Abstract
Historically, osteoporosis has not been considered a public health priority for the Hispanic population. However, recent data indicate that Mexican Americans are at increased risk for this chronic condition. Although it is well established that there is heterogeneity in social, lifestyle, and health-related factors among Hispanic subgroups, there are currently few studies on bone health among Hispanic subgroups other than Mexican Americans. The current study aimed to determine the prevalence of osteoporosis and low bone mass (LBM) among 953 Puerto Rican adults, aged 47 to 79 years and living on the US mainland, using data from one of the largest cohorts on bone health in this population: The Boston Puerto Rican Osteoporosis Study (BPROS). Participants completed an interview to assess demographic and lifestyle characteristics and bone mineral density measures. To facilitate comparisons with national data, we calculated age-adjusted estimates for osteoporosis and LBM for Mexican American, non-Hispanic white, and non-Hispanic black adults, aged ≥50 years, from the National Health and Nutrition Examination Survey (NHANES). The overall prevalence of osteoporosis and LBM were 10.5% and 43.3% for participants in the BPROS, respectively. For men, the highest prevalence of osteoporosis was among those aged 50 to 59 years (11%) and lowest for men ≥70 years (3.7%). The age-adjusted prevalence of osteoporosis for Puerto Rican men was 8.6%, compared with 2.3% for non-Hispanic white, and 3.9% for Mexican American men. There were no statistically significant differences between age-adjusted estimates for Puerto Rican women (10.7%), non-Hispanic white women (10.1%), or Mexican American women (16%). There is a need to understand specific factors contributing to osteoporosis in Puerto Rican adults, particularly younger men. This will provide important information to guide the development of culturally and linguistically tailored interventions to improve bone health in this understudied and high-risk population.
Keywords: OSTEOPOROSIS, GENERAL POPULATION STUDIES, DXA, AGING, EPIDEMIOLOGY
Introduction
According to recent prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), osteoporosis and low bone mass (LBM) affected more than 55.5% of US adults aged ≥50 years in 2013–2014.(1) Osteoporosis increases the risk of fracture, which can lead to decreased quality of life, disability, institutionalization, and excess mortality, making this disease an important contributor to public health burden.(2–4) In 2005, health care costs related to fracture exceeded $19 billion, and these are estimated to increase by 50% by 2025, as the US population ages.(5) The largest increase in fracture-related health care costs are expected to occur in Hispanics (from $754 million in 2005 to $2 billion in 2025),(5) one of the fastest growing segments of the US population.(6) Despite these projections, bone health remains relatively understudied in this population.
It is a commonly held belief that Hispanics have a lower risk of osteoporosis compared with non-Hispanic whites. However, recent data have shown that Hispanics may have a similar(7–9) or even higher prevalence of osteoporosis than non-Hispanic whites.(10,11) National data, adjusted to the age, sex, and race/ethnic distribution of the US population, using 2010 US Census data, indicate that Mexican American men and women have higher prevalences of osteoporosis than non-Hispanic white men and women (5.9% versus 3.9% and 20.4% versus 15.8%, respectively).(11) The vast majority of bone research among Hispanics has focused on Mexican Americans. However, there is evidence that bone status varies across Hispanic subgroups.(12) This highlights the need for research to accurately target specific groups at risk for LBM and osteoporosis.
More than 9% of the US Hispanic population is of Puerto Rican origin, one of the largest Hispanic subgroups in the United States, second only to Mexican Americans.(13) Little is known about the skeletal health of Puerto Rican adults and whether interventions and programs to improve bone health would benefit this underserved population. The primary objective of this study was to estimate the prevalence of osteoporosis and LBM among Puerto Rican adults, aged 47 to 79 years, living on the US mainland. Estimates from the Boston Puerto Rican Osteoporosis Study (BPROS) were compared to data for Mexican American, non-Hispanic white, and non-Hispanic black adults, aged 50 years and older, from the NHANES 2005–2010.
Subjects and Methods
Study population
This study included data from 953 participants from the BPROS and, for comparison, data from 4244 participants from NHANES (2005–2010). The BPROS is an ancillary study to the Boston Puerto Rican Health Study, a longitudinal investigation of health disparities experienced by Puerto Rican adults aged 45 to 75 years living in the Greater Boston area.(14) A detailed description of the recruitment methods for the Boston Puerto Rican Health Study is published elsewhere.(14) Briefly, year 2000 US Census data were used to identify census tracks with 25 or more Puerto Rican adults aged 45 to 75 years. Census blocks within identified tracks with 10 or more Hispanic adults aged 45 to 75 years were randomly selected for door-to-door enumeration. Each block was visited three to six times, on all days (including weekend days), and at all times of the day (including evenings). More than 77% of participants were recruited using this method. One participant per household was randomly selected to participate in the study. Additional participants were recruited through community efforts, including community events (9.8%), referrals from community organizations (7.2%), and calls to the study office from flyers distributed throughout the community and/or though advertisements on the radio and television (5.6%). Eligible participants included those who self-identified as Puerto Rican, could answer questions in English or Spanish, aged 45 to 75 years and living in the Greater Boston area. Exclusion criteria included: (i) any plans to move from the area within 2 years; (ii) low Mini Mental State Examination score (≤10); or (iii) inability to answer questions due to a serious health condition.(14) A total of 1504 Puerto Rican adults completed a baseline interview, and 1265 completed a 2-year follow-up interview. After completing the 2-year interview, participants were invited to participate in the BPROS. Of the 1267 participants who completed 2-year interviews, 973 were re-consented for the BPROS. Reasons for nonparticipation included: 205 were not interested, 13 moved from the area, 47 had difficulty scheduling the interview, 11 were lost to follow up, 2 did not participate for health issues, and 20 had died since their 2-year interview. Four participants did not complete the 2-year interview, but were consented for the BPROS. Those who declined to participate in the BPROS were older (60.9 years versus 58.7 years, p < 0.001) and were more likely to have type 2 diabetes (47.8% versus 40.4%, p = 0.03) compared with those who participated. There were no differences by sex (p = 0.91), smoking status (p = 0.16), physical activity score (p = 0.42), or activities of daily living (no versus some versus considerable impairment, p = 0.34).
Participants in the BPROS completed an interview and BMD measures, and provided a blood sample at the Metabolic Research Unit at the Jean Mayer US Department of Agriculture (USDA) Human Nutrition Research Center on Aging (HNRCA) at Tufts University. Participants provided written informed consent. The Institutional Review Boards at Tufts Medical Center, Tufts University, Northeastern University, and the University of Massachusetts Lowell approved this study.
The NHANES is a cross-sectional national survey conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC), to collect health and nutrition information on children and adults in the United States. The sample is selected using a complex, multilevel, probability-cluster sampling design and data are collected in biennial cycles using comprehensive questionnaires and medical examinations, as described in detail elsewhere.(15) The 2005–2006, 2007–2008, and 2009–2010 cycles were included in this study. A total of 4244 adults, aged 50 years and older and with valid BMD scans of the hip and of the lumbar spine (L2–L4), were included in this study to be comparable to the protocol and prevalence estimates from the BPROS.(16–18) Participants of NHANES provided written informed consent and the protocols were approved by the Research Ethics Review Board of the NCHS.
Measures of BMD
In the BPROS, BMD (g/cm2) of the hip and lumbar spine was measured using dual-energy X-ray absorptiometry (DXA) (GE-Lunar Model Prodigy scanner; GE Lunar, Madison, WI, USA) at the Bone Metabolism Laboratory at the HNRCA. All measures were obtained following standard procedures, and the right hip was routinely scanned unless the participant reported having a previous hip fracture or joint replacement. The root mean square precision was 1.31% for BMD measures of the femoral neck and 1.04% for the lumbar spine for this laboratory, as reported.(19) Each week, an external standard (aluminum spine phantom; Lunar Radiation Corp) was scanned to assess stability of the DXA measures (DXA acquisition software version 6.1 and analysis version 12.2). A total of 25 participants’ lumbar spine (L2–L4) measures and seven participants’ femoral neck measures were excluded from analyses, as determined by the study endocrinologist (BDH) to be inaccurate, after reviewing all scans with T-scores >4.0 to check for non-anatomical parts and for extraskeletal calcification.
In the NHANES, BMD of the hip and the lumbar spine were measured using a Hologic QDR 4500A fan-beam densitometer (Hologic Inc., Bedford, MA, USA) following the BMD examination protocol, as described.(20) Spine scans were analyzed using Apex version 3.0 software and femur scans using Discovery 12.4 software. Quality control was conducted routinely on all DXA machines.
Prevalence of osteoporosis and LBM
Osteoporosis was defined as T-score ≤ −2.5 (2.5 SD or more below peak bone mass) and LBM as T-score between −1.0 and −2.5 (between 1.0 and 2.5 SD below peak bone mass) at the femoral neck or lumbar spine, as defined by criteria from the World Health Organization.(21) Prevalence estimates of osteoporosis and LBM were completed for the full sample and by sex and age category (45–50 years, 50–59 years, 60–69 years, and 70–79 years) for the BPROS. For the BPROS and NHANES, T-scores for the lumbar spine were calculated using a reference group of 30-year-old non-Hispanic white females from the DXA manufacturer database,(22) and for the femoral neck using a reference group of 20-year-old to 29-year-old non-Hispanic white females from NHANES III.(23) Prevalence estimates of osteoporosis and LBM were calculated at the femoral neck and lumbar spine (L2–L4) for all participants from the BPROS and NHANES to be comparable to prevalence estimates from previous studies.(16–18)
Additional descriptive variables
At baseline and 2-year follow-up of the Boston Puerto Rican Health Study, questionnaires were administered to collect data on age, education, household income, and health and health behaviors (eg, physical activity, smoking, and alcohol consumption).(14) Educational attainment was categorized as <8th grade, 9th to 12th grade, and some college or higher. Poverty level was calculated using the annual poverty thresholds released by the U.S. Census Bureau.(24) A physical activity score was calculated based the number of hours spent in various activities from a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey(25,26) and the rate of oxygen consumption corresponding with each activity. Anthropometric measures were obtained in duplicate and an average of the two measures was used. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Diabetes was defined as fasting glucose >126 mg/dL or use of diabetes medications. Heart disease was defined based on self-report of heart disease, heart attack, or stroke.
During the BPROS study visit, fasting blood samples were collected by a bilingual certified phlebotomist with evacuated EDTA tubes. Samples were immediately centrifuged to separate plasma. Plasma 25-hydroxyvitamin D was measured using a 125I Radioimmunoassay procedure (DiaSorin, Inc., Stillwater, MN, USA; manufacturer procedures: 68100E).(27) The intraassay and interassay coefficients of variation (CVs) were 10.8% and 9.4%, respectively. Serum parathyroid hormone (PTH) and osteocalcin were measured on an Immulate 1000 analyzer by a two-site chemiluminescent enzyme-labeled immunometric assay(28–30) and an immunimetric assay,(31,32) respectively. The intraassay and interassay CVs were 5.5% and 7.9% for serum PTH and 2.8% and 3.9% for osteocalcin, respectively. Serum cross-linked N-telopeptides (NTx) were measured by competitive on-inhibition enzyme-linked immunosorbent assay (ELISA) on a BioTek Instruments Elx 808 Microplate Reader.(33,34) The intraassay and interassay CVs were 4.6% and 6.9%, respectively.
One hundred ancestry informative markers (AIMS) were selected if the allele frequency differed by at least 0.5 between any two of the three ancestral populations and were used to estimate genetic ancestry.(35) The 100 AIMS were distributed across the genome and were in linkage equilibrium in the three ancestral populations: West African, European, and native American. The QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) was used to isolate DNA, and IPLEX protocols for the multiplex PCR, single-base primer extension, and generation of mass spectra were used to genotype the AIMS.(35) Individual ancestry was calculated using two programs (STRUCTURE 2.2(36,37) and IAE3CI(38,39)) based on the genotypes of the AIMS in reference to the three ancestral populations.(40)
Data analysis
Data were analyzed using SAS (version 9.4; SAS Institute, Cary, NC, USA). Data are presented as mean and 95% CI for BMD of the femoral neck and lumbar spine, and prevalence estimates and standard error for osteoporosis and LBM, at either the femur neck or lumbar spine. Age-adjusted estimates for osteoporosis and LBM were obtained for Puerto Rican men and women. For comparison with the BPROS data, we calculated the age-adjusted prevalence estimates and 95% CI for osteoporosis and LBM for Mexican American, non-Hispanic white, and non-Hispanic black men and women using NHANES data (2005–2010). Sample weights were used, and all analyses accounted for the complex survey design. We examined the 95% CIs between the BPROS and NHANES groups to identify measurable differences between group means.
Results
The mean age of men was 58.2±7.8 years and women was 58.9±7.3 years (Table 1). Predominant European ancestry (56±16% and 57±15%) and lower African (28±16% and 28±15%) and native American ancestries (16±7% and 15±6%) were noted for men and women, respectively. The majority of participants had less than high school education and low income. Approximately 30% of men and 17% of women were current smokers and 45% of men and 29% of women consumed moderate to heavy amounts of alcohol. Approximately 40% of men and women had diabetes and 22% to 24% reported having heart disease. More than 44% of men and 68% of women reported having arthritis. Participants also tended to have low plasma vitamin D concentration (men: 18.6±7.0; women 20.1±7.5 ng/mL).
Table 1.
Characteristics of Participants in the Boston Puerto Rican Osteoporosis Study, by Sex
| Characteristic | Men (n = 258–265) |
Women (n = 666–686) |
|---|---|---|
| Age (years), mean SD | 58.2±7.8 | 58.9±7.3 |
| Education, % | 44.9 | 49.0 |
| Less than 8th grade | 44.9 | 49.0 |
| 9th to 12th grade (or GED) | 42.2 | 35.1 |
| Some college or higher | 12.9 | 15.9 |
| Below poverty level, % | 48.5 | 58.8 |
| Ancestry, mean±SD | ||
| European admixture | 56±16 | 57±15 |
| African admixture | 28±16 | 28±15 |
| Native American admixture | 16±7 | 15±6 |
| Physical activity score, mean±SD | 33.0±5.8 | 31.3±4.0 |
| Current smoker, % | 30.0 | 17.2 |
| Alcohol consumption, % | ||
| None within the past year | 54.7 | 70.7 |
| Moderate | 37.5 | 25.5 |
| Heavy | 7.8 | 3.8 |
| Body mass index (kg/m2), mean±SD | 30.1±5.6 | 33.0±6.8 |
| Diabetes, % | 40.7 | 40.6 |
| Heart disease, % | 24.2 | 22.9 |
| Arthritis, % | 44.2 | 68.7 |
| Plasma 25(OH) vitamin D (ng/mL), mean±SD | 18.6±7.0 | 20.1±7.5 |
| HRT use, % | – | 1.9 |
| Serum NTX (nmol BCE), mean±SD | 13.1±6.1 | 13.7±5.9 |
| Serum osteocalcin (ng/mL), mean±SD | 5.4±3.7 | 6.8±4.7 |
| Serum (pg/mL), PTH mean±SD | 51.7±30.6 | 54.4±30.6 |
GED=General Equivalency Diploma; HRT=hormone-replacement therapy.
In the full sample of Puerto Rican men and women, mean BMD of the femoral neck and lumbar spine decreased with increasing age categories (Table 2). Among men, mean femoral neck BMD was highest among the 45 to 49 year age group (1.05 g/cm2). In the older age groups, BMD of the femoral neck remained relatively similar (Table 2). Among women, mean femoral neck decreased across age categories (45–49 years: 1.01 g/cm2; 50–59 years: 0.93 g/cm2; 60–69 years: 0.88 g/cm2; and 70–79 years: 0.85 g/cm2). Among women, lumbar spine BMD decreased across all age categories (45–49 years: 1.27 g/cm2; 50–59 years: 1.14 g/cm2; 60–69 years: 1.11 g/cm2; and 70–79 years: 1.06 g/cm2). In contrast, mean lumbar spine BMD was lowest among men 50 to 59 years (1.18 g/cm2) and highest among men 70 to 79 years (1.31 g/cm2).
Table 2.
Mean Bone Mineral Density (g/cm2) of the Femoral Neck and the Lumbar Spine for Puerto Rican Adults From the Boston Puerto Rican Osteoporosis Study
| Femoral neck (g/cm2)
|
Lumbar spine (g/cm2)
|
|||
|---|---|---|---|---|
| n | Mean (95% CI) | n | Mean (95% CI) | |
| Full sample | ||||
| Age category | ||||
| 45–49 years | 85 | 1.023 (0.994,1.052) | 85 | 1.251 (1.217, 1.284) |
| 50–59 years | 430 | 0.950 (0.937, 0.963) | 422 | 1.154 (1.136, 1.171) |
| 60–69 years | 336 | 0.913 (0.898, 0.928) | 329 | 1.143 (1.123, 1.162) |
| ≥70 years | 100 | 0.889 (0.857, 0.921) | 95 | 1.123 (1.081, 1.165) |
| Men | ||||
| Age category | ||||
| 45–49 years | 32 | 1.050 (0.998, 1.103) | 32 | 1.226 (1.169, 1.282) |
| 50–59 years | 118 | 0.996 (0.969, 1.024) | 114 | 1.180 (1.146, 1.214) |
| 60–69 years | 96 | 0.987 (0.954, 1.020) | 91 | 1.232 (1.190. 1.273) |
| ≥70 years | 26 | 1.012 (0.953, 1.072) | 24 | 1.314 (1.239, 1.388) |
| Women | ||||
| Age category | ||||
| 45–49 years | 53 | 1.006 (0.971, 1.041) | 53 | 1.266 (1.224, 1.308) |
| 50–59 years | 312 | 0.933 (0.918, 0.947) | 308 | 1.144 (1.124, 1.164) |
| 60–69 years | 240 | 0.884 (0.869, 0.899) | 238 | 1.109 (1.088, 1.129) |
| ≥70 years | 74 | 0.846 (0.813, 0.879) | 71 | 1.058 (1.017, 1.099) |
The prevalences of osteoporosis and LBM were 10.5% and 43.3%, respectively for BPROS participants (Table 3). Women had higher prevalences of osteoporosis and LBM compared with men (11.2% versus 8.8% and 46.9% versus 34.4%, respectively). However, differences were only statistically significant for LBM (p < 0.001) and not for osteoporosis (p = 0.28). For women, the prevalence of osteoporosis increased across age categories (45–49 years: 3.8%; 50–59 years: 9.6%; 60–69 years: 10.8%; and 70–79 years: 24.3%). However, for men, the highest prevalence of osteoporosis was among those aged 50 to 59 years (11%) and lowest for men ≥70 years (3.7%). For men and women combined, the prevalence of LBM increased across age categories from 45 to 49 years to 60 to 69 years, but decreased for ages 70 to 79 years.
Table 3.
Prevalence of Osteoporosis and Low Bone Mass at Either the Femoral Neck or the Lumbar Spine for Puerto Rican Adults From the Boston Puerto Rican Osteoporosis Study
| Osteoporosis
|
LBM
|
||||
|---|---|---|---|---|---|
| n | Prevalence, % | SE | Prevalence, % | SE | |
| Full sample | |||||
| Age category | |||||
| 45–49 years | 85 | 4.7 | 2.3 | 22.4 | 4.6 |
| 50–59 years | 430 | 10.0 | 1.5 | 40.5 | 2.4 |
| 60–69 years | 337 | 10.1 | 1.6 | 51.9 | 2.7 |
| 70–79 years | 101 | 18.8 | 3.9 | 44.6 | 5.0 |
| Overall | 953 | 10.5 | 1.0 | 43.3 | 1.6 |
| Men | |||||
| Age category | |||||
| 45–49 years | 32 | 6.3 | 4.4 | 28.1 | 8.1 |
| 50–59 years | 118 | 11.0 | 2.9 | 34.8 | 4.4 |
| 60–69 years | 96 | 8.3 | 2.8 | 37.5 | 5.0 |
| 70–79 years | 27 | 3.7 | 3.7 | 29.6 | 9.0 |
| Overall | 273 | 8.8 | 1.7 | 34.4 | 2.9 |
| Women | |||||
| Age category | |||||
| 45–49 years | 53 | 3.8 | 2.6 | 18.9 | 5.4 |
| 50–59 years | 312 | 9.6 | 1.7 | 42.6 | 2.8 |
| 60–69 years | 241 | 10.8 | 2.0 | 57.7 | 3.2 |
| 70–79 years | 74 | 24.3 | 5.0 | 50.0 | 5.9 |
| Overall | 680 | 11.2 | 1.2 | 46.9 | 1.9 |
Figure 1 presents the age-adjusted prevalence of osteoporosis and LBM for men from the BPROS, and for Mexican American, non-Hispanic white, and non-Hispanic black men from NHANES 2005–2010. A greater percentage of BPROS men had osteoporosis compared with non-Hispanic white or non-Hispanic black men (8.6% versus 2.3% and 1.3%, respectively). Although the estimates for Puerto Rican men were more than double that of Mexican American men from NHANES (8.6% versus 3.9%), the CI values were large and between-group estimates were not significant. Puerto Rican, Mexican American, and non-Hispanic white men had similar prevalence estimates of LBM (34.6%, 38.3%, and 34.5%, respectively) and CI values overlapped. Non-Hispanic black men had the lowest estimate of LBM compared with the other groups.
Fig. 1.
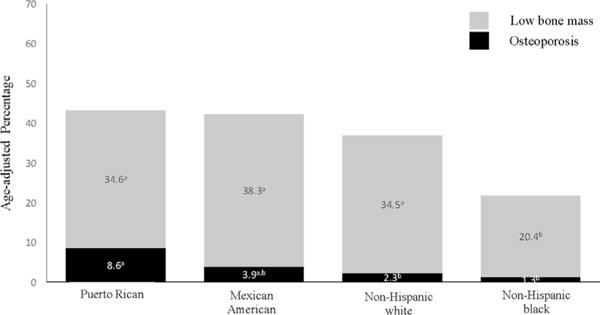
Age-adjusted prevalence of osteoporosis and LBM at either the femoral neck or lumbar spine for Puerto Rican men from the Boston Puerto Rican Osteoporosis Study and Mexican American, non-Hispanic white, and non-Hispanic black men from NHANES (2005–2010). Estimates with the same superscript signify overlap of 95% CI between groups.
Age-adjusted prevalence of osteoporosis was similar for Puerto Rican and non-Hispanic white women (10.7% versus 10.1%, respectively) (Fig. 2). Mexican American women had the highest prevalence of osteoporosis, at 16%, although CI values overlapped between this group and Puerto Rican women. Mexican American and non-Hispanic white women had similar age-adjusted estimates for LBM (50.1% and 51.5%, respectively). Approximately 46.7% of Puerto Rican women had LBM. Non-Hispanic black women had the lowest prevalence of osteoporosis (3.8%) and LBM (35.8%) compared with the other groups.
Fig. 2.
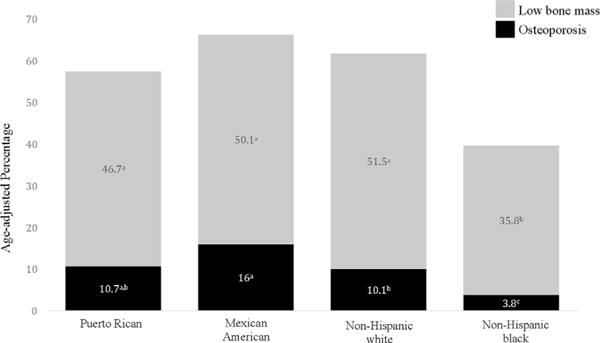
Age-adjusted prevalence of osteoporosis and LBM at either the femoral neck or lumbar spine for Puerto Rican women from the Boston Puerto Rican Osteoporosis Study and Mexican American, non-Hispanic white, and non-Hispanic black men from NHANES (2005–2010). Estimates with the same superscript signify overlap of 95% CI between groups.
Discussion
This study presents results from one of the largest cohort studies on bone health among Puerto Rican older adults living on the US mainland. Our findings indicate that the prevalence of osteoporosis and LBM among Puerto Rican adults was 10.5% and 43.4%, respectively. For women, the age-adjusted prevalence was 10.7% for osteoporosis and 46.7% for LBM. For men, the age-adjusted prevalence of osteoporosis and LBM was 8.6% and 34.6%, respectively. A greater percentage of Puerto Rican men from the BPROS had osteoporosis, compared with non-Hispanic white or non-Hispanic black men from NHANES; CI values overlapped between Puerto Ricans and Mexican Americans. Puerto Rican women had a similar estimate of osteoporosis, compared with non-Hispanic white women from NHANES.
The underlying belief has been that osteoporosis is not a major health concern for Hispanic adults.(41) In contrast, a study using national data found that the prevalence of osteoporosis was highest among Mexican American men [MA] and women (ages ≥50 years) compared with non-Hispanic white [NHW] and black [NHB] men (5.9% MA versus 3.9% NHW versus 1.3% NHB) and women (20.4% MA versus 15.8% NHW versus 7.7% NHB).(11) Based on these results, most bone research in Hispanics has focused on Mexican Americans. Given the heterogeneity among the Hispanic population for many factors, including sociodemographics, health behaviors, culture, and genetics,(42–44) it is imperative that bone health also be investigated among Hispanic subgroups individually. There are currently few data describing the prevalence of osteoporosis among Puerto Ricans; the second largest subgroup of Hispanics in the United States. One small study (n = 57) of Puerto Rican women (50–69 years) living on the island of Puerto Rico reported that 12% had osteoporosis at the lumbar spine and 8.7% at the femoral neck.(45) In our study of more than 900 men and women who were either first-generation or second-generation adults living on the US mainland, the prevalence of osteoporosis at either the femoral neck or lumbar spine was 10.7%; similar to the results observed among Puerto Rican women living on the island.
The current study also included men, where the age-adjusted prevalence of osteoporosis and LBM was 8.6% and 34.6%, respectively. These estimates of osteoporosis for Puerto Rican men are higher than those previously reported for non-Hispanic white or non-Hispanic black men, based on national data.(11) Comparison of our results from the BPROS to NHANES data showed that prevalence estimates were approximately two to three times greater for Puerto Rican men than noninstitutionalized men from the general US population. Further, the highest prevalence of osteoporosis among Puerto Rican men was noted for those aged 50 to 59 years (11.0%). This was a surprising finding, because previous studies have shown lower estimates of osteoporosis among men in this age range within the United States.(11,46) Osteoporosis has been typically shown to affect men later in life (≥70 years) whereas our study suggests that Puerto Rican men living in the Greater Boston area experience a higher burden of osteoporosis at a younger age, between 50 and 69 years.
The reason for higher estimates of osteoporosis in this population of younger Puerto Rican men is unknown and requires further exploration. One possible explanation for this phenomenon is that older Puerto Rican adults were more physically active during their lifetime compared with younger adults, which may offer protection for bone health.(47,48) A study using data from four US national surveillance systems reported that, among Hispanics (excluding Mexican Americans), the percentage of men and women who participated in occupational and transportation-related physical activities decreased with acculturation.(49) Approximately 47% of Hispanics living in the United States for <5 years reported transportation-related physical activity, compared to approximately 28% of Hispanics living in the United States for greater than 15 years. Dietary changes with acculturation may also be associated with the differences in bone health between younger Puerto Rican men and older Puerto Rican men in the current study. An earlier study using data from the BPROS showed that younger individuals followed a more Western-type dietary pattern (high in meat, processed meat, and French fries) compared with older Puerto Rican adults.(50) Further, adults who were the least acculturated were more likely to be following a traditional diet (rice, beans, and oils). Supporting these findings, a systematic review of 34 studies examining the acculturation-diet relation among Hispanics reported that more acculturated individuals consume less fruit and vegetables, rice and beans, and more sugar and sugar-sweetened beverages.(51) Furthermore, foreign-born individuals consumed more calcium compared to those born in the United States.(51) Dietary patterns that have been shown to be beneficial to bone are those high in fruit and vegetables(52) nuts, legumes, wine, and rice dishes,(53) whereas diets high in processed foods and red meat have been related to poorer bone health.(54) However, these studies have largely been conducted in non-Hispanic white adults. Although, additional research on the relationship between dietary patterns and bone health in this population are needed, the dietary patterns of younger, more acculturated Puerto Rican adults tend to include foods and beverages that may be detrimental to bone health.
In the current study, the age-adjusted prevalence of osteoporosis was similar for Puerto Rican women from the BPROS and for non-Hispanic white women from NHANES. Osteoporosis has traditionally been viewed as a condition affecting primarily non-Hispanic white women, because earlier studies indicated that BMD of Hispanics was intermediate between non-Hispanic whites and blacks.(55,56) Our findings, however, suggest that osteoporosis is currently a greater public health problem than previously appreciated in this population and, based on projections by Burge and colleagues,(5) will continue to grow, likely resulting in more fractures. A small study of postmenopausal women reported lower BMD at the spine, and poorer bone mechanical and microarchitecture, among 33 Caribbean-origin Hispanics, including Puerto Ricans, compared with 33 age-matched non-Hispanic white women, suggesting poorer bone quality in this population.(57) Combined with our findings of higher than expected prevalence of osteoporosis in Puerto Rican women, this is of concern and illustrates an urgent need for understanding the mechanisms underpinning poor bone health in this population.
Current clinical recommendations published by the National Osteoporosis Foundation (NOF) are available for screening, diagnosis, and treatment of osteoporosis in postmenopausal women and men ≥50 years. However, based on our results, recommendations to counsel on risk of osteoporosis and fracture and on health behaviors (diet and physical activity) to promote bone health should begin at an earlier age. Hispanics have been shown to have limited knowledge about osteoporosis and bone health, and to view osteoporosis as less important than other chronic conditions, such as heart disease and diabetes.(58) This population also engages in fewer health behaviors that promote bone health, such as participating in weight-bearing activities and taking calcium supplements.(58,59) Primary care visits should include education on the prevention of osteoporosis as part of routine care, particularly for this vulnerable population. Increasing knowledge and awareness about osteoporosis and risk factors for this chronic condition is imperative, as there is no cure. This may be a missed opportunity for primary prevention of osteoporosis, in an effort to prevent future fracture in this population. Further additional research is needed to determine whether early screening for osteoporosis in this population would be beneficial and cost-effective to inform future clinical recommendations.
This study had several strengths and limitations. To date, this is one of the largest studies of bone health among Puerto Rican adults living on the US mainland, although there were small sample sizes in some of age categories and for men, particularly for older men. Although bone measures were completed using the gold standard (DXA), this study did not examine fracture, which is clinically relevant, because self-reported measures were collected in the BPROS and are not a well-validated measure of fracture assessment for comparison across studies. Older patients may have better bone status versus younger patients because they are healthy enough to participate in the study.
In summary, this work contributes to a growing body of literature that indicates Hispanics, particularly Puerto Ricans, are at risk for osteoporosis and LBM. This is contrary to current public health standards which do not address the need for intervention for bone among Hispanic subgroups that have different lifestyle, acculturation, and sociodemographic influences. The current study indicates a need for additional research on the ethnic-specific factors contributing to osteoporosis in this high-risk and understudied population. This will provide valuable information to inform future culturally and linguistically appropriate interventions to reduce the public health burden of this prevalent chronic condition in this Hispanic subgroup.
Acknowledgments
This research was funded by the National Institutes of Health (P01 AG023394, P50 HL105185, R01 AG027087). SEN is supported by K01 AR067894. NCW is supported by K12 HS023009. We thank our skilled research team for their dedication to this project.
Authors’ roles: Study design: SEN, KLT, and BDH. Study conduct: SEN, KLT and BDH. Data collection: KLT and BDH. Data analysis: SEN and JLG. Data interpretation: SEN, KMM, BDH, KLT, and NCW. Manuscript preparation: SEN, KMM, BDH, and NCW. Approving final version of manuscript: SEN, KMM, NCW, KLT, BDH, and JLG. JLG and SEN than responsibility for the integrity of the data analysis.
Footnotes
Disclosures
BDH is the Vice President and on the Board of Trustees of the International Osteoporosis Foundation. NCW has research contracts from Amgen and serves as a consultant for Pfizer, Canada. SEN, KMM, JLG, and KLT report no conflicts of interest.
References
- 1.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28(6):1979–88. doi: 10.1007/s00198-017-3996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–51. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498–507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 4.Marks R, Allegrante JP, Ronald MacKenzie C, Lane JM. Hip fractures among the elderly: causes, consequences and control. Ageing Res Rev. 2003;2(1):57–93. doi: 10.1016/s1568-1637(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.US Census Bureau. Census Bureau Reports [Internet] Washington, DC: US Census Bureau; 2013. Asians Fastest-Growing Race or Ethnic Group in 2012. [cited 2017 Oct 30]. Release Number: CB13-112. Available from: https://www.census.gov/newsroom/press-releases/2013/cb13-112.html. [Google Scholar]
- 7.US Office of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: US Office of the Surgeon General; 2004. [cited 2017 Oct 30]. Available from http://www.ncbi.nlm.nih.gov/books/NBK45513/ [PubMed] [Google Scholar]
- 8.Morton DJ, Barrett-Connor E, Kritz-Silverstein D, Wingard DL, Schneider DL. Bone mineral density in postmenopausal Caucasian, Filipina, and Hispanic women. Int J Epidemiol. 2003;32(1):150–6. doi: 10.1093/ije/dyg024. [DOI] [PubMed] [Google Scholar]
- 9.Zingmond DS, Melton LJ, 3rd, Silverman SL. Increasing hip fracture incidence in California Hispanics, 1983 to 2000. Osteoporos Int. 2004;15(8):603–10. doi: 10.1007/s00198-004-1592-7. [DOI] [PubMed] [Google Scholar]
- 10.Looker AC, Borrud LG, Dawson-Hughes B, Shepherd JA, Wright NC. (NCHS Data Brief No. 93).Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005–2008. 2012 Apr; Available from: https://www.cdc.gov/nchs/data/databriefs/db93.pdf. [PubMed]
- 11.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the united states based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18(7):943–53. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 13.Motel S, Patten E. The 10 largest Hispanic origin groups: characteristics, rankings, top counties. Pew Research Hispanic Trends Project. 2012 Available from: http://www.pewhispanic.org/files/2012/06/The-10-Largest-Hispanic-Origin-Groups.pdf.
- 14.Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health and Nutrition Examination General Data Release Documentation [Internet] Atlanta, GA: Centers for Disease Control and Prevention and National Center for Health Statistics; 2015. [cited 2017 Oct 30]. Available from: https://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. [Google Scholar]
- 16.Bhupathiraju SN, Dawson-Hughes B, Hannan MT, Lichtenstein AH, Tucker KL. Centrally located body fat is associated with lower bone mineral density in older Puerto Rican adults. Am J Clin Nutr. 2011;94(4):1063–70. doi: 10.3945/ajcn.111.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B, Hannan MT, Tucker KL. Adherence to the 2006 American Heart Association Diet and Lifestyle Recommendations for cardiovascular disease risk reduction is associated with bone health in older Puerto Ricans. Am J Clin Nutr. 2013;98(5):1309–16. doi: 10.3945/ajcn.112.056267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noel SE, Arevalo S, Smith CE, et al. Genetic admixture and body composition in Puerto Rican adults from the Boston Puerto Rican Osteoporosis Study. J Bone Miner Metab. 2017;35(4):448–55. doi: 10.1007/s00774-016-0775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White J, Harris SS, Dallal GE, Dawson-Hughes B. Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom. 2003;6(2):159–62. doi: 10.1385/jcd:6:2:159. [DOI] [PubMed] [Google Scholar]
- 20.National Health and Nutrition Examination. Dual Energy X-ray Absorptiometry (DXA) Procedures Manual. Atlanta, GA: Centers for Disease Control and Prevention; 2007. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dexa.pdf. [Google Scholar]
- 21.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Kelly T. Bone mineral density reference databases for American men and women. J Bone Miner Res. 1990;5(Suppl 1):s249. [Google Scholar]
- 23.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 24.US Census Bureau. Housing and Household Economic Statistics Division. Poverty Thresholds. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html. Published August 11, 2017.
- 25.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 26.Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 27.Catalog: 68100E. Stillwater, MN: DiaSorin Inc; Diasorin (formerly Incstar) Corporation 25-Hydroxyvitamin D 125I RIA kit: 2001. [Google Scholar]
- 28.Armitage EK. Parathyrin metabolism and methods for assay. Clin Chem. 1986;32:418–24. [PubMed] [Google Scholar]
- 29.Lepage R, Whittom S, Bertrand S. Superiority of dynamic over static reference intervals for intact, midmolecule, and C-terminal parathyrin in evaluating calcemic disorders. Clin Chem. 1992;38:2129–35. [PubMed] [Google Scholar]
- 30.Document: PILKPP-9, 2004-02-24, Intact PTH and according to Immulate 1000 Operating Manual, Document #600467. Los Angeles, CA: Diagnostic Products Corporation (DCP); 2004. rev B1, revision 5.XX August 2003. [Google Scholar]
- 31.Document: PILKOC-7, 2002-02-12, Osteocalcin, and according to Immulate 1000 Operating Manual, Document #600467. Los Angeles, CA: Diagnostic Products Corporation (DCP); 2003. rev B1, revision 5.XX August 2003. [Google Scholar]
- 32.Fassbender WJ, Steinhauer B, Stracke H, Schumm-Draeger PM, Usadel KH. Validation of a new automated immunoassay for measurement of intact osteocalcin. Clin Chem. 2002;48:31–8. [PubMed] [Google Scholar]
- 33.Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997;43(11):2058–63. [PubMed] [Google Scholar]
- 34.Document: 9021/20047RT7006E-Osteomark NTx Serum – A quantitative measure of cross-linked N-telopeptides of Type I collagen (NTx) in human serum. Princeton, NJ: Wampole Laboratories (Former Ostex, Seattle, WA 98134); [Google Scholar]
- 35.Lai CQ, Tucker KL, Choudhry S, et al. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Hum Genet. 2009;125(2):199–209. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parra EJ, Kittles RA, Argyropoulos G, et al. Ancestral proportions and admixture dynamics in geographically defined African Americans living in South Carolina. Am J Phys Anthropol. 2001;114(1):18–29. doi: 10.1002/1096-8644(200101)114:1<18::AID-AJPA1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsai HJ, Choudhry S, Naqvi M, Rodriguez-Cintron W, Burchard EG, Ziv E. Comparison of three methods to estimate genetic ancestry and control for stratification in genetic association studies among admixed populations. Hum Genet. 2005;118(3–4):424–33. doi: 10.1007/s00439-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 40.Choudhry S, Coyle NE, Tang H, et al. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006;118(5):652–64. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 41.Bohannon AD, Hanlon JT, Landerman R, Gold DT. Association of race and other potential risk factors with nonvertebral fractures in community-dwelling elderly women. Am J Epidemiol. 1999;149(11):1002–9. doi: 10.1093/oxfordjournals.aje.a009744. [DOI] [PubMed] [Google Scholar]
- 42.Aponte J. Diabetes-related risk factors across Hispanic subgroups in the Hispanic health and nutritional examination survey (1982–1984) Public Health Nurs. 2009;26(1):23–38. doi: 10.1111/j.1525-1446.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 43.Neighbors CJ, Marquez DX, Marcus BH. Leisure-time physical activity disparities among Hispanic subgroups in the United States. Am J Public Health. 2008;98(8):1460–4. doi: 10.2105/AJPH.2006.096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siega-Riz AM, Sotres-Alvarez D, Ayala GX, et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99(6):1487–98. doi: 10.3945/ajcn.113.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haddock L. Prevalence of osteopenia and osteoporosis in a normal female Puerto Rican population. P R Health Sci J. 1997;16(3):241–4. [PubMed] [Google Scholar]
- 46.Cawthon PM, Shahnazari M, Orwoll ES, Lane NE. Osteoporosis in men: findings from the Osteoporotic Fractures in Men Study (MrOS) Ther Adv Musculoskelet Dis. 2016;8(1):15–27. doi: 10.1177/1759720X15621227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delvaux K, Lefevre J, Philippaerts R, et al. Bone mass and lifetime physical activity in Flemish males: a 27-year follow-up study. Med Sci Sports Exerc. 2001;33(11):1868–75. doi: 10.1097/00005768-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Ulrich CM, Georgiou CC, Gillis DE, Snow CM. Lifetime physical activity is associated with bone mineral density in premenopausal women. J Womens Health. 1999;8(3):365–75. doi: 10.1089/jwh.1999.8.365. [DOI] [PubMed] [Google Scholar]
- 49.Ham SA, Yore MM, Kruger J, Heath GW, Moeti R. Physical activity patterns among Latinos in the United States: putting the pieces together. Prev Chronic Dis. 2007;4(4):A92. [PMC free article] [PubMed] [Google Scholar]
- 50.Noel SE, Newby PK, Ordovas JM, Tucker KL. A traditional rice and beans pattern is associated with metabolic syndrome in Puerto Rican older adults. J Nutr. 2009;139(7):1360–7. doi: 10.3945/jn.109.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayala GX, Baquero B, Klinger S. A systematic review of the relationship between acculturation and diet among Latinos in the United States: implications for future research. J Am Diet Assoc. 2008;108(8):1330–44. doi: 10.1016/j.jada.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker KL, Chen H, Hannan MT, et al. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2002;76(1):245–52. doi: 10.1093/ajcn/76.1.245. [DOI] [PubMed] [Google Scholar]
- 53.McNaughton SA, Wattanapenpaiboon N, Wark JD, Nowson CA. An energy-dense, nutrient-poor dietary pattern is inversely associated with bone health in women. J Nutr. 2011;141(8):1516–23. doi: 10.3945/jn.111.138271. [DOI] [PubMed] [Google Scholar]
- 54.Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Bone mineral density and protein-derived food clusters from the Framingham Offspring Study. J Acad Nutr Diet. 2015;115(10):1605–1613.e1. doi: 10.1016/j.jand.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–94. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 56.Marcus R, Greendale G, Blunt BA, et al. Correlates of bone mineral density in the postmenopausal estrogen/progestin interventions trial. J Bone Miner Res. 1994;9(9):1467–76. doi: 10.1002/jbmr.5650090920. [DOI] [PubMed] [Google Scholar]
- 57.Zhou B, Wang J, Stein EM, et al. Bone density, microarchitecture and stiffness in Caucasian and Caribbean Hispanic postmenopausal American women. Bone Res. 2014;2:14016. doi: 10.1038/boneres.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geller SE, Derman R. Knowledge, beliefs, and risk factors for osteoporosis among African-American and Hispanic women. J Natl Med Assoc. 2001;93(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 59.Larkey LK, Day SH, Houtkooper L, Renger R. Osteoporosis prevention: knowledge and behavior in a southwestern community. J Community Health. 2003;28(5):377–88. doi: 10.1023/a:1025448730662. [DOI] [PubMed] [Google Scholar]


