Abstract
The essentiality of fatty acids was determined by the Burrs in the 1920s. Subsequently, it is commonly accepted that provision of linoleic (LA) and alpha-linolenic acids (ALA) prevents and reverses essential fatty acid deficiency (EFAD). Development of alternative injectable lipid emulsions (ILE) low in LA and ALA has raised concern about their ability to prevent EFAD. This review provides biochemical evidence coupled with observations from animal and human studies that aim to characterize which fatty acids are truly essential to prevent EFAD.
Retroconversion pathways and mobilization from body stores suggest that arachidonic and docosahexaenoic acids (ARA and DHA – the main derivatives of LA and ALA, respectively) also prevent EFAD. Our group first proposed the essentiality of ARA and DHA by feeding mice exclusively these fatty acids and proving that they prevent EFAD. Survival for 5 generations on this diet provides additional evidence that growth and reproductive capabilities are maintained. Moreover, the use of fish oil-based ILE, which contain minimal LA and ALA and abundant DHA and ARA, for treatment of intestinal failure-associated liver disease, does not result in EFAD. These findings challenge the essentiality of LA and ALA in the presence of ARA and DHA.
Evidence discussed in this review supports the idea that ARA and DHA can independently fulfill dietary essential fatty acid requirements. The imminent introduction of new ILE rich in ARA and DHA in the United States highlights the importance of understanding their essentiality, especially when provision of ALA and LA is below the established daily minimum requirement.
Keywords: Essential fatty acid, essential fatty acid deficiency, intestinal failure-associated liver disease, injectable lipid emulsion, parenteral nutrition
Before essential fatty acids (EFAs) were first described in the 1920s, fats were primarily considered a dense source of calories and absorptive substrates for dietary fat-soluble vitamins [1,2]. Fatty acids were not considered essential as it was believed that all fats could be synthesized from dietary carbohydrates [3]. Subsequent research identified the structural roles of fatty acids in cell membranes and the permeability barrier of the epidermis, and their role as precursors of prostaglandins [4,5]. Later work established that some fatty acids also affect gene expression by serving as ligands for different types of nuclear receptors, in addition to regulating cellular functions via their action on G-protein-coupled membrane receptors in the liver, brain, and bone [6–10].
Burr et al concluded that certain fatty acids were essential for adequate growth and reproduction in small animals. Linoleic acid (LA) from the omega (ω)-6 family was initially identified as the EFA, although the possibility that other fatty acids might serve a similar essential role was acknowledged [2]. A year later, the ω-3 fatty acid alpha-linolenic acid (ALA) was added to the list of EFAs after noting that it was also not synthesized de novo in rats and had to be provided in the diet [11,12]. The essentiality of ALA was initially questioned as it was less effective than LA in promoting weight gain, reproduction, and reversal of skin lesions in rats. There was also concern that ALA decreased the effectiveness of LA in preventing essential fatty acid deficiency (EFAD) by competitively inhibiting the enzymes involved in LA metabolism [3,13]. Elucidation of the functional properties of the main ALA metabolites, eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively), solidified its role as an EFA (Figure 1) [3,14]. To date, LA and ALA have been considered the EFAs. However, their downstream metabolites, LA-derived arachidonic acid (ARA) and ALA-derived DHA and EPA, are precursors to important mediators in the inflammatory response and other cellular processes (Figure 2).
Figure 1.
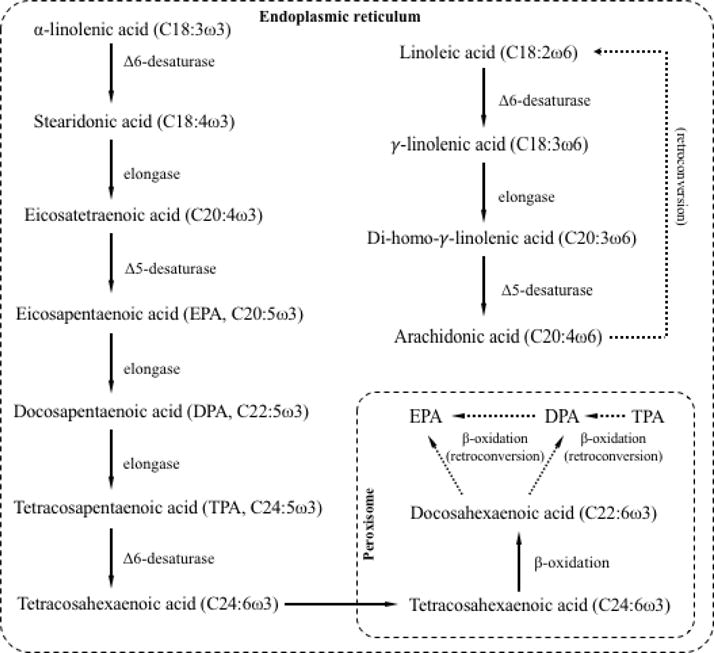
Elongation, desaturation, and β-oxidation reactions leading to the synthesis of DHA and ARA from ALA and LA, respectively, in mammalian cells. Dotted lines depict retroconversion via peroxisomal β-oxidation.
Figure 2.
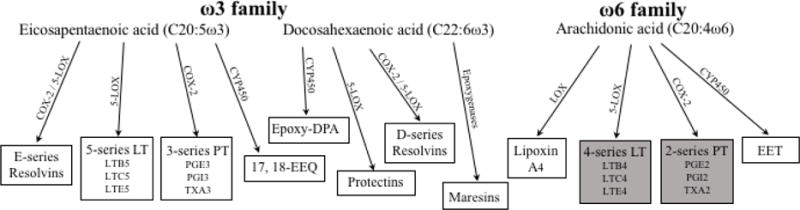
Pro-inflammatory (in gray blocks) and anti-inflammatory (in white blocks) metabolites of ARA, DHA, and EPA. Abbreviations: 17, 18-EEQ, 17(R), 18(S)-epoxyeicosaquatraenoic acid; COX, cyclooxygenase; CYP450, cytochrome P450 epoxygenases; EET, epoxyeicosatrienoic acids; Epoxy-DPA, epoxydocosapentaenoic acid; LOX, lipoxygenase; LT, leukotrienes; PG, prostaglandin; PT, prostanoids; TX, thromboxane. Figure adapted from Lee et al [55], de Roos et al [56], and Serhan et al [57].
Clinically, EFAD presents with growth retardation, reproductive failure, eczematous dermatitis, and alopecia [2,15–17]. Biochemically, the changes that occur in the setting of EFAD result from the competitive inhibition and differences in affinities of the enzymes in the metabolic pathways for the three families of polyunsaturated fatty acids (in order of affinity, ω-3 > ω-6 > ω-9) [18]. A reduction in ARA, a tetraenoic ω-6 fatty acid, results in an increase in the trienoic nonessential ω-9 Mead acid. Mead acid is synthesized in states of EFAD from the elongation and desaturation of oleic acid when both dietary LA and ALA are limited. The Holman index is used to diagnose biochemical EFAD. It is determined by calculating the triene (i.e., Mead acid) to tetraene (i.e., ARA) ratio in plasma. A triene:tetraene (T:T) ratio > 0.2 is considered biochemical EFAD, although signs and symptoms of EFAD are detected at ratios > 0.4 [17,19]. Thus, elevation of the Holman index precedes the development of clinical signs of EFAD, and may appear as early as 7-10 days following the restriction of EFAs, particularly when continuous fat-free feeding prevents mobilization of adipose tissue stores of LA [20]. The Holman index does not take ω-3 fatty acid status into consideration. EFAD also results in a reduction in protein utilization due to the limited availability of energy to support this process [21]. Should EFAD occur during the critical stages of development, the effect on protein metabolism can lead to permanent growth deficiencies.
EFA experts in the 1920s hypothesized that only a small EFA dose would be required to support growth [22]. Indeed, only 1-3% and 0.2-1% of the total daily caloric intake are needed from dietary LA and ALA, respectively [23]. The essentiality of fatty acids was further demonstrated in humans when fat-free parenteral nutrition (PN) was first used in the United States in the 1960s. Its use in patients with limited fat stores due to malnutrition led to clinical signs and symptoms previously described in rodents with EFAD [24]. Similar to animal models, provision of LA and ALA reversed EFAD in humans. For this reason, injectable lipid emulsions (ILE) administered with dextrose-amino acid PN formulations serve as both a rich source of non-protein calories and provide the EFAs.
Significant changes have been made to ILE since their inception. The main variation in each product lies in the modification and/or combination of different oil sources. Until recently, the only accessible ILE in the United States were soybean oil-based lipid emulsions (SOLE). However, soy allergy and a link between PN-associated cholestasis and SOLE have led to an increased interest in alternative ILE formulated with different oils [25]. Since the content of fatty acids in ILE varies by oil source, concern has been raised about the risk of EFAD in patients who require long-term (Table 1). For example, children receiving fish oil-based lipid emulsion (FOLE) monotherapy for intestinal failure-associated liver disease (IFALD) do not develop EFAD despite the limited amounts of ALA and LA in FOLE and the more substantial amounts of DHA and ARA [26]. Similarly, rodents fed a diet in which the only polyunsaturated fatty acids provided are DHA and ARA do not develop biochemical or clinical EFAD over many generations [27].
Table 1.
Oil sources and percentage of fatty acid content of currently available lipid injectable emulsions.
| Component | Intralipid | Omegaven | ClinoLipid/ClinOleic | SMOFlipid | Lipidem/Lipoplus® |
|---|---|---|---|---|---|
| Soybean Oil (%) | 100 | 20 | 30 | 40 | |
| MCT (%) | 30 | 50 | |||
| Olive Oil (%) | 80 | 25 | |||
| Fish Oil (%) | 100 | 15 | 10 | ||
| LA (%) | 50 | 4.4 | 18.5 | 21.4 | 24.5 |
| ALA (%) | 9 | 1.8 | 2 | 2.5 | 3.5 |
| EPA (%) | 0 | 19.2 | 0 | 3 | 3.5 |
| DHA (%) | 0 | 12.1 | 0 | 2 | 2.5 |
| ARA (%) | 0 | 1-4 | 0 | 0.15-0.6 | 0 |
Abbreviations: ALA, alpha-linolenic acid; ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; MCT: medium-chain triglycerides. Adapted from Anez-Bustillos et al [54].
Due to concerns regarding EFA delivery with FOLE monotherapy, our group has challenged the traditional notion that LA and ALA are the only two fatty acids that can fulfill EFA requirements. We previously suggested that the “true” essential fatty acids are the downstream metabolites of LA and ALA, ARA and DHA, respectively [28]. The purpose of this review is to discuss evidence available from animal and human studies that suggest that EFA requirements may be achieved by not only LA and ALA, but also by provision of their downstream metabolites.
Biochemical Background
The metabolic pathways that form ARA and DHA from LA and ALA, respectively, were described in the 1960s [29,30]. The synthesis of ARA (C20:4ω-6) and EPA (C20:5ω-3) in mammals involves alternating reactions by elongases and desaturases present in the cell’s endoplasmic reticulum. The transport of tetracosahexaenoic acid (C24:6ω-3) into peroxisomes followed by one cycle of β-oxidation leads to the production of DHA (C22:6ω-3) from EPA [31,32] (Figure 1). The same elongases and desaturases catalyze the reactions in the ω-3 and ω-6 pathways, with enzymes having higher affinity for substrates in the ω-3 pathway. For this reason, the amount of dietary ALA or LA can influence which fatty acids predominate through competitive inhibition [6]. Given that the Western diet is rich in ω-6 fatty acids, LA has become the prevailing EFA in human nutrition.
Another reason for LA predominance is that the synthesis of EPA and DHA from ALA is an inefficient process, especially in infants and critically ill patients [6]. Arterburn et al performed a cross-study meta-regression analysis on the concentration of ω-3 fatty acids in plasma phospholipids after ALA, EPA, and DHA supplementation [6]. ALA supplementation modestly elevated EPA but not DHA. EPA supplementation significantly increased EPA levels, but did not affect DHA levels. Conversely, DHA supplementation resulted in increases in both EPA and DHA levels. Retroconversion of DHA into upstream metabolites occurs in peroxisomes via β-oxidation reactions, which result in the formation of EPA and docosapentaenoic acid (C22:5ω-3, DPA) [33,34] (Figure 1). Retroconversion pathways are relatively minor unless there is dietary supplementation of DHA. Similarly, although ARA was thought to be an inadequate substrate for retroconversion, experiments in rodents have suggested that its conversion back to LA can be induced in vivo by providing ARA in the setting of EFAD [35]. ARA supplementation can also stimulate other tissues to mobilize LA and incorporate these into skin lipids, which can reverse the trans-epidermal water loss seen in rats with EFAD [36]. In healthy adults, adipose tissue contains approximately 10% of total fatty acids as LA, and slowly releases LA and ARA during post-prandial and absorptive periods. Thus, if fat is restricted and caloric intake is decreased, development of EFAD is delayed until tissue stores are depleted. In patients who receive 100% of calories as fat-free PN, biochemical EFAD can develop in as little as 2-4 weeks due to dextrose-mediated insulin secretion, which suppresses fatty acid mobilization from adipose tissue [37]. In the presence of protein malnutrition, the anabolic effect of continuous nutrition, either parenteral or enteral, may hasten the development of EFAD.
Animal Studies
Over 40 years before EFAD was described in humans, the condition had been well characterized in rodents [1]. Although fish oil is abundant in ω-3 fatty acids, there is still concern that low levels of LA may limit its ability to prevent EFAD. Until recently, the only question that remained is whether FOLE monotherapy can be safely used as a long-term fat source without resulting in EFAD. Our group has worked extensively in designing animal diets that differ in lipid source and content. Strijbosch et al initially demonstrated that menhaden fish oil (supplied in the diet at concentrations of at least 5%) contained enough ω-6 fatty acids in the form of ARA to support growth and avoid the development of EFAD [38]. Although the levels of LA in this oil are minimal compared to soybean oil, fish oil may provide adequate amounts of ARA to protect against EFAD. This is supported by research conducted approximately 40 years earlier suggesting that ARA is nearly three times more efficient and effective as an EFA than LA [39]. It has been shown that ARA has the same effects as LA in restoring the epidermal permeability barrier in rats with EFAD and may even substitute for each other [4], since ARA can be retroconverted to LA for this unique aspect of LA in EFA metabolism. Both the retroconversion of ARA and the mobilization of LA from other tissues appear to be the basis for the ability of ARA to prevent EFAD.
To determine the safety and effect of using varying ratios of DHA and ARA, Le et al formulated murine diets devoid of both LA and ALA and containing only DHA and ARA [40,41]. These experiments demonstrated that DHA and ARA, when constituting at least 2% and 0.1% of total dietary calories, respectively (20:1 diet), could prevent and reverse EFAD [41]. Interestingly, the 20:1 ratio mimics that of EPA and DHA to ARA present in oily, cold-water fish. The effect of this diet on growth and reproduction was also evaluated by feeding female mice (F0 generation) for 4 weeks with either a control diet, an EFA-deficient diet, or the 20:1 diet. F0 mice were subsequently bred and the offspring (F1 generation) were kept on the same diet as their progenitors until the age of 8 weeks. Although F1 mice receiving the 20:1 and EFA-deficient diets weighed significantly less than the control mice at weaning, those from the 20:1 group achieved the same weight as the controls by the end of the study period. This was not the case for animals receiving the EFA-diet, which remained significantly lower in weight than the other two groups. In addition, the same 20:1 diet prevented the development of hepatosteatosis, one of the main metabolic sequelae of EFAD. Subsequent experiments using the 20:1 diet have demonstrated its efficacy in sustaining growth, reproduction, and neurocognitive function in mice, even after maintaining this diet over multiple generations [27]. Animals provided a diet of EFA-devoid hydrogenated coconut oil as the sole source of fat were infertile after only 1 generation [27].
The intravenous administration of FOLE as the only source of fat has been extensively tested in the laboratory using a mouse model of hepatosteatosis and EFAD induced by the administration of PN. We developed this model by giving mice a liquid fat-free, high-carbohydrate diet ad libitum for 19 days [42]. Mice that receive intravenous SOLE (Intralipid®, Fresenius Kabi Uppsala, Sweden) do not develop EFAD, but do develop hepatosteatosis. Intravenous administration of the commercially-available FOLE Omegaven® (Fresenius Kabi, Bad Homburg, Germany) attenuates the development of hepatosteatosis and prevents EFAD [43]. The mechanisms by which FOLE prevents and treats PN-induced hepatic injury are beyond the scope of this review. However, these studies demonstrate that FOLE can be administered as the only fat source without the development of biochemical or clinical EFAD in mice.
Human Studies
SOLE has been the principal ILE available to practitioners since its approval in the United States in the 1970s. FOLE monotherapy was first used in the United States in a PN-dependent soy-allergic patient who developed EFAD while receiving fat-free PN [19]. The patient in this case report had recently undergone an allogeneic bone marrow transplant and his clinical course was complicated by the development of graft versus host disease and gastrointestinal hemorrhage. The combination of these comorbidities made it impossible to provide fat enterally, and the allergy to soy protein precluded the use of the only available ILE. In looking for soy-free alternatives, FOLE was identified and administered to the patient following emergency approvals by the Institutional Review Board and the United States Food and Drug Administration (FDA). After 10 days of FOLE monotherapy, the skin rash that had developed disappeared and T:T ratios which once were well above 0.2 normalized, suggesting that FOLE had adequate EFAs to treat EFAD.
Strategies such as lipid restriction and/or modification of oil sources have gained importance with the recognition that SOLE plays a critical role in the pathogenesis of IFALD [25]. Based on findings of our laboratory’s animal experiments, Gura et al first reported the use of FOLE for the reversal of cholestasis in two infants who had developed IFALD [44]. The efficacy and safety of FOLE monotherapy was further demonstrated in a larger number of patients compared to a historical cohort that received SOLE [45]. More importantly, the long-term effects of FOLE monotherapy were recently described [26]. During the nearly 12-year span since its introduction, over 250 patients have received FOLE monotherapy for the treatment of IFALD and none have developed EFAD. This includes patients who received no enteral calories and more than 30 patients that remained on such therapy for at least 3 years [46].
While the small amounts of LA and ALA in FOLE may be sufficient to prevent EFAD, data provided in this review suggests that the higher amounts of DHA, EPA, and ARA present in fish oil are as adequate as ALA and LA in preventing EFAD. Similar outcomes have been reported in adults with IFALD treated with FOLE. Although fatty acid profiles were not reported in these studies, no patient was reported to have clinical signs of EFAD [47,48]. The daily requirements to prevent EFAD in adults are lower than for children. In a study assessing the plasma fatty acid profiles in adult patients receiving home PN, Jeppesen et al concluded that 500 mL of 20% SOLE per week (1.4 g/kg per week in a 70 kg adult) was enough to prevent biochemical EFAD [49].
The use of lower T:T ratio values to characterize EFAD can be misleading. Clinical laboratories use normal ranges of EFAs generated from samples of healthy individuals eating a typical, ω-6-predominant diet in which up to 7% of energy is provided as LA. In such situations, a lower T:T ratio than 0.02 is expected [50]. In sick patients, often with poor dietary intake, and diet administered as dose PN or enteral tube feedings, LA is typically present in lower proportions [51]. Clinicians need to appreciate these differences when evaluating patients’ fatty acid profiles.
Presently, FOLE has not been approved by the FDA. Its use is only allowed under a compassionate protocol for the treatment of patients with IFALD. Our experience, coupled with that of many others across the country, has demonstrated that FOLE monotherapy is a safe alternative to SOLE for PN-dependent patients and does not increase the risk of EFAD [26,52].
The information reviewed here suggests a new definition for the role of DHA and ARA in preventing EFAD. This change in paradigm is first established by an understanding of their biochemical properties and further supported by animal studies involving strict control of dietary fatty acids across multiple generations, and clinical observations in PN-dependent children receiving FOLE monotherapy (Table 2). Nearly half a century ago, ILE were added to PN formulations to serve as a source of ALA and LA once the risk of EFAD in PN-dependent patients was recognized. The introduction of commercially available ILE with fewer “traditional” EFAs (ALA and LA) raised concern that their use may lead to EFAD. Such concerns that lead to excluding the use of alternative ILE, such as FOLE, may be detrimental to patients who would benefit from their use, such as children with IFALD [53].
Table 2.
Summary of findings supporting the roles of ARA and DHA in preventing EFAD.
| Biochemical | Enzymatic retroconversion of ARA and DHA to LA and ALA |
| Mobilization of LA from other body stores | |
| Improved biochemical efficiency of DHA and ARA | |
| Improved biochemical efficacy of DHA and ARA | |
|
| |
| Animal Data | DHA/ARA provided as sole source of FA resulting in: |
| - Normal growth | |
| - Normal reproduction | |
| - Normal neurocognitive function | |
| - Reversal of hepatic steatosis | |
| - No development of EFAD (clinical nor biochemically) | |
| Animals on diet devoid of ALA/LA bred for multiple generations | |
|
| |
| Human Data | FOLE provided to PN-dependent children with IFALD resulting in: |
| - Normal growth | |
| - Reversal of PN-induced cholestasis | |
| - No development of EFAD (clinical nor biochemically) | |
Abbreviations: ARA, arachidonic acid; DHA, docosahexaenoic acid; EFAD, essential fatty acid deficiency; FA, fatty acids; FOLE, fish oil-based lipid emulsion; IFALD, intestinal failure-associated liver disease; PN, parenteral nutrition.
The data reviewed here support the consideration of ARA and DHA as EFAs that can prevent EFAD independently of LA and ALA. Recognizing the essentiality of DHA and ARA is particularly important in settings where provision of ALA and LA is below the daily minimum requirement but adequate DHA and ARA is present, such as with the use of FOLE.
Acknowledgments
Research funding is provided by the Boston Children’s Hospital Surgical Foundation and the Vascular Biology Program within Boston Children’s Hospital (LA-B, DTD, MAB, GLF, and MP), the Corkin and Maher Family Fund, and the National Institutes of Health Grants 5T32HL007734 (MAB, DTD), and 1F32DK104525-01 (GLF).
LA-B, DTD, GLF, MAB, BRB, KMG, and MP contributed to the conception of the manuscript. LA-B, KMG, and MP drafted the initial version of the manuscript. All of the authors critically revised the manuscript and gave final approval of the submitted version.
Footnotes
Disclaimers:
A license agreement for the use of Omegaven® has been signed by Boston Children’s Hospital and Fresenius Kabi. Mark Puder and Kathleen Gura hold an issued patent on the treatment of parenteral nutrition-associated liver disease. They both serve on the Scientific Advisory Boards for Pronova-BASF and Sancilio and Company Inc. Kathleen Gura also serves on the Pharmaceutical Advisory Board for B. Braun USA.
References
- 1.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–67. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 2.Burr GO, Burr MM. On the nature and role of the fatty acids essential in nutrition. J Biol Chem. 1930;86:587–621. [Google Scholar]
- 3.Spector AA, Kim H-Y. Discovery of essential fatty acids. J Lipid Res. 2015;56:11–21. doi: 10.1194/jlr.R055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houtsmuller UMT, van der Beek A. Effects of topical application of fatty acids. Prog Lipid Res. 1981;20:219–24. doi: 10.1016/0163-7827(81)90041-2. [DOI] [PubMed] [Google Scholar]
- 5.Gramlich L, Meddings L, Alberda C, Wichansawakun S, Robbins S, Driscoll D, et al. Essential Fatty Acid Deficiency in 2015: The Impact of Novel Intravenous Lipid Emulsions. JPEN J Parenter Enteral Nutr. 2015;39:61S–6S. doi: 10.1177/0148607115595977. [DOI] [PubMed] [Google Scholar]
- 6.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:S1467–1476. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 7.Raptis DA, Limani P, Jang JH, Ungethüm U, Tschuor C, Graf R, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J Hepatol. 2014;60:625–32. doi: 10.1016/j.jhep.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Bey Y, Boularan C, Vural A, Huang N-N, Hwang I-Y, Shan-Shi C, et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS One. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornish J, MacGibbon A, Lin J-M, Watson M, Callon KE, Tong PC, et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008;149:5688–95. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- 10.Khan MZ, He L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology. 2017;113:639–51. doi: 10.1016/j.neuropharm.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Wesson LG, Burr GO. The metabolic rate and respiratory quotients of rats on a fat-deficient diet. J Biol Chem. 1931;91:525–39. [Google Scholar]
- 12.Burr G, Burr M, Miller E. On the fatty acids essential in nutrition. J Biol Chem. 1932;97:1–9. [Google Scholar]
- 13.Hume E, Nunn L, Smedley-Maclean I, Henderson Smith A. Studies of the essential unsaturated fatty acids in their relation to the fat-deficiency disease of rats. Biochem J. 1938;32:2162–77. doi: 10.1042/bj0322162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman RT. The slow discovery of the importance of omega 3 essential fatty acids in human health. J Nutr. 1998;128:427S–433S. doi: 10.1093/jn/128.2.427S. [DOI] [PubMed] [Google Scholar]
- 15.Aaes-Jorgensen E, Leppik EE, Hayes HW, Holman RT. Essential fatty acid deficiency. II. In adult rats J Nutr. 1958;66:245–59. doi: 10.1093/jn/66.2.245. [DOI] [PubMed] [Google Scholar]
- 16.Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976–83. doi: 10.1093/ajcn/29.9.976. [DOI] [PubMed] [Google Scholar]
- 17.Holman RT. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960;70:405–10. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 18.Holman R, Mohrhauer H. A hypothesis involving competitive inhibition in the metabolism of polyunsaturated fatty acids. Acta Chem Scand. 1963;17:S84–90. [Google Scholar]
- 19.Gura KM, Parsons SK, Bechard LJ, Henderson T, Dorsey M, Phipatanakul W, et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin Nutr. 2005;24:839–47. doi: 10.1016/j.clnu.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Wene JD, Connor WE, DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. J Clin Invest. 1975;56:127–34. doi: 10.1172/JCI108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry CJ, Ghusain-Choueiri A, Payne PR. Protein utilization, growth and survival in essential-fatty-acid-deficient rats. Br J Nutr. 1996;75:237–48. doi: 10.1079/bjn19960127. [DOI] [PubMed] [Google Scholar]
- 22.Osborne T, Mendel L. Feeding experiments with mixtures of foodstuffs in unusual proportions. Proc Natl Acad Sci. 1921;7:157–62. doi: 10.1073/pnas.7.6.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30:39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell MD, Jonsson HT, Othersen HB. Essential fatty acid deficiency in an infant receiving prolonged parenteral alimentation. J Pediatr. 1972;81:894–8. doi: 10.1016/s0022-3476(72)80539-0. [DOI] [PubMed] [Google Scholar]
- 25.Iyer KR, Spitz L, Clayton P. BAPS prize lecture: New insight into mechanisms of parenteral nutrition-associated cholestasis: role of plant sterols. British Association of Paediatric Surgeons J Pediatr Surg. 1998;33:1–6. doi: 10.1016/s0022-3468(98)90349-9. [DOI] [PubMed] [Google Scholar]
- 26.Nandivada P, Fell GL, Mitchell PD, Potemkin AK, O’Loughlin AA, Gura KM, et al. Long-Term Fish Oil Lipid Emulsion Use in Children With Intestinal Failure-Associated Liver Disease. JPEN J Parenter Enteral Nutr. 2016 doi: 10.1177/0148607116633796. [DOI] [PubMed] [Google Scholar]
- 27.Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11:1046–54. doi: 10.1111/acel.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le HD, Meisel JA, de Meijer VE, Gura KM, Puder M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81:165–70. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcel YL, Christiansen K, Holman RT. The preferred metabolic pathway from linoleic acid to arachidonic acid in vitro. Biochim Biophys Acta. 1968;164:25–34. doi: 10.1016/0005-2760(68)90067-2. [DOI] [PubMed] [Google Scholar]
- 30.Klenk E, Mohrhauer H. [Studies on the metabolism of polyenoic fatty acids in the rat] Hoppe Seylers Z Physiol Chem. 1960;320:218–32. doi: 10.1515/bchm2.1960.320.1.218. [DOI] [PubMed] [Google Scholar]
- 31.Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci. 2002;27:467–73. doi: 10.1016/S0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- 32.Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res. 1995;36:2471–7. [PubMed] [Google Scholar]
- 33.Sprecher H, Baykousheva S, Luthria D, Mohammed B. Differences in the regulation of biosynthesis of 20- versus 22-carbon polyunsaturated fatty acids. Prostaglandins Leukot Essent Fat Acids. 1995;52:99–101. doi: 10.1016/0952-3278(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 34.Brossard N, Croset M, Pachiaudi C, Riou JP, Tayot JL, Lagarde M. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am J Clin Nutr. 1996;64:577–86. doi: 10.1093/ajcn/64.4.577. [DOI] [PubMed] [Google Scholar]
- 35.Hansen HS, Jensen B, von Wettstein-Knowles P. Apparent in vivo retroconversion of dietary arachidonic to linoleic acid in essential fatty acid-deficient rats. Biochim Biophys Acta. 1986;878:284–7. doi: 10.1016/0005-2760(86)90158-x. [DOI] [PubMed] [Google Scholar]
- 36.Hansen HS, Jensen B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim Biophys Acta - Lipids Lipid Metab. 1985;834:357–63. doi: 10.1016/0005-2760(85)90009-8. [DOI] [PubMed] [Google Scholar]
- 37.Richardson TJ, Sgoutas D. Essential fatty acid deficiency in four adult patients during total parenteral nutrition. Am J Clin Nutr. 1975;28:258–63. doi: 10.1093/ajcn/28.3.258. [DOI] [PubMed] [Google Scholar]
- 38.Strijbosch RAM, Lee S, Arsenault DA, Andersson C, Gura KM, Bistrian BR, et al. Fish oil prevents essential fatty acid deficiency and enhances growth: clinical and biochemical implications. Metabolism. 2008;57:698–707. doi: 10.1016/j.metabol.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohrhauer H, Holman RT. The effect of dose level of essential fatty acids upon fatty acid composition of the rat liver. J Lipid Res. 1963;4:151–9. [PubMed] [Google Scholar]
- 40.Le HD, Meisel JA, de Meijer VE, Fallon EM, Gura KM, Nose V, et al. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. JPEN J Parenter Enteral Nutr. 2012;36:431–41. doi: 10.1177/0148607111414580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le HD, Fallon EM, Kalish BT, de Meijer VE, Meisel JA, Gura KM, et al. The effect of varying ratios of docosahexaenoic acid and arachidonic acid in the prevention and reversal of biochemical essential fatty acid deficiency in a murine model. Metabolism. 2013;62:499–508. doi: 10.1016/j.metabol.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alwayn IPJ, Javid PJ, Gura KM, Nosé V, Ollero M, Puder M. Do polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREPB-1 suppression or by correcting essential fatty acid deficiency. Hepatology. 2004;39:1176–7. doi: 10.1002/hep.20189. [DOI] [PubMed] [Google Scholar]
- 43.Alwayn IPJ, Gura K, Nosé V, Zausche B, Javid P, Garza J, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57:445–52. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 44.Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 45.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 46.de Meijer VE, Le HD, Meisel JA, Gura KM, Puder M. Parenteral Fish Oil as Monotherapy Prevents Essential Fatty Acid Deficiency in Parenteral Nutrition–dependent Patients. J Pediatr Gastroenterol Nutr. 2010;50:212–8. doi: 10.1097/MPG.0b013e3181bbf51e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venecourt-Jackson E, Hill SJ, Walmsley RS. Successful treatment of parenteral nutrition–associated liver disease in an adult by use of a fish oil–based lipid source. Nutrition. 2013;29:356–8. doi: 10.1016/j.nut.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Jurewitsch B, Gardiner G, Naccarato M, Jeejeebhoy KN. Omega-3–Enriched Lipid Emulsion for Liver Salvage in Parenteral Nutrition–Induced Cholestasis in the Adult Patient. J Parenter Enter Nutr. 2011;35:386–90. doi: 10.1177/0148607110382023. [DOI] [PubMed] [Google Scholar]
- 49.Jeppesen PB, Høy CE, Mortensen PB. Essential fatty acid deficiency in patients receiving home parenteral nutrition. Am J Clin Nutr. 1998;68:126–33. doi: 10.1093/ajcn/68.1.126. [DOI] [PubMed] [Google Scholar]
- 50.Siguel E. Essential fatty acid status in patients. JPEN J Parenter Enteral Nutr. 1997;21:243. doi: 10.1177/0148607197021004243. [DOI] [PubMed] [Google Scholar]
- 51.Abushufa R, Reed P, Weinkove C, Wales S, Shaffer J. Essential fatty acid status in patients on long-term home parenteral nutrition. JPEN J Parenter Enteral Nutr. 1995;19:286–90. doi: 10.1177/0148607195019004286. [DOI] [PubMed] [Google Scholar]
- 52.de Meijer VE, Gura KM, Meisel JA, Le HD, Puder M. Parenteral fish oil monotherapy in the management of patients with parenteral nutrition-associated liver disease. Arch Surg. 2010;145:547–51. doi: 10.1001/archsurg.2010.80. [DOI] [PubMed] [Google Scholar]
- 53.Goulet O, Lambe C. Intravenous lipid emulsions in pediatric patients with intestinal failure. Curr Opin Organ Transplant. 2017;22:142–8. doi: 10.1097/MOT.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 54.Anez-Bustillos L, Dao DT, Baker MA, Fell GL, Puder M, Gura KM. Intravenous Fat Emulsion Formulations for the Adult and Pediatric Patient: Understanding the Differences. Nutr Clin Pract. 2016;31:596–609. doi: 10.1177/0884533616662996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S, Gura KM, Kim S, Arsenault DA, Bistrian BR, Puder M. Current clinical applications of omega-6 and omega-3 fatty acids. Nutr Clin Pract. 2006;21:323–41. doi: 10.1177/0115426506021004323. [DOI] [PubMed] [Google Scholar]
- 56.de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–28. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta - Mol Cell Biol Lipids. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


