Abstract
Background
Focal embouchure dystonia impairs orofacial motor control in wind musicians and causes professional disability. A paucity of quantitative measures or rating scales impedes object assessment of treatment efficacy.
Objectives
We quantified specific features of focal embouchure dystonia using acoustic measures and developed a metric to assess severity across multiple domains of symptomatic impairment.
Methods
We recruited nine brass musicians with and six without embouchure dystonia. Four domains of symptomatic dysfunction in focal embouchure dystonia were identified: pitch inaccuracy, sound instability and tremor, sound breaks, and timing variability. Musicians performed sustained tones and sequences, and then acoustic variables within each domain were quantified. A composite brass acoustic severity score comprised of these variables was validated against clinical global impressions of severity.
Results
Musicians with dystonia performed worse in acoustic domains of pitch inaccuracy (Median: dystonia=100%, control=62%), instability (Median shimmer: dystonia=3%, control=2%), and breaks (Median: dystonia=0.34%, control=0.05%). Tremor in embouchure dystonia was 5–8 Hz, intermittent, and variable in amplitude. Rhythmic variability did not differ between groups. Participants with embouchure dystonia had different patterns of impairment across variables. Composite severity scores strongly predicted clinical global impression of severity (R2=0.95).
Conclusions
Acoustic variables distinguish musicians with embouchure dystonia from controls and reflect different types of symptomatic impairments. Our composite acoustic severity score predicts severity of clinical global impression for musicians with different patterns of symptomatic impairment and may provide a foundation for developing a clinical rating scale.
Keywords: Musician Dystonia, Task-Specific Dystonia, Clinical Assessment
Introduction
Dystonia is a movement disorder characterized by abnormal movements, postures, or both.1 Musicians have greater vulnerability to developing focal, task-specific dystonia affects playing their musical instrument, with an estimated prevalence of 1–2%,2,3 a 100-fold increase over the population at large.4 Musician dystonia typically leads to abandonment of public performance, often at the height of the performer’s career.5 Much of the research on musician dystonia has focused on hand involvement. Dystonia in brass musicians most commonly involves the embouchure, the positioning of orofacial muscles used to play a wind instrument. There are no effective treatments for focal embouchure dystonia (FED).6 A subset of affected individuals have progression of their symptoms to involve additional orofacial tasks,5,6 but the risk factors for generalization remain unknown.
One barrier to studying the natural history, prognosis, and treatment of FED is the lack of a validated, objective, quantitative assessment tool to measure its specific features and overall severity. Much of the dysfunction in FED is “heard” rather than “seen.” For this reason, quantification of acoustic measures may be preferable to kinematic approaches.
Previous work has quantified sound instability in FED.7 However, this measure reflects only one facet of impairment in FED and does not measure dysfunction across all symptomatic subtypes.5 Some musicians manifest delay or inaccuracy when initiating notes or sequences, which, while highly debilitating, are not reflected in sound instability.
Using acoustic analysis, we developed an FED assessment tool capable of measuring specific symptoms and quantifying overall severity across a broad range of phenotypes. This tool is suitable for use in rigorous mechanistic or clinical studies of FED.
Methods
Participants
Adult brass musicians with suspected or confirmed FED were recruited from the University of Rochester (Rochester, NY) and Washington University (St. Louis, MO) Movement Disorders Clinics, and via advertisement through music instructors, the Musicians with Dystonia program of the Dystonia Medical Research Foundation, and Musicians with Focal Embouchure Dystonia Facebook group. Brass musicians without dystonia were recruited from the community as a control group (MC). Study visits were conducted at the University of Rochester or Washington University.
Baseline information including age, sex, race, ethnicity, years of musical study, duration of FED symptoms, musical sophistication (Ollen Musical Sophistication Index, OMSI),8 and quality of life (QOL) using two validated instruments (Spirituality Index of Wellbeing9 and World Health Organization Quality of Life Assessment Instrument)10,11 was collected on all participants. Group assignment was confirmed with examination by an expert movement disorders neurologist (JWM or JSP). Diagnosis based upon published guidelines required a history of playing deterioration, examination revealing a typical pattern of impairment for FED and the absence of non-dystonic causes of dysfunction.12 Exclusion criteria included the presence of dystonia at rest, dystonia syndromes common in non-musicians, comorbid neurological disorders aside from tremor, or treatment with botulinum toxin within 6 months. Three movement disorders neurologists (JWM, SAN, JSP) independently evaluated audio recordings from all FED participants and assigned a clinical global impression (CGI) severity score on a 4-point Likert scale (0 = ‘none’, 1 = ‘mild’, 2 = ‘moderate’, and 3 = ‘severe.’) The three CGI scores were averaged to yield one score per participant.
The University of Rochester Research Subjects Review Board and the Washington University Human Subjects Protection Office approved this study. All participants provided informed consent and were compensated for travel and time.
Data Acquisition and Processing
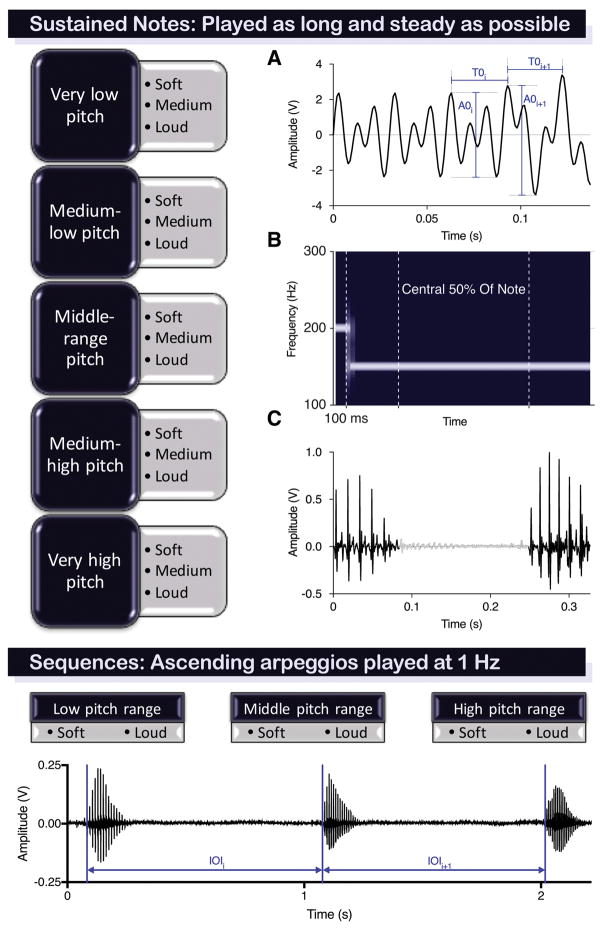
Participants performed custom-written musical exercises designed to test function across a range of playing speeds, pitch registers, and volumes. We analyzed data from two of these exercises (Figure 1). In the first, sustained tones were repeated at soft, medium, and loud volumes in low, middle, and high registers. Second, note sequences were repeated in all registers at soft and loud volumes. Musicians were instructed to attempt each note and sequence only once. In the event of multiple attempts, only the first was included in analyses. Sustained notes were analyzed for instability, inaccuracy, breaks, and tremor; sequences were analyzed for inter-onset interval (IOI) variability.
Figure 1.
Study design & key acoustic variables.
Top: Sustained tones were selected from the lowest pitch register of the instrument (very low pitch), highest pitch register of the instrument (very high pitch) and in between and each of the 5 pitches was played as long as possible at soft, moderate, and loud volumes. Each note was analyzed for measures of instability of the fundamental frequency, inaccuracy, and sound breaks. A: Diagram of the fundamental frequency period (T0) and amplitude. B: Inaccuracy is the F0 deviation from the initial 100ms and central 50% of each note. C: A sound break (gray) characterized by a loss of periodicity and amplitude during a sustained tone. Bottom: Evenly-spaced note sequences consisting of a one-octave ascending, articulated musical triad in each instrument’s low, middle, and high pitch registers were performed at soft and loud volumes. Inter-onset-intervals (IOIs) were measured between notes of each sequence. Subject mean IOI and IOI CoV were calculated from IOI values across all 6 sequence conditions.
High-fidelity audio was recorded using an Earthworks TC30 microphone (Earthworks Audio, Milford NH, USA), amplified with a Grace Design M101 preamp (Grace Design, Lyons, CO, USA), and sampled at 83 kHz with a Cambridge Electronic Design Power 1401 running Spike2 software (Cambridge Electronic Design Limited, Cambridge, England.) Analyses were performed offline using custom MATLAB code (MATLAB 2013b; Mathworks, Natick, MA, USA).
Note Detection
Individual note start- and stop-times were determined by generating a maximum amplitude envelope from a 6 ms sliding window, applying 60 ms boxcar smoothing, and detecting note maxima via peak detection on the smoothed envelope. Note onset and offset times were defined when the envelope amplitude crossed 10% of the note maximum above the mean background recording voltage. Only notes with a minimum duration of 800 ms were analyzed.
Detection of the Fundamental Frequency
The fundamental frequency (F0) of each sustained note was detected via a peak-picking method13,14 with an F0 analysis program developed by the authors. The signal was filtered using a fourth-order Butterworth (fc = 10 kHz). The program identified peaks with amplitude ≥80% of the maximum envelope and separation ≥0.36 ms to estimate the instantaneous period (T0) of the fundamental. A binned histogram function estimated mean F0 from inter-peak intervals. Peak detection was repeated using this estimate to refine peak separation criteria, with a required peak-to-peak frequency within 500 cents (5 semitones) of the estimated mean F0 and minimum amplitude of 40% of neighboring peak amplitudes. The latter peak detection process was then repeated using the refined T0 and peak amplitude values, identifying F0 peaks in every note. Peak accuracy was ensured through visual inspection with manual correction as needed. Instantaneous T0, F0, and peak-to-peak amplitude (A0) were measured for each cycle (Figure 1a). For cross-validation, each note’s median F0 was compared with the F0 obtained using an autocorrelation function (Spearman ρ = 1.000, p < 0.001).
Acoustic Instability
Acoustic instability was measured in amplitude and time domains using shimmer and jitter, standard measures of instability,15 which calculate cycle-to-cycle variability in A0 (shimmer) and T0 (jitter). These calculations were performed for each sustained note, excluding segments without a detectable F0 and excluding the first and last 150 ms to eliminate ramp-up and taper phases. Shimmer and jitter were expressed as percentages of the note mean T0 or A0 (Equations 1 and 2). The values were averaged across sustained notes to obtain a mean value for each variable for each subject.
| (1) |
| (2) |
Tremor
To assess whether instability reflected the presence of tremor and to characterize its features, the instantaneous A0s from each sustained note were linearly normalized to median A0, cropped to remove ramp-up and taper phases, concatenated across notes into a single A0 time series, and resampled to a uniform rate of 1000 Hz using linear interpolation. Notes shorter than 6 seconds were excluded to minimize the artifacts at note transitions. Subjects with fewer than 5 usable notes were excluded. Fast Fourier transform (FFT) and short-time Fourier transform (STFT) were performed on the concatenated A0 and assessed over the range of 3–20 Hz. The FFT output was normalized to total FFT amplitude and non-parametrically smoothed using a Gaussian kernel over a 300 ms window. A0 power spectral density (A0 PSD) was assessed over 3–8 Hz as a broad measure of low-frequency sound instability. STFT using a window size of 2s and 50% overlap was employed to qualitatively assess temporal aspects of tremor.
Inaccuracy
Inaccuracy was defined as the difference in pitch at the note onset compared to mid-note (Figure 1b). Mean F0 was calculated during the initial 100 ms (F0100) and central 50% of each note (F0mid). The absolute value of that frequency difference was converted to “cents” (percent of a semitone), a normalized measure of frequency (Equation 3). As instrument harmonic resonance frequency intervals increase exponentially with pitch, conversion to cents allows comparison of inaccuracy across notes in different pitch ranges. A mean note inaccuracy value was calculated for each subject.
| (3) |
Percentage Break Time
Breaks were defined as a decrease in signal amplitude below 5% of the median note amplitude with loss of a coherent fundamental frequency upon auditory and visual inspection of the waveform. Break starts were defined at the last cycle peak before loss of F0 and break ends at the first cycle peak upon return of F0 as illustrated in Figure 1c. For each note, the net break duration as a percentage of note length was calculated. Note values were averaged to yield subject break severity.
Inter-Note Timing of Sequential Notes
Subjects performed sequences of notes of equal duration and temporal spacing at regular pitch intervals (Figure 1). To control for individual differences in intrinsic rhythmic ability, all participants received metronome-pacing at 1 Hz through a single earbud. IOIs were calculated between note starts, defined when amplitude first exceeded 10% of peak note amplitude over the inter-note minimum. Rhythmic variability was measured as the IOI coefficient of variation.
COmposite BRass Acoustic Severity Score
A COmposite BRass Acoustic Severity (COBRAS) score was calculated for each subject from independent acoustic variables that differed significantly between FED and MC musicians. Based on these criteria, inaccuracy, break severity, and shimmer were included in COBRAS. Subject values for each of these variables were rank-ordered, averaged, and normalized to values ranging from 1–10 to obtain a COBRAS value for each subject (Equation 4). COBRAS were derived separately for the entire cohort of musicians (COBRASALL) and for FED musicians only (COBRASFED). COBRASALL was used for between group analyses and COBRASFED for within FED group analyses.
| (4) |
Statistical Analysis
Inter-group comparisons were performed for each measure using a nonparametric two-tail Mann-Whitney-U test. To assess variable independence, Spearman correlation coefficients were obtained between each pair of acoustic variables (jitter, shimmer, break severity, inaccuracy, and IOI CoV) with Bonferroni correction. Inter-rater reliability for CGI ratings was determined using intra-class correlation (ICC) to measure consistency among ratings and repeated-measures ANOVA. The relationship between CGI and COBRASFED was quantified using linear regression.
Results
Increased inaccuracy (Figure 2a), instability (Figure 2b–c, f), and break severity (Figure 2d) characterize FED. A composite measure that captures dysfunction across these domains predicts CGI severity.
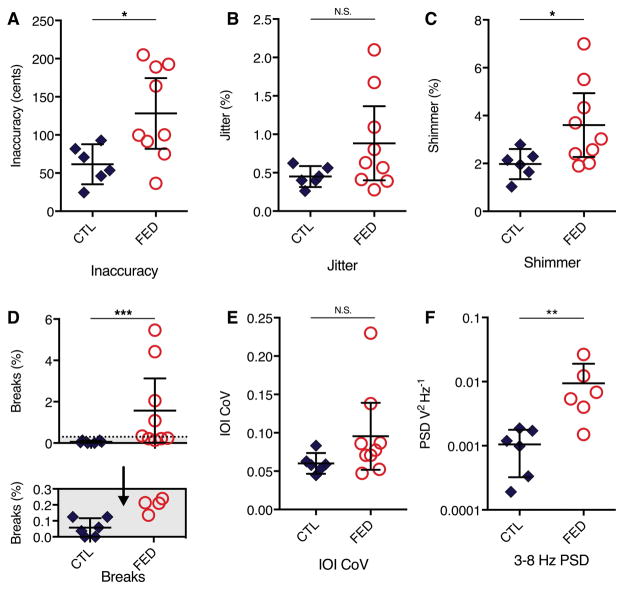
Figure 2.
Summary of group-level analyses for key acoustic variables.
Musicians with FED have increased attack inaccuracy (A), sound instability (B–C), break severity (D), and low-frequency instability of A0 (F) compared with MC musicians. IOI CoV (E) did not differ between groups, however two outliers in the FED group reflect individuals with difficulty initiating notes. All musicians with FED had breaks (D) though they occurred rarely in MC subjects, such that the best musician with FED had greater break severity than the most impaired MC. The shaded region from y = 0 to y = 0.3 is expanded below the plot to show the distribution of FED and MC subjects in this severity range. Error bars represent the 95% confidence interval. In all graphs, each data point represents a subject. *p<0.05, **p<0.01, ***p<0.001.
Participants
Nine brass musicians with, and six without FED enrolled in this study. FED and MC groups did not differ in age, sex, years of practice, musical sophistication or QOL. Table 1 summarizes group characteristics and statistical results. All FED participants were medication-naïve and not previously treated with botulinum toxin injections.
Table 1.
Demographic and clinical features of participants.
Groups did not significantly differ in age, sex, years of musical practice, musical sophistication (OMSI), or quality of life (SIWB, QOL-100). Higher values indicate greater likelihood of musical sophistication on the OMSI and better quality of life on the SIWB and QOL-100.
| Subject | Instrument | Age | Sex | Years of Practice | OMSI | SIWB | QOL-100 | Years of Symptoms | Dystonia Treatments | CGI |
|---|---|---|---|---|---|---|---|---|---|---|
| FED1 | Trombone | 22 | F | 8 | 0.67 | 4.3 | 59.0 | 1 | None | 1.5 |
| FED2 | Trombone | 25 | M | 15 | 0.60 | 4.7 | 81.5 | 3 | Retraining | 3 |
| FED3 | Horn | 26 | F | 15 | 0.80 | 5 | 82.8 | 4 | None | 1 |
| FED4 | Tuba | 33 | M | 18 | 0.98 | 4.7 | 79.8 | 4 | None | 1.5 |
| FED5 | Trombone | 56 | M | 54 | 1.00 | 4.2 | 78.8 | 34 | None | 3 |
| FED6 | Trombone | 57 | M | 46 | 0.98 | 4.9 | 81.3 | 9 | None | 1 |
| FED7 | Horn | 66 | F | 57 | 0.72 | 4.7 | 77.5 | 15 | None | 3 |
| FED8 | Horn | 68 | M | 59 | 0.96 | 5 | 85.5 | 27 | Retraining | 1.5 |
| FED9 | Horn | 70 | M | 61 | 0.94 | 4.5 | 82.5 | 10 | None | 3 |
| MC1 | Horn | 18 | F | 9 | 0.23 | 4.6 | 78.3 | |||
| MC2 | Horn | 27 | F | 16 | 0.98 | 4.8 | 92.8 | |||
| MC3 | Horn | 27 | M | 16 | 0.97 | 4.5 | 72.0 | |||
| MC4 | Horn | 28 | M | 22 | 0.98 | 3.3 | 63.3 | |||
| MC5 | Horn | 30 | F | 26 | 0.97 | 4 | 75.8 | |||
| MC6 | Trumpet | 63 | M | 53 | 0.99 | 4.5 | 82.0 | |||
| All Controls Mean (95% CI) | 32.2 (16–49) | 50% F, 50% M | 23.7 (7.4–40) | 0.85 (0.5–1.2) | 4.26 (3.7–4.9) | 77.3 (67–88) | ||||
| All FED Mean (95% CI) | 47.0 (32–63) | 33% F, 67% M | 35.8 (19–52) | 0.85 (0.7–1.0) | 4.65 (4.4–4.9) | 78.7 (73–85) | ||||
| Statistic | U = 18 | χ2 = 0.4 | U = 21 | U =20 | U = 15 | U = 20 | ||||
| P-value | 0.31 | 0.52 | 0.51 | 0.46 | 0.17 | 0.46 | ||||
Abbreviations: FED: Focal embouchure dystonia; MC: Musician control; OMSI: Ollen Musical Sophistication Index; SIWB: Spirituality Index of Well-Being; QOL-100: World Health Organization Quality of Life Assessment Instrument; CGI: Clinical Global Impression of severity.
Cyclic measures of instability are higher in FED
The FED group had higher instability in both shimmer and jitter (Figure 2b, c). On average, shimmer was 50% higher in FED (Mdn = 3.0%) versus MC (Mdn = 2.0%) groups, indicating impaired embouchure control over miniscule time scales, U = 9, p = 0.04. Jitter had a similar but not significant difference magnitude (Mdn = 0.6% FED, 0.4% MC, U = 14, p = 0.14). In both, MC participants clustered around a low value while FED participants had wide variability.
Tremor in FED is 5–8 Hz, intermittent, and variable across participants
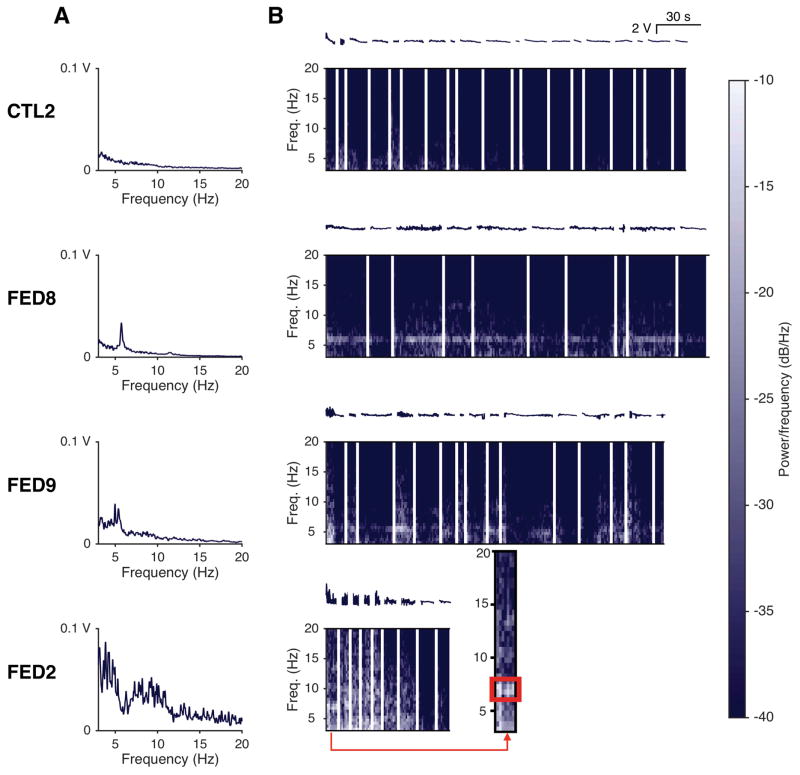
All 6 MC and 5 of 9 FED subjects had sufficient data for tremor analysis (Figure 3). All five FED subjects had a 5–8 Hz dystonic tremor during at least one note in the STFT, with 4 of 5 having a 5–8 Hz FFT peak. One FED participant had consistent, regular tremor at 6 Hz, while the remaining four FED subjects had intermittent tremor within and between notes. Dystonic tremor was task-specific and examination did not reveal visible tremor in other body regions during playing in all participants. Clinical impressions aligned with STFT and FFT findings in all subjects except FED2 (Figure 3), where raters perceived tremor in most notes while analyses identified only broad instability. Raters had high inter-rater reliability for detecting tremor (ICC r = 0.78, F(8,16) = 11.5, p < 0.0001). MC participants had no detectable tremor and lower A0 3–8 Hz PSD (Mdn = 0.006 FED, 0.001 MC; U = 2, p < 0.01) (Figure 2f).
Figure 3.
Tremor analyses in a MC & 3 FED musicians.
Normalized, concatenated note A0 time series were analyzed for tremor in the range of 3–20 Hz. A: FFT of A0 reveals a dominant tremor frequency in the range of 5–8 Hz with increased low-frequency FFT noise in some musicians with FED. B: STFT analysis of the A0 time series (above each spectrogram) depicts patterns of tremor. The representative control (top) has no dominant tremor frequency and minimal low-frequency noise. Each FED participant had a different pattern: regular, nearly continuous tremor (FED8), intermittent tremor with substantial low-frequency noise (FED9), or predominantly noise with a visible tremor band in one note only (FED2, inset). White spaces indicate concatenated note transitions.
Musicians with FED have greater attack inaccuracy
Attack inaccuracy (Figure 2a) measures difficulty in pitch initiation. On average, FED had nearly double the attack inaccuracy (Mdn = 100 c) of the MC group (Mdn = 62 c; U = 8, p = 0.03). The FED group had a wide range of severity with an apparent bimodal distribution. Of the four most inaccurate participants, three were outliers for instability, while the fourth had low instability despite high inaccuracy, reflecting this particular subject’s difficulty with initiating notes but relatively spared sound production once started.
Breaks are a characteristic feature of FED
Break severity quantifies the proportion of total note time interrupted by breaks. Every musician with FED had breaks, while MC participants had no or brief and infrequent breaks across all notes. Average break severity was nearly ten-fold higher in FED (Mdn = 0.34%) versus MC (Mdn = 0.048%) groups, U = 0, p = 0.001 (Figure 2D). Groups did not overlap, with the best FED participant having a higher break severity score than the worst control and outside the MC group 95% CI (Figure 2D, Inset).
Temporal control is preserved in most musicians with FED
Sequence IOI CoV (Figure 2e) measures temporal control. Mean IOI did not differ between groups (Mdn = 0.99 FED, 1.0 MC; U = 17, p = 0.27). IOI CoV was not quite significantly higher in FED (Mdn = 0.077) versus MC (Mdn = 0.058; U = 13, p = 0.11). Two FED participants were outliers with high IOI CoV. One was impaired across all variables. The other had selective dysfunction in IOI CoV and inaccuracy, reflecting a specific deficit in initiating notes.
COBRAS score distinguishes between FED and MC groups and predict clinical severity
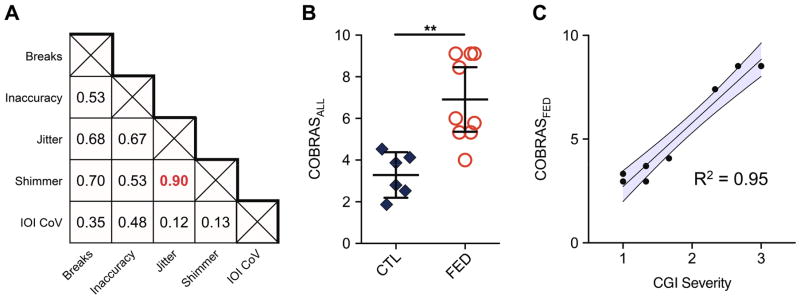
Average rank-order values of shimmer, inaccuracy, and break severity yielded composite severity scores across all subjects (COBRASALL) and in FED participants only (COBRASFED). Variables that were not independent (jitter, Figure 4a), not significantly different between groups (IOI CoV, jitter) or not obtainable on all subjects (A0 PSD) were not included. Musicians with FED had higher COBRASALL scores (Mdn = 6) than controls (Mdn = 3.3), U = 2, p = 0.001 (Figure 4b).
Figure 4.
Summary of composite score analyses.
A: Correlation matrix of values for key acoustic variables (breaks, inaccuracy, jitter, and shimmer) in musicians with FED. Spearman ρ values are shown for each pair and significant results (p<0.05, corrected) are denoted in bold. Jitter and shimmer were highly correlated in musicians with FED. B: COmposite BRass Acoustic Severity (COBRASALL) scores of global FED severity were calculated for FED and MC musicians. COBRASALL is significantly higher in the FED group. Only one FED subject falls within the 95% confidence interval of controls. C: Linear regression of COBRASFED and average CGI severity. COBRASFED is predictive of average CGI severity in musicians with FED. The shaded region represents the 95% confidence interval. **p<0.01
Average CGI severity scores from three experienced movement disorders neurologists (Table 1) validated the COBRASFED to reflect FED severity. Inter-rater reliability was high (ICC r = 0.75, F(8,16) = 10, p < 0.0001). COBRASFED strongly predicted CGI (F(1,7) = 139, p < .0001), with an R2 of 0.95 (Figure 4c).
Discussion
This study identifies specific measurable domains of dysfunction in FED and demonstrates a single, unified metric that correlates with CGI and captures multiple types of playing impairments in FED.
Many methods have been used in an attempt to quantify musician’s dystonia,16 however most utilize kinematic or EMG methodologies and are limited to hand dystonia. In comparison, our acoustic approach is quick, non-invasive, and reflects physiological and functional impairment. Though this acoustic approach cannot identify the specific muscles involved, it captures involvement of intraoral and deep muscles not measured with standard kinematic tools. One study quantified sound instability in FED7 but this metric was not compared to clinical impact and did not capture other aspects of FED. Several clinical phenotypes of playing dysfunction have been described in FED based on clinical examination and video review, with investigators emphasizing six features associated with specific symptoms and performance deficits (embouchure tremor, lip-pulling, lip-lock, jaw dystonia, tongue dystonia, and Meige syndrome).5 An ideal measure would incorporate all these phenotypic features. Here we identified specific and independent acoustic domains in FED (sound instability, pitch inaccuracy, and timing variability) and quantified acoustic variables within each domain. By sampling each instrument’s full dynamic- and pitch-ranges, we were able to characterize and measure FED severity across multiple phenotypes. While there is not a one-to-one correspondence between each acoustic domain and phenotype, a larger study may further identify specific associations between dysfunction acoustic domains and selective phenotypes.
All FED subjects in our study had abnormalities in multiple measures. While individuals with severe FED had impairment in all domains, dysfunction was limited to a subset of domains in mild participants. This suggests that different proportions of abnormalities in these domains may determine the clinical phenotype and severity. Breaks were the only domain in which all FED participants were impaired. Our sample size limits rigorous testing of the independence of these measures. However, the lack of significant correlations between measures in different domains suggests these variables are sufficiently independent to include in our overall severity score (Figure 4a).
This study supports the previous finding of increased sound instability in FED.7 Although shimmer and jitter correlate in FED subjects, they may still represent distinct biomechanical properties. Healthy brass musicians modulate volume and pitch independently, naturally compensating for changes in air pressure by adjusting aperture size at the lips or vice versa. Impairment of these mechanisms through abnormal sensory feedback or reduced motor control could drive the coupling of jitter and shimmer in participants with FED. Instability may be aperiodic or periodic (i.e. tremor) and instability may be due to either or both. Jitter and shimmer do not distinguish these types of instability, but tremor refers exclusively to periodic instability.
Dystonic tremor occurs in a body part affected by dystonia and may have irregular amplitude or occur intermittently.17 Previous quantifications of task-specific tremor in musicians identified increased 3–8 Hz frequency power, peaking around 5 Hz, in both string and brass musicians.18,19 Yet, analyses of musicians with focal hand dystonia have failed to show either dystonic tremor or tremor associated with dystonia.20 In our cohort, we used STFT, FFT, and PSD analyses to characterize tremor in the amplitude of the fundamental frequency. A dominant 5–8 Hz tremor was observed in four of five FED subjects and no controls. This tremor varied in amplitude and was intermittent in 3 subjects and continuous in 1. Breaks and aperiodic instability may contribute to the high 3–8 Hz power and give the perception of irregular tremor, even in the absence of a dominant tremor frequency. These results have commonalities with previous descriptions of dystonic tremor and task-specific embouchure tremor, suggesting shared mechanisms. The quantitative analyses used in this study may be employed to gather further insights into the nature of dystonic tremor and whether dystonic tremor has characteristic features that are independent of the body part affected.
Attack inaccuracy reflects impairment of correct movement initiation independent of sensory feedback. As auditory perception operates on a logarithmic scale, linear measures of pitch inaccuracy can create a mismatch between calculated and perceived impairment, particularly affecting high-range instruments. Accordingly, we used a logarithmic frequency scale (units of cents) for direct comparison between notes, finding that inaccuracy was greater in most FED participants, consistent with previous reports showing abnormal movement preparation21 and initiation22 in dystonia.
Our data show that sound breaks are characteristic of FED. This is a feature shared with laryngeal dystonia, where breaks during speech are characteristic and potentially pathognomonic.23 In our study, every individual with FED had breaks during sustained note blocks, typically involving multiple registers and volumes, while MC participants had either no breaks or a very brief break in extreme pitch registers. The loss of a resonant frequency despite continued airflow through the instrument suggests breaks may reflect a temporary collapse of the embouchure. This variable loss of coordination may be a defining characteristic of FED and merits further evaluation in a larger cohort.
Scale analysis has revealed increased sequence temporal variability in pianists with focal hand dystonia versus controls and has been used to quantify dystonia severity.24,25 We did not find significant group differences in temporal variability, but two FED participants were outliers with high IOI CoV. This measure may reflect lip-locking,5 a common manifestation of FED. However, it only captures temporal abnormalities within sequences and thus is not sensitive to lip-locking or other delays with playing initiation. Temporal abnormalities and lip-locking may be better-characterized by combining acoustic measures with kinematic or EMG analyses.
Our composite measure of severity, COBRASFED, highly correlated with expert-rated CGI severity, the current gold standard. This high correlation provides independent validation of our quantitative acoustic methods. Thus, our composite measure is suitable for use in mechanistic and clinical research on FED.
Our broader measure, the COBRASALL, distinguishes the FED and control groups and thus may be a useful tool for identifying the presence of FED in future studies. However, because COBRASALL uses scores that are rank-ordered across the entire study population and are non-linear in distribution, it is not useful for quantifying severity of FED. Future work in larger cohorts may lead to fixed reference values for each variable that can be used independent of the specific cohort properties.
Our study quantifies specific types of acoustic abnormalities in brass musician with FED. Furthermore, these measures can be combined into a single, unified measure of overall FED severity. However, there are also some limitations. As noted, IOI CoV did not distinguish FED from MC. Furthermore, it could not be used to measure temporal delays in the initiation of sustained tones or the first note of a sequence. A different method may facilitate quantification of initiation delays. While horns were overrepresented in the control group, its broad range overlaps with other brass instruments in the study and it has the closest harmonic spacing, predisposing to greater inaccuracy. Thus, the instrument composition of the MC group is unlikely to drive our results. Nevertheless, future work should include a wider sampling of musician controls. Characteristics such as age or years of study could be significant factors in performance on any of these variables, however our sample size did not allow for rigorous assessment of these potential relationships. We also do not know if the findings extend to woodwind instruments, which have a different mechanism of sound production.
In conclusion, brass musicians with FED have more breaks, inaccuracy, and acoustic instability than MC subjects. Our combined measure of these unique manifestations yields an objective, unified metric that predicts clinical severity across individuals with different types of playing impairment. Future studies may combine these acoustic measures with other quantitative data (clinical, kinematic, neurophysiologic, neuroimaging, etc.) to improve knowledge of FED pathophysiology, prognostication, and therapeutic targeting and provide a sensitive means for studying the natural history of FED and treatment response.
Acknowledgments
Funding sources: NIH TL1 TR000096, Schmidt Foundation for Integrative Brain Research, UR Provost Multidisciplinary Award, Geoffrey Waasdorp Pediatric Neurology Fund, Wilbur Smith Pediatric Neurology Fund, Barnes Jewish Hospital Foundation (Elliot Stein Family Fund) and the Barbara & Sam Murphy Fund.
We thank Avi Snyder, Seth Anderson, Molly Wilson, and Peter Kurau for their valuable discussions and contributions and Ralph Manchester for referring participants.
Footnotes
Authors’ Roles
Aimee E. Morris: Design, Analysis, Execution, Writing of the first draft
Scott A. Norris: Data generation, review and critique
Joel S. Perlmutter: Data generation, Interpretation, and Review and Critique
Jonathan W. Mink: Design, Data Generation, Interpretation, Review and Critique
Financial Disclosures of all authors (for the preceding 12 months)
MORRIS, AIMEE E.
Stock Ownership in medically-related fields - none
Intellectual Property Rights - none
Consultancies - none
Expert Testimony - none
Advisory Boards - none
Employment - University of Rochester
Partnerships - none
Contracts - none
Honoraria - none
Royalties - none
Grants - none
Other - none
NORRIS, SCOTT A.
Stock Ownership in medically-related fields - none
Intellectual Property Rights - none
Consultancies - none
Expert Testimony - none
Advisory Boards - none
Employment – Washington University School of Medicine, Saint Louis, MO
Partnerships - none
Contracts - none
Honoraria - none
Royalties - none
Grants - NINDS RO1 NS058714
Other - NIH LRP award and renewal
PERLMUTTER, JOEL S.
Stock Ownership in medically-related fields - none
Intellectual Property Rights - none
Consultancies - none
Expert Testimony - Consultant for Lowis & Gellen, LLP, The Expert Institute, Arnold Todaro & Welch, and Riverstone Claims Management, LLC
Advisory Boards - Scientific Advisory Board of the American Parkinson Disease Association; co-chair of the Scientific Advisory Committee of the Parkinson Study Group, member of the Medical and Scientific Advisory Committee of the Dystonia Medical Research Foundation, chair of Scientific Advisory and Publications Committee for ENROLL (an international Huntington Disease study), and chair of the Standards Committee of the Huntington Study Group.
Employment - Washington University in Saint Louis
Partnerships - none
Contracts - none
Honoraria – American Academy of Neurology, Stanford University, NIH, World Parkinson Congress, Parkinson Disease Foundation, Huntington Study Group, University of Rochester (for Parkinson Study Group)
Royalties - none
Grants - NIH/NINDS/NCATS/NIA (NS41509, NS075321, NS058714, NS092865, NS077946, NS097437, ES021488, AG050263, NS097799, NS098020, U54TR001456, U10NS077384), Department of Defense 12219880, the American Parkinson Disease Association (APDA), Greater St. Louis Chapter of the APDA, Michael J Fox Foundation, Barnes Jewish Hospital Foundation (Elliot Stein Family Fund, Oertli Fund), The Fixel Foundation, Barbara & Sam Murphy Dystonia Fund, CHDI and Huntington Disease Society of America.
Other – none
MINK, JONATHAN W
Stock Ownership in medically-related fields - none
Intellectual Property Rights - none
Consultancies - Consultant for Biomarin, Inc; Censa, Inc; Xonovo, Inc; Abide Therapeutics, Inc.
Expert Testimony - none
Advisory Boards - Scientific Advisory Board for the Tourette Association of America
Employment - University of Rochester
Partnerships - none
Contracts - Abeona, Inc.
Honoraria - American Academy of Neurology (Associate Editor of Neurology)
Royalties - Elsevier, Inc.
Grants - NIH, Batten Disease Support and Research Association, Batten Research Alliance
Other - none
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord [Internet] 2013;28(7):863–73. doi: 10.1002/mds.25475. [cited 2014 Mar 22] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23649720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altenmüller E, Jabusch HC. Focal dystonia in musicians: Phenomenology, pathophysiology and triggering factors. Eur J Neurol. 2010;17(SUPPL 1):31–6. doi: 10.1111/j.1468-1331.2010.03048.x. [DOI] [PubMed] [Google Scholar]
- 3.Konaka K, Mochizuki H. Questionnaire survey of musician’s dystonia among students of a music college. Rinsho Shinkeigaku. 2015;55(4):263–5. doi: 10.5692/clinicalneurol.55.263. [DOI] [PubMed] [Google Scholar]
- 4.Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord [Internet] 2012;27(14):1789–96. doi: 10.1002/mds.25244. [cited 2014 Mar 31] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23114997. [DOI] [PubMed] [Google Scholar]
- 5.Frucht SJ. Embouchure dystonia--Portrait of a task-specific cranial dystonia. Mov Disord [Internet] 2009;24(12):1752–62. doi: 10.1002/mds.22550. [cited 2014 Apr 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19562760. [DOI] [PubMed] [Google Scholar]
- 6.Termsarasab P, Frucht SJ. Evaluation of embouchure dysfunction: Experience of 139 patients at a single center. Laryngoscope [Internet] 2016;126(6):1327–1333. doi: 10.1002/lary.25723. Available from: http://doi.wiley.com/10.1002/lary.25723. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Furuya S, Morise M, Iltis P, Altenmüller E. Quantification of instability of tone production in embouchure dystonia. Parkinsonism Relat Disord [Internet] 2014:8–11. doi: 10.1016/j.parkreldis.2014.08.007. [cited 2014 Oct 22] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25172127. [DOI] [PubMed]
- 8.Ollen JE. A criterion-related validity test of selected indicators of musical sophistication using expert ratings. 2006. [Google Scholar]
- 9.Daaleman T, Frey B. The spirituality index of well-being: a new instrument for health-related quality-of-life research. Ann Fam Med. 2004;2(5):499–503. doi: 10.1370/afm.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonomi A, Patrick D, Bushnell D, Martin M. Validation of the United States’ version of the World Health Organization Quality of Life (WHOQOL) instrument. J Clin Epidemiol. 2000;53(1):13–7. doi: 10.1016/s0895-4356(99)00123-7. [DOI] [PubMed] [Google Scholar]
- 11.Bonomi A, Patrick D. User’s manual and interpretation guide for the United States Version of the World Health Organization Quality of Life (WHOQOL) instrument. 1997. [DOI] [PubMed] [Google Scholar]
- 12.Frucht SJ. Embouchure dystonia: a video guide to diagnosis and evaluation. J Clin Mov Disord [Internet] 2016;3(1):10. doi: 10.1186/s40734-016-0035-x. [cited 2017 Nov 3] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4912816/pdf/40734_2016_Article_35.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreiman J, Gabelman B, Gerratt BR. Perception of vocal tremor. J Speech Lang Hear Res [Internet] 2003;46(1):203–14. doi: 10.1044/1092-4388(2003/016). Available from: http://www.ncbi.nlm.nih.gov/pubmed/12647899. [DOI] [PubMed] [Google Scholar]
- 14.Vieira MN, Mcinnes FR, Jack MA. On the influence of laryngeal pathologies on acoustic and electroglottographic jitter measures a) J Acoust Soc Am. 2002;111(2):1045–55. doi: 10.1121/1.1430686. [DOI] [PubMed] [Google Scholar]
- 15.Brockmann M, Drinnan MJ, Storck C, Carding PN. Reliable jitter and shimmer measurements in voice clinics: the relevance of vowel, gender, vocal intensity, and fundamental frequency effects in a typical clinical task. J Voice [Internet] 2011;25(1):44–53. doi: 10.1016/j.jvoice.2009.07.002. [cited 2014 Oct 22] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20381308. [DOI] [PubMed] [Google Scholar]
- 16.Peterson DA, Berque P, Jabusch H-C, Altenmüller E, Frucht SJ. Rating scales for musician’s dystonia: the state of the art. Neurology [Internet] 2013;81(6):589–98. doi: 10.1212/WNL.0b013e31829e6f72. [cited 2014 Jun 17] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23884039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A. Is tremor in dystonia a phenotypic feature of dystonia? Neurology. 2015;84(10):1053–9. doi: 10.1212/WNL.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 18.Lee A, Chadde M, Altenmüller E, Schoonderwaldt E. Characteristics of Task-specific Tremor in String Instrument Players. Tremor Other Hyperkinet Mov (NY) [Internet] 2014;4:198. doi: 10.7916/D86Q1V9W. [cited 2014 Jul 21] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4053556&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Voget J, Furuya S, Morise M, Altenmüller E. Quantification of sound instability in embouchure tremor on the time-varying fundamental frequency. J Neural Transm [Internet] 2016;123(5):515–21. doi: 10.1007/s00702-016-1533-6. [cited 2016 Aug 10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/27023201. [DOI] [PubMed] [Google Scholar]
- 20.Lee A, Schoonderwaldt E, Chadde M, Altenmüller E. Analysis of dystonic tremor in musicians using empirical mode decomposition. Clin Neurophysiol [Internet] 2015;126(1):147–53. doi: 10.1016/j.clinph.2014.04.013. Available from: http://dx.doi.org/10.1016/j.clinph.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci. 2012;35(3):478–85. doi: 10.1111/j.1460-9568.2011.07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banja JD, DeJong G. Impairment of movement initiation and execution but not preparation in idiopathic dystonia. Exp Brain Res. 2001;140(4):460–8. doi: 10.1007/s002210100847. [DOI] [PubMed] [Google Scholar]
- 23.Roy N, Whitchurch M, Merrill RM, Houtz D, Smith ME. Differential diagnosis of adductor spasmodic dysphonia and muscle tension dysphonia using phonatory break analysis. Laryngoscope [Internet] 2008;118(12):2245–53. doi: 10.1097/MLG.0b013e318184577c. [cited 2014 Oct 23] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19029863. [DOI] [PubMed] [Google Scholar]
- 24.Jabusch H-C, Vauth H, Altenmüller E. Quantification of focal dystonia in pianists using scale analysis. Mov Disord [Internet] 2004;19(2):171–80. doi: 10.1002/mds.10671. [cited 2014 Oct 23] Available from: http://www.ncbi.nlm.nih.gov/pubmed/14978672. [DOI] [PubMed] [Google Scholar]
- 25.Furuya S, Altenmüller E. Finger-specific loss of independent control of movements in musicians with focal dystonia. Neuroscience [Internet] 2013;247:152–63. doi: 10.1016/j.neuroscience.2013.05.025. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23707706. [DOI] [PubMed] [Google Scholar]