Abstract
Objective
Bilateral cavernous sinus dural arteriovenous fistula (CSdAVF) is very rare, even in Asian countries. The research intended to present clinical and radiologic outcomes of treating such fistulas through endovascular embolization.
Materials and Methods
Data was obtained from 220 consecutive patients, with CSdAVF, who were treated from January 2004 to December 2015. Bilateral CSdAVF was identified in 17 patients (7.7%). The clinical and radiologic outcomes of the fistulas were assessed with an emphasis on the technical aspects of treatment.
Results
At the time of treatment, 7 and 10 patients presented with bilateral and unilateral symptoms, respectively. In the former cases, 4 patients had progressed from unilateral to bilateral symptoms. Bilateral fistulas were treated with a single-stage transvenous embolization (TVE) in 15 patients, via bilateral inferior petrosal sinuses (IPS) (n = 9) and unilateral IPS (n = 6). In the other 2 patients with one-sided dominance of shunting, only dominant fistula was treated. Two untreated lesions were found on follow-up to have spontaneously resolved after treatment of the dominant contralateral fistula. Of the 34 CSdAVF lesions, complete occlusion was achieved in 32 lesions after TVE. Seven patients (41.2%) developed worsening of cranial nerve palsy after TVE. During the follow-up period, 4 patients obtained complete recovery, whereas the other 3 remained with deficits.
Conclusion
With adjustments of endovascular procedures to accommodate distinct anatomical configurations, endovascular treatment for bilateral CSdAVF can achieve excellent angiographic occlusion results. However, aggravation of symptoms after TVE may occur frequently in bilateral CSdAVF. In the patients with one-sided dominance of shunt, treatment of only dominant fistula might be an alternative option.
Keywords: Cavernous sinus, Dural fistula, Arteriovenous fistula, Embolization, Bilateral, Transvenous embolization, Endovascular treatment
INTRODUCTION
A dural arteriovenous fistula (dAVF) is defined as an abnormal arteriovenous connection confined to a specific region of the pachymeninges (1,2,3). The cavernous, transverse, and sigmoid sinuses are the most frequently involved (1,4,5). Cavernous sinus dAVF (CSdAVF) is relatively more common in Asian than in Western countries (6). Apart from a few lesions that spontaneously regress (10%), high-risk lesions associated with cortical venous drainage, progressive visual loss, neurologic deficits, hemorrhage, and those associated with intolerable diplopia, severe headache, and severe cosmetic disfigurement require treatment to halt abnormal shunting (7). Although embolization devices and new embolic materials have been developed and improved, endovascular treatment of CSdAVF remains technically challenging. Bilateral CSdAVF is very rare, even in Asian countries. Only a few cases have been reported. The purpose of this study was to report the clinical and radiological outcomes of patients with bilateral CSdAVF, who were treated by endovascular management, with a focus on the clinical and angio-anatomical features characteristic of bilateral CSdAVF, and technical approaches to treatment based on angiographic anatomical configurations.
MATERIALS AND METHODS
Patient Population
From January 2004 through December 2015, a total of 220 patients with CSdAVF were treated by endovascular interventions at 2 hospitals affiliated with Seoul National University (Seoul National University Hospital and Seoul National University Bundang Hospital). A total of 17 patients with bilateral CSdAVF (34 lesions), who were identified from this population at the time of treatment, were included in this study (13 females, 4 males; mean age, 64.9 years, range 44–76 years). Therapeutic alternatives were discussed with both the neurological, neurosurgical, and neurointerventional teams in a multidisciplinary decision-making process. Informed consent was obtained from every patient after comprehensive consultation. The study protocol was approved by the Institutional Review Board.
Angiographic Evaluation and Endovascular Procedure
Cavernous sinus dAVF was confirmed by the following biplane systems: Integris V, Allura Clarity (Philips Medical System, Best, The Netherlands), or Innova IGS 630 biplane system (GE Healthcare, Wauwatosa, WI, USA). Bilateral internal and external carotid angiographic exploration was performed to assess the feeding arteries, location, and extent of fistulous sites, as well as venous parameters (drainage paths and patterns). Bilaterality of the fistula was defined as the coexistence of fistulas in both CS at initial diagnosis (synchronous type). It was confirmed through the careful investigation of sequential images in the initial conventional angiography, selective venography performed immediately before the transvenous embolization (TVE), and a completion angiography performed immediately after unilateral embolization. The relationship between the clinical presentations and the angiographic finding was considered in determining therapeutic options and a route of approach were determined after thorough angiographic investigation of vascular anatomy. Treatment was chosen in patients with an aggressive lesion by venous reflux including ocular symptoms (chemosis, proptosis, periorbital pain, and/or eyelid swelling), secondary glaucoma, venous hypertension (by cortical reflux), diplopia by cranial nerve (CN) deficits, etc. In patients where the bilateral shunts were symmetric and symptoms were associated with venous reflux, both lesions were treated. In a few patients with one-sided dominance of shunting, only the dominant fistula related to main symptoms was treated and follow-up was recommended. All lesions were categorized as focal or diffuse type, depending on whether the fistulous sites (mural channels) were limited in scope or diffusely involved the CS. Each lesion was classified as proliferative, restrictive, and late restrictive based on the degree and pattern of prominent arteriovenous shunting and venous flow (classifications of Suh et al.) (8). Cognard classification (9) was applied as well. Antiplatelet agents were not prescribed before the procedure. However, after placement of the femoral sheath, a systemic heparinization was administered (single 2000-International Unit injection).
The transvenous approach was preferred for every case. Access through the ipsilateral or patent inferior petrosal sinuses (IPS) was usually the first choice. If that route was unsuccessful, another venous route was attempted. The route was based on the pattern of venous drainage. Pushable fiber coils were used for all TVE. A microcatheter for delivering the pushable coil was placed in the CS and near the fistulous site. After confirmation of the AVF configuration by selective angiography, an embolization was performed. A transarterial embolization was attempted when a transvenous coil embolization failed to occlude the fistula or the fistula recurred.
Angiographic Outcome and Follow-Up
Immediate angiographic results after endovascular embolization were classified, according to degree of shunting as follows: complete occlusion (no shunting), near-complete occlusion (small residual shunting with marked reduction in volume and velocity), and partial occlusion (large residual shunting with slight reduction or no change in volume and velocity) (7). Ipsilateral carotid compression for at least 2 weeks was recommended for patients who obtained near-complete or partial occlusion. A follow-up digital subtraction angiography (DSA) was subsequently performed 1-month post-procedure to confirm progressive occlusion or the need for further treatment. Clinical follow-ups performed at 1 and 6 months post-treatment were advised for patients with completely occluded fistulas. Additional imaging studies such as DSA or magnetic resonance angiography were recommended for any patients with aggravated clinical symptoms.
Clinical outcomes were assessed according to degree of improvement after treatment. They were as follows: improvement, no change, and aggravation. Assessments were performed while the patient was in the hospital and at outpatient clinics at 1 and 6 months post-treatment. In patients suffering from post-procedural worsening of CN palsy, a steroid was administered for 1 week.
RESULTS
Demographics and Clinical Presentation
Among 220 patients with CSdAVF, bilateral lesions were observed in 17 patients (7.7%). General characteristics of the cohort are summarized in Table 1. All cases of bilateral CSdAVF were idiopathic and without a history of trauma. Eight patients had hypertension and two had diabetes. At the time of treatment, 7 and 10 patients presented with bilateral and unilateral symptoms or signs, respectively. Among these patients with bilateral findings, bilateral orbital involvement such as proptosis and conjunctival injection was found in 4. This was followed by bilateral tinnitus in 2 and bilateral CN palsy in 1 patient (CN III plus VI). Among 4 patients with bilateral orbital findings, unilateral CN palsy was combined in 3 (CN VI). Four patients with bilateral symptoms had progressed from unilateral to bilateral symptoms. The other 3 patients had bilateral symptoms or signs at onset. Of the 10 patients with unilateral findings, a unilateral CN palsy occurred in 8 (CN VI, 7; III plus IV, 1). This was followed by unilateral orbital symptoms in 7, headache in 3, and unilateral tinnitus in 2 patients. All patients experienced multiple symptoms (> 2) in combination. No patient presented an intracerebral hemorrhage (ICH). Most of the patients (n = 15) visited the hospital within 3 months of onset.
Table 1. Summary of Patients' Data (n = 17).
| Characteristics | Cases, n (%) |
|---|---|
| Age, mean (range), year | 64.9 (44–76) |
| Cause | |
| Idiopathic (non-traumatic) | 17 (100) |
| Sex | |
| Male | 4 (23.5) |
| Female | 13 (76.5) |
| Hypertension | 8 (47.1) |
| Diabetes | 2 (11.8) |
| Symptom laterality | |
| Unilateral | 10 (47.1) |
| Bilateral | 7 (35.3) |
| Shunt predominance | |
| Symmetric | 15 (88.2) |
| Asymmetric (one-side predominance) | 2 (11.8) |
| Patency of IPS | |
| Bilateral IPS patent | 10 (58.8) |
| Unilateral IPS occlusion | 6 (35.3) |
| Bilateral IPS occlusion | 1 (5.9) |
| Cognard classification (n = 34) | |
| I | 6 (17.6) |
| IIa | 20 (58.8) |
| IIa+IIb | 8 (23.5) |
| Fistula type (n = 34) | |
| Focal | 2 (5.9) |
| Diffuse | 32 (94.1) |
| Transvenous approaching route | |
| Bilateral via each ipsilateral IPS | 9 (52.9) |
| Bilateral via unilateral IPS | 6 (35.3) |
| Unilateral via ipsilateral IPS | 1 (5.9) |
| Unilateral via ipsilateral FV | 1 (5.9) |
| Procedural occlusion outcome (n = 32) | |
| Complete occlusion | 25 (78.1) |
| Nearly complete occlusion | 7 (21.9) |
| Additional transarterial embolization | 0002 |
| Follow-up occlusion result | |
| Complete occlusion | 34 (100) |
| Paradoxical worsening after transvenous embolization | 7 (41.2) |
FV = facial vein, IPS = inferior petrosal sinus
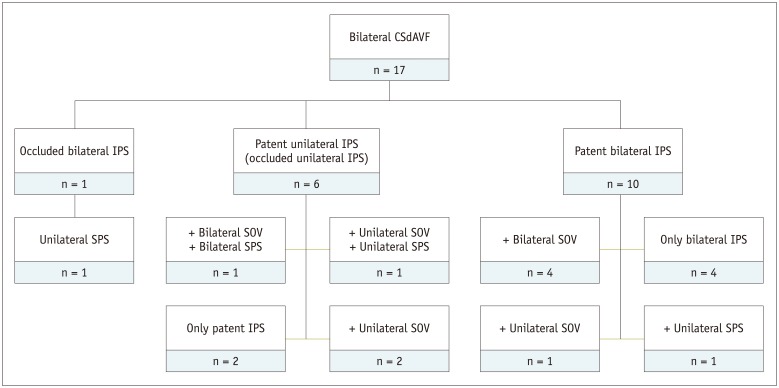
Conventional diagnostic angiography revealed bilateral patent IPSs in 10 patients, unilateral patent IPS in 6 patients, and bilateral occluded IPSs in 1 patient. The main pattern of any venous drainage is summarized in Fig. 1. Most of the patients (n = 15, 88.2%) showed bilateral symmetrical shunting. Two patients (11.8%) had one-sided predominance. Among the 34 fistulas, 20 (58.8%) were classified as Cognard type IIa, 8 (23.5%) as type IIa+b, and 6 (17.6%) as type I. Diffuse-type CSdAVFs predominated (32 diffuse, 2 focal). Based on Suh classification, 20 (58.8%) lesions were diffuse proliferative, 12 (35.3%) were restrictive, and 2 (5.9%) were late restrictive.
Fig. 1. Main patterns of venous drainage in patient cohort (n = 17), according to patency of IPS.
CSdAVF = cavernous sinus dural arteriovenous fistula, IPS = inferior petrosal sinus, SOV = superior ophthalmic vein, SPS = superior petrosal sinus
Procedural and Follow-Up Outcome
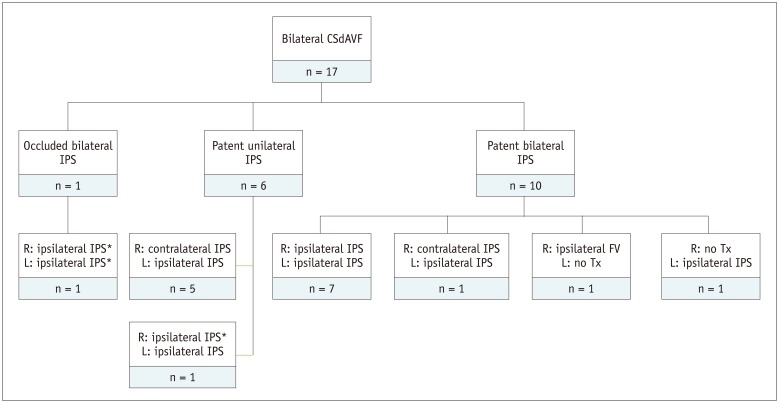
Bilateral fistulas were treated with a single-stage transvenous approach in 15 patients, via bilateral IPSs (n = 9) and unilateral IPS (n = 6) (Fig. 2). In the 2 patients with one-sided dominance of shunting, only unilateral fistula with dominancy was treated via ipsilateral IPS (n = 1) and ipsilateral facial vein (n = 1). In the latter, because both IPS was patent but narrowed and the course of ipsilateral facial vein was smooth and not tortuous, a facial vein was first used for approaching route. Among 32 lesions except 2 untreated lesions, an approach via the IPS was used for 31 lesions, of which 28 IPSs were patent and 3 were occluded. The 3 occluded IPSs were breached with microdevices. The approaching routes of TVE, according to patency of the IPS was summarized in Fig. 3.
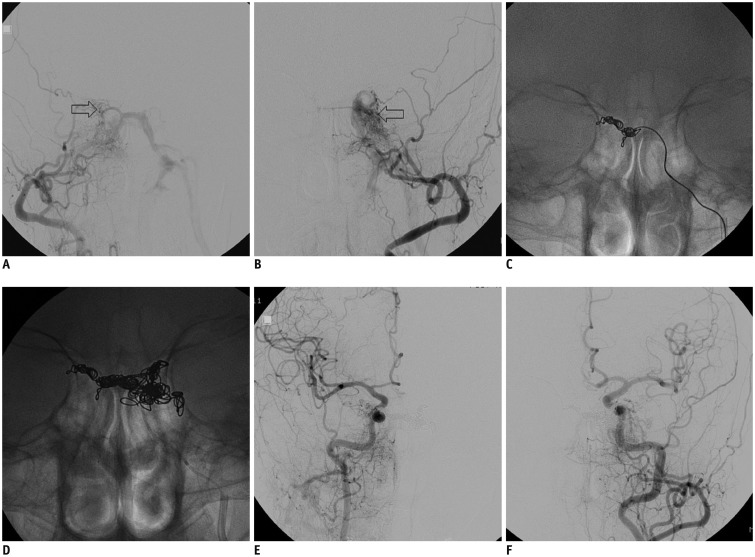
Fig. 2. 62-year-woman with 2-month history of diplopia.
A, B. Bilateral CSdAVF shown by both external carotid artery angiograms (arrows indicates fistulas). Occlusion of right IPS and main venous drainage of left IPS. Fistula was supplied by several dural branches of both internal and external carotid arteries, including accessory meningeal artery, internal maxillary artery, middle meningeal artery, and others. C, D. Transvenous embolization of right CSdAVF was performed first through contralateral IPS and then left side fistula was treated through ipsilateral IPS. E, F. Angiogram after procedure shows near-complete occlusion of blood shunting through either fistula.
Fig. 3. Approaching routes of transvenous embolization, according to patency of IPS.
*Cases that occluded IPS was breached with microdevices. FV = facial vein, L = left, R = right, Tx = treatment
Among 32 lesions treated by TVE, complete occlusion was achieved in 25 and a near-complete occlusion with small residual shunting was achieved in 7, immediately after TVE. Follow-up assessments found that 6 lesions with near-complete occlusion displayed progressive occlusion. One lesion showed sustained shunting of blood via the fistula. One of the completely occluded lesions recurred on day 6 after treatment. Additional transarterial embolization using glue was performed for 2 lesions (1 recurrent fistula and 1 remained fistula) and both were cured. Two untreated lesions were found on follow up to have spontaneously resolved after treatment of the dominant contralateral fistula. None of the patients sustained any type of procedure-related mortality or morbidity.
After the treatment, symptoms in 10 patients completely resolved. Seven patients (41.2%) developed unilateral worsening of CN palsy after TVE. During the follow-up period (mean 33.7 ± 22.8 months, range 5–87 months), 3 patients remained with deficit despite partial improvement. The other 4 obtained complete recovery.
DISCUSSION
Dural AVF is a well-known pathoanatomical and clinical entity and represents 10% to 15% of all intracranial arteriovenous shunting diseases (10). The incidence of multiplicity is regarded as infrequent in literature (11). According to van Dijk et al. (11), multiple dAVFs were found in 8.1% of patients with intracranial dAVF. Although their study was the largest, consisting of 20 patients, patients with bilateral CSdAVF were not included. To the best of the researchers' knowledge, there have been only a few case reports of bilateral CSdAVF (12,13,14,15). This study constitutes the largest published series on bilateral CSdAVF, revealing that 7.7% of all cases of CSdAVF occurring in a 12-year period in an Asian country, were bilateral.
The pathogenesis of bilateral CSdAVF (as in multiple dAVFs) remains unclear. The researchers hypothesize as follows: 1) unilateral CSdAVF started by a specific cause such as trauma, and contralateral CSdAVF developed as subsequent consequence of initial dAVF; 2) bilateral CSdAVF developed simultaneously because of a single cause; 3) bilateral CSdAVF developed respectively from 2 unassociated causes. Venous thrombosis and venous hypertension were recently accepted as major triggers of dAVF formation (16). Venous hypertension that occurs because of an outflow obstruction diminishes cerebral perfusion and promotes neoangiogenesis (17,18). Although venous drainage in the region of the CS should confer a sump effect, serial thrombotic events may develop within the CS and draining veins, including the ipsilateral IPS (7).
Venous hypertension induced by ipsilateral CS shunt flow may impact the contralateral CS through the anterior and posterior intercavernous sinuses. The phenomenon can be enhanced in cases of thrombosis in or occlusion of the IPS (or within the CS). As a result, a unilateral CSdAVF might subsequently induce a dAVF near the contralateral CS. Ha et al. (19) reported 14 multiple dAVF, 7 of which were metachronous. They proposed that a change in the sinus (sinus thrombosis or thrombolysed sinus), because of initial dAVF, could play a prominent role in triggering or inducing additional dAVF at another site. The research findings support their postulation as follows: 1) many of the patients presented with unilateral symptoms or signs, although they had bilateral shunt lesions; and 2) many of the patients presenting with bilateral symptoms or sign initially had unilateral symptoms or sign that progressed to include the contralateral side. The other 2 hypotheses might also be applicable, in cases with multiple synchronous dAVFs and simultaneous onset of bilateral symptoms or signs. Patients with CSdAVF present with various manifestations, including tinnitus, CN palsy, ophthalmological problems, headache, and ICH.
The clinical presentation in CSdAVF does not depend exclusively on fistula flow; instead, venous drainage from the fistulas may play an important role in the development of symptoms (9,20,21). The clinical findings in patients with bilateral CSdAVF were like patients with unilateral lesions. However, only 7 of this research's 17 patients presented with bilateral findings and the other patients had unilateral manifestations. The clinical presentations of the patients in this study were also related to venous drainage patterns (Fig. 1). Van Dijk et al. (11) reported that a higher percentage of patients with multiple intracranial dAVFs had cortical venous drainage than patients with single lesions.
Cortical venous drainage, reportedly, is associated with a high risk of hemorrhage. Based on the findings in the study cohort, bilateral CSdAVF seems to be different from multiple dAVFs at other sites. It may be due to the multiple venous connections to the CS, which include the intercavernous sinus, ophthalmic vein, petrosal vein, pterygoid plexus, etc.
Among various interventional approaches to the CS, IPS is the most ideal transvenous approach for treating CSdAVF. In this patient series, most of the lesions (except 1, approached via the ipsilateral facial vein) were accessed through the IPS. Among the 31 lesions treated via the IPS, an ipsilateral approach was performed for 25 lesions and a contralateral approach was performed for 6. Regarding the patency of the IPS, 28 lesions were accessed through a patent IPS. Three lesions were approached by breaching an occluded ipsilateral IPS. Because the transvenous route through the ipsilateral IPS provides a relatively straight course and is seen as the shortest route to the CS, it is generally preferred. However, the ipsilateral IPS is sometimes occluded, thus impeding delivery of the microcatheter to the CS. Rhim et al. (7) reported a 55.3% success rate for breaching an occluded IPS, and Benndorf et al. (22) and Cho et al. (6) reported that the microguidewire looping technique enabled breaching of an occluded IPS for treating a CSdAVF. The approach through an ipsilateral and patent IPS was also preferred at our institution and achieved excellent outcomes.
Patients can develop new or worsening symptoms or signs, after undergoing successful TVE for CSdAVF. Jung et al. (23) reported that the rate of paradoxical worsening after TVE was 12.5%, and many patients (77.8%) fully recovered. Other studies reported similar outcomes (24,25,26). The postprocedural worsening might be due to progressive thrombosis of the superior ophthalmic vein, the CS, and their tributaries; clot propagation; mass effect due to the coil; or direct injury to the nerve by the coil or microwire/microcatheter (24,27,28,29). In this study, worsening of CN palsy after TVE occurred in 41.2% (n = 7). All the patients developed unilateral worsening. Moreover, 42.9% (n = 3) of them had permanent deficits. The worsening of cranial palsy after TVE for bilateral CSdAVF may be more common than after treatment of unilateral CSdAVF, and permanent deficit because of the worsening may also be more common. Precise reasons that postprocedural worsening are more common in bilateral CSdAVF are not known, but it may be affected by the fact that the bilateral lesions were diffuse type and coil volume occupying CS to get rid of the fistula were a bit massive. Thus, this resulted in compressing the CN by mass effect. In addition, relatively wide indication of treatment in this institution might make an impact on the results. In patients with one-sided dominance of shunt, treatment of only the dominant fistula may be an alternative option to avoid the postprocedural symptom aggravation, based on that the contralateral non-dominant lesions unrelated to main symptoms were simultaneously regressed after treatment of main ipsilateral fistula.
In conclusion, by adjustment of endovascular procedures to accommodate distinct anatomic configurations, endovascular intervention for bilateral CSdAVF can achieve results like those achieved by endovascular treatment of unilateral CSdAVF. This namely includes angiographic evidence of complete occlusion. However, postprocedural aggravation of signs and symptoms may occur more frequently after TVE of bilateral CSdAVF. In the patients with one-sided dominance of shunt, treatment of only dominant fistula might be an alternative option.
References
- 1.Cawley CM, Barrow DL, Dion JE. Treatment of lateral-sigmoid and sagittal sinus dural arteriovenous malformations. In: Winn HR, Youmans JR, editors. Youmans neurological surgery Volume 2. 5th ed. Philadelphia, PA: WB Saunders; 2004. pp. 2283–2291. [Google Scholar]
- 2.Collice M, D'Aliberti G, Talamonti G, Branca V, Boccardi E, Scialfa G, et al. Surgical interruption of leptomeningeal drainage as treatment for intracranial dural arteriovenous fistulas without dural sinus drainage. J Neurosurg. 1996;84:810–817. doi: 10.3171/jns.1996.84.5.0810. [DOI] [PubMed] [Google Scholar]
- 3.Hwang H, La YK, Baek MS, Baik K, Suh SH, Kim WJ. Dural arteriovenous fistula manifested as rapid progressive dementia successfully treated by endovascular embolization only. Neurointervention. 2017;12:50–53. doi: 10.5469/neuroint.2017.12.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnwell SL, Halbach VV, Dowd CF, Higashida RT, Hieshima GB, Wilson CB. A variant of arteriovenous fistulas within the wall of dural sinuses. Results of combined surgical and endovascular therapy. J Neurosurg. 1991;74:199–204. doi: 10.3171/jns.1991.74.2.0199. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi K, Kowada M. Anterior fossa dural arteriovenous malformation supplied by bilateral ethmoidal arteries. Surg Neurol. 1994;41:56–64. doi: 10.1016/0090-3019(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 6.Cho YD, Rhim JK, Yoo DH, Kang HS, Kim JE, Cho WS, et al. Transvenous microguidewire looping technique for breach of ipsilateral inferior petrosal sinus occlusions en route to cavernous sinus dural arteriovenous fistulas. Interv Neuroradiol. 2016;22:590–595. doi: 10.1177/1591019916653251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhim JK, Cho YD, Park JJ, Jeon JP, Kang HS, Kim JE, et al. Endovascular treatment of cavernous sinus dural arteriovenous fistula with ipsilateral inferior petrosal sinus occlusion: a single-center experience. Neurosurgery. 2015;77:192–199. doi: 10.1227/NEU.0000000000000751. discussion 199. [DOI] [PubMed] [Google Scholar]
- 8.Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, et al. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke. 2005;36:1134–1139. doi: 10.1161/01.STR.0000166194.82027.63. [DOI] [PubMed] [Google Scholar]
- 9.Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 10.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071–1078. doi: 10.1148/93.5.1071. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk JM, TerBrugge KG, Willinsky RA, Wallace MC. Multiplicity of dural arteriovenous fistulas. J Neurosurg. 2002;96:76–78. doi: 10.3171/jns.2002.96.1.0076. [DOI] [PubMed] [Google Scholar]
- 12.Dabus G, Batjer HH, Hurley MC, Nimmagadda A, Russell EJ. Endovascular treatment of a bilateral dural carotid-cavernous fistula using an unusual unilateral approach through the basilar plexus. World Neurosurg. 2012;77:201.e5–201.e8. doi: 10.1016/j.wneu.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Chaloupka JC, Goller D, Goldberg RA, Duckwiler GR, Martin NA, Viñuela F. True anatomical compartmentalization of the cavernous sinus in a patient with bilateral cavernous dural arteriovenous fistulae. Case report. J Neurosurg. 1993;79:592–595. doi: 10.3171/jns.1993.79.4.0592. [DOI] [PubMed] [Google Scholar]
- 14.Courtheoux P, Labbe D, Hamel C, Lecoq PJ, Jahara M, Théron J. Treatment of bilateral spontaneous dural carotid-cavernous fistulas by coils and sclerotherapy. Case report. J Neurosurg. 1987;66:468–470. doi: 10.3171/jns.1987.66.3.0468. [DOI] [PubMed] [Google Scholar]
- 15.Diez Lobato R, Escudero L, Lamas E. Bilateral dural arteriovenous fistula in the region of the cavernous sinus. Neuroradiology. 1978;15:39–43. doi: 10.1007/BF00327444. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey L, Konstas AA, Pile-Spellman J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg. 2014;121:64–75. doi: 10.1016/j.clineuro.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Chung SJ, Kim JS, Kim JC, Lee SK, Kwon SU, Lee MC, et al. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis. 2002;13:79–88. doi: 10.1159/000047755. [DOI] [PubMed] [Google Scholar]
- 18.Kojima T, Miyachi S, Sahara Y, Nakai K, Okamoto T, Hattori K, et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surg Neurol. 2007;68:277–284. doi: 10.1016/j.surneu.2006.10.075. discussion 284. [DOI] [PubMed] [Google Scholar]
- 19.Ha SY, Kwon YS, Kim BM, Kim DI, Kim DJ. Clinical and angiographic characteristics of multiple dural arteriovenous shunts. AJNR Am J Neuroradiol. 2012;33:1691–1695. doi: 10.3174/ajnr.A3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 21.Viñuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984;60:976–984. doi: 10.3171/jns.1984.60.5.0976. [DOI] [PubMed] [Google Scholar]
- 22.Benndorf G, Bender A, Lehmann R, Lanksch W. Transvenous occlusion of dural cavernous sinus fistulas through the thrombosed inferior petrosal sinus: report of four cases and review of the literature. Surg Neurol. 2000;54:42–54. doi: 10.1016/s0090-3019(00)00260-3. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Kwon BJ, Chu K, Noh Y, Lee ST, Cho YD, et al. Clinical and angiographic factors related to the prognosis of cavernous sinus dural arteriovenous fistula. Neuroradiology. 2011;53:983–992. doi: 10.1007/s00234-010-0805-3. [DOI] [PubMed] [Google Scholar]
- 24.Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006;27:2078–2082. [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis AI, Tomsick TA, Tew JM., Jr Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239–244. doi: 10.1227/00006123-199502000-00001. discussion 244-245. [DOI] [PubMed] [Google Scholar]
- 26.Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678–692. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 27.Sergott RC, Grossman RI, Savino PJ, Bosley TM, Schatz NJ. The syndrome of paradoxical worsening of dural-cavernous sinus arteriovenous malformations. Ophthalmology. 1987;94:205–212. doi: 10.1016/s0161-6420(87)33472-4. [DOI] [PubMed] [Google Scholar]
- 28.Nagy ZZ, Németh J, Süveges I, Lányi F. A case of paradoxical worsening of dural-sinus arteriovenous malformation syndrome after neurosurgery. Eur J Ophthalmol. 1995;5:265–270. doi: 10.1177/112067219500500412. [DOI] [PubMed] [Google Scholar]
- 29.Nishino K, Ito Y, Hasegawa H, Kikuchi B, Shimbo J, Kitazawa K, et al. Cranial nerve palsy following transvenous embolization for a cavernous sinus dural arteriovenous fistula: association with the volume and location of detachable coils. J Neurosurg. 2008;109:208–214. doi: 10.3171/JNS/2008/109/8/0208. [DOI] [PubMed] [Google Scholar]