Abstract
Objective
To evaluate the early changes in the apparent diffusion coefficient (ADC) of the salivary glands during radiotherapy (RT) and their association with the degree of xerostomia at 6 months after RT in patients with nasopharyngeal carcinoma (NPC).
Materials and Methods
We enrolled 26 patients with NPC who underwent RT. Each patient underwent diffusion-weighted MRI of the salivary glands at rest and with gustatory stimulation within 1 week before RT and 2 weeks after the beginning of RT. The ADC at rest (ADCR) and increase and increase rate with stimulation (ADCI, ADCIR) of the submandibular and parotid glands were calculated. The differences in the variables' values between 2 weeks after the beginning of RT and baseline (ΔADCR, ΔADCI, and ΔADCIR) were compared to the degree of xerostomia at 6 months after RT.
Results
The ADCR of the submandibular and parotid glands were both significantly higher at 2 weeks after the beginning of RT than found at baseline (both p < 0.01). The ADCI and ADCIR for the parotid glands were both significantly lower at 2 weeks after the beginning of RT than found at baseline (both p < 0.01). ΔADCI and ΔADCIR of the parotid glands were associated with the degree of xerostomia at 6 months after RT (r = −0.61 and −0.72, both p < 0.01).
Conclusion
The ADCs of the salivary glands change early during RT. The differences in the ADC increase and increase rate of the parotid glands between 2 weeks after the beginning of RT and baseline were associated with the degree of xerostomia at 6 months after RT.
Keywords: Diffusion-weighted imaging, MR imaging, Parotid gland, Dry mouth, Nasopharyngeal carcinoma, Head and neck, Radiation-induced complication, Quality of life
INTRODUCTION
Xerostomia is a common radiation-induced complication in patients with nasopharyngeal carcinoma (NPC) after a radical course of radiotherapy (RT) (1,2). Persistent xerostomia, due to damage to the salivary glands, causes difficulties in mastication and swallowing and enhances the risk of dental problems, subsequently degrading the quality of life in long-term survivors; however, we have no effective control methods (3,4). The early detection and prevention of xerostomia would be of great value in practice.
The development of xerostomia likely depends on various prognostic factors: radiation dose distribution, demographics, and tumor and treatment-related factors (5). Many studies aimed at improving the predictive models for baseline subjective scores and patient-dependent risks, but the results were discrepant (6,7). The mechanism of radiation-induced xerostomia might be complicated. Building a predictive model that summarizes all factors would be difficult.
A diffusion-weighted MRI (DWI) is commonly performed in patients with head and neck diseases (8). The parameters of the apparent diffusion coefficient (ADC) can reflect the microstructures or pathophysiologic conditions of tissues, even during the early stages of the disease (9). Some studies found that the ADC of the parotid glands changed (i.e., increased or decreased in different studies) after gustatory stimulation compared to the baseline ADC (10,11). Several studies investigated the diffusional alternations in the salivary glands after RT (11,12). Although these findings differed from each other, the ADC values certainly could reflect the function of the salivary glands and the grade of xerostomia after RT in some ways. To the best of our knowledge, the early changes in the ADC of the salivary glands during RT and their association with xerostomia after RT has not been documented. In this study, we sought to evaluate the early changes in the ADC (2 weeks after the beginning of RT) of the salivary glands and their association with the xerostomia degree at 6 months after RT.
MATERIALS AND METHODS
Patients
From June 2014 to December 2015, 26 consecutive patients with NPC who were only undergoing intensity-modulated RT (IMRT) were enrolled in another project where the changes over time in the ADC of the salivary glands during RT was observed. None of the patients had a history of salivary gland disease prior to RT. No patient received any treatment for related clinical symptoms of xerostomia during or after RT. Here, we retrospectively analyzed these data to investigate any relationships to the degree of xerostomia at 6 months after RT. The Institutional Ethics Committee approved the study. An informed consent form was signed by each patient before enrollment.
Radiotherapy
All patients were fixed with a thermoplastic mask. The simulation CT images (slice thickness 3 mm) ranged from the vertex cranii to 5 cm below the clavicular heads. All patients received IMRT with a simultaneous integrated boost. The gross tumor volume (GTV) included the primary NPC and lymph node-involved tumors exhibited by contrast-enhanced imaging. Clinical target volume 1 (CTV 1), as the high-risk region, was outlined by adding a 5-mm margin to GTV, which contained the inferior sphenoid sinus, clivus, basis cranii, nasopharynx, ipsilateral parapharyngeal space, posterior third of the nasal cavity, maxillary sinuses, and level II, III, and Va lymph nodes. CTV 2, as the low-risk region, included the lower neck under the cricothyroid membrane. A 3 mm margin was also suitable in regions close to the brainstem, optic nerves, and optic chiasm. All CTVs were outlined according to the Radiation Therapy Oncology Group's (RTOG) recommendations (13). The planning target volume (PTV) was bounded by appending a 5 mm margin to the CTV in all directions.
The prescribed RT dose was 66 Gy/30 fractions, 60 Gy/30 fractions, and 54 Gy/30 fractions to the planning gross tumor volume, PTV 1 and PTV 2, respectively, at 5 fractions per week. After a course of RT, the total dose was 36.45 ± 5.62 Gy (mean ± standard deviation; range 25.21–48.83 Gy) to the parotid glands and 55.71 ± 5.13 Gy (range 49.35–69.22 Gy) to the submandibular glands.
Imaging Protocol
Each patient underwent MRI within one week prior to RT and two weeks after the beginning of RT. All patients were asked to fast for at least 4 hours before the examination. All MRI examinations involved a 3T MR system (Achieva 3.0T; Philips Healthcare, Eindhoven, The Netherlands). The patients were imaged supine with a 16-channel head-neck array coil. An Axial and sagittal T1-weighted spin-echo sequence (repetition time [TR] 400 ms, echo time [TE] 10.5 ms, slice thickness 4 mm, slice interval 1 mm, field of view [FOV] 22 × 22 cm, matrix 136 × 109) and fast spin-echo T2-weighted images were obtained first as routine procedure. The T2-weighted sequence (TR 5563 ms, TE 46 ms, slice thickness 4 mm, slice interval 1 mm, FOV 22 × 22 cm, matrix 136 × 109) was used as a reference for the following DWI examinations to improve the outlining of the salivary glands. The images ranged from the basis cranii to the undersurface of the submandibular glands and contained the whole volume of the salivary glands.
An axial spin-echo echo-planar DWI sequence was then conducted (TR 5563 ms, TE 46 ms, matrix 136 × 109, b-values 0, 200, 500, 800 s/mm2, number of signal averages 2, scan time 87 seconds); the imaging FOV, slice thickness, and interval were equal to those used in the T2-weighted imaging. After a DWI sequence obtained at rest, six 100-mg tablets of ascorbic acid were placed in the patients' mouths. The patients were asked not to chew them. Immediately after, the DWI was repeated 10 times with 13-second intervals between the contiguous sequences.
Data Acquisition And Analysis
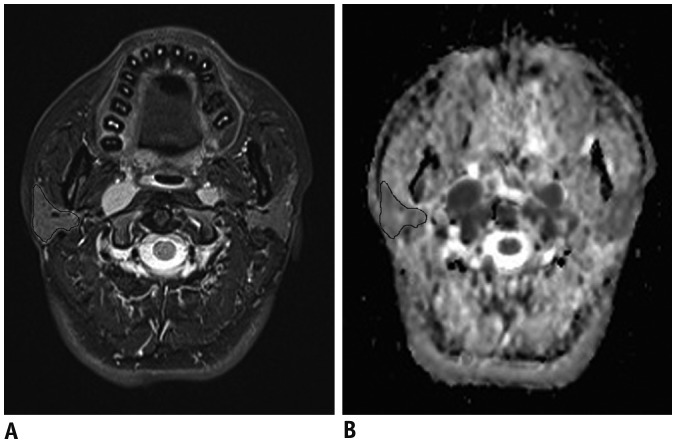
The ADC maps for all DWI images were constructed and transferred automatically to a workstation (Extended MR Workspace 2.6.3.4; Philips Healthcare). Three adjacent sections containing the biggest volumes of each salivary gland were used for analysis. In the T2-weighted images, the region of interest (ROI) on each section was outlined by hand such that it contained as much of the gland parenchyma as possible. These ROIs were copied to the matching ADC maps automatically for ADC calculations (Fig. 1).
Fig. 1. Measurement of ADC of salivary glands.
T2WI image (A) and corresponding ADC map (B) for 60-year-old male patient with nasopharyngeal carcinoma before radiotherapy. After placing ROI in manner that enclosed as much of gland parenchyma as possible in reference to T2WI, ROI was automatically copied to corresponding ADC map for further analysis. ADC = apparent diffusion coefficient, ROI = region of interest, T2WI = T2-weighted imaging
The ADC values at rest (ADCR) and increase and increase rate with stimulation (ADCI, ADCIR) for the bilateral submandibular and parotid glands were calculated. The ADCI was defined as the ADC difference between the peak value during stimulation and the baseline value at rest. The ADCIR was defined as the ratio of ADCI to the baseline value at rest. The differences in the absolute values of these variables between 2 weeks after the beginning of RT and baseline (ΔADCR, ΔADCI, and ΔADCIR) were compared with the degree of xerostomia at 6 months after RT. The clinical degree of xerostomia before and at 6 months after RT was assessed according to the morbidity scoring system developed by the RTOG/European Organization for Research and Treatment of Cancer (EORTC) (14).
Statistical Analysis
All data were presented as a percentage (%) or median (interquartile range). A Wilcoxon paired test was used to compare the ADC values of the salivary glands at rest and during stimulation as well as before and 2 weeks after the beginning of RT. Spearman's Rank-Order Correlations were used to test the associations between gender, age, ΔADCR, ΔADCI, ΔADCIR, and RT dose of the parotid and submandibular glands and the degree of xerostomia at 6 months after RT. The level of statistical significance was set at p < 0.05. Statistical analyses involved the use of the SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
For the 26 patients (17 men; mean age, 51 years [range 37–63]), at rest, the ADCR (× 10−3 mm2/s) of the parotid and submandibular glands were both significantly higher at 2 weeks after the beginning of RT than it was before RT (1.35 [1.20–1.52] vs. 0.94 [0.88–0.96], p < 0.01; 1.57 [1.47–1.69] vs. 1.24 [1.18–1.29], p < 0.01) (Fig. 2). The ADC of the parotid glands increased and then decreased in fluctuation, and the peak value appeared within the first 6 minutes after the beginning of stimulation both before and after RT. The ADCI (× 10−3 mm2/s) and ADCIR (%) values for the parotid glands after stimulation were both significantly lower at 2 weeks after the beginning of RT than they were before RT (0.27 [0.21–0.33] vs. 0.36 [0.32–0.42], p < 0.01; 22.17 [16.30–25.20] vs. 38.36 [32.92–46.79], p < 0.01). The ADC for the submandibular glands also increased slightly after stimulation both before and after RT, but the ADCI (× 10−3 mm2/s) and ADCIR (%) did not differ significantly (0.13 [0.07–0.16] vs. 0.15 [0.10–0.19], p = 0.19; 12.31 [8.48–17.23] vs. 16.24 [11.18–18.29], p = 0.43).
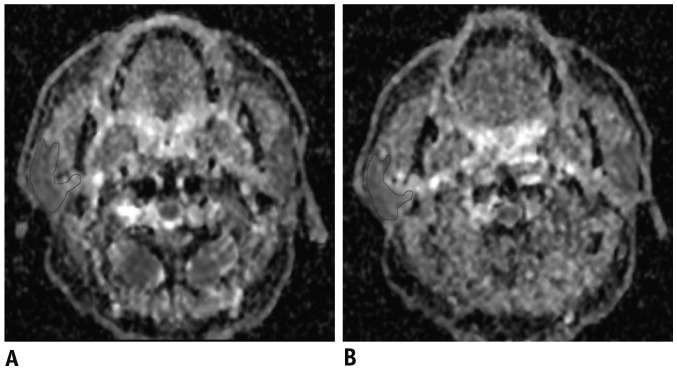
Fig. 2. ADC maps of parotid glands of 53-year-old male patient with nasopharyngeal carcinoma before (A) and during (B) RT.
Mean ADC of parotid glands at rest was 0.89 × 10−3 mm2/s before RT and increased to 1.24 × 10−3 mm2/s during RT. RT = radiotherapy
Before RT, xerostomia assessed by the RTOG/EORTC morbidity score was grade 0 for all patients. At 6 months after RT, xerostomia varied across patients: grade 0 for 1 patient (3.85%), grade 1 for 12 patients (46.15%), grade 2 for 11 patients (42.30%) and grade 3 for 2 patients (7.70%). The spearman correlation coefficient indicated that ΔADCI and ΔADCIR of the parotid glands were associated with the degree of xerostomia (r = −0.61, p < 0.01; r = −0.72, p < 0.01). The ΔADCR of the parotid glands was also significantly related to xerostomia (p = 0.04) but the correlation coefficient was as small as 0.41. At the same time, the ΔADCR of the submandibular glands, gender, age and RT dose to the parotid and submandibular glands was not significantly related to the degree of xerostomia at 6 months after RT (r = 0.35, 0.21, 0.49, 0.51, and 0.57, respectively, all p > 0.05).
DISCUSSION
The DWI of the salivary glands at rest and with gustatory stimulation has been valuable for visualizing the diffusional alterations of the salivary glands before and after RT in patients with NPC (11). We wondered whether the early changes in the ADC of the salivary glands of such patients during RT were associated with xerostomia. Here, we evaluated the association of early changes (2 weeks after the beginning of RT) in the ADC of the salivary glands during RT with the degree of xerostomia at 6 months after RT. Because the parotid gland mainly secretes saliva in stimulated conditions while the submandibular gland mainly works at rest, some studies only investigated the ADC changes of the parotid gland with stimulation (11). In this study, we investigated the ADC changes of the parotid and submandibular glands. Our preliminary results indicated that the mean ADC values for the parotid and submandibular glands at rest were both significantly higher at 2 weeks after the beginning of RT than they were before RT. The ADC increase and increase rate of the parotid glands with stimulation during RT were both significantly lower than they were before RT. The ADC increase and increase rate of the submandibular glands did not alter significantly. We hypothesized that the sensitivity to radiation might be different between the parotid and submandibular glands. The differences in the ADC increase and increase rate of the parotid glands were associated with the degree of xerostomia at 6 months after RT.
In previous studies, the ADC values of the salivary glands at rest varied greatly. The ADC of the parotid glands varied from 0.28 to 2.42 × 10−3 mm2/s even in healthy volunteers (15,16). In the present study, the ADC for the parotid and submandibular glands at rest was 0.94 (0.88−0.96) and 1.24 (1.18−1.29) × 10−3 mm2/s. Some authors believed that this discrepancy was likely attributed to the b values used because previous research showed that the ADC of the parotid gland is negatively affected by b values (10). The ADC is affected also by pulse sequences, accelerating factors, histological components, fat saturations, and bulk motions (17,18). Moreover, some authors insisted that high b values ought to be used when assessing the diffusion of the salivary glands (11) because the ADC values of obtained biological tissues at low b values were thought to reflect the fast water motion of the blood and saliva flows known as perfusion (10); whereas, the high b values were thought to reflect the slow water motion known as diffusion. The contribution of perfusion to the ADC decreases with increasing b values (19). However, Cho et al. (20) found that salivary flow rates were severely reduced in patients who had undergone RT. We used low and high b values in this study to reflect both the perfusion and diffusion changes of the salivary glands after RT. We found increased ADC values for salivary glands during stimulation before RT, and the reaction to stimulation still occurred 2 weeks after the beginning of RT, which is similar to that found in previous studies (11,21).
The ADC changes of the salivary glands induced by radiation have been examined by some authors, but the results differed greatly. Zhang et al. (16) first reported reduced parotid ADC after irradiation. However, some later research found significantly increased parotid ADC after RT (11,12,22). The b-values were different in these studies. In our study, we found that the ADC of the salivary glands increased after RT. Our results aligned with previous studies. Zhang et al. (11) also found the increase between the maximum and the baseline was greater after RT than that found before RT. They attributed this finding to increased water contents in the damaged parotid glands and delayed emptying of saliva. However, we found that the ADC increase and increase rate of the parotid glands with stimulation were both significantly lower during RT than that found before RT. We performed MRI during instead of after RT. Perhaps the secretion is damaged while the salivary duct system remains normal in the early stages while undergoing RT. Kato et al. (21) found that the mean increase rate was significantly smaller in patients with primary xerostomia than it was for those without it. We supposed that the decrease in ADC increase rate of the parotid glands during RT may have the same mechanism as primary xerostomia. This indicated the response of the salivary glands to stimulation was damaged during RT. Moreover, we found that the differences in the ADC increase and increase rate for the parotid glands between during and before RT were associated with the degree of xerostomia at 6 months after RT.
The present study had some limitations. First, the follow-up period after RT was insufficient. The degree of xerostomia varied over time after RT (23), and we only investigated ADC variables with xerostomia at 6 months after RT. So the follow-up period should be increased to assess late side effects. Second, we used a different b-value than other studies. Although some authors thought that high b values should be used when evaluating salivary glands after RT (11), we reasoned that perfusion alterations of the salivary glands after RT should not be neglected. The standard DWI protocol has not been established, so comparing these results is difficult. Finally, our sample size was small.
In this study, we demonstrated the ADC changes of the salivary glands with gustatory stimulation during the early stages while receiving RT. The difference in the ADC increase and increase rate of the parotid glands between 2 weeks after the beginning of RT and baseline was associated with the degree of xerostomia at 6 months after RT. The relationship between the early changes in the ADC of the salivary glands during RT and the degree of xerostomia should be verified by further investigations.
Footnotes
This study was supported by the Projects of Medical and Health Technology Development Program in Shandong Province, People's Republic of China (Grant No 2015WS0158).
References
- 1.Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008;44:617–627. doi: 10.1016/j.oraloncology.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Talmi YP, Horowitz Z, Bedrin L, Wolf M, Chaushu G, Kronenberg J, et al. Quality of life of nasopharyngeal carcinoma patients. Cancer. 2002;94:1012–1017. [PubMed] [Google Scholar]
- 3.Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26:796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- 4.Jellema AP, Slotman BJ, Doornaert P, Leemans CR, Langendijk JA. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:751–760. doi: 10.1016/j.ijrobp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 5.van de Water TA, Bijl HP, Westerlaan HE, Langendijk JA. Delineation guidelines for organs at risk involved in radiation-induced salivary dysfunction and xerostomia. Radiother Oncol. 2009;93:545–552. doi: 10.1016/j.radonc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Grégoire V, De Neve W, Eisbruch A, Lee N, Van den, Van Gestel D. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist. 2007;12:555–564. doi: 10.1634/theoncologist.12-5-555. [DOI] [PubMed] [Google Scholar]
- 7.Leek H, Albertsson M. Pilocarpine treatment of xerostomia in head and neck patients. Micron. 2002;33:153–155. doi: 10.1016/s0968-4328(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 8.Xu XQ, Choi YJ, Sung YS, Yoon RG, Jang SW, Park JE, et al. Intravoxel incoherent motion MR imaging in the head and neck: correlation with dynamic contrast-enhanced MR imaging and diffusion-weighted imaging. Korean Journal of Radiology. 2016;17:641–649. doi: 10.3348/kjr.2016.17.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–630. doi: 10.1148/radiol.2202010063. [DOI] [PubMed] [Google Scholar]
- 10.Thoeny HC, De Keyzer F, Boesch C, Hermans R. Diffusion-weighted imaging of the parotid gland: influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging. 2004;20:786–790. doi: 10.1002/jmri.20196. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Ou D, Gu Y, He X, Peng W, Mao J, et al. Diffusion-weighted MR imaging of salivary glands with gustatory stimulation: comparison before and after radiotherapy. Acta Radiol. 2013;54:928–933. doi: 10.1177/0284185113491089. [DOI] [PubMed] [Google Scholar]
- 12.Dirix P, De Keyzer F, Vandecaveye V, Stroobants S, Hermans R, Nuyts S. Diffusion-weighted magnetic resonance imaging to evaluate major salivary gland function before and after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:1365–1371. doi: 10.1016/j.ijrobp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 15.Sumi M, Takagi Y, Uetani M, Morikawa M, Hayashi K, Kabasawa H, et al. Diffusion-weighted echoplanar MR imaging of the salivary glands. AJR Am J Roentgenol. 2002;178:959–965. doi: 10.2214/ajr.178.4.1780959. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Murata Y, Ishida R, Ohashi I, Yoshimura R, Shibuya H. Functional evaluation with intravoxel incoherent motion echo-planar MRI in irradiated salivary glands: a correlative study with salivary gland scintigraphy. J Magn Reson Imaging. 2001;14:223–229. doi: 10.1002/jmri.1177. [DOI] [PubMed] [Google Scholar]
- 17.Chang HC, Juan CJ, Chiu HC, Cheng CC, Chiu SC, Liu YJ, et al. Effects of gender, age, and body mass index on fat contents and apparent diffusion coefficients in healthy parotid glands: an MRI evaluation. Eur Radiol. 2014;24:2069–2076. doi: 10.1007/s00330-014-3265-z. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ, Lee YH, Chang HC, Huang TY, Chiu HC, Wang CW, et al. A potential risk of overestimating apparent diffusion coefficient in parotid glands. PLoS One. 2015;10:e0124118. doi: 10.1371/journal.pone.0124118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 20.Cho MA, Ko JY, Kim YK, Kho HS. Salivary flow rate and clinical characteristics of patients with xerostomia according to its aetiology. J Oral Rehabil. 2010;37:185–193. doi: 10.1111/j.1365-2842.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 21.Kato H, Kanematsu M, Toida M, Kawaguchi T, Shibata T, Kajita K, et al. Salivary gland function evaluated by diffusion-weighted MR imaging with gustatory stimulation: preliminary results. J Magn Reson Imaging. 2011;34:904–909. doi: 10.1002/jmri.22729. [DOI] [PubMed] [Google Scholar]
- 22.Studer G, Kirilova A, Jaffray D, Dawson L, Lockwood G, Bayley A, et al. Major salivary gland function: diffusion-weighted MRI (DWI) assessment before, during and after radiation therapy. Int J Radiat Oncol Biol Phys. 2005;63:S361. [Google Scholar]
- 23.Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57:61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]