Abstract
Background
Antiplatelet therapy with clopidogrel is recommended to reduce cardiovascular events in patients with peripheral artery disease (PAD); however, clopidogrel efficacy has not been adequately studied in this patient population. Therefore, we aimed to determine the effects of cilostazol therapy on platelet reactivity among PAD patients on clopidogrel.
Methods
We performed a cross-sectional pilot study of 46 Puerto Rican patients diagnosed with PAD. The cohort was divided based on use of clopidogrel and cilostazol (n = 24) or clopidogrel alone (n = 22). Platelet function was measured ex vivo using the VerifyNow P2Y12 assay. Genomic DNA was extracted from peripheral blood samples using the QIAamp DNA Blood Midi Kit, which was subjected to candidate variant genotyping (CYP2C19, ABCB1, PON1 and P2RY12) using TaqMan quantitative polymerase chain reaction assays. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Among all enrolled patients, 18 (39%) had high on-treatment platelet reactivity (HTPR). The mean platelet reactivity was 207 ± 53 (range, 78–325) with higher P2Y12 reaction units in the non-cilostazol group, 224 ± 45 vs. 191 ± 55 on the cilostazol group (p = 0.03). No significant differences were observed in the clinical or genetic variables between the two groups. A multiple regression analysis determined that history of diabetes mellitus (p= 0.03), use of cilostazol (p = 0.03) and hematocrit (p = 0.02) were independent predictors of platelet reactivity.
Conclusions
In Puerto Rican PAD patients on clopidogrel therapy, history of diabetes mellitus, use of cilostazol and hematocrit are independent predictors of platelet reactivity. Adjunctive cilostazol therapy may enhance clopidogrel efficacy among PAD patients with HTPR.
Keywords: cilostazol, clopidogrel, peripheral artery disease, platelet reactivity
Introduction
Antiplatelet therapy (APT) with aspirin or clopidogrel alone is currently recommended to reduce major adverse cardiovascular events (MACE) such as acute myocardial infarction (AMI), stroke and cardiovascular death in patients with peripheral artery disease (PAD) [1]. Moreover, in symptomatic patients undergoing extremity revascularization, dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is indicated to prevent limb-related events (restenosis and stent thrombosis) [1]. Unfortunately, up to 83% of patients treated with DAPT have high on-treatment platelet reactivity (HTPR) to clopidogrel, which is a risk factor for MACE [2]. This inter-individual variation in platelet reactivity has been associated with several clinical and genetic variables including body mass index, diabetes mellitus (DM), concomitant use of some drugs, smoking status and variant alleles in the ABCB1, P2RY12, PON1, CES1 and B4GALT2 genes [3–9]. Importantly, even though the effects of HTPR on clinical adverse outcomes have been extensively described in patients with coronary artery disease (CAD) undergoing percutaneous coronary interventions [10], the utility of HTPR in predicting MACE and guiding APT in PAD has been poorly explored.
Cilostazol, a selective phosphodiesterase 3 inhibitor, is well known for its positive antiplatelet, anti-inflammatory, anti-oxidant and ischemia-reperfusion injury protective effects [11, 12]. When added to standard DAPT, cilostazol inhibits the adenosine diphosphate (ADP)-induced platelet aggregation, leading to enhanced antiplatelet effects and reduced platelet reactivity on clopidogrel [13]. The pharmacokinetic and pharmacodynamic interaction of clopidogrel and cilostazol has not been fully elucidated, but current evidence suggests a mechanism linked to CYP2C19 and/or CYP3A5 genotypes [14].
Because triple antiplatelet therapy with cilostazol, clopidogrel and aspirin is considered as an alternative regimen to achieve adequate platelet inhibition in CAD patients with HTPR [15], studying this interaction in the PAD population is warranted. Consequently, we aimed to determine the effect of cilostazol therapy on platelet reactivity among patients with PAD on clopidogrel.
Materials and methods
Study design and ethics
This was a multicenter cross-sectional study of Puerto Rican patients receiving APT who were recruited from January to February 2017. The study was approved by the institutional review board (Protocol No. A4070416), and it was conducted in accordance with the Declaration of Helsinki in compliance with Good Clinical Practice. Verbal and written informed consent was obtained from all participants included in the study.
Patient population and data collection
A total of 46 patients with PAD of Hispanic Puerto Rican descent on clopidogrel plus cilostazol or clopidogrel monotherapy were consecutively recruited from all geographic regions of the island. A single physician gathered patient information from the medical record. The study included Puerto Rican Hispanics >21 years old who were receiving 75 mg/day maintenance dose of clopidogrel with or without cilostazol 100 mg/day for at least seven consecutive days. Co-administration of aspirin with clopidogrel was permitted but not required for study inclusion. Exclusion criteria included current or recent therapy with other ADP receptor antagonists, patients receiving glycoprotein inhibitors, inherited or acquired platelet function disorder, malignancy, HIV-positive/AIDS patients, creatinine clearance <15 mL/min and patients on hemodialysis, hematocrit (Hct) ≤25% and platelet count <100,000/mm3. The cohort was divided into patients on clopidogrel plus cilostazol (n = 24) and patients on clopidogrel monotherapy (n = 22). An initial blood sample (2 mL) was collected from each participant for platelet function and DNA testing. Blood was collected from a peripheral vein, and platelet reactivity was assessed within 4 h of blood sampling. Platelet function was measured ex vivo using the United Stated Food and Drug Administration-approved point-of-care VerifyNow P2Y12 analyzer following the manufacturer’s instructions (Accumetrics Inc. San Diego, CA, USA). Genomic DNA was extracted using the QIAamp DNA Blood Midi Kit (QIAGEN Inc., Valencia, CA, USA) following the manufacturer’s protocol. TaqMan Genotyping Assay Reagent kits for allelic discrimination (Applied Biosystems, Foster City, CA, USA) were used to genotype all candidate variants, following the manufacturer’s instructions. A full description of this method can be found elsewhere [16, 17]. High platelet reactivity was defined as P2Y12 reaction unit (PRU) values ≤230.
Statistical analyses
Continuous variables were compared using the two-tailed Student’s t-test, and categorical data were assessed using either the Chi Squared (χ2) or Fisher’s exact test as appropriate. Comparison of minor allele frequencies between our cohort and reference populations was performed using a z-test for population proportions. The Hardy-Weinberg equilibrium (HWE) test was applied as a quality control for genotyping; deviation from the HWE was estimated using a χ2 goodness-of-fit test with one degree of freedom. Simple linear regression analysis was performed to determine the association between all measurements and platelet reactivity (refer to Table 1 for a full list of all the studied variables). A forward stepwise multiple regression analysis and backward stepwise elimination approach were performed to determine the predictors of platelet reactivity on clopidogrel and the total contribution of cilostazol therapy toward PRU values. Variables were included in the final multiple regression model if they were significantly associated with platelet reactivity in the study cohort (i.e. p <0.05, entry criteria). Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and values of p <0.05 were considered statistically significant.
Table 1.
Baseline characteristics of the study patients according to cilostazol therapy.
| Characteristics | No cilostazol (n = 22) |
Cilostazol (n = 24) |
All patients (n = 46) |
p-Value |
|---|---|---|---|---|
| Medical history | ||||
| Age, years | 72 ± 8 | 71 ± 9 | 72 ± 9 | 0.56 |
| Females | 11 (50) | 18 (75) | 29 | 0.08 |
| BMI, kg/m2 | 28 ± 5 | 29 ± 8 | 28 ± 7 | 0.73 |
| Hypertension | 21 (95) | 22 (92) | 43 | 1.00 |
| DM | 17 (77) | 18 (75) | 35 | 0.86 |
| Dyslipidemia | 18 (82) | 20 (83) | 38 | 1.00 |
| Active smoker | 5 (23) | 5 (21) | 10 | 0.88 |
| Platelet reactivity (PRU) | 224 ± 45 | 191 ± 55 | 207 ± 53 | 0.03 |
| Laboratory results | ||||
| WBC, ×103/μL | 7.5 ± 2.0 | 8.9 ± 2.5 | 8.3 ± 2.4 | 0.04 |
| Hgb, g/dL | 13.2 ± 2.2 | 13.0 ± 1.5 | 13.1 ± 1.8 | 0.74 |
| Hct, % | 39.1 ± 6.0 | 38.8 ± 3.8 | 39.0 ± 5.0 | 0.83 |
| Platelet count, ×103/μL | 249 ± 86 | 252 ± 78 | 251 ± 81 | 0.89 |
| BUN, mg/dL | 22 ± 8 | 20 ± 7 | 21 ± 8 | 0.34 |
| Creatinine, mg/dL | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.23 |
| Concomitant therapy | ||||
| Aspirin | 11 (50) | 15 (63) | 26 | 0.39 |
| PPIs | 3 (14) | 5 (21) | 8 | 0.70 |
| Statins | 17 (77) | 19 (79) | 36 | 0.88 |
| CCBs | 7 (32) | 7 (29) | 14 | 1.00 |
| Genotypes | ||||
| CYP2C19*2 (G681A) (rs4244285) | ||||
| Wild | 17 (77) | 17 (71) | 34 (74) | 0.61 |
| Mutant | 5 (23) | 7 (29) | 12 (26) | |
| CYP2C19*17(C806T) (rs12248560) | ||||
| Wild | 13 (59) | 20 (83) | 33 (72) | 0.10 |
| Mutant | 9 (41) | 4 (17) | 13 (28) | |
| ABCB1 (C3435T) (rs1045642) | ||||
| Wild | 8 (36) | 7 (29) | 15 (33) | 0.60 |
| Mutant | 14 (64) | 17 (71) | 31 (67) | |
| PON1 (A575G) (rs662) | ||||
| Wild | 5 (23) | 3 (13) | 8 (17) | 0.45 |
| Mutant | 17 (77) | 21 (87) | 38 (83) | |
| P2RY12 (T744C) (rs2046934) | ||||
| Wild | 17 (77) | 20 (83) | 37 (80) | 0.72 |
| Mutant | 5 (23) | 4 (17) | 9 (20) |
Values are mean ± SD or n (%). BMI, Body mass index; WBC, white blood cells; Hgb, hemoglobin; BUN: blood urea nitrogen. The alleles CYP2C19*3 and CYP2C19*4 were not observed in the study population. p-Values indicate significance of effects of genotype groups on the results of comparisons between the cilostazol and non-cilostazol groups.
Results
Study population
In our study cohort, 18 patients (39%) had HTPR. The study group characteristics are described in Table 1. No significant differences in baseline characteristics were identified including common comorbid conditions, laboratory results, concomitant use of aspirin (ASA), proton pump inhibitors (PPIs), statins or calcium channel blockers (CCBs) and genotypes. Notably, there was a significant difference in mean platelet reactivity between the two studied groups, with a higher PRU in the non-cilostazol group (224 ± 45; range: 78–285) than in the cilostazol group (191 ± 55; range: 146–325) (p = 0.03).
Genotype characteristics
Genotyping results are illustrated in Table 1 [CYP2C19*3 and CYP2C19*4 loss-of-function (LoF) alleles are not represented as they were not detected in the cohort]. A total of 22% of the participants were carriers of at least one copy of the CYP2C19*2 LoF allele. No significant differences were observed between frequencies of the different variants detected in the study cohort. Because of the low frequency of homozygosity for the tested variant alleles (i.e. CYP2C19*2, 0.04; CYP2C19*17, 0.02, ABCB1, 0.17; PON1, 0.22 and P2RY12, 0.0), carriers of at least one variant allele were compared to wild-type individuals with no significant differences observed between the clopidogrel and clopidogrel plus cilostazol groups. In addition, the observed variant allele frequencies in the study population were consistent with those from the Puerto Rican cohort included in the 1000 Genomes Project (p >0.05) [18]. No significant departure from HWE was found for all of the genotyped variants.
Determinants of platelet reactivity
The overall mean platelet reactivity on clopidogrel was 207 ± 53 (range: 78–325). No significant differences in platelet reactivity were detected between smokers and non-smokers, PPI and no PPI and carriers of CYP2C19*2 or CYP2C19*17 and wild type (Figure 1). However, diabetic patients had higher platelet reactivity than non-diabetic patients (p = 0.03). In a simple linear regression analysis, history of DM was positively associated with platelet reactivity (p = 0.03), while concomitant use of cilostazol and Hct had a negative significant effect (p = 0.03, p = 0.02; respectively). No genetic variants or other clinical factors including smoking or PPI use correlated with platelet reactivity. Additionally, cilostazol was found to determine 8% of the total variability in platelet reactivity. Moreover, after adjusting for possible confounders and interactions among history of DM, Hct and cilostazol use, they all remained as independent predictors of platelet reactivity on clopidogrel (Table 2).
Figure 1.
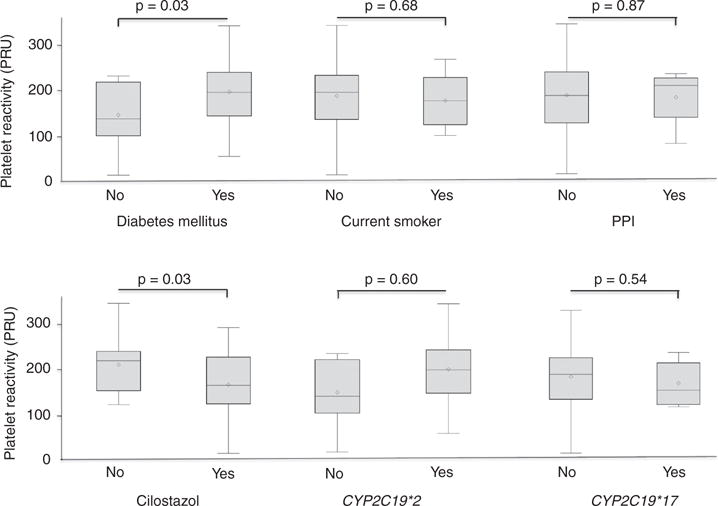
Association of important clinical and genetic characteristics with platelet reactivity.
Table 2.
Stepwise multiple regression analysis to determine the best predictors of platelet reactivity.
| Independent variables | Coefficient | Standard error | t | p-Value |
|---|---|---|---|---|
| DM | 44.41 | 16.94 | 2.62 | 0.01 |
| Hct | −4.27 | −1.48 | −2.89 | 0.01 |
| Cilostazol | −33.54 | 15.08 | −2.22 | 0.03 |
Discussion
To better inform medical decision-making for PAD patients undergoing antiplatelet treatment, we conducted a pilot study that assessed the effect of cilostazol on platelet reactivity among PAD patients on clopidogrel therapy. To the best of our knowledge, this is the first study of this kind conducted in Hispanic patients diagnosed with PAD. Accordingly, our results have potential clinical implications for this particular population.
PAD affects an estimated 202 million people world-wide [19]. PAD patients generally have a poor prognosis because they often experience an increased risk of cardiovascular death, non-fatal myocardial infarction and stroke within 5 years, with symptoms ranging from intermittent claudication to tissue loss [19, 20]. There is a lack of consensus regarding the APT to be used in patients with PAD, and little is known on the benefits of DAPT. Despite a slightly higher risk of severe bleeding, DAPT with aspirin and clopidogrel has been previously found to be associated with a reduced rate of major leg amputations following revascularization [21]. Furthermore, based on a systematic review and network meta-analysis of 49 available randomized controlled trials comparing different antiplatelet drugs in 34,518 patients, clopidogrel may be the preferred antiplatelet agent in treating PAD patients [21]. Nevertheless, PAD patients are often excluded from antiplatelet pharmacokinetic, pharmacodynamic and pharmacogenetic studies, and, to date, it is uncertain which approach is best to use when monotherapy fails.
In this study, we found that concomitant use of cilostazol was associated with platelet reactivity in PAD patients, with a significantly higher PRU in the non-cilostazol vs. the cilostazol group (224 ± 45 vs. 191 ± 55, p = 0.03). Of note, some preliminary studies on the impact of adjunctive cilostazol therapy on platelet reactivity in patients undergoing coronary stenting or with AMI have been performed [13, 22]; however, no studies have been reported to date in PAD patients. For example, in the ACCEL-RESISTANCE (i.e. Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) trial, the addition of 100 mg/day of cilostazol to DAPT reduced the rate of HTPR and improved platelet inhibition compared to high maintenance doses of clopidogrel (150 mg/day) [13].
Similarly, the ACCELAMI2C19 (i.e. Adjunctive Cilostazol Versus High Maintenance-Dose Clopidogrel in Acute Myocardial Infarction [AMI] Patients According to CYP2C19 Polymorphism) trial also showed that adjunctive cilostazol therapy significantly diminished the rate of HTPR in AMI patients with CYP2C19 LoF variants [23]. Therefore, DAPT plus cilostazol may benefit those patients who are CYP2C19 poor metabolizers [14]. However, in the present work, no significant differences were found between wild type and carriers of CYP2C19*2 with regard to platelet reactivity in PAD patients. Accordingly, CYP2C19 genotype was not an independent predictor of HTPR in this study, which was likely due to the small size of this PAD patient cohort. Nevertheless, the similar genetic profile between the studied groups allowed us to test the direct impact of cilostazol on residual platelet reactivity without the interaction/confounding effect of CYP2C19 and other potential pharmacogenetic variants.
Another finding from this study was the confirmation that a history of DM was an independent predictor of platelet reactivity on clopidogrel. Similar to other patient populations, DM is a significant factor that can impair the antiplatelet response to clopidogrel [7]. It has been suggested that the reduced response to clopidogrel among patients with DM, resulting in HTPR, is due in part to a higher percentage of circulating immature platelets as well as to the loss of responsiveness to insulin [7, 24, 25]. Notably, we recently reported that DM is an independent predictor of HTPR (odds ratio = 3.27; 95% confidence interval = 1.20–8.96; p < 0.05) in a heterogeneous cohort of cardiovascular Hispanic patients on clopidogrel therapy, which, together with four other clinical factors, explained 28% of the total variation in PRUs [3]. This is particularly relevant as Puerto Ricans are disproportionately affected by type 2 DM compared with other ethnic groups [26].
Interestingly, Hct showed a significant inverse correlation with platelet reactivity in our study cohort (p = 0.02). Kakouros et al. [27] have suggested that this association is more likely an in vitro phenomenon inherent to the platelet function assay, indicating that the VerifyNow P2Y12 assay may have limitations in patients with anemia. Despite this effect, a correction of PRUs for this lab result is not currently recommended [28].
There are some limitations to the present analysis. First, the small sample size precluded any stratification by CYP2C19 genotype between and within the studied subgroups. Additionally, we were unable to test a genotype-driven pharmacokinetic interaction between carriers of the CYP2C19*2 and CYP3A5*3 variants among Hispanic PAD patients on cilostazol plus clopidogrel. A previous study suggested that cilostazol might overcome clopidogrel resistance caused by CYP2C19 poor metabolizers, but only in subjects with the CYP3A5*3/*3 genotype [14]. It is postulated that patients with a CYP3A5*3/*3 genotype have decreased clearance of the active metabolite of clopidogrel, in effect compensating for the decreased formation of the active metabolite in patients carrying CYP2C19*2. Further studies in larger PAD and cardiovascular patient cohorts are warranted to confirm or refute this hypothesis.
Conclusions
In Puerto Rican PAD patients on clopidogrel APT, history of DM, adjunctive cilostazol therapy and Hct are independent predictors of platelet reactivity. The addition of cilostazol to DAPT may enhance the antiplatelet effect of clopidogrel in patients with HTPR. Further studies are now warranted to determine the clinical significance of adjunctive cilostazol therapy and pharmacogenetic variants on cardiovascular outcomes in PAD patients with HTPR.
Acknowledgments
Research funding: This study was funded by the National Institute of Health (NIH) Award Numbers U54MD007600, R25MD007607, TL1TR001434-3, S21MD001830, SC1HL123911 and K23GM104401. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Dagmar F. Hernandez-Suarez, Medicine Division, University of Puerto Rico School of Medicine, Medical Sciences Building, PO Box 365067, San Juan 00936-5067, Puerto Rico.
Hector Núñez-Medina, Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
Stuart A. Scott, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Angel Lopez-Candales, Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
Jose M. Wiley, Division of Cardiovascular Diseases Montefiore Medical Center/Albert Einstein College of Medicine, New York, NY, USA
Mario J. Garcia, Division of Cardiovascular Diseases Montefiore Medical Center/Albert Einstein College of Medicine, New York, NY, USA
Kyle Melin, Department of Pharmacy Practice, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico.
Karid Nieves-Borrero, Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
Christina Rodriguez-Ruiz, Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
Lorraine Marshall, Medicine Division, University of Puerto Rico School of Medicine, San Juan, Puerto Rico.
Jorge Duconge, Pharmaceutical Sciences Department, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico.
References
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–508. doi: 10.1016/j.jacc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Leunissen TC, Peeters Weem SM, Urbanus RT, den Ruijter HM, Moll FL, Asselbergs FW, et al. High on-treatment platelet reactivity in peripheral arterial disease: a pilot study to find the optimal test and cut off values. Eur J Vasc Endovasc Surg. 2016;52:198–204. doi: 10.1016/j.ejvs.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Suarez DF, Scott SA, Tomey MI, Melin K, Lopez-Candales A, Buckley CE, et al. Clinical determinants of clopidogrel responsiveness in a heterogeneous cohort of Puerto Rican Hispanics. Ther Adv Cardiovasc Dis. 2017;11:235–41. doi: 10.1177/1753944717718718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 5.Gagne JJ, Bykov K, Choudhry NK, Toomey TJ, Connolly JG, Avorn J. Effect of smoking on comparative efficacy of antiplatelet agents: systematic review, meta-analysis, and indirect comparison. Br Med J. 2013;347:f5307. doi: 10.1136/bmj.f5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karathanos A, Geisler T. Monitoring aspirin and clopidogrel response: testing controversies and recommendations. Mol Diagn Ther. 2013;17:123–37. doi: 10.1007/s40291-013-0022-y. [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64:1005–14. doi: 10.1016/j.jacc.2014.06.1170. [DOI] [PubMed] [Google Scholar]
- 8.Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–32. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott SA, Collet JP, Baber U, Yang Y, Peter I, Linderman M, et al. Exome sequencing of extreme clopidogrel response phenotypes identifies B4GALT2 as a determinant of on-treatment platelet reactivity. Clin Pharmacol Ther. 2016;100:287–94. doi: 10.1002/cpt.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52:1128–33. doi: 10.1016/j.jacc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, et al. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv. 2010;3:17–26. doi: 10.1161/CIRCINTERVENTIONS.109.880179. [DOI] [PubMed] [Google Scholar]
- 12.Koh JS, Kim IS, Tantry US, Yoon SE, Park Y, Cho SY, et al. Pharmacodynamic efficacy and safety of adjunctive cilostazol loading to clopidogrel and aspirin loading: the results of the ACCEL-LOADING (Accelerated Platelet Inhibition by Cilostazol Loading) study. Int J Cardiol. 2014;174:129–32. doi: 10.1016/j.ijcard.2014.03.081. [DOI] [PubMed] [Google Scholar]
- 13.Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity. J Am Coll Cardiol. 2009;53:1101–9. doi: 10.1016/j.jacc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Lim Y, Oh M, Ghim JL, Kim EY, Kim DH, et al. The pharmacokinetic and pharmacodynamic interaction of clopidogrel and cilostazol in relation to CYP2C19 and CYP3A5 genotypes. Br J Clin Pharmacol. 2016;81:301–12. doi: 10.1111/bcp.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BK, Lee SW, Park SW, Kim IS, Seo MK, Kwak CH, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity. Am J Cardiol. 2007;100:610–4. doi: 10.1016/j.jacc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16.De LaVega FM, Lazaruk KD, Rhodes MD, Wenz MH. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan® SNP Genotyping Assays and the SNPlex™ Genotyping System. Mutat Res. 2005;573:111–35. doi: 10.1016/j.mrfmmm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 17.TaqMan® SNP Genotyping Assays Protocol, Applied Biosystem. 2010 Available at: http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_042998.pdf. Accessed November 22, 2017.
- 18.The 1000 Genomes Project Consortium. A map of human genome variation from population scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 20.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–42. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsanos K, Spiliopoulos S, Saha P, Diamantopoulos A, Karunanithy N, Krokidis M, et al. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: a systematic review and network meta-analysis. PLoS One. 10:e0135692. doi: 10.1371/journal.pone.0135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panchal HB, Shah T, Patel P, Albalbissi K, Molnar J, Coffey B, et al. Comparison of on-treatment platelet reactivity between triple antiplatelet therapy with cilostazol and standard dual antiplatelet therapy in patients undergoing coronary interventions: a meta-analysis. J Cardiovasc Pharmacol Ther. 2013;18:533–43. doi: 10.1177/1074248413495971. [DOI] [PubMed] [Google Scholar]
- 23.Kim IS, Jeong YH, Park Y, Park KS, Yun SE, Park JR, et al. Platelet inhibition by adjunctive cilostazol versus high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype. JACC Cardiovasc Interv. 2011;4:381–91. doi: 10.1016/j.jcin.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Angiolillo DJ, Bernardo E, Ramirez C, Costa MA, Sabaté M, Jimenez-Quevedo P, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48:298–304. doi: 10.1016/j.jacc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Grove EL, Hvas AM, Kristensen SD. Immature platelets in patients with acute coronary syndromes. Thromb Haemost. 2009;101:151–6. [PubMed] [Google Scholar]
- 26.Pérez CM, Soto-Salgado M, Suárez E, Guzmán M, Ortiz AP. High prevalence of diabetes and prediabetes and their coexistence with cardiovascular risk factors in a Hispanic community. J Immigr Minor Health. 2015;17:1002–9. doi: 10.1007/s10903-014-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakouros N, Kickler TS, Laws KM, Rade JJ. Hematocrit alters VerifyNow P2Y12 assay results independently of intrinsic platelet reactivity and clopidogrel responsiveness. J Thromb Haemost. 2013;11:1814–22. doi: 10.1111/jth.12376. [DOI] [PubMed] [Google Scholar]
- 28.Janssen PW, Bergmeijer TO, Godschalk TC, Le TT, Breet NJ, Kelder JC, et al. The effect of correcting VerifyNow P2Y12 assay results for hematocrit in patients undergoing percutaneous coronary interventions. J Thromb Haemost. 2017;15:618–23. doi: 10.1111/jth.13642. [DOI] [PubMed] [Google Scholar]


