and initiation of fiber differentiation.
Interestingly, a genome level search of PITX3 binding motifs has led to the identification of MIP as a direct target of this protein. Indeed, chromatin immunoprecipitation, reporter assays and knockdown in zebrafish has demonstrated that PITX3 is necessary for transcriptionally activating MIP expression by binding to a regulatory site that is conserved in human and fish (Sakazume et al., 2007; Sorokina et al., 2011). Moreover, functional analysis by electrophoretic mobility shift assay (EMSA) and luciferase reporter assays revealed defective DNA-binding and transactivation rendered by specific mutations in human PITX3 proteins (p.11Lys>Glu, p.13Ser>Asn, p.Ser192AlafsX117, p.Gly220ProfsX95) (Sakazume et al., 2007; Sorokina et al., 2011; Verdin et al., 2014).
Future Prospects
In this article, we provide a comprehensive and updated summary of variants in four transcription factors genes FOXE3, HSF4, MAF and PITX3 that are commonly associated with ocular defects, including cataract, in addition to other developmental defects. Schematic of the variants in relation to the cDNA/gene are depicted at the nucleotide level as is their effect on the predicted protein. This serves to inform on variant distribution and frequency in the context of the protein and its domain(s), while the tables convey clinical details. Additionally, the conservation of the amino acid residues, which are affected by these mutations, across various vertebrate species is presented, and biochemical assays delineating the functional relevance of the variants are described. Importantly, this update reveals the frequently identified mutations and their correlation with specific phenotypes. For example, the FOXE3 gene has distinct variants that are linked to either ocular defects or thoracic aortic aneurysms and acute aortic dissections. Thus, this mutation update for FOXE3, HSF4, MAF and PITX3 provides key information to clinicians and developmental geneticists that may impact genetic investigation as well as diagnosis and prognosis of patients with these ocular defects.
In particular, this update highlights how animal models have provided rich information on understanding the function of these proteins in normal development and the effect of their mutation/deficiency on pathology. In some cases, the mouse mutant models have revealed an unexpected function, for example MAF’s role in mechanoreceptor cell function. Thus, these animal models may help investigate new subtle/previously undiagnosed defects in human patients. Also, some of the spontaneous or ENU mutations in mice have shed light into the function of these proteins. For example, while no variant in the transactivation domain of MAF has been described so far in human, a mutation in this region of the protein has been identified in the mouse. Additionally, mutations in the regulatory regions of transcription factor genes such as PAX6 are linked to human ocular defects (Kleinjan et al., 2001; Kleinjan and van Heyningen, 2005). Efforts will be needed to characterize the transcriptional regulation of FOXE3, HSF4, MAF, and PITX3 in animal models, which will facilitate the identification of regulatory mutations in these genes in human. Further, additional molecular studies on these mouse mutants employing chromatin immunoprecipitation followed by next-gen sequencing (ChIP-seq) and RNA-sequencing in combination with the bioinformatics tools such as iSyTE (integrated Systems Tool for Eye gene discovery) (Kakrana et al. 2018) will provide comprehensive information on the range of target genes regulated by FOXE3, HSF4, MAF and PITX3. These studies will also inform on the inter-connectedness of the gene regulatory network of these transcription factors, in support of which there is already some evidence. For example, FOXE3 may be regulated by PITX3, which itself may be downstream of MAF. Finally, although majority of this research has used germline targeted gene deletion in mouse, generation of conditional mutant alleles and the advent of CRISPR technology (Cong et al., 2013) will expedite the process of generating customized mouse mutant models for specific human mutations identified in these genes, in turn leading to a comprehensive understanding of their function in development.
Supplementary Material
Acknowledgments
Research in the Lachke laboratory is supported by the National Eye Institute of the National Institutes of Health under Award Number R01EY021505 and a University of Delaware Research Foundation, Inc. Strategic Initiatives (UDRF-SI) Grant to SAL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SAL is a Pew Scholar in Biomedical Sciences.
Footnotes
Disclosure statement: The authors have no conflicts of interest to declare.
References
- Agrawal SA, Anand D, Siddam AD, Kakrana A, Dash S, Scheiblin DA, Dang CA, Terrell AM, Waters SM, Singh A, Motohashi H, Yamamoto M, et al. Compound mouse mutants of bZIP transcription factors Mafg and Mafk reveal a regulatory network of non-crystallin genes associated with cataract. Hum Genet. 2015;134:717–735. doi: 10.1007/s00439-015-1554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Aslam M, Muenster D, Horsch M, Khan MA, Carlsson P, Beckers J, Graw J. Pitx3 directly regulates Foxe3 during early lens development. Int J Dev Biol. 2013;57:741–751. doi: 10.1387/ijdb.130193jg. [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed J, Alkuraya FS. Novel recessive BFSP2 and PITX3 mutations: insights into mutational mechanisms from consanguineous populations. Genet Med Off J Am Coll Med Genet. 2011;13:978–981. doi: 10.1097/GIM.0b013e31822623d5. [DOI] [PubMed] [Google Scholar]
- Ali M, Buentello-Volante B, McKibbin M, Rocha-Medina JA, Fernandez-Fuentes N, Koga-Nakamura W, Ashiq A, Khan K, Booth AP, Williams G, Raashid Y, Jafri H, et al. Homozygous FOXE3 mutations cause non-syndromic, bilateral, total sclerocornea, aphakia, microphthalmia and optic disc coloboma. Mol Vis. 2010;16:1162–1168. [PMC free article] [PubMed] [Google Scholar]
- Anjum I, Eiberg H, Baig SM, Tommerup N, Hansen L. A mutation in the FOXE3 gene causes congenital primary aphakia in an autosomal recessive consanguineous Pakistani family. Mol Vis. 2010;16:549–555. [PMC free article] [PubMed] [Google Scholar]
- Aymé S, Philip N. Fine-Lubinsky syndrome: a fourth patient with brachycephaly, deafness, cataract, microstomia and mental retardation. Clin Dysmorphol. 1996;5:55–60. doi: 10.1097/00019605-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Behnam M, Imagawa E, Chaleshtori ARS, Ronasian F, Salehi M, Miyake N, Matsumoto N. A novel homozygous mutation in HSF4 causing autosomal recessive congenital cataract. J Hum Genet. 2016;61:177–179. doi: 10.1038/jhg.2015.127. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis PJ, Prescott Q, Waseem NH, Moore AT, Bhattacharya SS. A novel 1-bp deletion in PITX3 causing congenital posterior polar cataract. Mol Vis. 2011;17:1249–1253. [PMC free article] [PubMed] [Google Scholar]
- Berry V, Yang Z, Addison PKF, Francis PJ, Ionides A, Karan G, Jiang L, Lin W, Hu J, Yang R, Moore A, Zhang K, et al. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4) J Med Genet. 2004;41:e109. doi: 10.1136/jmg.2004.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Pontikos N, Moore A, Ionides ACW, Plagnol V, Cheetham ME, Michaelides M. A novel missense mutation in HSF4 causes autosomal-dominant congenital lamellar cataract in a British family. Eye (Lond) 2017 doi: 10.1038/eye.2017.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidinost C, Matsumoto M, Chung D, Salem N, Zhang K, Stockton DW, Khoury A, Megarbane A, Bejjani BA, Traboulsi EI. Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci. 2006;47:1274–1280. doi: 10.1167/iovs.05-1095. [DOI] [PubMed] [Google Scholar]
- Blixt A, Landgren H, Johansson BR, Carlsson P. Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev Biol. 2007;302:218–229. doi: 10.1016/j.ydbio.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerbäck S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Brémond-Gignac D, Bitoun P, Reis LM, Copin H, Murray JC, Semina EV. Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol Vis. 2010;16:1705–1711. [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genes N Y N 2000. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, et al. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet. 2002;31:276–278. doi: 10.1038/ng921. [DOI] [PubMed] [Google Scholar]
- Burdon KP, McKay JD, Wirth MG, Russell-Eggit IM, Bhatti S, Ruddle JB, Dimasi D, Mackey DA, Craig JE. The PITX3 gene in posterior polar congenital cataract in Australia. Mol Vis. 2006;12:367–371. [PubMed] [Google Scholar]
- Chassaing N, Causse A, Vigouroux A, Delahaye A, Alessandri J-L, Boespflug-Tanguy O, Boute-Benejean O, Dollfus H, Duban-Bedu B, Gilbert-Dussardier B, Giuliano F, Gonzales M, et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet. 2014;86:326–334. doi: 10.1111/cge.12275. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Q, Cabrera PE, Zhong Z, Sun W, Jiao X, Chen Y, Govindarajan G, Naeem MA, Khan SN, Ali MH, Assir MZ, et al. Molecular Genetic Analysis of Pakistani Families With Autosomal Recessive Congenital Cataracts by Homozygosity Screening. Invest Ophthalmol Vis Sci. 2017;58:2207–2217. doi: 10.1167/iovs.17-21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon V, L’Honoré A, Ouimette J-F, Dumontier E, van den Munckhof P, Drouin J. A muscle-specific promoter directs Pitx3 gene expression in skeletal muscle cells. J Biol Chem. 2007;282:33192–33200. doi: 10.1074/jbc.M706119200. [DOI] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Cui X, Wang L, Zhang J, Du R, Liao S, Li D, Li C, Ke T, Li DW-C, Huang H, Yin Z, Tang Z, et al. HSF4 regulates DLAD expression and promotes lens de-nucleation. Biochim Biophys Acta. 2013;1832:1167–1172. doi: 10.1016/j.bbadis.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Doucette L, Green J, Fernandez B, Johnson GJ, Parfrey P, Young T-L. A novel, non-stop mutation in FOXE3 causes an autosomal dominant form of variable anterior segment dysgenesis including Peters anomaly. Eur J Hum Genet EJHG. 2011;19:293–299. doi: 10.1038/ejhg.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakova L, Stranecky V, Ulmanova O, Hlavova E, Trkovr M, Vincent AL, Liskova P. Segregation of aon of aSegregation of arkov P, Young T-L. 2011*) CRYGD variant in a family with dominantly inherited congenital cataracts. Mol Biol Rep. 2017 doi: 10.1007/s11033-017-4121-4. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, Roux A-F, Smith T, Antonarakis SE, Taschner PEM. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- Finzi S, Li Y, Mitchell TN, Farr A, Maumenee IH, Sallum JMF, Sundin O. Posterior polar cataract: genetic analysis of a large family. Ophthalmic Genet. 2005;26:125–130. doi: 10.1080/13816810500229124. [DOI] [PubMed] [Google Scholar]
- Forshew T, Johnson CA, Khaliq S, Pasha S, Willis C, Abbasi R, Tee L, Smith U, Trembath RC, Mehdi SQ, Moore AT, Maher ER. Locus heterogeneity in autosomal recessive congenital cataracts: linkage to 9q and germline HSF4 mutations. Hum Genet. 2005;117:452–459. doi: 10.1007/s00439-005-1309-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S-I, Kato K, Yonemura S, Inouye S, Nakai A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23:4297–4306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Oshima K, Shinkawa T, Wang BB, Inouye S, Hayashida N, Takii R, Nakai A. Analysis of HSF4 binding regions reveals its necessity for gene regulation during development and heat shock response in mouse lenses. J Biol Chem. 2008;283:29961–29970. doi: 10.1074/jbc.M804629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Jing Z, Bhat AM, Lee J, Nagaoka Y, Deng SX, Jiang M, Bhat SP. Expression of the HSF4 DNA binding domain-EGFP hybrid gene recreates early childhood lamellar cataract in transgenic mice. Invest Ophthalmol Vis Sci. 2014;55:7227–7240. doi: 10.1167/iovs.14-14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RL, O’Sullivan J, Ashworth J, Bhaskar S, Williams S, Biswas S, Kehdi E, Ramsden SC, Clayton-Smith J, Black GC, Lloyd IC. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. 2014;121:2124–2137-2. doi: 10.1016/j.ophtha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Grimm C, Chatterjee B, Favor J, Immervoll T, Löster J, Klopp N, Sandulache R, Graw J. Aphakia (ak), a mouse mutation affecting early eye development: fine mapping, consideration of candidate genes and altered Pax6 and Six3 gene expression pattern. Dev Genet. 1998;23:299–316. doi: 10.1002/(SICI)1520-6408(1998)23:4<299::AID-DVG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Nicholson L, Scott CI. Apparently new syndrome of congenital cataracts, sensorineural deafness, Down syndrome-like facial appearance, short stature, and mental retardation. Am J Med Genet. 1996;61:382–386. doi: 10.1002/(SICI)1096-8628(19960202)61:4<382::AID-AJMG14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hansen L, Eiberg H, Rosenberg T. Novel MAF mutation in a family with congenital cataract-microcornea syndrome. Mol Vis. 2007;13:2019–2022. [PubMed] [Google Scholar]
- Hansen L, Mikkelsen A, Nürnberg P, Nürnberg G, Anjum I, Eiberg H, Rosenberg T. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest Ophthalmol Vis Sci. 2009;50:3291–3303. doi: 10.1167/iovs.08-3149. [DOI] [PubMed] [Google Scholar]
- He S, Pirity MK, Wang W-L, Wolf L, Chauhan BK, Cveklova K, Tamm ER, Ashery-Padan R, Metzger D, Nakai A, Chambon P, Zavadil J, et al. Chromatin remodeling enzyme Brg1 is required for mouse lens fiber cell terminal differentiation and its denucleation. Epigenetics Chromatin. 2010;3:21. doi: 10.1186/1756-8935-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Limi S, McGreal RS, Xie Q, Brennan LA, Kantorow WL, Kokavec J, Majumdar R, Hou H, Edelmann W, Liu W, Ashery-Padan R, et al. Chromatin remodeling enzyme Snf2h regulates embryonic lens differentiation and denucleation. Development. 2016;143:1937–1947. doi: 10.1242/dev.135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A. 2006;103:45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H-Y, Chang K-H, Nichols J, Li M. Homeodomain protein Pitx3 maintains the mitotic activity of lens epithelial cells. Mech Dev. 2009;126:18–29. doi: 10.1016/j.mod.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D-Y, Ardayfio P, Kang UJ, Semina EV, Kim K-S. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Iseri SU, Osborne RJ, Farrall M, Wyatt AW, Mirza G, Nürnberg G, Kluck C, Herbert H, Martin A, Hussain MS, Collin JRO, Lathrop M, et al. Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat. 2009;30:1378–1386. doi: 10.1002/humu.21079. [DOI] [PubMed] [Google Scholar]
- Islam L, Kelberman D, Williamson L, Lewis N, Glindzicz MB, Nischal KK, Sowden JC. Functional analysis of FOXE3 mutations causing dominant and recessive ocular anterior segment disease. Hum Mutat. 2015;36:296–300. doi: 10.1002/humu.22741. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Lu L, Wang X, Chesler EJ, Carps E, Qi S, Gu J, Williams RW. The ldis1 lens mutation in RIIIS/J mice maps to chromosome 8 near cadherin 1. Mol Vis. 2004;10:577–587. [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GCM. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- Javadiyan S, Craig JE, Sharma S, Lower KM, Casey T, Haan E, Souzeau E, Burdon KP. Novel missense mutation in the bZIP transcription factor, MAF, associated with congenital cataract, developmental delay, seizures and hearing loss (Aymé-Gripp syndrome) BMC Med Genet. 2017;18:52. doi: 10.1186/s12881-017-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez NL, Flannick J, Yahyavi M, Li J, Bardakjian T, Tonkin L, Schneider A, Sherr EH, Slavotinek AM. Targeted “next-generation” sequencing in anophthalmia and microphthalmia patients confirms SOX2, OTX2 and FOXE3 mutations. BMC Med Genet. 2011;12:172. doi: 10.1186/1471-2350-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Gangalum RK, Bhat AM, Nagaoka Y, Jiang M, Bhat SP. HSF4 mutation p.Arg116His found in age-related cataracts and in normal populations produces childhood lamellar cataract in transgenic mice. Hum Mutat. 2014;35:1068–1071. doi: 10.1002/humu.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA. iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res. 2018 doi: 10.1093/nar/gkx837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K. Multiple mechanisms and functions of maf transcription factors in the regulation of tissue-specific genes. J Biochem (Tokyo) 2007;141:775–781. doi: 10.1093/jb/mvm105. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Ke T, Wang QK, Ji B, Wang X, Liu P, Zhang X, Tang Z, Ren X, Liu M. Novel HSF4 mutation causes congenital total white cataract in a Chinese family. Am J Ophthalmol. 2006;142:298–303. doi: 10.1016/j.ajo.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Moody SA, Jamrich M. A novel fork head gene mediates early steps during Xenopus lens formation. Dev Camb Engl. 1999;126:5107–5116. doi: 10.1242/dev.126.22.5107. [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil K, Welch J, Baker-Lange K. Syndrome of congenital cataracts, sensorineural deafness, Down syndrome-like facial appearance, short stature, and mental retardation: two additional cases. Am J Med Genet A. 2007;143A:2581–2587. doi: 10.1002/ajmg.a.31990. [DOI] [PubMed] [Google Scholar]
- Khan SY, Vasanth S, Kabir F, Gottsch JD, Khan AO, Chaerkady R, Lee M-CW, Leitch CC, Ma Z, Laux J, Villasmil R, Khan SN, et al. FOXE3 contributes to Peters anomaly through transcriptional regulation of an autophagy-associated protein termed DNAJB1. Nat Commun. 2016;7:10953. doi: 10.1038/ncomms10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A. 1999;96:3781–3785. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DA, van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DA, Seawright A, Schedl A, Quinlan RA, Danes S, van Heyningen V. Aniridia-associated translocations, DNase hypersensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum Mol Genet. 2001;10:2049–2059. doi: 10.1093/hmg/10.19.2049. [DOI] [PubMed] [Google Scholar]
- Kuang S-Q, Medina-Martinez O, Guo D-C, Gong L, Regalado ES, Reynolds CL, Boileau C, Jondeau G, Prakash SK, Kwartler CS, Zhu LY, Peters AM, et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest. 2016;126:948–961. doi: 10.1172/JCI83778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Ho JWK, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML, Park PJ, Maas RL. iSyTE: integrated Systems Tool for Eye gene discovery. Invest Ophthalmol Vis Sci. 2012;53:1617–1627. doi: 10.1167/iovs.11-8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren H, Blixt A, Carlsson P. Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci. 2008;49:4269–4277. doi: 10.1167/iovs.08-2243. [DOI] [PubMed] [Google Scholar]
- Li D, Wang S, Ye H, Tang Y, Qiu X, Fan Q, Rong X, Liu X, Chen Y, Yang J, Lu Y. Distribution of gene mutations in sporadic congenital cataract in a Han Chinese population. Mol Vis. 2016;22:589–598. [PMC free article] [PubMed] [Google Scholar]
- Liang L, Liegel R, Endres B, Ronchetti A, Chang B, Sidjanin DJ. Functional analysis of the Hsf4(lop11) allele responsible for cataracts in lop11 mice. Mol Vis. 2011;17:3062–3071. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu H, Tang J, Lin Q, Sun Y, Wang C, Yang H, Khan MR, Peerbux MW, Ahmad S, Bukhari I, Zhu J. Whole Exome Sequencing Identifies a Novel Mutation in the PITX3 Gene, Causing Autosomal Dominant Congenital Cataracts in a Chinese Family. Ann Clin Lab Sci. 2017;47:92–95. [PubMed] [Google Scholar]
- Liu L, Zhang Q, Zhou L, Tang Z. A novel HSF4 mutation in a Chinese family with autosomal dominant congenital cataract. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2015;35:316–318. doi: 10.1007/s11596-015-1430-5. [DOI] [PubMed] [Google Scholar]
- Lv H, Huang C, Zhang J, Liu Z, Zhang Z, Xu H, You Y, Hu J, Li X, Wang W. A novel HSF4 gene mutation causes autosomal-dominant cataracts in a Chinese family. G3 Bethesda Md. 2014;4:823–828. doi: 10.1534/g3.113.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Jamieson RV, Perveen R, Glenister PH, Griffiths R, Boyd Y, Glimcher LH, Favor J, Munier FL, Black GCM. A dominant mutation within the DNA-binding domain of the bZIP transcription factor Maf causes murine cataract and results in selective alteration in DNA binding. Hum Mol Genet. 2003;12:585–594. [PubMed] [Google Scholar]
- Ma AS, Grigg JR, Ho G, Prokudin I, Farnsworth E, Holman K, Cheng A, Billson FA, Martin F, Fraser C, Mowat D, Smith J, et al. Sporadic and Familial Congenital Cataracts: Mutational Spectrum and New Diagnoses Using Next-Generation Sequencing. Hum Mutat. 2016;37:371–384. doi: 10.1002/humu.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean HE, Kim JI, Glimcher MJ, Wang J, Kronenberg HM, Glimcher LH. Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev Biol. 2003;262:51–63. doi: 10.1016/s0012-1606(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE, Rollins J, Kadambi S, Joseph J, Rahman T, Wink J, Benayed R, Paigen B, et al. The orphan G protein-coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Proc Natl Acad Sci U S A. 2008;105:2088–2093. doi: 10.1073/pnas.0705657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Shah R, Jamrich M. Pitx3 controls multiple aspects of lens development. Dev Dyn Off Publ Am Assoc Anat. 2009;238:2193–2201. doi: 10.1002/dvdy.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-N, Zhang Y, Moskophidis D, Mivechi NF. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genes N Y N 2000. 2004;40:205–217. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane T, Mizobe N, Hayashibe H, Nakazawa S. A variant of Fine-Lubinsky syndrome: a Japanese boy with profound deafness, cataracts, mental retardation, and brachycephaly without craniosynostosis. Clin Dysmorphol. 2002;11:195–198. doi: 10.1097/00019605-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Narumi Y, Nishina S, Tokimitsu M, Aoki Y, Kosaki R, Wakui K, Azuma N, Murata T, Takada F, Fukushima Y, Kosho T. Identification of a novel missense mutation of MAF in a Japanese family with congenital cataract by whole exome sequencing: a clinical report and review of literature. Am J Med Genet A. 2014;164A:1272–1276. doi: 10.1002/ajmg.a.36433. [DOI] [PubMed] [Google Scholar]
- Niceta M, Stellacci E, Gripp KW, Zampino G, Kousi M, Anselmi M, Traversa A, Ciolfi A, Stabley D, Bruselles A, Caputo V, Cecchetti S, et al. Mutations Impairing GSK3-Mediated MAF Phosphorylation Cause Cataract, Deafness, Intellectual Disability, Seizures, and a Down Syndrome-like Facies. Am J Hum Genet. 2015;96:816–825. doi: 10.1016/j.ajhg.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Kawane K, Watanabe-Fukunaga R, Fukuyama H, Ohsawa Y, Uchiyama Y, Hashida N, Ohguro N, Tano Y, Morimoto T, Fukuda Y, Nagata S. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–1074. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Blixt A, Churchill A, Martinsson T, Enerbäck S, Carlsson P. Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- Perveen R, Favor J, Jamieson RV, Ray DW, Black GCM. A heterozygous c-Maf transactivation domain mutation causes congenital cataract and enhances target gene activation. Hum Mol Genet. 2007;16:1030–1038. doi: 10.1093/hmg/ddm048. [DOI] [PubMed] [Google Scholar]
- Plaisancié J, Ragge NK, Dollfus H, Kaplan J, Lehalle D, Francannet C, Morin G, Colineaux H, Calvas P, Chassaing N. FOXE3 mutations: genotype-phenotype correlations. Clin Genet. 2017 doi: 10.1111/cge.13177. [DOI] [PubMed] [Google Scholar]
- Rajaram N, Kerppola TK. Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract. Mol Cell Biol. 2004;24:5694–5709. doi: 10.1128/MCB.24.13.5694-5709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Semina EV. Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol. 2011;22:314–324. doi: 10.1097/ICU.0b013e328349412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Schneider A, Bardakjian T, Stoler JM, Melancon SB, Semina EV. FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A. 2010;152A:582–590. doi: 10.1002/ajmg.a.33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Dev Camb Engl. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Rosemann M, Ivashkevich A, Favor J, Dalke C, Hölter SM, Becker L, Rácz I, Bolle I, Klempt M, Rathkolb B, Kalaydjiev S, Adler T, et al. Microphthalmia, parkinsonism, and enhanced nociception in Pitx3 (416insG) mice. Mamm Genome Off J Int Mamm Genome Soc. 2010;21:13–27. doi: 10.1007/s00335-009-9235-0. [DOI] [PubMed] [Google Scholar]
- Saboo US, Penke D, Mahindrakar A, Uddaraju M, Sankurathri C, Gong X, Xing C, Mootha VV. Exome sequencing reveals novel homozygous FOXE3 mutation in microphthalmos with staphylomatous malformation. Ophthalmic Genet. 2017;38:295–297. doi: 10.1080/13816810.2016.1217549. [DOI] [PubMed] [Google Scholar]
- Sajjad N, Goebel I, Kakar N, Cheema AM, Kubisch C, Ahmad J. A novel HSF4 gene mutation (p.R405X) causing autosomal recessive congenital cataracts in a large consanguineous family from Pakistan. BMC Med Genet. 2008;9:99. doi: 10.1186/1471-2350-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Imaki J, Yoshida K, Ogata A, Matsushima-Hibaya Y, Kuboki Y, Nishizawa M, Nishi S. Rat maf related genes: specific expression in chondrocytes, lens and spinal cord. Oncogene. 1997;14:745–750. doi: 10.1038/sj.onc.1200869. [DOI] [PubMed] [Google Scholar]
- Sakazume S, Sorokina E, Iwamoto Y, Semina EV. Functional analysis of human mutations in homeodomain transcription factor PITX3. BMC Mol Biol. 2007;8:84. doi: 10.1186/1471-2199-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Hawkins RK. Dysgenetic lens (dyl)--a new gene in the mouse. Invest Ophthalmol Vis Sci. 1979;18:642–645. [PubMed] [Google Scholar]
- Sato K, Miyoshi F, Yokota K, Araki Y, Asanuma Y, Akiyama Y, Yoh K, Takahashi S, Aburatani H, Mimura T. Marked induction of c-Maf protein during Th17 cell differentiation and its implication in memory Th cell development. J Biol Chem. 2011;286:14963–14971. doi: 10.1074/jbc.M111.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum Mol Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Shi X, Bosenko DV, Zinkevich NS, Foley S, Hyde DR, Semina EV, Vihtelic TS. Zebrafish pitx3 is necessary for normal lens and retinal development. Mech Dev. 2005;122:513–527. doi: 10.1016/j.mod.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Shi X, Cui B, Wang Z, Weng L, Xu Z, Ma J, Xu G, Kong X, Hu L. Removal of Hsf4 leads to cataract development in mice through down-regulation of gamma S-crystallin and Bfsp expression. BMC Mol Biol. 2009;10:10. doi: 10.1186/1471-2199-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Luo Y, Howley S, Dzialo A, Foley S, Hyde DR, Vihtelic TS. Zebrafish foxe3: roles in ocular lens morphogenesis through interaction with pitx3. Mech Dev. 2006;123:761–782. doi: 10.1016/j.mod.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Shi Y, Shi X, Jin Y, Miao A, Bu L, He J, Jiang H, Lu Y, Kong X, Hu L. Mutation screening of HSF4 in 150 age-related cataract patients. Mol Vis. 2008;14:1850–1855. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. Molecular Genetics of Cataract. Prog Mol Biol Transl Sci. 2015;134:203–218. doi: 10.1016/bs.pmbts.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res. 2017;156:95–102. doi: 10.1016/j.exer.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui N, Beltaief O, BenHamed S, M’Rad R, Maazoul F, Ouertani A, Chaabouni H, Hejtmancik JF. A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci. 2004;45:2716–2721. doi: 10.1167/iovs.03-1370. [DOI] [PubMed] [Google Scholar]
- Smidt MP, van Schaick HS, Lanctôt C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci U S A. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokina EA, Muheisen S, Mlodik N, Semina EV. MIP/Aquaporin 0 represents a direct transcriptional target of PITX3 in the developing lens. PloS One. 2011;6:e21122. doi: 10.1371/journal.pone.0021122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye Lond Engl. 2007;21:1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- Summers KM, Withers SJ, Gole GA, Piras S, Taylor PJ. Anterior segment mesenchymal dysgenesis in a large Australian family is associated with the recurrent 17 bp duplication in PITX3. Mol Vis. 2008;14:2010–2015. [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xiao X, Li S, Guo X, Zhang Q. Exome sequencing of 18 Chinese families with congenital cataracts: a new sight of the NHS gene. PloS One. 2014;9:e100455. doi: 10.1371/journal.pone.0100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell EC, Zilinski CA, Hashimoto R, Shah R, Lane ME, Jamrich M. Regulation and function of foxe3 during early zebrafish development. Genes N Y N 2000. 2008;46:177–183. doi: 10.1002/dvg.20380. [DOI] [PubMed] [Google Scholar]
- Talamas E, Jackson L, Koeberl M, Jackson T, McElwee JL, Hawes NL, Chang B, Jablonski MM, Sidjanin DJ. Early transposable element insertion in intron 9 of the Hsf4 gene results in autosomal recessive cataracts in lop11 and ldis1 mice. Genomics. 2006;88:44–51. doi: 10.1016/j.ygeno.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe M, Sasai N, Nagata K, Liu XD, Liu PC, Thiele DJ, Nakai A. The mammalian HSF4 gene generates both an activator and a repressor of heat shock genes by alternative splicing. J Biol Chem. 1999;274:27845–27856. doi: 10.1074/jbc.274.39.27845. [DOI] [PubMed] [Google Scholar]
- Ullah E, Nadeem Saqib MA, Sajid S, Shah N, Zubair M, Khan MA, Ahmed I, Ali G, Dutta AK, Danda S, Lao R, Ling-Fung Tang P, et al. Genetic analysis of consanguineous families presenting with congenital ocular defects. Exp Eye Res. 2016;146:163–171. doi: 10.1016/j.exer.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Valleix S, Niel F, Nedelec B, Algros M-P, Schwartz C, Delbosc B, Delpech M, Kantelip B. Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet. 2006;79:358–364. doi: 10.1086/505654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanita V, Singh D, Robinson PN, Sperling K, Singh JR. A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am J Med Genet A. 2006;140:558–566. doi: 10.1002/ajmg.a.31126. [DOI] [PubMed] [Google Scholar]
- Varnum DS, Stevens LC. Aphakia, a new mutation in the mouse. J Hered. 1968;59:147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- Verdin H, Sorokina EA, Meire F, Casteels I, de Ravel T, Semina EV, De Baere E. Novel and recurrent PITX3 mutations in Belgian families with autosomal dominant congenital cataract and anterior segment dysgenesis have similar phenotypic and functional characteristics. Orphanet J Rare Dis. 2014;9:26. doi: 10.1186/1750-1172-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Maeda YY, Watanabe K, Oshio T, Ueda T, Takahashi G, Yokohama M, Saito J, Seki Y, Takahama S, Ishii R, Shitara H, et al. A deletion in a cis element of Foxe3 causes cataracts and microphthalmia in rct mice. Mamm Genome Off J Int Mamm Genome Soc. 2011;22:693–702. doi: 10.1007/s00335-011-9358-y. [DOI] [PubMed] [Google Scholar]
- Wada K, Matsushima Y, Tada T, Hasegawa S, Obara Y, Yoshizawa Y, Takahashi G, Hiai H, Shimanuki M, Suzuki S, Saitou J, Yamamoto N, et al. Expression of truncated PITX3 in the developing lens leads to microphthalmia and aphakia in mice. PloS One. 2014;9:e111432. doi: 10.1371/journal.pone.0111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Jürgens G, Küttner F, Seifert E, Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, Birchmeier C. The transcription factor c-Maf controls touch receptor development and function. Science. 2012;335:1373–1376. doi: 10.1126/science.1214314. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chauhan BK, Cveklova K, Cvekl A. Transcriptional regulation of mouse alphaB- and gammaF-crystallin genes in lens: opposite promoter-specific interactions between Pax6 and large Maf transcription factors. J Mol Biol. 2004;344:351–368. doi: 10.1016/j.jmb.2004.07.102. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Tissue-specific regulation of the mouse alphaA-crystallin gene in lens via recruitment of Pax6 and c-Maf to its promoter. J Mol Biol. 2005;351:453–469. doi: 10.1016/j.jmb.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Large Maf Transcription Factors: Cousins of AP-1 Proteins and Important Regulators of Cellular Differentiation. Einstein J Biol Med EJBM. 2007;23:2–11. doi: 10.23861/ejbm20072347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklová K, Duncan MK, Pestell RG, Chepelinsky AB, et al. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O’Brien C, de Felipe C, Semina E, Li M. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.