Abstract
Tumor necrosis factor-α (TNFα) and the angiotensin system are involved in inflammatory diseases and may contribute to acute kidney injury. We investigated the mechanisms by which TNFα-converting enzyme (TACE) contributes to lipopolysaccharide (LPS)-induced renal inflammation and the effect of TACE inhibitor treatment on LPS-induced cellular injury in human renal proximal tubule epithelial (HK-2) cells. Mice were treated with LPS (10 mg/kg, i.p.) and HK-2 cells were cultured with or without LPS (10 µg/ml) in the presence or absence of a type 1 TACE inhibitor (1 µM) or type 2 TACE inhibitor (10 µM). LPS treatment induced increased serum creatinine, TNFα, and urinary neutrophil gelatinase-associated lipocalin. Angiotensin II type 1 receptor, mitogen activated protein kinase (MAPK), and TACE increased, while angiotensin-converting enzyme-2 (ACE2) expression decreased in LPS-induced acute kidney injury and LPS-treated HK-2 cells. LPS induced reactive oxygen species and the down-regulation of ACE2, and these responses were prevented by TACE inhibitors in HK-2 cells. TACE inhibitors increased cell viability in LPS-treated HK-2 cells and attenuated oxidative stress and inflammatory cytokines. Our findings indicate that LPS activates renin angiotensin system components via the activation of TACE. Furthermore, inhibitors of TACE are potential therapeutic agents for kidney injury.
Keywords: Acute kidney injury, Angiotensin-converting enzyme-2, Lipopolysaccharide, Mitogen activated protein kinase, Oxidative stress, TNFα-converting enzyme
INTRODUCTION
Lipopolysaccharide (LPS), also known as endotoxin, is a cell wall component of gram-negative bacilli. It provokes phenotypes mimicking sepsis in animal models and exerts toxicity in various organs, such as the heart, lung, liver, and kidney [1,2,3,4]. Accordingly, LPS may be useful for studies of the mechanisms underlying sepsis-induced kidney injury.
Tumor necrosis factor-α (TNFα) has crucial roles in inflammatory diseases, including sepsis [5]. The disintegrin and metalloproteinase (ADAM) family member ADAM17, also termed TNFα-converting enzyme (TACE), has emerged as a major shedding enzyme. It is constitutively expressed in heart and lung cells and is the pivotal shedding enzyme mediating acute lung inflammation in a cell-specific manner [6]. In a heart failure animal model, angiotensin II-induced TACE expression increases angiotensin converting enzyme 2 (ACE2) shedding [7]. In particular, ACE2 may play a critical role via Ang-II degradation or Ang-(1-7) generation. ACE2 modulates the kidney response to injury in diverse settings, including ischemia reperfusion injury, unilateral ureteral obstruction, and diabetic nephropathy [8,9,10]. However, it is not clear whether the effects of TACE in inflammatory kidney injury and sepsis are associated with ACE2 and the renin angiotensin system (RAS).
In this study, we investigated the roles of TACE and ACE2 in kidney injury. Furthermore, we evaluated the potential function of TACE inhibitors in the prevention of LPS-induced oxidative stress and cell injury.
METHODS
Animals
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Animal Care Regulations (ACR) Committee of Chonnam National University Medical School (CNU IACUCH-2017-44). Male mice of the C57BL/6 strain were used for in vivo experiments. All mice weighed 20 g at the start of the experiments. LPS (LPS from Escherichia Coli 0111:B4, Sigma Chemical, St. Louis, MO, 10 mg/kg, n=8) was administered intraperitoneally. Mice were maintained on a standard rodent diet and allowed free access to drinking water. On 12 h after LPS injection, mice were anesthetized with isoflurane. Blood samples were collected from the carotid artery and analyzed for creatinine (Exocell, Philadelphia, PA, USA), TNFα and neutrophil gelatinase-associated lipocalin (NGAL, R&D, Minneapolis, MN, USA).
Cell culture and reagents
Human renal proximal tubular epithelial cells (HK-2 cells, American Type Culture Collection, Manassas, VA) were cultured and passaged every 3–4 days. The detailed condition and media used for cell culture was previously described [11]. The cells were treated with or without LPS (10 µg/ml) for 8 h. The control cells were treated with a buffer solution alone. TAPI1 (1 µM for 30 min pre-trement, calbiochem, San Diego, CA, USA) or TAPI2 (10 µM for 1 h pre-treatment, Cayman, Ann Arbor, MI, USA) were used as a TACE inhibitor.
Protein extraction and semiquantitative immunoblotting
The kidney cortex was homogenized in ice-cold isolation solution containing 0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride (pH 7.2). The homogenates were centrifuged at 1,000 g for 15 min at 4℃ to remove whole cells, nuclei, and mitochondria, and the total protein concentration was measured (Pierce BCA protein assay kit, Pierce, Rockford, IL). All samples were adjusted with isolation solution to normalize the protein concentrations, solubilized at 65℃ for 15 min in SDS-containing sample buffer, and the stored at −20℃. HK-2 cells were harvested, washed with cold PBS and resuspended in lysis buffer (20 mM Tris-HCl, pH 7.4, 0.01 mM EDTA, 150 mM NaCl, 1 mM PMSF, 1 µg/ml leupeptin, 1 mM Na3VO4) and prepared for immunoblotting. The detailed procedure followed previously described methods [11].
Primary antibodies
Angiotensin converting enzyme (ACE), and angiotensin II type 1 receptor (AT-1R) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), ACE2 (R&D, Minneapolis, MN, USA), TACE (Millipore), heme oxygenase-1 (HO-1, Abcam, Inc., Cambridge, MA, USA), angiotensin II/III (Novus Biologicals, Littleton CO, USA), anti-p-p38, anti-total p38, anti-c-Jun N-terminal kinase (JNK), anti-p-JNK, anti-caspase-3, anti-cleaved caspase-3, (Cell Signaling Technology, Danvers, MA), anti-Bcl-2, and anti-Bax (Cell Signaling Technology, Beverly, MA, USA), and β-actin (Sigma-Aldrich, St. Louis, MO, USA) antibodies were purchased.
Real-time PCR
To quantify mRNA levels, total RNA was extracted from HK-2 cells using TRIzol reagent (Invitrogen). cDNA was then reverse transcribed from 1 µg samples of total RNA using QuantiTect Reverse Transcription kit (Qiagen Science, Maryland, USA). Real-time PCR was performed using QuantiTect SYBR Green PCR master mix (Qiagen Science, Maryland, USA) and a Rotor-Gene TM 3000 Detector System (Corbette research, Mortlake, New South Wales, Australia). Real time-PCR primer sequences were as follows: for human GAPDH, 5′-GACATCAAGAAGGTGGTGAA-3′ (F) and 5′-TGTCATACCAGGAAATGAGC-3′; for human IL-6, 5′-TCAATGAGGAGACTTGCCTG-3′ (F) and 5′-GATGAGTTGTCATGTCCT GC-3′ (R); for TNFα, 5′-GGCTCCAGGCGGTGCTTG-3′ (F) and 5′- GGGCTACAGGCTTGTCACTCG-3′ (R); for iNOS, 5′-ACGTGCGTTACTCCACCAACA-3′ (F) and 5′-CATAGCGGATGAGCTGAGCATT-3′ (R); for MCP-1, 5′-CAGCCAGATGCAATCAATGC-3′ (F) and 5′-GTGGTCCATGGAATCCTGAA-3′ (R). Data from the reaction were collected and analyzed with the appropriate software package from Corbett Research.
Intracellular level of reactive oxygen species (ROS)
HK2 cells were cultured in 24-well plates until they reached confluence. Cells were pre-treated with TAPI1 (1 µM for 30 min pre-trement, Calbiochem) and TAPI2 (10 µM for 1hr pretreatment, Cayman) and then treated with 10 µg/ml of LPS for 8 h. At the end of the experimental periods, cells were preloaded with 10 µM 2′, 7′-dichlorofluorescein diacetate (DCF-DA; Molecular Probes) for 30 min at 37℃. Fluorescence intensity was analyzed by a fluorescence reader (Fluoroscan Ascent FL; Lab systems, Helsinki, Finland) using 485 nm excitation and 538 nm emission filter. HK2 cells were cultured on a 6-well plate for DCF-DA staining. Cells were pre-treated with TAPI1 (1 µM for 30 min) and TAPI2 (10 µM for 1 h) and then treated with 10 µg/ml of LPS for 8 h. Cells were washed twice with hanks balanced salt solution (HBSS) and incubated with HBSS (without phenol red) containing DCF-DA for 30 min at 37℃ in dark. The images were obtained with a fluorescence microscope (Nikon, Tokyo, Japan).
Cell viability assay
The viability of HK2 cells was determined by using WTS kit, according to manufacturer's instructions. Prepared HK2 cells were plated in 96-well microplates and incubated with serum-free DMED media for 24 h. The cells pre-treated with TAPI-1 (1 µM for 30 min) and TAPI-2 (10 µM for 1 h) and then treated with 10 µg/ml of LPS for 8 h. To exam cell viability, 10 µL of CCK8 reagent was added into each well of the 96-well plate. After incubation of 4 h, the absorbance of the plate was read at 492 nm using a microplate reader.
TACE activity
Kidney TACE activities were assessed using fluorescent assay protocols involving 20 µM 7-methoxycoumarin-PLAQAV-(2,4-dinitrophenyl)-RSSSR-NH2 (R&D Systems) and 20 µM 7-methoxycoumarin-YVADAPK-(2,4-dinitrophenyl)-OH (R&D Systems) as fluorogenic substrates for TACE, as shown before [12].
Immunofluorescence
HK2 cells were cultured on chamber slide (Nalge Nunc International, Rochester, NY, USA) and treated with TAPI1 (1 µM for 30 min) and TAPI2 (10 µM for 1 h) and then treated with 10 µg/ml of LPS for 8 h. The primary antibody rabbit anti-TACE was incubated with the cells overnight at 4℃ in a humidified chamber. After washes, secondary antibody conjugated with Alexa Flour 568-labeled (red) goat anti-rabbit IgG (1:200dilution; Invitrogen, Carlsbad, CA, USA) was incubated with chamber slide for 1 h at room temperature. Stained cells were visualized using confocal laser microscope (LSM 510, Carl Zeiss, Germany), as previously described [13]. The sections were stained with DAPI (Invitrogen) for nuclear counterstaining.
Annexin V/propidium iodide staining assay
HK-2 apoptosis assessed by using an apoptosis detection kit (Koma Biotech, Seoul, Korea). After exposure to LPS for 8 h in the presence or absence of TAPI1 (1 µM for 30 min) and TAPI2 (10 µM for 1 h), HK-2 cells were harvested and washed with precooled PBS and re-suspended in a binding buffer containing fluorescein isothiocyanate (FITC)-conjugated annexin-V protein and PI. Annexin-V binding and PI staining were determined by a FACSCalibur™ flow cytometry (Becton Dickinson, San Jones, CA, USA). Apoptotic cells were defined as PI-negative and Annexin V-FITC positive [14].
DAPI staining
Apoptotic nuclei were detected using the DNA-specific fluorescent dye 40-6-diamidino-2-phenylindole (DAPI) (Invitrogen). After exposure to LPS for 8 h in the presence or absence of TAPI1 (1 µM for 30 min) and TAPI2 (10 µM for 1 h), cells were fixed with 3% paraformaldehyde and washed with PBS. DAPI was added to the fixed cells for 5 min, after which they were examined by fluorescence microscopy (Nikon, Tokyo, Japan). The number of cells with apoptotic bodies was counted in 5 randomly chosen fields at 200 magnification and the percent apoptosis was calculated.
Statistical analysis
Results are expressed as mean±SEM of at least 3 independent experiments. Multiple comparisons between groups were made by one-way analysis of variance and post-hoc Tukey's honestly significant difference test. Differences with values of p<0.05 were considered significant.
RESULTS
Effect of LPS on serum creatinine, TNFα, and NGAL
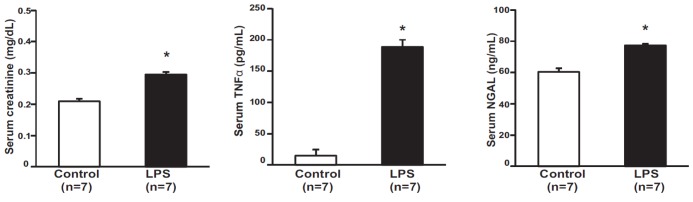
The total body weight (20.3±0.32 g in controls, 19.6±0.23 mg/dl in LPS-treated mice, p=0.13) and kidney weight (0.19±0.003 g in controls, 0.20±0.004 g in LPS-treated mice, p=0.12) of mice were not different among the groups. Serum creatinine levels were elevated in LPS-treated mice compared with those in control mice (0.209±0.007 mg/dl in controls, 0.295±0.008 mg/dl in LPS-treated mice, p<0.05). Serum TNFα levels were elevated in LPS-treated mice compared with those in control mice (14.8±9.74 pg/ml in controls, 188.6±11.41 pg/ml in LPS-treated mice, p<0.01). Serum NGAL levels were elevated in LPS-treated mice compared with those in control mice (60.34±2.36 ng/ml in controls, 77.22±0.99 ng/ml in LPS-treated mice, p<0.01) (Fig. 1).
Fig. 1. Effect of LPS on serum creatinine, TNFα, and NGAL.
Serum creatinine, tumor necrosis factor-α (TNFα), and urinary neutrophil gelatinase-associated lipocalin (NGAL) were higher in lipopolysaccharide (LPS)-treated mouse than in control mice (n=7). Results are presented as mean±SEM of three individual experiments. *p<0.05 vs. control.
Effect of LPS on renin-angiotensin system
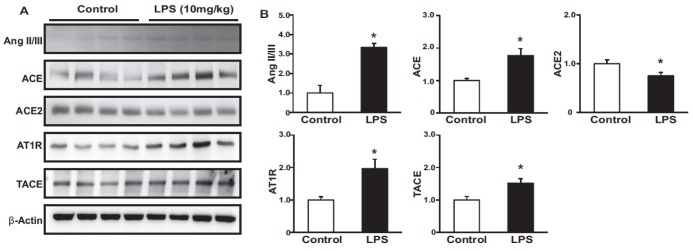
Immunoblotting analysis showed that the administration of LPS induced the expression of Ang II/III, ACE, AT1R and TACE, while LPS reduced the expression of ACE2 in vivo (Figs. 2A and B).
Fig. 2. Effects of LPS on the renin-angiotensin system.
Protein expression levels of Ang II/III, ACE, ACE2, AT1R, and TACE were higher, while that of ACE2 was lower in LPS-treated mice than in controls (A, B). Results are presented as mean±SEM. *p<0.05 vs. control.
Effect of LPS on oxidative stress, inflammatory pathway and apoptosis
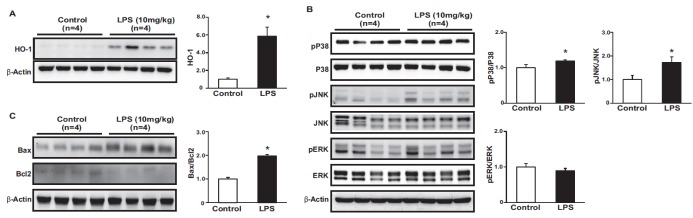
To evaluate LPS-induced oxidative stress, we measured HO-1 levels in kidney tissues, and found that the administration of LPS induced the expression of HO-1. We also measured MAPKase phosphorylation to evaluated inflammatory pathway (Fig. 3A). The phosphorylation of p38 and JNK increased in the kidneys of LPS-treated mice (Fig. 3B). The protein expression levels of proapoptotic Bax and anti-apoptotic Bcl-2 were measured by immunoblotting. Bax levels were higher, whereas Bcl2 levels were lower in LPS-treated mice than in control mice. Accordingly, the ratio of Bax to Bcl2 was increased in LPS-treated mice (Fig. 3C).
Fig. 3. Effects of LPS on the oxidative stress, inflammation and apoptosis.
The protein expression of HO-1 were higher in LPS-treated mice compared to control (A). The phosphorylation level of p38 and JNK was increased in LPS-treated mice compared to control (B). Protein expression of Bax was higher, while Bcl2 was lower in LPS-treated mice than in control. Ratio of Bax to Bcl2 was increased in LPS-treated mice (C). Results are presented as mean±SEM. *p<0.05 vs. control.
Effects of TACE inhibitors on oxidative stress, cell proliferation and inflammation
To assess the degree of oxidative stress in the kidney, DCF-DA staining was performed (Figs. 4A and B). LPS induced marked ROS generation compared to ROS levels in the controls. Immunoblotting analysis showed that the administration of LPS induced the expression of HO-1 in HK-2 cells, and this effect was attenuated by TAPI1 and TAPI2 pretreatment (Fig. 4C). We examined cell proliferation using a WST-1 proliferation assay. LPS-treated HK-2 cells showed markedly decreased cell proliferation compared to that of control cells, and proliferation improved by TAPI1 and TAPI2 pretreatment (Fig. 4D). We also investigated the expression of IL-6 and TNFα, which are key inflammatory cytokines. As shown in Fig. 5, LPS treatment significantly induced renal IL-6 and TNFα mRNA expression, while these changes were attenuated with TAPI-1and TAPI-2 pretreatment (Figs. 5A and B). Increased expression of certain chemokines and adhesion molecules such as MCP-1, which can activate, recruit, or induce the transmigration of inflammatory cells. The expression of these factors was induced by LPS treatment and attenuated by TAPI1 and TAPI2 pretreatment (Fig. 5C). mRNA expression of iNOS, which is known as oxidative stress marker was increased in LPS-treated HK2 cell, which was attenuated by TAPI1 and TAPI2 pretreatment (Fig. 5D).
Fig. 4. ROS generation detected using the ROS-sensitive fluorescent dye DCF.
LPS caused an increase in DCF fluorescence after incubation for 8 h, which was attenuated by a TACE type 1 inhibitor (TAPI1) and TACE type 2 inhibitor (TAPI2) (A, B). Protein expression of HO-1 increased in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 (C). A cell proliferation assay using WST-1 showed a marked decreased in cell proliferation in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 (D). *p<0.05 vs. control. #p<0.05 vs. LPS.
Fig. 5. Real-time PCR.
Effects of TAPI1 and TAPI2 on the IL-6, TNFα, iNOS and MCP-1 in LPS-treated HK-2 cells. mRNA expression levels of IL-6, TNFα, iNOS and MCP-1 were higher in LPS-treated HK-2 cells than control, and were attenuated by TAPI1 and TAPI2. Results are presented as mean±SEM. *p<0.05 vs. control. #p<0.05 vs. LPS.
Effect of TACE inhibitors on RAS and TACE in LPS-treated HK2 cells
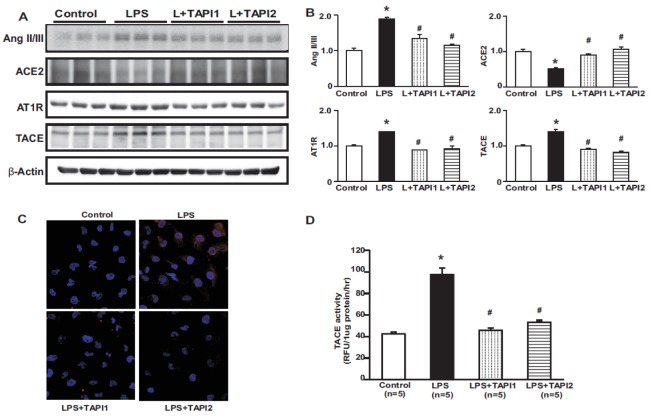
TACE is a sheddase capable of proteolytically cleaving and releasing membrane-bound proteins. In the heart, TACE mediates ACE2 shedding after Ang II stimulation and contributes to cardiac injury [7]. We therefore examined whether TACE is involved in the loss of ACE2 in the kidney. TAPI1 and TAPI2 treatment attenuated Ang II/III, AT1R, and TACE expression and reversed the reduction in ACE2 expression in LPS-treated HK-2 cells (Figs. 6A and B). Immunofluorescence staining for TACE also showed increased immunoreactivity in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 treatment (Fig. 6C). TACE activity was also markedly increased in LPS-treated HK-2 cells, and this was counteracted by TAPI1 and TAPI2 (Fig. 6D).
Fig. 6. Effects of TAPI1 and TAPI2 on the renin-angiotensin system in LPS-treated HK-2 cells.
Protein expression levels of Ang II/III, AT1R, and TACE were higher in LPS-treated HK-2 cells than in controls, and were attenuated by TAPI1 and TAPI2. The protein expression of ACE2 was decreased in LPS-treated HK-2 cells compared to control, which was counter-regulated by TAPI1 and TAPI2 (A, B). Immunofluorescence for TACE shows increased expression in LPS-treated HK-2 cells (C). TACE activity was increased in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 treatment (D). Results are presented as mean±SEM. *p<0.05 vs. control. #p<0.05 vs. LPS.
Effects of TACE inhibitors on the MAPK pathway and apoptosis in LPS-treated HK-2 cells
JNK, and p38 MAPK phosphorylation levels increased in the LPS-treated HK-2 cells, and were attenuated by TAPI1 and TAPI2 (Figs. 7A and B). LPS increased the ratio of Bax/Bcl-2 protein expression in HK-2 cells, and this increase was counter-regulated by TAPI1 and TAPI2 pre-treatment (Figs. 8A and B). Annexin-V and PI staining were used to detect apoptotic changes in LPS-treated HK-2 cells; double-stained cells were analyzed by flow cytometry. HK-2 cells treated with LPS for 8 h exhibited a progressive increase in annexin-V(+)/PI(−) staining (apoptotic cells), and this was prevented by TAPI-1 and TAPI-2 (Fig. 8C).
Fig. 7. Effects of LPS on the MAPK pathway in LPS-treated HK-2 cells.
Expression of pP38 tended to increase in LPS-treated mice and phosphorylation of P38, c-Jun N-terminal kinase (pJNK), and extracellular signal-regulated kinase (pERK 1/2) increased in LPS-treated HK-2 cells, and was attenuated by TAPI1 and TAPI2 treatment. Increased phosphorylation of ERK 1/2 was not affected by TAPI1 treatment. Results are presented as mean±SEM of three individual experiments. *p<0.05 vs. control. #p<0.05 vs. LPS.
Fig. 8. Effects of LPS on apoptosis.
Protein expression of Bax was increased, while protein expression of Bcl2 was decreased in LPS treated HK-2 cells compared to controls (A). The ratio of Bax to Bcl2 was increased in LPS-treated HK-2 cells, and was attenuated by TAPI1 and TAPI2 (B). DAPI staining shows increased apoptotic cells in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 treatment (C). FACS showed apoptotic cells in LPS-treated HK-2 cells, which was attenuated by TAPI1 and TAPI2 treatment (D). *p<0.05 vs. control. #p<0.05 vs. LPS.
DISCUSSION
In this study, we demonstrated that LPS administration evoked kidney injury, characterized by increased creatinine, TNFα, and NGAL. It induced RAS alterations, such as increased Ang II/III and decreased ACE2, as well as increased TACE. Treatment with TACE inhibitors attenuated HK-2 cell injury via the inhibition of oxidative stress, the MAPK pathway, and apoptosis. These findings suggest that TACE inhibitor treatment prevents LPS-induced kidney injury and may be useful as a therapeutic agent targeting kidney injury.
TNF-α, a pleiotropic pro-inflammatory cytokine, mediates inflammation, cell activation, and cell migration [15]. TACE, also known as ADAM-17, is a member of the ADAM family of proteases, which are implicated in various inflammatory diseases, including arthritis, diabetes, cancer, multiple sclerosis, and Alzheimer's disease [16,17,18]. Therefore, TACE inhibition is an attractive strategy for controlling the level of active TNFα for the treatment of inflammatory disorders [19]. Ectodomain shedding describes the proteolytic cleavage of transmembrane proteins to their soluble forms [20]. TACE was the first mammalian sheddase discovered [21] and is the primary physiological sheddase responsible for cleaving membrane TNF to its soluble form [22]. ROS have been implicated in the up-regulation of TACE shedding activity in a variety of experimental systems [23,24,25,26]. According to Zhang et al., in an immature monocyte cell line, PMA-induced ROS and reactive nitrogen species attack the cysteinyl thiol of the prodomain of TACE, nullifying its inhibitory effect [23,24]. ROS have also emerged as essential effectors of a variety of intracellular signaling pathways, such as MAPK cascades [27,28]. Inhibition of p38 MAPK attenuates the shedding of some TACE substrates [29]. Therefore, we hypothesized that LPS induces ROS, which may mediate the up-regulation of TACE activity and that the inhibition of TACE may prevent this process. As expected, TAPI1, which inhibits MMPs and TACE and blocks the shedding of several cell surface proteins [30], and TAPI2, a broad-spectrum inhibitor of TACE, inhibited ROS generation, the phosphorylation of ERK, JNK, and p38, and apoptosis. ROS metabolism is thought to play an important role in all known types of programmed cell death [31]. Specifically, the imbalance between cell survival and death, a key feature of many degenerative and inflammatory diseases, may be caused by aberrant ROS turnover, a process that regulates the crosstalk between NF-κB and MAPKs [32].
Apoptosis is programmed cell death caused by alterations of the mitochondrial membrane potential. Among various molecules involved in the apoptotic process, caspases play important roles during the initiation and effector phases of apoptotic cell death. Caspase-3 is a prototypical caspase and an important protease in the execution of apoptosis [33]. Moreover, the Bcl-2 family of proteins, including Bax and Bcl-2, are key regulators in the early stages of the apoptotic pathway. In the present study, HK-2 cells treated with LPS showed a significant increase in caspase-3 activity and the Bax/Bcl-2 ratio compared to those of untreated control cells; these effects were attenuated by concomitant TAPI1 and TAPI2 treatment. These findings suggest that TAPI contributes to the attenuation of LPS-induced apoptosis via the regulation of the Bax/Bcl-2 ratio.
At an early stage of apoptosis, phosphatidylserine is translocated to the external surface from the inner surface of the cell membrane. Annexin V is a Ca2+-dependent phospholipid-binding protein; it binds strongly to phosphatidylserine on the cell membrane. Externalization of phosphatidylserine can be assessed by measuring the extent of FITC-annexin V binding. In contrast, membrane integrity is compromised in necrotic cell death and can be assessed by PI staining, which indicates a modification of cell permeability, a sign of necrosis [34]. We found that HK-2 cells treated with LPS exhibited a significant progressive increase in annexin V+/PI− staining, indicating that LPS induced apoptosis. TAPI pretreatment reduced this expression, suggesting that it has antiapoptotic properties. These results were confirmed by the increased nuclear staining, indicating increased nuclear condensation, in cells treated with LPS than in controls. TAPI pretreatment attenuated LPS-induced apoptosis.
Increased ROS generation is closely associated with renal tubular cell apoptosis [35]. ROS may disrupt mitochondrial membrane permeability, resulting in the release of apoptotic factors, such as cytochrome c [36]. LPS is a strong inducer of ROS production; accordingly, we examined the effect of TAPI on LPS-mediated ROS production using fluorescent DCF-DA. LPS-treatment strongly induced ROS production, whereas TAPI treatment prevented ROS generation in LPS treated HK-2 cells.
Based on our results and those of other studies, we conclude that LPS activates RAS components via the activation of TACE, which is attenuated by TACE inhibitors in HK-2 cells.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2017M3A9E8023001), by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2016R1A2B4007870), by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT &Future Planning (2014M3C1A3053036), the Korean Government Research Foundation Grant (NRF-2016R1C1B2011883), Chonnam National University Hospital Biomedical Reserch Institute Grant (CRI16018-1), and by a grant (CRI14072-3) of the CNUH-GIST.
Footnotes
Author contributions: E.H.B. and H.S.C. performed the animal experiments. I.J.K. and H.Y.K. performed cell-based study. I.S.K., M.S.K. and S.W.K. supervised and coordinated the study. E.H.B. and C.S.K. wrote the manucript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Kiemer AK, Müller C, Vollmar AM. Inhibition of LPS-induced nitric oxide and TNF-alpha production by alpha-lipoic acid in rat Kupffer cells and in RAW 264.7 murine macrophages. Immunol Cell Biol. 2002;80:550–557. doi: 10.1046/j.1440-1711.2002.01124.x. [DOI] [PubMed] [Google Scholar]
- 2.Goraca A, Józefowicz-Okonkwo G. Protective effects of early treatment with lipoic acid in LPS-induced lung injury in rats. J Physiol Pharmacol. 2007;58:541–549. [PubMed] [Google Scholar]
- 3.Goraca A, Piechota A, Huk-Kolega H. Effect of alpha-lipoic acid on LPS-induced oxidative stress in the heart. J Physiol Pharmacol. 2009;60:61–68. [PubMed] [Google Scholar]
- 4.Olesen ET, de Seigneux S, Wang G, Lütken SC, Frøkiaer J, Kwon TH, Nielsen S. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol Dial Transplant. 2009;24:2338–2349. doi: 10.1093/ndt/gfp011. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 6.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol. 2012;91:472–485. doi: 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest. 2012;92:650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- 10.Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, Hu A, Pan J, Konvalinka A, Oudit GY, Scholey JW, John R. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS One. 2013;8:e71433. doi: 10.1371/journal.pone.0071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol. 2010;298:F301–F313. doi: 10.1152/ajprenal.00471.2009. [DOI] [PubMed] [Google Scholar]
- 12.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res. 2005;97:380–390. doi: 10.1161/01.RES.0000178789.16929.cf. [DOI] [PubMed] [Google Scholar]
- 13.Kim CS, Joo SY, Lee KE, Choi JS, Bae EH, Ma SK, Kim SH, Lee J, Kim SW. Paricalcitol attenuates 4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. PLoS One. 2013;8:e63186. doi: 10.1371/journal.pone.0063186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamai L, Falcieri E, Marhefka G, Vitale M. Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry. 1996;23:303–311. doi: 10.1002/(SICI)1097-0320(19960401)23:4<303::AID-CYTO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 16.Asai M, Hattori C, Szabó B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003;301:231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- 17.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci. 2009;56:148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Bahia MS, Silakari O. Tumor necrosis factor alpha converting enzyme: an encouraging target for various inflammatory disorders. Chem Biol Drug Des. 2010;75:415–443. doi: 10.1111/j.1747-0285.2010.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 2010;293:925–937. doi: 10.1002/ar.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Kolls JK, Oliver P, Good D, Schwarzenberger PO, Joshi MS, Ponthier JL, Lancaster JR., Jr Activation of tumor necrosis factor-alpha-converting enzyme-mediated ectodomain shedding by nitric oxide. J Biol Chem. 2000;275:15839–15844. doi: 10.1074/jbc.M000604200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Oliver P, Lancaster JR, Jr, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 25.Pietri M, Schneider B, Mouillet-Richard S, Ermonval M, Mutel V, Launay JM, Kellermann O. Reactive oxygen species-dependent TNF-alpha converting enzyme activation through stimulation of 5-HT2B and alpha1D autoreceptors in neuronal cells. FASEB J. 2005;19:1078–1087. doi: 10.1096/fj.04-3631com. [DOI] [PubMed] [Google Scholar]
- 26.Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, Sunnarborg SW. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol Biol Cell. 2009;20:5236–5249. doi: 10.1091/mbc.E08-12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blouin E, Halbwachs-Mecarelli L, Rieu P. Redox regulation of beta2-integrin CD11b/CD18 activation. Eur J Immunol. 1999;29:3419–3431. doi: 10.1002/(SICI)1521-4141(199911)29:11<3419::AID-IMMU3419>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 29.Pedron T, Girard R, Chaby R. TLR4-dependent lipopolysaccharide-induced shedding of tumor necrosis factor receptors in mouse bone marrow granulocytes. J Biol Chem. 2003;278:20555–20564. doi: 10.1074/jbc.M203551200. [DOI] [PubMed] [Google Scholar]
- 30.Lovejoy B, Welch AR, Carr S, Luong C, Broka C, Hendricks RT, Campbell JA, Walker KA, Martin R, Van Wart H, Browner MF. Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nat Struct Biol. 1999;6:217–221. doi: 10.1038/6657. [DOI] [PubMed] [Google Scholar]
- 31.Rosenau C, Emery D, Kaboord B, Qoronfleh MW. Development of a high-throughput plate-based chemiluminescent transcription factor assay. J Biomol Screen. 2004;9:334–342. doi: 10.1177/1087057103261446. [DOI] [PubMed] [Google Scholar]
- 32.Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev. 2007;220:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 34.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]