Abstract
Purpose of review
This review article discusses the evolution of extracellular volume (ECV) quantification using both cardiovascular magnetic resonance (CMR) and computed tomography (CT).
Recent findings
Visualizing diffuse myocardial fibrosis is challenging and until recently, was restricted to the domain of the pathologist. CMR and CT both use extravascular, extracellular contrast agents, permitting ECV measurement. The evidence base around ECV quantification by CMR is growing rapidly and just starting in CT. In conditions with high ECV (amyloid, oedema and fibrosis), this technique is already being used clinically and as a surrogate endpoint. Non-invasive diffuse fibrosis quantification is also generating new biological insights into key cardiac diseases.
Summary
CMR and CT can estimate ECV and in turn diffuse myocardial fibrosis, obviating the need for invasive endomyocardial biopsy. CT is an attractive alternative to CMR particularly in those individuals with contraindications to the latter. Further studies are needed, particularly in CT.
Keywords: Extracellular volume, Computed tomography, Cardiovascular magnetic resonance, Tissue characterization
Introduction
Myocardial fibrosis is a frequently unwanted, common end point in the majority of pathological mechanisms affecting heart muscle. It can occur as focal scarring due to replacement fibrosis following myocyte death (apoptosis, autophagy or necrosis) or as diffuse fibrosis due to expansion of the collagen fibre network around individual myocytes or myocyte bundles [1]. The best non-invasive technique for visualising focal fibrosis is cardiovascular magnetic resonance (CMR) using late gadolinium enhancement (LGE), because of the high contrast, high spatial resolution and whole heart coverage. Although resultant image quality is currently reduced, myocardial fibrosis can also be assessed with cardiac computed tomography (CT)—both use an extracellular, extravascular contrast agent that lingers in extracellular water in areas of scar, due to a higher volume of distribution and slower kinetics. Visualization of diffuse fibrosis until now has remained challenging and limited to the domain of the pathologist, who was able to measure the extracellular matrix (ECM) directly on histological sections using stains specific for connective tissue [2]. Coupled with this, no useful blood biomarkers of myocardial fibrosis are currently extant. In the last 7 years, the same contrast agents have begun to be used to measure diffuse interstitial expansion (as well as focal scar), by measuring the extracellular volume (ECV). This review will focus on the quantification of ECV using the two most commonly used contrast agents: gadolinium and iodine based.
Development of an Extracellular Contrast Agent
An extracellular contrast agent has a key set of properties: (1) homogeneous distribution; (2) high water, but no fat solubility; (3) not adsorbed, actively transported, protein-bound or metabolized; (4) non-toxic, stable and freely cleared from the body and (5) readily measurable. Iodine and gadolinium compounds both fulfil these requirements as contrast agents. They diffuse rapidly and passively from the vascular space into extracellular tissue, but not into the intracellular space—leading to the term ‘extracellular, extravascular contrast agent’. Following an intravenous bolus, they enter the myocardium down a concentration gradient (‘wash-in phase’), and later, while being cleared, they return to the blood pool down the reverse concentration gradient (‘wash-out phase’). This occurs over seconds to minutes in healthy myocardium, but in scar tissue (focal or diffuse), these pharmacokinetics are delayed due to changes in coronary flow rates, capillary permeability, functional capillary density and the presence of a dense, hydrated collagen matrix [3]. In addition, the increased volume of extracellular water present in scar compared to normal myocardium means total accumulation is higher. The combined result is that, at a certain time ‘late’ after a bolus, there is more contrast agent in scar than in the blood or remote myocardium and measurable signal is therefore changed.
In CMR, gadolinium-based contrast agents (GBCAs) are used due to their unique magnetic properties (gadolinium is a paramagnetic metal with the most unpaired electrons) [4]. They are particularly efficient T1-relaxing agents, resulting in increased signal on T1-weighted images and typically appearing bright on a T1 inversion recovery image. The relaxation rate (R1 or 1/T1) is directly proportional to the concentration of gadolinium. In CT, non-ionic iodinated contrast agents have become the most commonly used contrast agents [4, 5]; they are water-soluble, extracellular, extravascular contrast agents, which are not metabolized and are excreted by the kidneys [6, 7]. CT attenuation values (represented as Hounsfield units, HU) are directly proportional to the concentration of iodine.
Extracellular Volume Imaging by CMR
Until recently, the gold standard of diagnosing diffuse fibrosis was endomyocardial biopsy, which is invasive (carrying risk) and is prone to sampling errors. This has led to the development of new, non-invasive techniques to better quantify diffuse fibrosis. CMR allows non-invasive tissue characterization of the myocardium and as such, is being increasingly used to identify the aetiology of a range of cardiomyopathies. LGE is the mainstay of this myocardial characterization and allows the detection of focal fibrosis [3, 8]. This technique combined with functional imaging is the main reason that CMR is so useful clinically. LGE imaging relies on the delayed post-contrast difference in T1 between areas of fibrosis (more gadolinium, shorter T1) and healthy myocardium (less gadolinium, longer T1) [9], making it ideal for identifying focal areas of fibrosis. In diffuse fibrosis, this relative difference is lost, so conventional LGE imaging struggles—being a difference test where the operator selects one ‘normal’ tissue to null making all other tissues ‘bright’[9, 10••]. GBCAs change tissue T1; however, the native (non-contrast) T1 also changes with pathology. Advances in CMR sequences now permit its quantification via T1 mapping, which offers absolute values of T1, rather than relative differences in signal intensity. [10••]
Native T1 describes the signal in the whole of the measured myocardium and therefore represents a composite signal from all species present—this signal is swamped by iron or gadolinium if present and in their absence is measuring the signal of both the cardiac myocytes and the ECM [10••]. Therefore, fibrosis/oedema/amyloid and associated water increase T1, and conversely increased cellularity (athleticism), iron (thalassemia) or fat (Anderson Fabry disease) decrease T1 [10••, 11•, 12•].
The use of extracellular GBCAs in CMR offers the opportunity to quantify the extracellular (i.e. interstitial) space, relative to the intracellular (i.e. myocyte) space, which is the essence of ECV quantification. It dichotomises the myocardium into myocytes and matrix. In conjunction with myocardial volume, ECV can be used to calculate the relative volumes of each compartment. It is expressed as a volume fraction and provides us with unique insights into the pathophysiology of a range of myocardial diseases [10••].
T1 mapping and ECV may provide an advantage over conventional LGE imaging, by enabling us to more accurately quantify diffuse fibrosis and potentially detect early fibrosis-related changes not always detectable by LGE [13]. Indeed, increases in ECV seen on CMR are associated with an increased mortality and may be as important to prognosis as left ventricular ejection fraction [13, 14••]. In-depth discussion of the advantages and limitations of native T1 mapping are beyond the scope of this review and have been described elsewhere [8, 11•]; instead, this review will focus on ECV imaging, which combines pre- and post-contrast images.
Evolution of ECV by CMR
Initial validation in humans utilizing CMR to quantify ECV (ECVCMR) and in turn diffuse myocardial fibrosis was performed in 2010 by Flett et al. [15]. They employed a technique they termed ‘equilibrium contrast CMR’, which involved an initial bolus of GBCA, followed by a continuous infusion to achieve an equilibrium of contrast between the blood pool and the myocardium [15]. They estimated the blood volume of distribution from 1-haematocrit and then used CMR to measure the pre- and post-contrast equilibrium T1 [15]. Using the formula below, they then calculated the ECV.
They validated this technique by direct comparison with histological fibrosis quantification using picrosirius red staining on surgical biopsy samples from patients undergoing aortic valve replacement for aortic stenosis (AS, n = 18) and myomectomy for hypertrophic cardiomyopathy (HCM, n = 8) and showed excellent correlation (combined r2 = 0.80) [15]. Similar studies correlating ECVCMR with histology have been reproduced in patients with aortic and mitral regurgitation [16], dilated cardiomyopathy (DCM) or ischaemic heart disease (IHD) awaiting cardiac transplantation [17], heart failure [18] and myocarditis [19]. See Table 1 for more details.
Table 1.
Histological validation of ECV by modality
| Reference | Year | Population | Number | Finding |
|---|---|---|---|---|
| ECV by CMR | ||||
| Flett et al. [15] | 2010 | AS/HCM | 26 | Strong correlation with histological fibrosis in AS (r2 = 0.86) and HCM (r2 = 0.62). |
| White et al. [21] | 2013 | AS | 18 | Bolus only and infusion ECV measurements correlated with histological CVF (r2 = 0.69 and 0.71). |
| Miller et al. [17] | 2013 | DCM/IHD (transplant) | 6 | Significant linear relationship with histological CVF using either the 10- or 15-min post-contrast T1 (p < 0.001). |
| De Meester et al. [16] | 2015 | AS/AR/MR | 31 | Strong correlation with the magnitude of histological fibrosis (r = 0.78). |
| Kammerlander et al. [18] | 2016 | Mixed HF | 36 | Significant correlation with histological CVF (r = 0.493). |
| Lurz et al. [19] | 2016 | Myocarditis | 129 | ECV adequately estimated the degree of LV fibrosis percentage only in patients without inflammation (r = 0.72) and not in those with inflammation (r = 0.24). |
| ECV by CT | ||||
| Bandula et al. [34] | 2013 | AS | 23 | Strong correlation with histological measures of fibrosis (r = 0.71). |
| Yoon et al. [43] | 2015 | Hepatic fibrosis | 141 | Significant correlation with histological hepatic fibrosis staging (r = 0.493). |
AS aortic stenosis, AR aortic regurgitation, CVF collagen volume fraction, DCM dilated cardiomyopathy, HCM hypertrophic cardiomyopathy, HF heart failure, IHD ischemic heart disease, MR mitral regurgitation
A significant barrier to the adoption of the ECVCMR technique was the use of the primed infusion protocol. This involved the patient being removed from the scanner after conventional LGE imaging and being given another bolus of GBCA, followed by a 15-min pause and then an infusion. The patient would then be returned to the scanner any time between 45 and 80 min after the bolus for repeat T1 measurement [15].
A bolus-only approach was proposed by Schelbert et al., who demonstrated in 10 volunteers that myocardial ECVCMR could be reliably measured 15–20 min after a single bolus of GBCA [20]. Further work by White et al., this time in 147 subjects, demonstrated a strong correlation between 15-min bolus only and infusion ECVCMR measurement (r2 = 0.97) [21]. They did note that when the ECV was > 40%, the bolus only technique consistently measured a higher ECV than the infusion [21]. Finally, the validation of ECVCMR as part of a split-dose protocol (e.g. as part of stress perfusion) by McDiarmid et al. further increased the potential clinical utility of the technique [22]; however, there are suggestions that ECVCMR values may differ depending on the dose of GBCA used [23].
Most recently, a synthetic ECV can be automatically generated during scanning, in which the haematocrit of blood is inferred from the T1 of the blood pool (as the relationship between haematocrit and R1 [1/T1Blood] is linear), removing the need for a blood test [24]. It has also been replicated at 1.5 and 3T on other scanner platforms [25]. The key advantage of this technique is the simplification of the ECV workflow—by removing the need for blood tests to measure haematocrit, which is burdensome in busy departments, is a source of user error and introduces reporting delay. Implementation of inline synthetic ECV tools (with instantaneous ECV maps) would reduce the barriers to clinical use of ECV and potentially increase quality of care as review is immediately available.
ECV by CMR in Clinical Practice
Normal ECV values depend on the field strength, T1 mapping sequence and scanner manufacturer, but range between 20 and 26%, and appear to be slightly higher in women compared to men [26]. With the exception of cardiac amyloidosis and oedema [27], increases in myocardial ECV are generally due to an increased collagen volume fraction (CVF)—making it a marker of fibrosis [8, 10••]. For example, acute myocardial infarction (MI) results in some of the highest ECV values (58.5 ± 7.6%) and chronic MI is not far behind (51 ± 8%) [26, 28]. Diffuse fibrosis, however, rarely increases beyond 40%. Cardiac amyloidosis, which is characterized by the extracellular deposition of misfolded protein, produces large increases in ECV (greater than any other non-ischemic cardiomyopathy) in the region of 46.6 ± 7% [26, 27, 29].
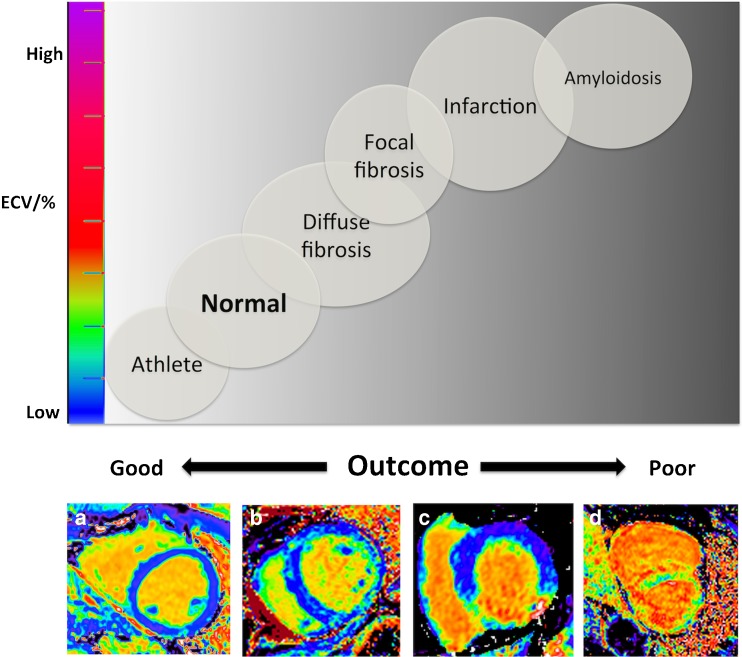
ECV is also mildly elevated in both hypertrophic cardiomyopathy (29.1 ± 0.5%) and dilated cardiomyopathy (28 ± 0.4%) [26]. On the other hand, Anderson Fabry disease appears to have a similar ECV to healthy volunteers (25.0 ± 2.3%), at least in the early stages of the disease [8, 26]. For an overview of ECV variability in health and disease, see Fig. 1 and Table 2.
Fig. 1.
Extracellular volume fraction (ECV) variability and outcome at 1.5T by myocardial pathologies. Top panel depicts ECV and associated outcome across health and disease with increasing ECV on the y-axis and outcome on the x-axis. Bottom panel shows four exemplar ECV maps of a healthy volunteer with normal ECV of 24% (a), a patient with aortic stenosis with mild ECV elevation at 30% (b), a patient with an inferior myocardial infarct (c), and a patient with AL cardiac amyloidosis with an ECV of 50% and the poorest outcome (d). (Adapted from Ugander 2014) [86]
Table 2.
Overview of ECV imaging in myocardial disease
| Process | Disease | Number of patients | ECV |
|---|---|---|---|
| Athletic hypertrophy | |||
| Physiological hypertrophy [12•] | 30 | ↓ | |
| Fibrosis | |||
| Focal | Myocardial infarction [26, 28] | 56 | ↑↑↑ |
| Diffuse | Aortic stenosis [26, 61, 62] | 136 | — /↑ |
| Systolic heart failure [63] | 40 | ↑ | |
| Diastolic heart failure [63] | 62 | ↑ | |
| Hypertrophic cardiomyopathy [64, 65] | 102 | ↑ | |
| Non-ischemic dilated cardiomyopathy [65, 66] | 116 | ↑ | |
| Mitochondrial cardiomyopathy [67] | 1 | ↑ | |
| Diabetes [14••] | 231 | ↑ | |
| Hypertensive heart disease [68, 69] | 89 | – | |
| Obesity [70] | 21 | ↑ | |
| Congenital heart disease [71] | 14 | ↑ | |
| Inflammation | |||
| Rheumatoid arthritis [72] | 39 | ↑↑ | |
| Systemic sclerosis [73, 74] | 49 | ↑↑ | |
| Systemic lupus erythematosus [75] | 33 | ↑↑ | |
| Oedema | |||
| Regional | Myocarditis [76, 77] | 135 | ↑ |
| Global | Anti-synthetase syndrome [78] | 1 | ↑↑ |
| Chronic systemic capillary leak syndrome [79] | 6 | ↑↑ | |
| Acute cardiac allograft rejection [80] | 22 | – | |
| Infiltration | |||
| Amyloid | AL amyloid [81] | 100 | ↑↑↑ |
| TTR amyloidosis [82] | 102 | ↑↑↑ | |
| Glycosphingolipid | Anderson-Fabry disease [83] | 31 | – |
| Toxins | |||
| Uraemia in chronic kidney disease [84] | 43 | ↑ | |
| Anthracycline-toxicity [85] | 30 | — /↑ | |
Reference list is non-exhaustive—several other references may exist that are not listed here
— No significant change; ↑ Significant increase; ↓ Significant decrease.
(Adapted from Captur et al. Heart 2016, Sep 15;102 (18):1429–35, with permission from BMJ Publishing Group Ltd.) [87].
Evolution of ECV by CT
ECV imaging by cardiac CT (ECVCT) lags behind the CMR field, but is potentially an attractive alternative. ECVCT relies upon the same principle as ECVCMR and is calculated using the following formula:
where ΔHU is the change in Hounsfield unit attenuation pre- and post-contrast (i.e. HUpost-contrast − HUpre-contrast) [30–32].
Early data was presented in abstract form by Ugander et al. in 2011, which showed, in dogs that underwent coronary occlusion and reperfusion (n = 10), that ECVCT and ECVCMR correlated well (R2 = 0.80, p < 0.001), with a small mean difference between ECVCMR and ECVCT (3 ± 9%) [33]. Myocardial ECVCT was first validated in humans by Nacif et al. in 2012 [30]. They compared ECVCMR and ECVCT in 24 subjects (both healthy volunteers and those with heart failure) and found good correlation between the two (r = 0.82, p < 0.001) [30]. Post-contrast images were taken after a 10-min delay, copying the exact parameters of the initial pre-contrast calcium score scan. Overall radiation dose was low (< 2 mSv) [30]. The average duration of ECVCT in this study was 13 ± 1.5 min, compared to 47 ± 5 min for ECVCMR [30]. ECVCMR results were slightly lower (28.6 ± 4.4%) compared to ECVCT (31.6 ± 5.1%) [30]. The same group went on to compare ECVCT in healthy volunteers and those with either systolic or diastolic heart failure [31]. They used a similar protocol, only this time, taking the post-contrast images after a shorter 7-min delay [31]. They found that the ECV was significantly higher in participants with systolic heart failure (41 ± 6%) compared to healthy subjects (33 ± 2%) and those with diastolic heart failure (35 ± 5%) [31].
Bandula et al. in the same year validated ECVCT against the gold standard—invasive endomyocardial biopsy, as well as ECVCMR in 23 patients with severe aortic stenosis [34]. This time, they used an initial bolus of contrast agent, followed by a slow infusion to achieve equilibrium. They found that ECVCT showed significant correlation with both histological measures of fibrosis (r = 0.71, p < 0.001) and ECVCMR (r = 0.73) [34]. We subsequently compared 5- and 15-minute time points post-contrast bolus for equilibrium cardiac CT in 53 patients (26 with systemic amyloidosis and 27 with aortic stenosis) [32]. We demonstrated that ECVCT at 5-minute post-contrast showed a stronger correlation with ECVCMR than at 15-minute post-contrast (r2 = 0.85 compared to 0.74) [32]. We think this is because, although earlier imaging risks non-equilibration, iodine is a weaker contrast agent than GBCAs, so the higher signal-to-noise ratio of earlier imaging outweighs this. ECVCT was consistently higher in those patients with confirmed cardiac amyloid compared to those with aortic stenosis (54 ± 11% compared to 28 ± 4%, p < 0.001) and was able to discriminate between patients with definite cardiac amyloid and those with aortic stenosis in all cases [32]. ECVCT also tracked various important clinical parameters including reduced 6-min walk test distance and increasing NT-proBNP, as well as amyloid burden in transthyretin-related amyloid—when measured semi-quantitatively by 99mTc-3,3-diphsphono-1,2-propanodicarboxylic acid (DPD) scintigraphy [32].
Work is also being done using dual-energy CT to quantify myocardial ECV, which is an attractive concept, as it would potentially avoid mis-registration errors associated with the separate pre- and post-contrast scans, by obviating the need for the pre-contrast scan [35, 36]. Hong et al. used dual-energy CT to estimate myocardial ECV in doxorubicin-induced dilated cardiomyopathy in rabbits [36]. They showed equivalent ECV results by dual-energy CT from 3 up to 20 minute post-contrast administration [36]. ECV values were significantly higher at 6, 12 and 16 weeks after starting twice weekly doxorubicin injections than at baseline (35.3, 41.9, 42.1% vs. 28.5%) [36]. ECV measured by dual-energy CT showed excellent correlation with ECVCMR (r = 0.888, p < 0.001) and with collagen volume fraction on histology (r = 0.925, p < 0.001) [36]. Work has also been done in humans using dual energy CT—involving 30 subjects (7 healthy, 23 with hypertrophic or dilated cardiomyopathy, amyloidosis or sarcoidosis) [37]. The post-contrast dual-energy CT scan was performed at 12 minute and results for ECV were compared with CMRECV and showed good agreement. Those participants with disease had significantly higher myocardial ECV by dual-energy CT compared to healthy subjects (p < 0.01) [37].
More recently, synthetic ECV has also been successfully implemented in CT, where the haematocrit of blood is inferred from the attenuation of the blood pool (as the relationship between haematocrit and HU is also linear), simplifying the ECV workflow and allowing instantaneous display of ECV maps [38].
CT versus CMR for Myocardial ECV Quantification
While the evidence base for ECVCMR may be larger and experience greater, the use of CT in this regard does have some distinct advantages. Likely to prove the biggest advantage is that ECVCT measures the direct effect of iodine-based contrast agents on the measured signal (through the effect of iodine on x-ray absorption), whereas ECVCMR relies on measuring the effect of GBCAs on protons (therefore making two assumptions—the first is that the relaxivity of tissues compared to blood is the same and the second is that water is rapidly exchanged between intra- and extracellular compartments). Also, common contraindications to CMR such as claustrophobia and pacemaker implantation (the latter being not uncommon in patients with for example cardiac amyloid—where ECV quantification could prove helpful) do not apply for CT.
ECVCMR is likely to be costly, both financially and in terms of patient throughput—with scans taking up to 60 min, whereas CT scans are significantly faster and more widely available. Indeed, CT availability is only likely to increase, particularly in the UK, given the expanded role for CT coronary angiography in the 2016 update of the National Institute for Health and Care Excllence clinical guideline (CG95) on the assessment of chest pain of recent onset [39]. CT also offers higher spatial resolution particularly inplane and allows isotropic reconstruction. Of course, these potential advantages should be weighed up against the risks of ionizing radiation from CT, especially in younger patients. Furthermore, CT is also prone to artefacts, e.g. beam hardening and streak artefacts that may hamper the evaluation of myocardial ECV. Finally, in delayed acquisitions, iodine contrast media provide less signal compared to GBCAs and differentiation between myocardium and left ventricular cavity is hampered, particularly when the myocardium is thinned (for example in dilated cardiomyopathy).
GBCAs have been associated with the development of nephrogenic systemic fibrosis, seen with linear chelates rather than macrocyclic and in patients with significantly impaired renal function (eGFR < 30 mL/min/1.73 m2) [40]. These linear agents have also been associated with brain deposition, a currently evolving story (but apparently not seen with macrocyclic chelates [41]. Iodinated contrast agents have in turn been associated with contrast-induced nephropathy and pre-existing chronic kidney disease (eGFR < 60 mL/min/1.73 m2) is the most important risk factor for this [42].
It is important to bear in mind while considering the pros and cons of both of these modalities that relative to the current gold standard for diagnosing diffuse fibrosis of invasive endomyocardial biopsy, both offer very attractive alternatives.
ECVCT in Clinical Practice—from Research Tool to Clinical Application
For clinical utilization, there needs to be standardized protocols in place for performing ECVCT. Furthermore, we need to employ this technique to better diagnose and understand disease processes and the effect of treatment on ECV (for example as a surrogate end point in drug trials). This has been implemented in the T1 mapping consensus statement, with a second version to be published in mid-2017 [10••]. For ECVCT to become a technique used in clinical practice, key steps need to be implemented—similar to developments in the CMR community, in particular standardization with phantom work, multicentre clinical data in health and disease, a consensus statement by national/international organizations (e.g. EACVI/SCCT) and adoption by all major manufacturers.
ECV Quantification beyond the Myocardium
The potential for ECVCT and ECVCMR extends beyond the myocardium. Yoon et al. used CT to estimate liver ECV in order to measure hepatic fibrosis and validated this against histological hepatic fibrosis staging in 141 participants (r = 0.493, p < 0.001) [43]. Bandula et al. also demonstrated elevated ECVCMR in the liver, spleen and skeletal muscle in patients with systemic amyloidosis, which tracked semi-quantitative amyloid burden in the liver and spleen by serum amyloid P component (SAP) scintigraphy [44]. Similar work has been performed by Yeung et al. using ECVCT [45]. They showed that in patients with hepatic and splenic amyloid, there was a significantly higher ECV compared to patients without liver and spleen involvement (p < 0.0005) [45]. They were also able to show that increases in ECVCT positively correlated with the grade of hepatic and splenic uptake on SAP scintigraphy (r = 0.758 for liver, r = 0.867 for spleen) [45].
Future Outlook for CT
Currently, a poorer signal-to-noise ratio than CMR and the use of ionizing radiation have impeded wider application of myocardial tissue characterization by CT. Nevertheless, the possibility of providing an assessment of coronary anatomy, coronary flow and myocardial tissue characterization in a single modality is an attractive concept, with huge implications for imaging workflow.
Beyond the optimization of dual-energy CT with minimization of image artefacts, radiation dose and iodinated contrast dose (e.g. using low-energy monochromatic imaging [46]), more advanced technologies are on the horizon. Spectral CT imaging exploits the different K-edge behaviour of different tissues (calcium, blood, fat and myocardium) [47]. This technology goes beyond the two-photon energy levels used in dual-energy CT, and utilizes energy-sensitive photon-counting detectors to obtain greater tissue information by differentiating photons at different energy levels. Early pre-clinical data suggests that spectral CT may improve image quality over conventional CT by eliminating beam hardening [48].
Future Outlook for CMR
The T1 mapping field has been rapidly advancing to the point of widespread clinical utility. Since the first T1 quantification with the original modified look-locker imaging (MOLLI) in 2004 [49], new MOLLI variants, ShMOLLI (a shortened variation with long T1 advantages) [50], saturation recovery variants such as SASHA (offering complete heart rate insensitivity) [51] or hybrid approaches [52–54] have been developed, and incremental developments such as respiratory motion correction [55] have gradually increased accuracy and precision [52, 56•]. ECV maps are now routine in some centres [57], but ECV development and standardization are still on-going and will require global approaches. Quality control systems, commercial sequences, mega-registries (e.g. the Global CMR Registry, HCM Registry and UK Biobank) are in progress, and will provide high volumes and new insights into the currently most active CMR research area [58, 59]. On the horizon, MR fingerprinting may offer more rapid multi-parametric tissue characterization in the future by providing myocardial T1, T2, and Proton Spin Density in a single breath-hold [60].
Conclusion
Myocardial ECV is important, with increases related to myocardial fibrosis, cardiac amyloid or oedema, which in turn are associated with an increased mortality. Quantification of ECV enables the detection of diffuse myocardial fibrosis, which would otherwise potentially be missed using conventional LGE imaging, making it a useful addition to the armamentarium of myocardial characterization techniques.
CT and CMR can be used to estimate myocardial ECV and in turn diffuse myocardial fibrosis, without the need for invasive endomyocardial biopsy. Each modality has strengths and weaknesses, with CT an attractive alternative to CMR particularly in those with contraindications to the latter. Further studies are needed in this field, especially ECVCT, where the evidence base is less robust.
Abbreviations
- AS
Aortic stenosis
- AR
Aortic regurgitation
- CMR
Cardiovascular magnetic resonance
- CT
Computed tomography
- CVF
Collagen volume fraction
- DCM
Dilated cardiomyopathy
- ECM
Extracellular matrix
- ECV
Extracellular volume
- ECVCMR
Extracellular volume quantified by cardiovascular magnetic resonance
- ECVCT
Extracellular volume quantified by computed tomography
- HCM
Hypertrophic cardiomyopathy
- HF
Heart Failure
- GBCAs
Gadolinium based contrast agents
- IHD
Ischaemic heart disease
- LGE
Late gadolinium enhancement
- MI
Myocardial infarction
- MR
Mitral regurgitation
- SAP
Serum amyloid P component
Funding
Paul R. Scully is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/16/31/32185). James C. Moon is directly and indirectly supported by the University College London Hospitals NIHR Biomedical Research Centre and Biomedical Research Unit at Barts Hospital, respectively. Thomas Treibel was supported by doctoral research fellowship from the National Institute of Health Research (NIHR; DRF-2013-06-102).
Compliance with Ethical Standards
Conflict of Interest
Paul R. Scully, James C. Moon, and Thomas A. Treibel declare that they have no conflict of interest.
Gorka Bastarrika reports other from Siemens Healthcare, General Electric, and Bayer.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cardiac PET, CT, and MRI
Contributor Information
Paul R. Scully, Email: paul.scully@bartshealth.nhs.uk
Gorka Bastarrika, Email: bastarrika@unav.es.
James C. Moon, Email: james.moon@bartshealth.nhs.uk
Thomas A. Treibel, Phone: +44 20 3465 6115, Email: thomas.treibel@bartshealth.nhs.uk
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KR, Sutton MGSJ, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979;128:79–85. doi: 10.1002/path.1711280205. [DOI] [PubMed] [Google Scholar]
- 3.Kim RJ, Albert TSE, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM, for the Gadoversetamide Myocardial Infarction Imaging Investigators Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117:629–637. doi: 10.1161/CIRCULATIONAHA.107.723262. [DOI] [PubMed] [Google Scholar]
- 4.Lusic H, Grinstaff MW. X-ray-computed tomography contrast agents. Chem Rev. 2013;113:1641–1666. doi: 10.1021/cr200358s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliva MR, Mortele KJ. Iodinated contrast agents for cardiac CT. In: Di Carli MF, Lipton MJ, editors Card PET PETCT Imaging, New York, NY: Springer New York; 2007, p. 83–93. doi:10.1007/978-0-387-38295-1_6.
- 6.Nicol E, Stirrup J, Kelion AD, Padley SPG. Cardiovascular computed tomography. Oxford: OUP Oxford; 2011. [Google Scholar]
- 7.Deray G. Dialysis and iodinated contrast media. Kidney Int. 2006;69:S25–S29. doi: 10.1038/sj.ki.5000371. [DOI] [PubMed] [Google Scholar]
- 8.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2017;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson. 2012;14:54. doi: 10.1186/1532-429X-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 12.McDiarmid AK, Swoboda PP, Erhayiem B, Lancaster RE, Lyall GK, Broadbent DA, Dobson LE, Musa TA, Ripley DP, Garg P, Greenwood JP, Ferguson C, Plein S. Athletic cardiac adaptation in males is a consequence of elevated myocyte mass. Circ Cardiovasc Imaging. 2016;9:e003579. doi: 10.1161/CIRCIMAGING.115.003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–664. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 16.de Meester Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from modified look-locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. doi: 10.1186/s12968-015-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJM, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 18.Kammerlander AA, Marzluf BA, Zotter-Tufaro C, Aschauer S, Duca F, Bachmann A, Knechtelsdorfer K, Wiesinger M, Pfaffenberger S, Greiser A, Lang IM, Bonderman D, Mascherbauer J. T1 Mapping by CMR Imaging. JACC Cardiovasc Imaging. 2016;9:14–23. doi: 10.1016/j.jcmg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Lurz P, Luecke C, Eitel I, Fohrenbach F, Frank C, Grothoff M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis. J Am Coll Cardiol. 2016;67:1800–1811. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR. JACC Cardiovasc Imaging. 2013;6:955–962. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 22.McDiarmid AK, Swoboda PP, Erhayiem B, Ripley DP, Kidambi A, Broadbent DA, et al. Single bolus versus split dose gadolinium administration in extra-cellular volume calculation at 3 Tesla. J Cardiovasc Magn Reson. 2015;17:6. doi: 10.1186/s12968-015-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballeros M, Bartolomé P, Fernández González Ó, Greiser A, García del Barrio L, Pueyo J, Bastarrika G. Effect of contrast dose in the quantification of myocardial extra-cellular volume in adenosine stress/rest perfusion cardiac magnetic resonance examinations. Acta Radiol. 2017;58:809–815. doi: 10.1177/0284185116674501. [DOI] [PubMed] [Google Scholar]
- 24.Treibel TA, Fontana M, Maestrini V, Castelletti S, Rosmini S, Simpson J, Nasis A, Bhuva AN, Bulluck H, Abdel-Gadir A, White SK, Manisty C, Spottiswoode BS, Wong TC, Piechnik SK, Kellman P, Robson MD, Schelbert EB, Moon JC. Automatic measurement of the myocardial Interstitium. JACC Cardiovasc Imaging. 2016;9:54–63. doi: 10.1016/j.jcmg.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Fent GJ, Garg P, Foley JRJ, Swoboda PP, Dobson LE, Erhayiem B, Treibel TA, Moon JC, Greenwood JP, Plein S. Synthetic myocardial extracellular volume fraction. JACC Cardiovasc Imaging. 2017;10:1402–1404. doi: 10.1016/j.jcmg.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 27.Banypersad SM, Sado DM, Flett AS, Gibbs SDJ, Pinney JH, Maestrini V, Cox AT, Fontana M, Whelan CJ, Wechalekar AD, Hawkins PN, Moon JC. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6:34–39. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 28.Ugander M, Oki AJ, Hsu L-Y, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1:e000364. doi: 10.1161/JAHA.111.000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A, Sibley CT, Lima JAC, Liu S, Bluemke DA. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264:876–883. doi: 10.1148/radiol.12112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nacif MS, Liu Y, Yao J, Liu S, Sibley CT, Summers RM, Bluemke DA. 3D left ventricular extracellular volume fraction by low-radiation dose cardiac CT: assessment of interstitial myocardial fibrosis. J Cardiovasc Comput Tomogr. 2013;7:51–57. doi: 10.1016/j.jcct.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treibel TA, Bandula S, Fontana M, White SK, Gilbertson JA, Herrey AS, Gillmore JD, Punwani S, Hawkins PN, Taylor SA, Moon JC. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015;9:585–592. doi: 10.1016/j.jcct.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ugander M, Chen MY, Chen B, Bagi PS, Hsu L-Y, Kellman P, et al. Abstract 16534: contrast enhanced CT and MRI measures of extracellular volume fraction confirm presence of peri-infarct edema in acute myocardial infarction. Circulation. 2011;124:A16534. [Google Scholar]
- 34.Bandula S, White SK, Flett AS, Lawrence D, Pugliese F, Ashworth MT, Punwani S, Taylor SA, Moon JC. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. 2013;269:396–403. doi: 10.1148/radiol.13130130. [DOI] [PubMed] [Google Scholar]
- 35.Graser A, Johnson TRC, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. 2009;19:13–23. doi: 10.1007/s00330-008-1122-7. [DOI] [PubMed] [Google Scholar]
- 36.Hong YJ, Kim TK, Hong D, Park CH, Yoo SJ, Wickum ME, Hur J, Lee HJ, Kim YJ, Suh YJ, Greiser A, Paek MY, Choi BW. Myocardial characterization using dual-energy CT in doxorubicin-induced DCM. JACC Cardiovasc Imaging. 2016;9:836–845. doi: 10.1016/j.jcmg.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Lee H-J, Im DJ, Youn J-C, Chang S, Suh YJ, Hong YJ, Kim YJ, Hur J, Choi BW. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. 2016;280:49–57. doi: 10.1148/radiol.2016151289. [DOI] [PubMed] [Google Scholar]
- 38.Treibel TA, Fontana M, Steeden JA, Nasis A, Yeung J, White SK, Sivarajan S, Punwani S, Pugliese F, Taylor SA, Moon JC, Bandula S. Automatic quantification of the myocardial extracellular volume by cardiac computed tomography: synthetic ECV by CCT. J Cardiovasc Comput Tomogr. 2017;11:221–226. doi: 10.1016/j.jcct.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence (NICE). Chest pain of recent onset: assessment and diagnosis: clinical guideline [CG95] 2016. [PubMed]
- 40.Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment—report of 33 cases. Radiology. 2009;250:371–377. doi: 10.1148/radiol.2502080498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015;275:803–809. doi: 10.1148/radiol.14140364. [DOI] [PubMed] [Google Scholar]
- 42.Lameire N, Adam A, Becker CR, Davidson C, McCullough PA, Stacul F, et al. Baseline renal function screening. Am J Cardiol. 2006;98(6):21–26. doi: 10.1016/j.amjcard.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JH, Lee JM, Klotz E, Jeon JH, Lee K-B, Han JK, Choi BI. Estimation of hepatic extracellular volume fraction using multiphasic liver computed tomography for hepatic fibrosis grading. Investig Radiol. 2015;50:290–296. doi: 10.1097/RLI.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 44.Bandula S, Banypersad SM, Sado D, Flett AS, Punwani S, Taylor SA, Hawkins PN, Moon JC. Measurement of tissue interstitial volume in healthy patients and those with amyloidosis with equilibrium contrast-enhanced MR imaging. Radiology. 2013;268:858–864. doi: 10.1148/radiol.13121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung J, Sivarajan S, Treibel TA, Rosmini S, Fontana M, Gillmore JD, Hawkins PN, Punwani S, Moon JC, Taylor SA, Bandula S. Measurement of liver and spleen interstitial volume in patients with systemic amyloid light-chain amyloidosis using equilibrium contrast CT. Abdom Radiol. 2017;42:2646–2651. doi: 10.1007/s00261-017-1194-4. [DOI] [PubMed] [Google Scholar]
- 46.Scheske JA, O’Brien JM, Earls JP, Min JK, LaBounty TM, Cury RC, et al. Coronary artery imaging with single-source rapid kilovolt peak–switching dual-energy CT. Radiology. 2013;268:702–709. doi: 10.1148/radiol.13121901. [DOI] [PubMed] [Google Scholar]
- 47.Boussel L, Coulon P, Thran A, Roessl E, Martens G, Sigovan M, Douek P. Photon counting spectral CT component analysis of coronary artery atherosclerotic plaque samples. Br J Radiol. 2014;87:20130798. doi: 10.1259/bjr.20130798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahmi R, Eck BL, Levi J, Fares A, Dhanantwari A, Vembar M, Bezerra HG, Wilson DL. Quantitative myocardial perfusion imaging in a porcine ischemia model using a prototype spectral detector CT system. Phys Med Biol. 2016;61:2407–2431. doi: 10.1088/0031-9155/61/6/2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 50.Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened modified look-locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Saturation TRB. Recovery single-shot acquisition (SASHA) for myocardial T1 mapping: SASHA for T1 mapping. Magn Reson Med. 2014;71:2082–2095. doi: 10.1002/mrm.24878. [DOI] [PubMed] [Google Scholar]
- 52.Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272:683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta BB, Chen X, Bilchick KC, Salerno M, Epstein FH. Accelerated and navigator-gated look-locker imaging for cardiac t1 estimation (ANGIE): development and application to T1 mapping of the right ventricle: ANGIE: accelerated and navigator-gated look-locker imaging for cardiac T1 estimation. Magn Reson Med. 2015;73:150–160. doi: 10.1002/mrm.25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kvernby S, Warntjes MJB, Haraldsson H, Carlhäll C-J, Engvall J, Ebbers T. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson. 2014;16:102. doi: 10.1186/s12968-014-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue H, Greiser A, Zuehlsdorff S, Jolly M-P, Guehring J, Arai AE, Kellman P. Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med. 2013;69:1408–1420. doi: 10.1002/mrm.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramer CM, Appelbaum E, Desai MY, Desvigne-Nickens P, DiMarco JP, Friedrich MG, et al. Hypertrophic cardiomyopathy registry: the rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J. 2015;170:223–230. doi: 10.1016/j.ahj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petersen SE, Matthews PM, Bamberg F, Bluemke DA, Francis JM, Friedrich MG, Leeson P, Nagel E, Plein S, Rademakers FE, Young AA, Garratt S, Peakman T, Sellors J, Collins R, Neubauer S. Imaging in population science: cardiovascular magnetic resonance in 100,000 participants of UK biobank—rationale, challenges and approaches. J Cardiovasc Magn Reson. 2013;15:46. doi: 10.1186/1532-429X-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton JI, Jiang Y, Chen Y, Ma D, Lo W-C, Griswold M, Seiberlich N. MR fingerprinting for rapid quantification of myocardial T1, T2, and proton spin density: cardiac MR fingerprinting for T1, T2, and M 0 mapping. Magn Reson Med. 2017;77:1446–1458. doi: 10.1002/mrm.26216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chin CWL, Semple S, Malley T, White AC, Mirsadraee S, Weale PJ, Prasad S, Newby DE, Dweck MR. Optimization and comparison of myocardial T1 techniques at 3T in patients with aortic stenosis. Eur Heart J-Cardiovasc Imaging. 2014;15:556–565. doi: 10.1093/ehjci/jet245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A, Horsfield MA, Bekele S, Khan JN, Greiser A, McCann GP. Myocardial T1 and extracellular volume fraction measurement in asymptomatic patients with aortic stenosis: reproducibility and comparison with age-matched controls. Eur Heart J-Cardiovasc Imaging. 2015;16:763–770. doi: 10.1093/ehjci/jev007. [DOI] [PubMed] [Google Scholar]
- 63.Su M-YM, Lin L-Y, Tseng Y-HE, Chang C-C, Wu C-K, Lin J-L, Tseng WYI. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, Gonzalez A, Lopez B, Diez J, Jerosch-Herold M, Kwong RY. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–422. doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Barison A, Del Torto A, Chiappino S, Aquaro GD, Todiere G, Vergaro G, et al. Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J Cardiovasc Med. 2015;16:681–687. doi: 10.2459/JCM.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 67.Lee KH, Park HS, Park CH, Kim K-H, Chung H, Kim TH, Rim SJ, Choi EY. Extracellular volume imaging and quantitative T2 mapping for the diagnosis of mitochondrial cardiomyopathy. Circulation. 2014;130:1832–1834. doi: 10.1161/CIRCULATIONAHA.114.010779. [DOI] [PubMed] [Google Scholar]
- 68.Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, Epstein FH, Kramer CM, Salerno M. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging. 2015;8:172–180. doi: 10.1016/j.jcmg.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treibel TA, Zemrak F, Sado DM, Banypersad SM, White SK, Maestrini V, Barison A, Patel V, Herrey AS, Davies C, Caulfield MJ, Petersen SE, Moon JC. Extracellular volume quantification in isolated hypertension—changes at the detectable limits? J Cardiovasc Magn Reson. 2015;17:74. doi: 10.1186/s12968-015-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah RV, Abbasi SA, Neilan TG, Hulten E, Coelho-Filho O, Hoppin A, Levitsky L, de Ferranti S, Rhodes ET, Traum A, Goodman E, Feng H, Heydari B, Harris WS, Hoefner DM, McConnell JP, Seethamraju R, Rickers C, Kwong RY, Jerosch-Herold M. Myocardial tissue remodeling in adolescent obesity. J Am Heart Assoc. 2013;2:e000279. doi: 10.1161/JAHA.113.000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, Moon JC. Diffuse myocardial fibrosis in the systemic right ventricle of patients late after mustard or Senning surgery: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J-Cardiovasc Imaging. 2013;14:963–968. doi: 10.1093/ehjci/jet014. [DOI] [PubMed] [Google Scholar]
- 72.Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, Wordsworth PB, Neubauer S, Karamitsos TD. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis. JACC Cardiovasc Imaging. 2015;8:526–536. doi: 10.1016/j.jcmg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 73.Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16:21. doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barison A, Gargani L, De Marchi D, Aquaro GD, Guiducci S, Picano E, et al. Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur Heart J-Cardiovasc Imaging. 2015;16:74–80. doi: 10.1093/ehjci/jeu167. [DOI] [PubMed] [Google Scholar]
- 75.Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, et al. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging. 2013;6:295–301. doi: 10.1161/CIRCIMAGING.112.000151. [DOI] [PubMed] [Google Scholar]
- 76.Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. doi: 10.1161/CIRCIMAGING.114.003073. [DOI] [PubMed] [Google Scholar]
- 77.Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis. JACC Cardiovasc Imaging. 2014;7:667–675. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Sado DM, Kozor R, Corr L, Moon JC. Global myocardial edema in antisynthetase syndrome detected by cardiovascular magnetic resonance mapping techniques. Circulation. 2016;133:e25–e26. doi: 10.1161/CIRCULATIONAHA.115.017430. [DOI] [PubMed] [Google Scholar]
- 79.Ertel A, Pratt D, Kellman P, Leung S, Bandettini P, Long LM, Young M, Nelson C, Arai AE, Druey KM. Increased myocardial extracellular volume in active idiopathic systemic capillary leak syndrome. J Cardiovasc Magn Reson. 2015;17:76. doi: 10.1186/s12968-015-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller CA, Naish JH, Shaw SM, Yonan N, Williams SG, Clark D, Bishop PW, Ainslie MP, Borg A, Coutts G, Parker GJM, Ray SG, Schmitt M. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson. 2014;16:52. doi: 10.1186/s12968-014-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD, Lachmann HJ, Wechalekar AD, Hawkins PN, Moon JC. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244–251. doi: 10.1093/eurheartj/ehu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado D, White SK, Bulluck H, Herrey AS, Hawkins PN, Moon J. AL and ATTR cardiac amyloid are different: native T1 mapping and ECV detect different biology. J Cardiovasc Magn Reson. 2014;16:P341. doi: 10.1186/1532-429X-16-S1-P341. [DOI] [Google Scholar]
- 83.Thompson RB, Chow K, Khan A, Chan A, Shanks M, Paterson I, Oudit GY. T1 mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ Cardiovasc Imaging. 2013;6:637–645. doi: 10.1161/CIRCIMAGING.113.000482. [DOI] [PubMed] [Google Scholar]
- 84.Edwards NC, Moody WE, Yuan M, Hayer MK, Ferro CJ, Townend JN, Steeds RP. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol. 2015;115:1311–1317. doi: 10.1016/j.amjcard.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ugander, M; 17th Annual SCMR Scientific Sessions [conference presentation]. New Orleans, LA, USA; January 16–19, 2014. [DOI] [PMC free article] [PubMed]
- 87.Captur G, Manisty C, Moon JC, Cardiac MRI. Evaluation of myocardial disease. Heart. 2016;102(18):1429–1435. doi: 10.1136/heartjnl-2015-309077. [DOI] [PubMed] [Google Scholar]