Abstract
Objectives
Studies that rely on self-report to investigate the relationship between hormonal contraceptive use and HIV acquisition and transmission, as well as other health outcomes, could have compromised results due to misreporting. We determined the frequency of misreported hormonal contraceptive use among African women with and at risk for HIV.
Study design
We tested 1,102 archived serum samples from 664 African women who had participated in prospective HIV prevention studies. Using a novel high-performance liquid chromatography mass-spectrometry assay, we quantified exogenous hormones for injectables (medroxyprogesterone acetate or norethisterone), oral contraceptives (OC) (levonorgestrel or ethinyl estradiol), and implants (levonorgestrel or etonogestrel) and compared them to self-reported use.
Results
Among women reporting hormonal contraceptive use, 258/358 (72%) of samples were fully concordant with self-report, as were 642/744 (86%) of samples from women reporting no hormonal contraceptive use. However, 42/253 (17%) of samples from women reporting injectable use, 41/66 (62%) of samples from self-reported OC users, and 3/39 (8%) of samples from self-reported implant users had no quantifiable hormones. Among self-reported non-users, 102/744 (14%) had ≥1 hormone present. Concordance between self-reported method and exogenous hormones did not differ by HIV status.
Conclusion
Among African women with and at risk for HIV, testing of exogenous hormones revealed agreement with self-reported contraceptive use for most women. However, unexpected exogenous hormones were identified among self-reported hormonal contraceptive users and non-users, and an important fraction of women reporting hormonal contraceptive use had no hormones detected; absence of oral contraceptive hormones could be due, at least in part, to samples taken during the hormone-free interval. Misreporting of hormonal contraceptive use could lead to biased results in observational studies of the relationship between contraceptive use and health outcomes.
Implications
Research studies investigating associations between hormonal contraceptive use and HIV should consider validating self-reported use by objective measures; because both over- and under-reporting of use occurs, potential misclassification based on self-report could lead to biased results in directions that cannot be easily predicted.
Keywords: hormonal contraceptives, injectable contraceptive, implant, HIV
Introduction
Associations between use of hormonal contraceptive methods and the transmission or acquisition of HIV[1–4], bacterial vaginosis[5,6], and other sexually transmitted infections[7–9] have been topics of substantial interest for decades. Of particular concern is a possible increase in HIV acquisition and transmission among women using the injectable contraceptive DMPA (depot medroxyprogesterone acetate) [10]. Such research in general has relied on self-reported hormonal contraceptive use, rarely validated by biomarkers[11]. However, like many sensitive topics, self-reported hormonal contraceptive use is subject to possible misclassification due to social desirability bias, a need for privacy from sexual partners, confusion about methods, recall difficulties, and potentially intentional misreporting because of desire to participate in research studies in which contraceptive use is a requirement[12–14]. Misclassification of women with respect to their hormonal contraceptive use could produce biased results in epidemiologic studies. In this analysis, we examined the degree of discordance between self-reported hormonal contraceptive use and exogenous hormones in serum from a cohort of HIV-uninfected and infected African women.
Materials & Methods
Data for this analysis came from three prospective studies (Partners in Prevention HSV/HIV Transmission Study, Couples Observation Study, and Partners PrEP Study) [15–20]. Briefly, women were members of HIV-serodiscordant heterosexual couples in seven African countries (Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, Zambia) who were followed between 2004 and 2013.
This analysis includes a randomly selected subset of HIV-uninfected women who did not acquire HIV and HIV-infected women who did not transmit HIV to their male partner, but who had a risk profile similar to women who acquired/transmitted HIV during the study follow-up, thereby representing a high-risk population [21]. A total of 843 women were initially selected for this analysis; each woman contributed up to two samples. Of the initial 1,419 samples, we excluded: 50 samples from women with surgical contraception or IUD use (only copper IUDs without hormones were available in these settings); 188 samples from women 40 years old or older; and 79 samples from women who were pregnant at the time of sample collection. However, samples from the first visit at which the pregnancy was detected were not excluded as these women may have been particularly likely to misreport their contraceptive use.
Contraceptive use was not a study requirement, but all study sites offered multiple contraceptive methods on site and women could choose to obtain methods from the site or other providers. Women self-reported contraceptive methods by standard interviewer-administered questionnaires at regular study visits and women in the analysis could switch between methods.
Blood samples were taken at quarterly visits and stored at −80°C. We used a validated, high-performance liquid chromatography-heated electrospray ionization-tandem triple quadrupole mass spectrometry (LC-MS/MS) assay to simultaneously test for five exogenous hormones, as well as progesterone (P4) [22]. We expected the following exogenous hormones for each self-reported method (Table 1): medroxyprogesterone acetate (MPA) or norethisterone (NET), components of two different injectable contraceptives; levonorgestrel (LNG) or ethinyl estradiol (EE2), components of oral contraceptives (OC); and LNG or etonogestrel (ENG), components of contraceptive implants. The lower limit of quantification (LLQ) for MPA, ENG, and LNG was 0.02 ng/mL; for EE2 and P4, 0.01 ng/mL; and for NET, 0.04 ng/mL. We set results below LLQ to half the LLQ value for analysis.
Table 1.
Classification of Quantifiable Hormone Results
| Self-reported Method | Only Expected Hormones | Expected & Unexpected Hormones | Only Unexpected Hormones | No Hormones |
|---|---|---|---|---|
| Injectable | Only MPA or NET | MPA or NET, plus LNG, ENG, or EE2 | Only LNG, ENG, or EE2 | None |
| Oral | Only LNG or EE2 | LNG or EE2, plus MPA, NET, or ENG | Only MPA, NET, or ENG | None |
| Implant | Only LNG or ENG | LNG or ENG, plus MPA, NET, or EE2 | Only MPA, NET, or EE2 | None |
| None | None | -- | Any detected | -- |
For each sample, we compared self-reported hormonal contraceptive use to exogenous hormones as quantified by LC-MS/MS; the quantified hormones were not used to assign contraceptive use. We categorized the quantified hormones as expected, unexpected, both expected and unexpected, or no exogenous hormones for each method, as described above (Table 1). We compared the quantification of any expected exogenous hormone (versus no expected exogenous hormone) as a binary outcome by HIV status using generalized estimating equation (GEE) models to account for repeated observations, with a Poisson distribution and exchangeable correlation matrix. We also calculated the proportion of samples with evidence of ovulation (P4≥3ng/mL). Among all users and separately for all non-users, we modeled ovulation by presence of expected exogenous hormones using the same GEE model as above; due to small samples sizes, we were unable to model each method independently. Samples from pregnant women were excluded from all analyses involving P4, as pregnancy alters P4 levels. All analyses were conducted with SAS 9.4 (SAS Institute).
Results
We analyzed a total of 1,102 samples from 664 women. Most samples (952, 86%) were from married women and 323 (29%) were from women age under 25 years old (Table 2). Roughly a third of samples (413, 37%) were from HIV-infected women. The majority of samples were taken when no hormonal contraceptive method was reported (744, 68%); injectable use was reported for 253 (23%), OC use was reported for 66 (6%), and implant use was reported for 39 (4%) of samples.
Table 2.
Characteristics of 664 Women Contributing Samples
| Self-Reported Method | ||||
|---|---|---|---|---|
| Injectable, % (n) | OC, % (n) | Implant, % (n) | None, % (n) | |
| N Samples | 23.0% (253) | 6.0% (66) | 3.5% (39) | 67.5% (744) |
| Age | ||||
| <25 | 22.5% (57) | 30.3% (20) | 15.4% (6) | 32.3% (240) |
| 25–29 | 25.3% (64) | 28.8% (19) | 25.6% (10) | 28.5% (212) |
| 30–34 | 29.2% (74) | 30.3% (20) | 28.2% (11) | 26.6% (198) |
| 35–39 | 22.9% (58) | 10.6% (7) | 30.8% (12) | 12.6% (94) |
| Married | 87.7% (222) | 90.9% (60) | 100% (39) | 84.8% (631) |
| >8 years education | 32.4% (82) | 21.2% (14) | 23.1% (9) | 37.0% (275) |
| Recent Condomless Sex | 19.4% (49) | 31.8% (21) | 28.2% (11) | 15.1% (112) |
| Sexual Partners Outside of Study Partner | 2.4% (6) | 1.5% (1) | 0% (0) | 3.9% (29) |
| Pregnant | 1.2% (3) | 1.5% (1) | 0% (0) | 3.2% (24) |
| HIV-infected | 35.6% (90) | 36.4% (24) | 10.3% (4) | 40.0% (295) |
| Study | ||||
| Couples Observational Study | 2.0% (5) | 1.5% (1) | 0% (0) | 6.7% (50) |
| Partners in Prevention HSV/HIV Transmission Study | 42.7% (108) | 36.4% (24) | 2.6% (1) | 66.7% (496) |
| Partners PrEP Study | 55.3% (140) | 62.1% (41) | 97.4% (38) | 26.6% (198) |
For women reporting hormonal contraceptive use, only the expected exogenous hormones were quantified in 258 (72%) of samples, including 205 (81%) of samples from injectable users, 18 (27%) of samples from OC users, and 35 (90%) of samples from implant users (Table 3). However, unexpected hormones were also quantified. Specifically, 6 (2%) of samples from self-reported injectable users, 7 (11%) OC users, and 1 (3%) of implant users had an unexpected exogenous hormone quantified. In addition, no exogenous hormones were quantified in 42 (17%) of self-reported injectable user samples, 41 (62%) of OC user samples, and 3 (8%) of implant user samples (Figure 1; Figure S1). When restricted to the first sample per woman, results were similar.
Table 3.
Quantifiable Exogenous Hormones by Self-Reported Hormonal Contraceptive Method
| Self-Reported Method | Only Expected Hormones(s), % (n) | Expected & Unexpected Hormones, % (n) | Only Unexpected Hormone(s), % (n) | No Hormones, % (n) |
|---|---|---|---|---|
| Injectable | 81.0% (205) | 1.6% (4) | 0.8% (2) | 16.6% (42) |
|
| ||||
| HIV-infected | 87.8% (79) | 0% (0) | 0% (0) | 12.2% (11) |
| HIV-uninfected | 77.3% (126) | 2.5% (4) | 1.2% (2) | 19.0% (31) |
|
| ||||
| OC | 27.3% (18) | 6.1% (4) | 4.5% (3) | 62.1% (41) |
|
| ||||
| HIV-infected | 29.2% (7) | 8.3% (2) | 12.5% (3) | 50.0% (12) |
| HIV-uninfected | 26.2% (11) | 4.8% (2) | 0% (0) | 69.0% (29) |
|
| ||||
| Implant | 89.7% (35) | 2.6% (1) | 0% (0) | 7.7% (3) |
|
| ||||
| HIV-infected | 75.0% (3) | 25.0% (1) | 0% (0) | 0% (0) |
| HIV-uninfected | 91.4% (32) | 0% (0) | 0% (0) | 8.6% (3) |
|
| ||||
| None* | 86.3% (642) | N/A | 13.7% (102) | N/A |
|
| ||||
| HIV-infected | 85.4% (252) | N/A | 14.6% (43) | N/A |
| HIV-uninfected | 86.9% (390) | N/A | 13.1% (59) | N/A |
|
| ||||
| Total | 81.7% (900) | 0.8% (9) | 9.7% (107) | 7.8% (86) |
For non-users, expected hormones=none.
Figure 1. Quantities of Exogenous Hormones by Self-Reported Hormonal Contraceptive Use.
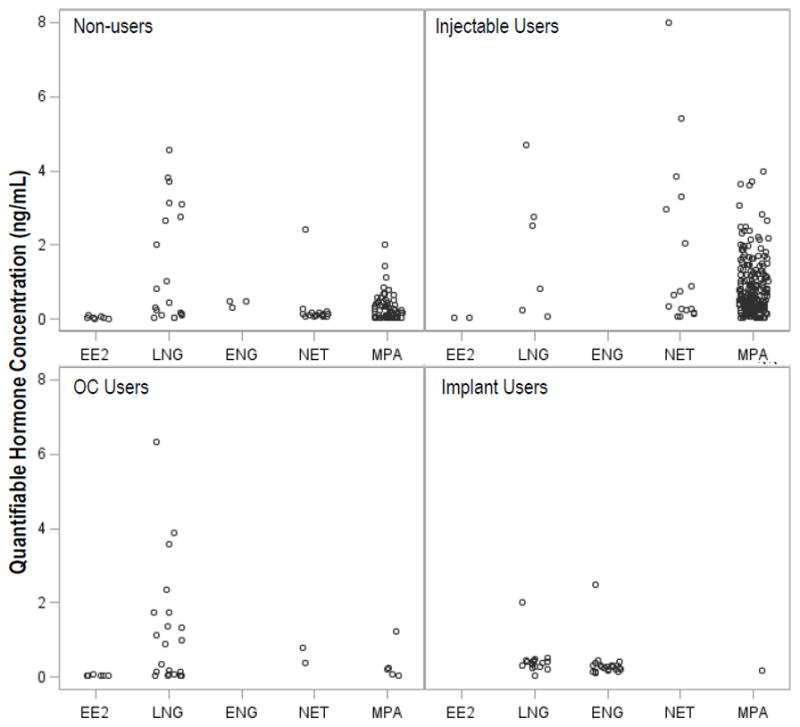
The concentration of quantifiable hormones are presented for each women, according to their self-reported hormonal contraceptive use at the time of the sample, for those with quantifiable hormones; women may be represented by more than one symbol if more than one hormone was detected in the sample. The expected hormones were as follows: for injectables users, medroxyprogesterone acetate (MPA) and norethisterone (NET); for oral contraceptive (OC) users, levonorgestrel (LNG) and ethinyl estradiol (EE2); and for implant users, LNG and etonogestrel (ENG). The lower limit of quantification (LLQ) for MPA, ENG, and LNG was 0.02 ng/mL; for EE2 and P4, 0.01 ng/mL; and for NET, 0.04 ng/mL. Results below LLQ were set to half the LLQ value for analysis.
Among the 744 samples collected from women reporting no hormonal contraceptive use, 642 (86%) had no hormones quantified (Table 3). However, of the 102 samples with an exogenous hormone quantified, 68 (67%) had MPA, 16 (16%) had NET, 7 (7%) had EE2, 19 (19%) had LNG, and 3 (3%) had ENG identified, including ten samples that had multiple hormones (Figure S2).
Of the 116 samples across all methods with an unexpected exogenous hormone quantified, 89 had data on previous hormonal contraceptive use available and of these 30 (34%) had self-reported a method in the six months prior that could explain the hormones identified. Predominantly, these had quantified MPA or NET and reported prior injectable use (n=28). One sample had quantified ENG and reported prior implant use and one sample had quantified LNG and reported prior OC use.
When we stratified results by HIV status (Table 3), no significant differences were noted in quantification of any expected hormones (versus no expected hormone) between HIV-infected and HIV-uninfected women (p=0.92 among non-users, p=0.19 among injectable users, p=0.58 among OC users, and p=0.08 among implant users).
Among all non-pregnant hormonal contraceptive method users with the expected exogenous hormones quantified, only 12/266 (5%) of the samples showed evidence of ovulation (Table 4). When there were no expected hormones, the proportion with ovulatory P4 levels was significantly higher, 25/88 (28%, p<0.0001). Furthermore, among all non-pregnant OC users, 11/65 (17%) had no exogenous hormones quantified and had evidence of ovulation, possibly distinguishing them from women on the placebo week. Among self-reported non-users, 17/102 (17%) of samples with any quantifiable exogenous hormones had evidence of ovulation, compared to 193/618 (31%) of samples with no quantifiable hormones (p=0.005).
Table 4.
| Quantifiable Hormones | Self-Reported Method | |||
|---|---|---|---|---|
| Injectable, % (n) | OC, % (n) | Implant, % (n) | None***, % (n) | |
| Only Expected Hormones | 3.9% (8) | 11.1% (2) | 5.7% (2) | 31.2% (193) |
| Expected & Unexpected Hormones | 0% (0) | 0% (0) | 0% (0) | -- |
| Only Unexpected Hormones | 0% (0) | 0% (0) | -- | 16.8% (17) |
| No Hormones | 35.0% (14) | 27.5% (11) | 0% (0) | -- |
|
| ||||
| Total | 8.8% (22) | 20.0% (13) | 5.1% (2) | 29.2% (210) |
Excludes pregnant women
P4≥3ng/mL
For non-users, expected hormones=none.
Finally, three women reported injectable use when a pregnancy was detected, only one of whom had quantifiable MPA, at 0.025 ng/mL. In addition, one woman had a pregnancy detected while reporting OC use, with no quantifiable exogenous hormones.
Discussion
In this large study of African women with and at risk for HIV, we tested serum samples for multiple hormones to objectively assess self-reported hormonal contraceptive use. We identified self-reported use that could not be verified by serum levels as well as unreported use that was identified by serum results. Overall, 82% of samples were perfectly concordant with self-reported hormonal contraceptive use. However, 17% of samples from women reporting injectable use, 8% of samples from reported implant users, and 62% of samples from reported OC users had no exogenous hormones detected, and unexpected exogenous hormones were also detected in 2–11% of samples. Among self-reported non-users, 14% had at least one exogenous hormone present. These results illustrate the potential for both under- and over-reporting of hormone use and emphasize the limitations of self-report for epidemiologic analyses of the relationship between hormone exposure and health outcomes, such as HIV acquisition.
A recent study, limited to self-reported contraceptive non-users from Zimbabwe, found 27% had detectable exogenous hormones, mostly related to OC or injectable use [14]. In a qualitative component, they also found that women misrepresented their contraceptive use in order to be eligible to participate in research studies and access services[14]. Similarly, in a U.S.-based study of oral-contraceptive users, non-use was associated with poverty and the authors posited that participants may have joined the study for its benefits[23]. For the present analysis, hormonal contraceptive use was not a criterion for participation in the studies, which may explain the lower rate of misreporting among non-users that we found compared to the Zimbabwe study. However, our results broadly agree that hormonal contraceptive use may go underreported.
In addition, some women may report hormonal contraceptives when they are not using them, and we found such over-reporting of use for women who reported oral, injectable, and implantable hormonal methods. Even among users of longer-acting methods, where adherence should have limited impact, we found evidence of overreporting. In addition, 67% of samples from self-reported OC users had no LNG nor EE2. While one explanation for this lack of hormone detection is the hormone free interval of OC use, 17% of samples from reported OC users had no expected hormones while also having ovulatory P4 levels. For comparison, a study of consistent OC users in the U.S. found ovulation (by ultrasound) in 2.7% of participants[24]. Although poor compliance may still be a factor, the true proportion of misreporting among OC users is likely between 17% and 67%. One study in the U.S. found 9–13% of OC users had undetectable LNG and EE at some point during follow-up [25], while another found that 17% of OC users were inconsistent or non-users, by LNG [24].
We found evidence that quantification of unexpected hormones in some cases may have been due to prior hormonal contraceptive use, particularly of injectable methods, which are known to have a long half-life [26,27]; additional work understanding the pharmacokinetics of these drugs in African populations is still needed. However, only one-third of samples had a prior method reported that could possibly explain the discordant results; LNG and ENG have shorter half-lives and prior use is less likely to explain unexpected presence of these hormones[28,29]. Women also may have taken exogenous hormones for non-contraceptive purposes, such as treating menorrhagia, which could explain some results, and we did not specifically collect data on hormone use for other reasons. Finally, a total of four women were pregnant yet reported hormonal contraceptive use; this may be due to either misreporting (3 of the 4 had no quantifiable exogenous hormones) or contraceptive failure.
The effect of this misreporting on the results of an epidemiological study is not immediately clear. To fully understand the direction of bias in studies of hormonal contraception and HIV risk, for example, the sensitivity of self-report within each contraceptive method stratified by HIV-acquisition status and the proportion of women in each strata would be needed. If misreporting of hormonal contraceptives is the same among women who acquire HIV and those who do not, then the reported increases in risk among DMPA users would be underestimated.[1] On the other hand, if misreporting differs between women who acquire HIV and those who do not (known as differential misclassification), results may be overestimated or underestimated [30,31]. This supports the need for well-done clinical trials to answer the question of whether hormonal contraception increases the risk of HIV acquisition.[32]
This study has several limitations. We did not have data on the exact composition and date of initiation for each contraceptive method, nor on last menses. In this region, we believe that OC was limited to LNG/EE2 combinations; while it is possible that some women could have had access to NET- or ENG-based OC, there were only two OC users with detectable NET in our sample and none with ENG. Hormonal contraceptive use was not a requirement for these studies; rates of misreporting may differ (and potentially be much higher) in studies with such requirements. Likewise, the rates of misreporting may depend on the prevalence of each method and may not be generalizable to settings with a different contraceptive mix. Additionally, serum progesterone is a marker of presumed ovulation [33], but sampling without regard to the date of last menstrual period may have missed some ovulations; however, ultrasound for ovulation confirmation was not a part of this study.
The strengths of this study include the ability to test for a range of hormonal contraceptive methods and endogenous hormones simultaneously using highly sensitive laboratory techniques. The P4 results demonstrate that hormones were identified at biologically relevant concentrations, as evidence of ovulation was more common when exogenous hormones were not quantified. These data underscore the value of the LC-MS/MS assay, which provides quantitative data on a range of exogenous and endogenous hormones, providing objective data to supplement self-reported use [22]. In addition, we had a large sample size, including women representing many African countries and throughout the reproductive age range.
In conclusion, for studies evaluating the biological relationship between contraceptive use and acquisition and transmission of HIV and other sexually transmitted infections, reliance on self-reported hormonal contraceptive use will be limited by some degree of misreporting and could result in misclassification. Importantly, use of hormones may be either under- or over- reported, and even women reporting use of a particular hormonal contraceptive may have been exposed to other exogenous hormones. Studies using hormonal contraception as an inclusion or exclusion criteria may benefit from objective measures to screen women, or at least retrospective testing to identify a subgroup fully meeting the entry criteria. Studies examining associations with hormonal contraceptive methods should use objective methods to verify self-reported use and, in particular, determine if misclassification is differential, thus quantifying the impact of exposure misclassification.
Supplementary Material
Acknowledgments
Funding: This work was supported by the US Centers for Disease Control and Prevention (U48 DP 005013 SIP 14-023), the Eunice Kennedy Shriver National Institute of Child Health and Development of the US National Institutes of Health (R21 HD074439), and the University of Washington Center for AIDS Research (P30 AI027757). The Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC) is supported by NIH Grant P51 OD011092 awarded to ONPRC. The studies from which the samples for this analysis were drawn were supported by the Bill & Melinda Gates Foundation (OPP1056051, 26469, and 41185). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
We thank the women who participated in these studies.
Partners in Prevention HSV/HIV Transmission Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins
Study sites and site principal investigators: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Jairam R. Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly R. Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baeten JM, Lavreys L, Overbaugh J. The Influence of Hormonal Contraceptive Use on HIV-1 Transmission and Disease Progression. Clin Infect Dis. 2007;45:360–9. doi: 10.1086/519432. [DOI] [PubMed] [Google Scholar]
- 2.Ralph LJ, McCoy SI, Shiu K, Padian NS. Does hormonal contraceptive use increase women’s risk of HIV acquisition? A meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–9. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360–90. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013 Febr;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 5.van de Wijgert JHHM, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS Lond Engl. 2013;27:2141–53. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 6.Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PloS One. 2013;8:e73055. doi: 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin HL, Jr, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: Results from a prospective study. Am J Obstet Gynecol. 2001;185:380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 8.Gursahaney PR, Meyn LA, Hillier SL, Sweet RL, Wiesenfeld HC. Combined hormonal contraception may be protective against Neisseria gonorrhoeae infection. Sex Transm Dis. 2010;37:356–60. doi: 10.1097/OLQ.0b013e3181d40ff1. [DOI] [PubMed] [Google Scholar]
- 9.Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Hormonal contraceptive use and risk of sexually transmitted infections: a systematic review. Contraception. 2006;73:154–65. doi: 10.1016/j.contraception.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS Lond Engl. 2016;30:2665–83. doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall KS, White KO, Reame N, Westhoff C. Studying the use of oral contraception: a review of measurement approaches. J Womens Health. 2002–2010;19:2203–10. doi: 10.1089/jwh.2010.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart GS, Grimes DA. Social desirability bias in family planning studies: a neglected problem. Contraception. 2009;80:108–12. doi: 10.1016/j.contraception.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Polis CB, Westreich D, Balkus JE, Heffron R. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. AIDS Lond Engl. 2013;27:S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achilles SL, Mhlanga FG, Musara P, Poloyac SM, Chirenje ZM, Hillier SL. Misreporting of contraceptive hormone use among clinical research participants. Contraception. 2016;94:417–8. doi: 10.1016/j.contraception.2016.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahle EM, Hughes JP, Lingappa JR, John-Stewart G, Celum C, Nakku-Joloba E, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1 serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr. 2013;62:339–47. doi: 10.1097/QAI.0b013e31827e622d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. The Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS ONE. 2011;6:e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Hormonal contraceptive use and risk of HIV-1 transmission: a prospective cohort analysis. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackelprang RD, Baeten JM, Donnell D, Celum C, Farquhar C, de Bruyn G, et al. Quantifying Ongoing HIV-1 Exposure in HIV-1–Serodiscordant Couples to Identify Individuals With Potential Host Resistance to HIV-1. J Infect Dis. 2012;206:1299–308. doi: 10.1093/infdis/jis480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blue SW, Winchell AJ, Kaucher AV, Lieberman R, Gilles C, Pyra M, et al. Simultaneous quantification of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception. doi: 10.1016/j.contraception.2018.01.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012;85:465–9. doi: 10.1016/j.contraception.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Westhoff CL, Torgal AH, Mayeda ER, Stanczyk FZ, Lerner JP, Benn EKT, et al. Ovarian Suppression in Normal-weight and Obese Women During Oral Contraceptive Use: A Randomized Controlled Trial. Obstet Gynecol. 2010;116:275–83. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 25.Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR, Foegh M. Self-reported and verified compliance in a phase 3 clinical trial of a novel low-dose contraceptive patch and pill. Contraception. 2015;91:204–10. doi: 10.1016/j.contraception.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 26. [accessed February 24, 2017];Depo-Provera [Summary of Product Characteristics] n.d http://www.medicines.org.uk/emc/medicine/11121.
- 27. [accessed February 24, 2017];Noristerat 200mg, solution for intramuscular injection - Summary of Product Characteristics (SPC) - (eMC) n.d http://www.medicines.org.uk/emc/medicine/1835/SPC/Noristerat+200mg,+solution+for+intramuscular+injection.
- 28. [accessed March 29, 2017];IMPLANON™ (etonogestrel implant) [drug label] n.d https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=10208.
- 29. [accessed April 7, 2017];Jadelle [drug label] n.d https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020544s010lbl.pdf.
- 30.Baena A, Garcés-Palacio IC, Grisales H, Baena A, Garcés-Palacio IC, Grisales H. The effect of misclassification error on risk estimation in case-control studies. Rev Bras Epidemiol. 2015;18:341–56. doi: 10.1590/1980-5497201500020005. [DOI] [PubMed] [Google Scholar]
- 31.Höfler M. The effect of misclassification on the estimation of association: a review. Int J Methods Psychiatr Res. 2005;14:92–101. doi: 10.1002/mpr.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cates W Evidence for Contraceptive Options and HIV Outcomes (ECHO) Consortium. Research on hormonal contraception and HIV. Lancet Lond Engl. 2014;383:303–4. doi: 10.1016/S0140-6736(14)60097-0. [DOI] [PubMed] [Google Scholar]
- 33.Roos J, Johnson S, Weddell S, Godehardt E, Schiffner J, Freundl G, et al. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept Reprod Health Care Off J Eur Soc Contracept. 2015;20:438–50. doi: 10.3109/13625187.2015.1048331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


