Abstract
Glucocorticoid (GC) signaling in thymocytes counters negative selection and promotes the generation of a self-tolerant yet antigen-responsive T-cell repertoire. Whereas circulating GC are derived from the adrenals, GC are also synthesized de novo in the thymus. The significance of this local production is unknown. Here we deleted 11β-hydroxylase (Cyp11b1), the enzyme that catalyzes the last step of GC biosynthesis, in thymic epithelial cells (TEC) or thymocytes. Like glucocorticoid receptor (GR)-deficient T cells, T cells from mice lacking TEC-derived but not thymocyte-derived GC proliferated poorly to alloantigen, had a reduced anti-viral response, and exhibited enhanced negative selection. Strikingly, basal expression of GC-responsive genes in thymocytes from mice lacking TEC-derived GC was reduced to the same degree as in GR-deficient thymocytes, indicating that at steady state the majority of biologically-active GC are paracrine in origin. These findings demonstrate the importance of extra-adrenal GC even in the presence of circulating adrenal-derived GC.
Introduction
Generation of a competent but self-tolerant T cell antigen-specific repertoire takes place in the thymus. The fate of CD4+CD8+ (double positive, or DP) thymocytes is determined by recognition of self peptides presented by MHC molecules (self-pMHC). DP cells with TCRs that do not recognize self-pMHC presented by cortical thymic epithelial cells (cTEC) die “by neglect”. Those that recognize self-pMHC enter the medulla where they encounter migratory dendritic cells (DC), some of which present self-pMHC derived from peripheral tissues, medullary TEC (mTEC) in which the autoimmune regulator (Aire) drives expression of tissue-restricted antigens, and resident DC bearing peptides transferred from mTEC (1). DP cells having TCRs with strong avidity for self-pMHC die (negative selection) whereas those with intermediate avidity survive (positive selection) and populate the periphery (2, 3).
Glucocorticoids (GC) are steroid hormones that bind the glucocorticoid receptor (GR), a ligand-dependent transcription factor that translocates to the nucleus and regulates transcription by binding to its response elements or other transcription factors. GC potently downregulate the production of pro-inflammatory cytokines, chemokines, and prostaglandins, and antagonize NF-κB and AP-1 (4). GC also inhibit transcriptional activity of Nur77 (5), a TCR-induced transcription factor implicated in thymocyte negative selection (6, 7). We previously suggested that by blunting TCR signals at a distal step (i.e. in the nucleus), GC could raise the threshold of avidity for self-pMHC above which negative selection takes place, allowing positive selection of TCRs that would otherwise be negatively selected (8). Evidence for an effect of GC on thymocyte selection was initially obtained from fetal thymic organ cultures in which negative selection of TCR-transgenic thymocytes was increased by pharmacologic inhibition of local GC production (9). This was subsequently supported by in vivo studies in which GR expression was reduced by the expression of an antisense transgene (10-12). The best evidence has been obtained with mice in which the GR was deleted in thymocytes (13). T cells from these mice responded normally to repertoire-independent TCR stimuli, but had diminished responses to immunization with foreign antigen, infection with lymphocytic choriomeningitis virus (LCMV) Armstrong strain, and culture with allogeneic APC, indicating a decrease in the avidity with which the repertoire recognized pMHC (13). Alterations of the TCR repertoire were confirmed by analysis of TCR Vβ CDR3 sequences.
Although circulating GC are primarily produced in the adrenal cortex, the thymus is itself a site of synthesis (14-19). Cultured mouse and chicken TECs express GC-synthetic enzymes and secrete steroid intermediates and GC themselves, production being highest at birth when adrenal production of GC is lowest (14, 17, 20). Direct measurement of thymus GC found corticosterone and its precursor steroid concentrations to be higher than in blood, particularly shortly after birth, confirming thymic GC synthesis in vivo (19). In addition to TEC, it has been proposed that thymocytes themselves are a source of GC, especially later in life (18). The functional contribution of extra-adrenal GC synthesis in the thymus, or any tissue for that matter, is unknown. To address this, we conditionally deleted Cyp11b1 (P450 c11b1), the enzyme that catalyzes the conversion of biologically inactive precursors to active GC, in TEC or thymocytes, and characterized the results in thymocytes and T cells.
Materials and Methods
Mice
C57BL/6 (B6) and the congenic strains B10.A and Rag2–/–, AND TCR-transgenic mice (21), β-actin-FLPe (22), FoxN1-Cre-transgenic mice (23), and β-actin-Cre mice were obtained from Jackson Laboratory. Lck-Cre–transgenic mice were obtained from Taconic. Nr3c1 (GR) exon 3 conditionally targeted mice were described (13). A conditional Cyp11b1 allele with loxp sites flanking exons 3-5 was generated by recombineering (24) (Supplemental Fig. 1A) and Cyp11b1-floxed mice were generated using C57BL/6 ES cells. The Neo cassette was removed by crossing floxed mice with β-actin-FLPe transgenic mice. All mice used in this study were backcrossed for at least 6 generations onto B6. Primer sequences used for genotyping are provided in Supplemental Table 1.
Antibodies
Anti-CD3 (145-2C11) and anti-CD28 (37.51) were from BD Pharmingen. For flow cytometry, antibodies recognizing CD45.2 (104), CD4 (RM4-5), and PD-1 (J43) were from eBioScience, recognizing Helios (22F6) from BioLegend, and recognizing Bim (C34C5) from Cell Signaling Technology. Antibodies against EpCAM (G8.8), MHC-II (M5/114.15.2), CD8α (53-6.7), and TCRβ (H57), as well as Annexin V, were from BD Pharmingen.
Measurement of corticosterone
Corticosterone was measured by chemiluminescence ELISA (Arbor Assays).
Cell culture and T cell proliferation
T cells were cultured in RPMI 1640 (Biofluids) supplemented with 10% heat-inactivated calf serum (Sigma), 100 mg/ml gentamicin, 4 mM glutamine, and 50 μM 2-mercaptoethanol. To measure T cell proliferation, 3 × 104 (anti-CD3/CD28) or 1.5 × 105 (alloantigen) purified lymph node T cells were cultured in triplicate in a total volume of 200 μl complete medium with 0.5 μg/ml plate-bound anti-CD28 and the indicated amounts of plate-bound anti-CD3, or with the indicated numbers of irradiated B10.A splenocytes in 96-well plates. After 48 (anti-CD3/anti-CD28) or 72 (alloantigen) hr wells were pulsed overnight with [3H]-thymidine and harvested. Incorporation of radioactivity was determined using a 1450 Microbeta liquid scintillation counter (Wallac).
Cell purification and flow sorting
Thymic epithelial cells were isolated by digestion of minced thymi and enrichment with discontinuous percoll as described (25). Genomic DNA from sorted TECs (Epcam+, MHC-II+, and CD45.2−) and DP thymocytes (TCRβ+, CD4+CD8α+) was purified using a DNeasy kit (Qiagen). Sorts were performed with a FACSAria II or a FACSAria Fusion (BD Bioscience). T cells used in proliferation assays were purified from lymph nodes using Dynabead Untouched Mouse T cell kits (Invitrogen).
RT-PCR
Total RNA was isolated with an RNeasy Mini kit (Qiagen) and cDNA generated with Superscript RT (Invitrogen). Real-time PCR was performed with SYBR Green PCR mix (Applied Biosystems) using a QuantStudio 6 (Applied Biosystems). The results are relative to HPRT expression. Primer sequences used for real-time PCR are provided in Supplemental Table 1.
Viral infection
LCMV Armstrong was obtained from Dorian McGavern (NINDS). Mice were inoculated i.p. with 2 × 105 PFU. Splenocytes were stained with APC-labeled class I tetramers containing LCMV peptides gp33, gp276, and np396 (NCI tetramer core facility).
LPS treatment in vivo
LPS from E. coli (Sigma #L2880) was solubilized in PBS and injected i.p. at a dose of 3 μg/gram body weight. Control mice were injected with PBS alone.
Statistical analysis
Unless otherwise indicated, statistical analyses were performed using GraphPad Prism software and an unpaired 2-tailed Student’s t test. P values 0.05 or less were considered significant. Averaged results of multiple experiments are presented as the arithmetic mean ± SEM.
Results
Generation and functional characterization of a conditional Cyp11b1 allele
Mice with global deletion of exons 3-7 of CYP11B1 have markedly diminished adrenal corticosterone production (26). To address the role of thymus-derived GC in thymocyte development, we generated mice in which CYP11B1 could be disrupted in a tissue-specific manner. Mice were generated in which LoxP sites flanked CYP11B1 exons 3-5 (Cyp11b1fl/fl mice, Supplemental Fig. 1A). Deletion of these exons results in early termination of translation after an open reading frame containing the first 135 of 501 amino acids followed by 5 out-of-frame residues (Supplemental Fig. 1B). These mice were crossed with animals expressing actin-Cre to delete CYP11B1 in the entire germline (Cyp11b1-/- mice). Cultured wild-type but not Cyp11b1-/- adrenal cells synthesized substantial amounts of corticosterone, which was prevented by the Cyp11b1 inhibitor metyrapone (Fig. 1A). Plasma corticosterone levels in Cyp11b1-/- mice were < 50% of WT levels, similar to the reduction reported for Cyp11b1 exon 3-7-targeted mice (26), but were not statistically significantly different from levels in Cyp11b1foxN1-Cre animals (Fig. 1B). Systemic GC increase in response to an acute stress such as LPS (27). Intraperitoneal injection of LPS increased plasma corticosterone levels in WT but not Cyp11b1-/- mice (Fig. 1C). Finally, Cyp11b1-/- mice exhibited adrenal hyperplasia (Fig. 1D), characteristic of impaired glucocorticoid production (26).
FIGURE 1. Lack of corticosterone production in Cyp11b1 exon 3-5-/- (Cyp11b1-/- mice) mice.
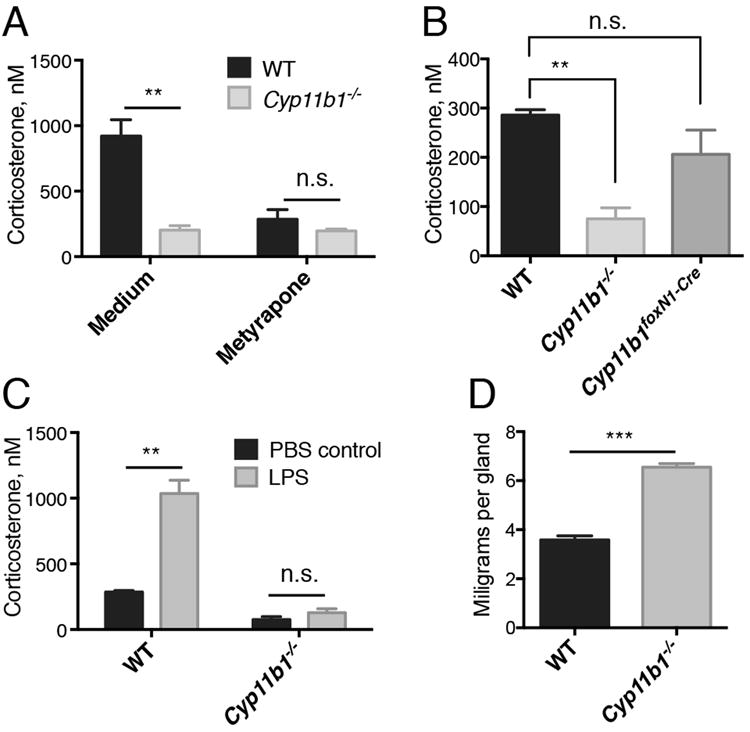
(A) Corticosterone concentrations in the supernatants of adrenals from WT and Cyp11b1-/- mice cultured for three days in the absence or presence of metyrapone. Adrenals from each mouse were cultured in the absence or presence of 200 μg/ml metyrapone. (B) Corticosterone levels in plasma from WT, Cyp11b1foxN1-Cre, and Cyp11b1-/- mice. (C) Corticosterone levels in plasma from WT and Cyp11b1-/- mice 3 hr after injection LPS or PBS alone. (D) Cyp11b1-/- mice exhibit adrenal hyperplasia. Weights of adrenals from WT and Cyp11b1-/- mice. All data in this figure are shown as the mean ± SEM with n=3. *P < 0.05, **P < 0.005.
TEC, not thymocytes, are the major source of thymic GC
Cyp11b1 was deleted in TEC or thymocytes by crossing Cyp11b1fl/fl mice with mice expressing Cre knocked into the FoxN1 locus (23) (Cyp11b1foxN1-Cre mice) or expressed as a transgene driven by the proximal lck promoter (Cyp11b1lck-Cre mice) (28). The tissue specificity of deletion was shown by PCR of genomic DNA from sort-purified cells, which demonstrated that Cyp11b1 exon 4 was absent in Cyp11b1foxN1-Cre TEC but not DP thymocytes, with the opposite being true for Cyp11b1lck-Cre cells (Fig. 2A). Because stress-induced elevations in adrenal-derived GC can cause acute thymic involution (29) it is assumed that the thymus also responds to circulating levels at steady-state. However, the relative contributions of systemic versus paracrine GC have never been experimentally addressed. To do this, the constitutive expression of two GC-responsive genes, Gilz and Lad1 (30) was used as a read-out of GC signaling in freshly-isolated thymocytes. In GR-deficient thymocytes (GRlck-Cre), Gilz and Lad1 mRNA levels were reduced 40-50% compared to WT controls (Fig. 2B). Gilz and Lad1 expression were similarly reduced in Cyp11b1-/- thymocytes, as expected, and also in Cyp11b1foxN1-Cre thymocytes, in which only TEC-synthesized GC are absent. In contrast, this reduction was not observed in thymocytes of Cyp11b1lck-Cre mice, or in Cyp11b1 heterozygous (Cyp11b1fl/+,actin-Cre) thymocytes. Thymocyte expression of the GC-unresponsive gene Calm3 was similar across genotypes. Differences in Gilz and Lad1 expression were not due to GC hyporesponsiveness, as they were induced by exogenous GC in all GR-sufficient thymocytes (Supplemental Fig. 1C). Together, these data show that biologically-active thymus GC are synthesized de novo by TEC, not thymocytes, in situ. Furthermore, under basal conditions, thymocyte GC signaling is primarily driven by TEC- rather than adrenal-derived GC.
FIGURE 2. Reduced glucocorticoid-dependent gene expression in Cyp11b1foxN1-Cre thymocytes.
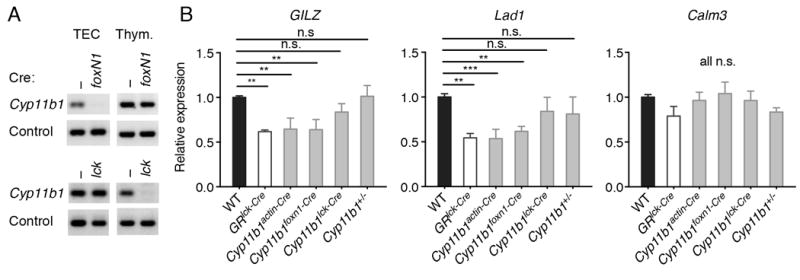
(A) Cre-mediated disruption of Cyp11b1. Genomic DNA from sorted TECs and DP thymocytes from WT and Cyp11b1foxN1-Cre (N1-Cre) and Cyp11b1lck-Cre (lck-Cre) mice was analyzed by PCR for the presence of CYP11B1 exon 4. Control primers were specific for the H-2A locus (41). One representative pair of three sets of mice for each Cre is shown. (B) mRNA levels of GC-sensitive and -insensitive genes in Cyp11b1foxN1-Cre thymocytes. Relative mRNA levels in thymocytes either freshly isolated or after 3 hr of treatment with 100 nM corticosterone were determined by RT-PCR. Significance was determined by 1-way ANOVA, followed by Fisher’s LSD multiple comparison (each mutant vs control, n =4 to 8). *P < 0.05, **P < 0.005, *** P < 0.0005.
Negative selection is enhanced in the absence of TEC-derived GC
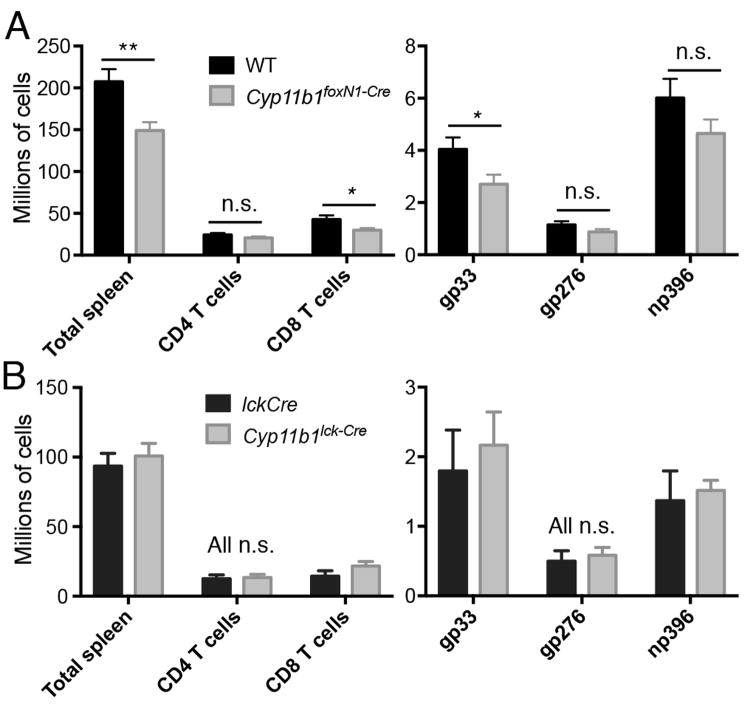
Deletion of the GR in immature thymocytes results in the negative selection of cells that otherwise would have been positively selected (13). One example was the reduction in DP and CD4+ thymocytes in AND mice whose transgenic TCR normally promotes positive selection of CD4+ T cells in H-2b animals. To prevent rearrangement of endogenous TCRα, recombinase-activating gene 2 (RAG-2) was deleted by crossing AND with RAG2-/- mice. As observed with AND GRlck-Cre mice whose thymocytes cannot respond to GC (13), there was a reduction in the number of DP and CD4+ SP thymocytes in Cyp11b1foxN1-Cre AND mice compared to Cyp11b1fl/fl AND controls (Fig. 3A). Among the molecules upregulated during negative selection are PD-1, Helios, and the pro-apoptotic Bcl-2 family member Bim (31). Furthermore, Annexin V binds to cells actively undergoing apoptosis (32). We examined pre-selection thymocytes (DP), TCR-signaled DP thymocytes (TCRhi, (33)), a population of cells enriched for those undergoing negative selection (CD4low/CD8low “double dull” (34-36)), and mature thymoytes (CD4+ SP). We found that double dull, and to a lesser extent SP, thymocytes from Cyp11b1foxN1-Cre AND mice had a larger fraction of BimhiPD-1+ cells compared to Cyp11b1fl/fl AND controls (Fig. 3B). Strikingly, the fraction of apoptotic cells (HelioshiAnnexin V+) was increased in Cyp11b1foxN1-Cre thymocytes, most notably in the double dull subset. Therefore, loss of TEC-derived GC mice resulted in increased negative selection of thymocytes that normally undergo positive selection.
FIGURE 3. Increased negative selection of thymocytes developing in the absence of corticosterone production by TECs.
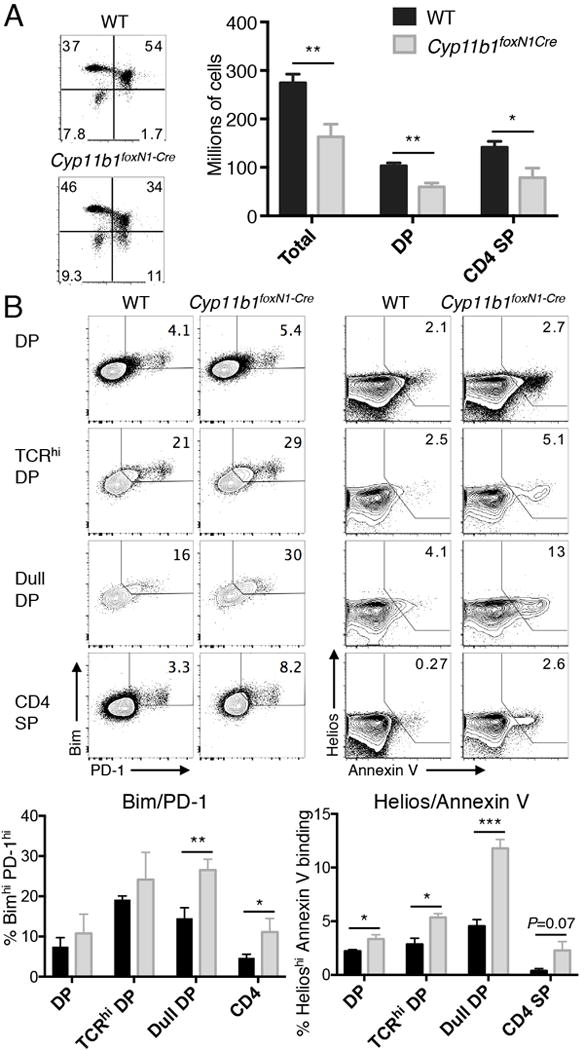
(A, left panels) CD4 versus CD8 profiles of representative 5 week-old AND TCR WT and Cyp11b1foxN1-Cre thymi. The numbers represent the percentages in each quadrant. (A, right panel) Total thymocytes and subsets from five week-old WT (n=10) and Cyp11b1foxN1-Cre (n=6) Rag2-/- mice. (B) Increased indicators of negative selection in Cyp11b1foxN1-Cre AND TCR thymocytes. PD-1 and Bim expression (upper left panels) and Helios expression and Annexin V-binding (upper right panels) are shown in the indicated subsets of WT and Cyp11b1foxN1-Cre AND TCR thymocytes. Shown below each are the means and SEM of three (Bim/PD-1) and four (Helios/Annexin V) mice. *P < 0.05, **P < 0.005, ***P < 0.0005.
Cyp11b1foxN1-Cre T cells have decreased response to allo- and foreign antigens
A hallmark of the changed repertoire in GR-deficient T cells is a decreased allogenic response (13). If paracrine production by TEC is the major source of intrathymic GC, Cyp11b1foxN1-Cre T cells would also be expected to have a reduced response to allogeneic APC. To test this, H-2b T cells were cultured with irradiated H-2a splenocytes. Whereas Cyp11b1foxN1-Cre T cells responded normally to stimulation with plate-bound anti-CD3/anti-CD28, the response of Cyp11b1foxN1-Cre T cells to allogeneic stimulation was blunted (Fig. 4A). A possible contribution of thymocyte-synthesized GC on the TCR repertoire was addressed by parallel experiments using Cyp11b11ck-Cre T cells as responders. The response of WT and Cyp11b11ck-Cre T cells was identical to both anti-CD3/anti-CD28 and allogeneic APC (Fig. 4B).
FIGURE 4. The repertoires of Cyp11b1foxN1-Cre T cells, but not of Cyp11b1lck-Cre T cells, were weakened.
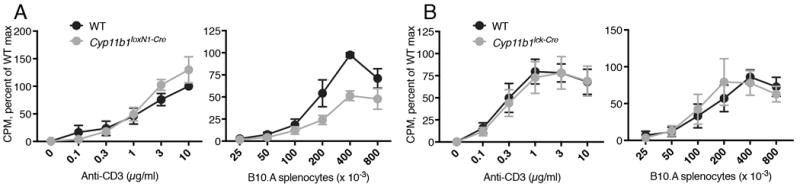
Cyp11b1foxN1-Cre T cells proliferate normally (A) to cross-linked CD3 (anti-CD3/CD28) but not (B) to alloantigen. Cyp11b1lck-Cre T cells proliferate normally (C, D) to TCR cross-linking and alloantigen. Data are presented as the averaged percent of WT maximum from four (Cyp11b1foxN1-Cre) and four (Cyp11b1lck-Cre) independent experiments. Data are shown as mean ± SEM.
Similar to alloantigen, the altered TCR repertoire in GRlck-Cre caused T cells to respond less well when mice were infected with LCMV Armstrong strain (13), which elicits a robust CD8+ T cell response that peaks at seven days. WT and Cyp11b1foxN1-Cre mice were infected with the LCMV and seven days later splenic T cells were characterized. Cyp11b1foxN1-Cre mice had a 15% and 30% reduction in CD4+ and CD8+ T cell subsets, respectively, compared to WT (Fig. 5A). There was a decrease in the number of LCMV gp33-specific CD8+ T cells in spleens of Cyp11b1foxN1-Cre mice as measured by peptide-MHC I tetramer binding. There was also a decrease in LCMV np396-reactive CD8 T cells, although the reduction did not achieve statistical significance. In contrast, targeting Cyp11b1 expression in thymocytes did not affect the response to LCMV (Fig. 5B). Thus, T cells that developed in the absence of TEC- but not thymocyte-provided GC have a reduced ability to respond to pMHC.
FIGURE 5. Reduced CD8+ T cell response in LCMV-infected Cyp11b1foxN1-Cre but not Cyp11b1lck-Cre mice.
Mice were infected with LCMV Armstrong and splenocytes were analyzed 7 days later. Shown are (left) numbers of splenocytes and T cells and (right) numbers of MHC class I tetramer+ CD8 + T cells from mice of the indicated genotypes (WT, n=12, Cy11b1foxN1-Cre, n=11; lckCre alone, n=6, Cyp11b1lck-Cre, n=6). Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
Discussion
It was long assumed that GC acting on the thymus were derived from the circulation. However, the discovery that TEC can produce GC (14, 16) raised the possibility that the locally-derived product was biologically active. In fact, blockade of glucocorticoid production in fetal thymic organ culture resulted in increased TCR-mediated activation and enhanced negative selection (15). To assess the relative contribution of local versus systemic GC in the thymus, we quantified the expression of GC-responsive genes in thymocytes at steady-state. Remarkably, the lack of local synthesis reduced expression of these genes to the same levels as in GR-deficient thymocytes, which cannot respond to GC at all. This implies that the bulk of biologically active GC in the thymus under basal conditions are supplied by TEC in a paracrine manner. This discrepancy with the classical understanding of endocrine glucocorticoid signaling may be explained by enhanced bioavailability. Interaction between thymocytes and TECs is a prerequisite for the TCR-pMHC interactions underlying selection. Paracrine delivery via cell-cell contact raises the possibility that GC pass directly from TECs to thymocytes without diffusion and dilution into the extracellular space. In addition, hormone delivered directly to thymocytes would bypass carrier proteins. Approximately 80-90% of plasma GC are bound by the corticosteroid-binding globulin (CBG) and another 5-10% by albumin, leaving only 5% free and available to signal by entering the cell and binding the GR (37). GC that pass directly from TEC to thymocytes would therefore have a much higher effective concentration than those delivered via the blood.
It has been reported that thymocytes express the complete set of steroidogenic enzymes and can produce measurable corticosterone in vitro (18, 38, 39). Thymocytes from adult (14-22 weeks of age) mice produced more GC than those from younger mice, leading to the suggestion that thymocytes supplant TECs for GC production later in life (18). We and others were unable to detect thymocyte Cyp11b1 activity in vitro (data not shown and 40). We were, however, able test for a role for thymocyte-produced GC genetically by deleting Cyp11b1 in thymocytes and T cells. Expression of GC-sensitive genes in Cyp11b1lck-Cre thymocytes was normal, indicating that GC levels sensed by thymocytes at the population level were normal. As indicated by the response to allogeneic APC and LCMV, the repertoire of Cyp11b1lck-Cre T cells was also normal.
The data in this report demonstrate a paracrine mode of action by GC produced in the thymus, where effects of GC have previously been ascribed to hormonal control via adrenal production. Awareness of the impact of locally-produced GC could aid development of targeted therapies addressing thymocyte development.
Supplementary Material
Acknowledgments
We thank Bei Dong for expert technical assistance and Dorian McGavern for the gift of LCMV.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- GC
glucocorticoids
- Cyp
cytochrome P450
- Cyp11b1
11β-hydroxylase
- GR
glucocorticoid receptor
- TEC
thymic epithelial cells
- DP
double positive thymocytes
References
- 1.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton R. Molecular mechanisms of glucocorticoid action: what is important. Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philips A, Maira M, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol. 1997;17:5952–5959. doi: 10.1128/mcb.17.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fassett MS, Jiang W, D’Alise AM, Mathis D, Benoist C. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci U S A. 2012;109:3891–3896. doi: 10.1073/pnas.1200090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu QN, Baldwin TA. Differential roles for Bim and Nur77 in thymocyte clonal deletion induced by ubiquitous self-antigen. J Immunol. 2015;194:2643–2653. doi: 10.4049/jimmunol.1400030. [DOI] [PubMed] [Google Scholar]
- 8.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 9.Vacchio MS, Lee JY, Ashwell JD. Thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting TCR-mediated thymocyte activation. J Immunol. 1999;163:1327–1333. [PubMed] [Google Scholar]
- 10.King LB, Vacchio MS, Dixon K, Hunziker R, Margulies DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;3:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 11.Tolosa E, King LB, Ashwell JD. Thymocyte glucocorticoid resistance alters positive selection and inhibits autoimmunity and lymphoproliferative disease in MRL-lpr/lpr mice. Immunity. 1998;8:67–76. doi: 10.1016/s1074-7613(00)80459-8. [DOI] [PubMed] [Google Scholar]
- 12.Lu FW, Yasutomo K, Goodman GB, McHeyzer-Williams LJ, McHeyzer-Williams MG, Germain RN, Ashwell JD. Thymocyte resistance to glucocorticoids leads to antigen-specific unresponsiveness due to “holes” in the T cell repertoire. Immunity. 2000;12:183–192. doi: 10.1016/s1074-7613(00)80171-5. [DOI] [PubMed] [Google Scholar]
- 13.Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122:2384–2394. doi: 10.1172/JCI63067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vacchio MS, Ashwell JD. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med. 1997;185:2033–2038. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazirandeh A, Xue Y, Rafter I, Sjövall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J. 1999;13:893–901. doi: 10.1096/fasebj.13.8.893. [DOI] [PubMed] [Google Scholar]
- 17.Lechner O, Wiegers GJ, Oliveira-Dos-Santos AJ, Dietrich H, Recheis H, Waterman M, Boyd R, Wick G. Glucocorticoid production in the murine thymus. Eur J Immunol. 2000;30:337–346. doi: 10.1002/1521-4141(200002)30:2<337::AID-IMMU337>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Qiao S, Chen L, Okret S, Jondal M. Age-related synthesis of glucocorticoids in thymocytes. Exp Cell Res. 2008;314:3027–3035. doi: 10.1016/j.yexcr.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology. 2014 doi: 10.1210/en.2013-1606. en20131606. [DOI] [PubMed] [Google Scholar]
- 20.Lechner O, Dietrich H, Wiegers GJ, Vacchio M, Wick G. Glucocorticoid production in the chicken bursa and thymus. Int Immunol. 2001;13:769–776. doi: 10.1093/intimm/13.6.769. [DOI] [PubMed] [Google Scholar]
- 21.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 23.Gordon J, Xiao S, Hughes B, Su DM, Navarre SP, Condie BG, Manley NR. Specific expression of lacZ and cre recombinase in fetal thymic epithelial cells by multiplex gene targeting at the Foxn1 locus. BMC Dev Biol. 2007;7:69. doi: 10.1186/1471-213X-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 26.Mullins LJ, Peter A, Wrobel N, McNeilly JR, McNeilly AS, Al-Dujaili EA, Brownstein DG, Mullins JJ, Kenyon CJ. Cyp11b1 null mouse, a model of congenital adrenal hyperplasia. J Biol Chem. 2009;284:3925–3934. doi: 10.1074/jbc.M805081200. [DOI] [PubMed] [Google Scholar]
- 27.Li CC, Munitic I, Mittelstadt PR, Castro E, Ashwell JD. Suppression of Dendritic Cell-Derived IL-12 by Endogenous Glucocorticoids Is Protective in LPS-Induced Sepsis. PLoS Biol. 2015;13:e1002269. doi: 10.1371/journal.pbio.1002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 29.Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- 30.van der Laan S, Sarabdjitsingh RA, Van Batenburg MF, Lachize SB, Li H, Dijkmans TF, Vreugdenhil E, de Kloet ER, Meijer OC. Chromatin immunoprecipitation scanning identifies glucocorticoid receptor binding regions in the proximal promoter of a ubiquitously expressed glucocorticoid target gene in brain. J Neurochem. 2008;106:2515–2523. doi: 10.1111/j.1471-4159.2008.05575.x. [DOI] [PubMed] [Google Scholar]
- 31.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 33.Kearse KP, Takahama Y, Punt JA, Sharrow SO, Singer A. Early molecular events induced by T cell receptor (TCR) signaling in immature CD4+ CD8+ thymocytes: increased synthesis of TCR-alpha protein is an early response to TCR signaling that compensates for TCR-alpha instability, improves TCR assembly, and parallels other indicators of positive selection. J Exp Med. 1995;181:193–202. doi: 10.1084/jem.181.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351:150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- 35.Lucas B, Germain RN. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- 36.Sant’Angelo DB, Lucas B, Waterbury PG, Cohen B, Brabb T, Goverman J, Germain RN, Janeway CA. A molecular map of T cell development. Immunity. 1998;9:179–186. doi: 10.1016/s1074-7613(00)80600-7. [DOI] [PubMed] [Google Scholar]
- 37.Bae YJ, Kratzsch J. Corticosteroid-binding globulin: modulating mechanisms of bioavailability of cortisol and its clinical implications. Best Pract Res Clin Endocrinol Metab. 2015;29:761–772. doi: 10.1016/j.beem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Qiao S, Tuckermann J, Okret S, Jondal M. Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J. 2010;24:5043–5051. doi: 10.1096/fj.10-168724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology. 2009;150:4163–4169. doi: 10.1210/en.2009-0195. [DOI] [PubMed] [Google Scholar]
- 40.Rocamora-Reverte L, Reichardt HM, Villunger A, Wiegers G. T-cell autonomous death induced by regeneration of inert glucocorticoid metabolites. Cell Death Dis. 2017;8:e2948. doi: 10.1038/cddis.2017.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164:786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.