Abstract
The use of cardiovascular progenitor cells (CPCs) to repair damaged myocardium has been the focus of intense research. Previous reports have shown that pretreatments, including hypoxia, improve cell function. However, the age-dependent effects of short-term hypoxia on CPCs, and the role of signaling in these effects, are unknown. Cloned neonatal and adult CPCs expressing Isl1, c-Kit, KDR, PDGFRA, and CXCR4, were preconditioned using hypoxia (1% O2 for six hours). Intracellular signaling pathway changes were modeled using Ingenuity Pathway Analysis (IPA), while qRT-PCR, flow cytometry, and immunoblotting were used to measure pathway activation. Cellular function, including survival, cell cycle, and invasion, were evaluated using a TUNEL assay, flow cytometry, and a Transwell® invasion assay, respectively. IPA predicted, and RT-PCR and flow cytometry confirmed, that the PI3K/AKT pathway was activated following short-term hypoxia. Heat shock protein (HSP) 40 expression increased significantly in both age groups, while HSP70 expression increased only in neonatal CPCs. Neonatal CPC invasion and survival improved after hypoxia pre-treatment, while no effect was observed in cell cycling and developmental status. Prostaglandin receptor expression was enhanced in neonatal cells. Prior to transplantation, hypoxic preconditioning enhances CPC function, including invasion ability and pro-survival pathway activation.
Keywords: Cardiovascular progenitor cells, hypoxia, Akt, Isl1, c-Kit
Introduction
Cardiovascular disease is a global crisis accounting for approximately one out of every three deaths worldwide [1]. Patients hospitalized for heart failure typically experience a significant loss of cardiac function. Traditional treatments fail to address this issue and, as a result, the long-term outlook of these patients is abysmal [2]. The potential benefits of endogenous cardiac stem cell transplantation as a means of stimulating repair in heart tissue are under investigation with encouraging results in clinical trials [3-5]. In these trials, several cell types, such as cardiac c-Kit cells, cardiopoietic stem cells, induced pluripotent stem cell-derived cardiomyocytes, and cardiosphere-derived cells, have been applied to treat ischemic and chronic heart failure [6]. Despite improvements in cardiac function following transplantation, cells fail to engraft and differentiate into new myocardium [7]. This may be circumvented by the use of progenitor types that are not restricted to endothelial or cardiomyocyte lineages and instead exhibit multipotency. Furthermore, autologous stem cells derived from adult patients are known to lack the functional potency of their neonatal counterparts [8].
Due to the correlation between incidence of cardiovascular disease and age, the application of autologous stem cell-based therapies for treatment of cardiovascular injury may be limited in older adults. The effectiveness of cell-based treatments for the heart may be significantly improved by use of a novel cell type or pretreatment method, such as hypoxic exposure, that alters the function of adult-derived cardiac stem cells to mirror that of the more capable neonatal stem cells. Several populations of resident cardiac stem cells have been identified within the human heart and are promising candidates for use in future studies [9].
A novel cardiac progenitor cell population that co-expresses Isl1 and c-Kit and is capable of self-renewal, expansion, and differentiation into all three major cell types of the heart [10,11], has been recently isolated from human cardiac tissue and characterized as reported in our previous study [8]. In doing so, we found that CPCs isolated from human tissue can be screened for the co-expression of Isl1 and c-Kit, and further selected for the expression of other early cardiovascular developmental markers, such as KDR and PDGFRA, and the chemokine receptor CXCR4. In order to identify the optimal cell type for clinical practice, further investigation of various cardiac progenitors, which have a critical role in heart formation [12-14], is required. Using other models, previous studies have established that hypoxia treatment can boost cellular function through intracellular signaling pathways [15-19]. However, the effects of low oxygen tension on early cardiac progenitor cell (CPC) function have yet to be elucidated. Accordingly, we assessed the impact of short-term hypoxia (six hours at 1.0% O2) on the in vitro biology of clonal CPCs. Historically, in other cell types, Protein kinase B (Akt) expression increases in response to short-term hypoxia [20] and is well known to play vital roles in numerous cell functions [21]. We therefore tested the hypothesis that short-term hypoxia upregulates Akt phosphorylation in early CPCs and is correlated with enhanced cell function in vitro.
Materials and methods
Isolation and culture of early CPCs
The Institutional Review Board of Loma Linda University approved the protocol for use of tissue that was discarded during cardiovascular surgery, without identifiable private information, for this study with a waiver of informed consent. CPCs were isolated from cardiac tissue of neonates (1 day-1 month) or adults (57-72 years), as previously described [8]. Briefly, atrial tissue was cut into small clumps (approximately 1.0 mm3) then enzymatically digested using collagenase (Roche, Indianapolis, IN) at a working concentration of 1.0 mg/mL. The resulting solution was then passed through a 40-µm cell strainer. Cells were cloned in a 96-well plate by limiting dilution to a final concentration of 0.8 cells per well to create populations for expansion. Cells were then screened for the co-expression of Isl1 and c-Kit. Clonal CPC cultures were screened for other markers of early cardiac development (KDR and PDGFRA) and then supplemented with growth media comprised of 10% fetal bovine serum (Thermo Scientific, Waltham, MA), 100 µg/mL Penicillin-Streptomycin (Life Technologies, Carlsbad, CA), 1.0% minimum essential medium non-essential amino acids solution (Cat no. 11120052, Life Technologies, Carlsbad, CA), and 22% endothelial cell growth media (Lonza, Basel, Switzerland) in Medium 199 (Life Technologies, Carlsbad, CA).
Hypoxic preconditioning
CPCs were placed in a Heracell™ 150 tri-gas incubator (Thermo Scientific, Waltham, MA) set to 1.0% O2, 5.0% CO2, and 94% N2 for six hours. Control CPC conditions included an environment composed of 21% O2, 5.0% CO2, and 74% N2. Cells were then immediately processed for analysis to avoid prolonged exposure to normoxic conditions.
Quantitative real-time PCR
RNA was isolated from CPCs using TRIzol® reagent (Life Technologies, Carlsbad, CA) and purified using the RNeasy® Mini Kit (Qiagen, Valencia, CA). cDNA was prepared with Superscript III (Life Technologies, Carlsbad, CA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in triplicate using Go-Taq® qPCR Mastermix (Promega, Madison, WI) and the iCycler iQ™5 PCR Thermal Cycler (Bio-Rad, Hercules, CA) following a protocol of 94°C for 10 minutes, 94°C for 15 seconds, 52-58°C (depending on the primer) for 60 seconds, and 72°C for 30 seconds for a total of 45 cycles. Primers used in experiment are listed in Table S1. Relative gene expression data were analyzed using the comparative CT method [22].
Ingenuity Pathway Analysis (IPA)
Gene expression changes in hypoxia pre-treated neonatal CPCs were broadly assessed using the RT2 profiler PCR array for the human EGF/PDGF signaling pathway (Qiagen, Valencia, CA). Conserved fold changes in the expression of genes included on this array plate across three clones were then processed using a core analysis in IPA (Qiagen, Valence, CA). All potential pathways whose-log (p-value) score was greater than 2.5 (i.e., p < 0.005) were included, except for those pathway categories associated with cancer, cellular immune response, disease-specific pathways, ingenuity toxicity list pathways, neurotransmitters and other nervous system signaling, pathogen-influenced signaling, and xenobiotic metabolism.
Flow cytometry
Progenitor cell populations were fluorescently labeled with antibodies as recommended by their respective manufacturers and then analyzed using a MACSQuant® analyzer (Miltenyi Biotec, Auburn, CA). Quantification of data was performed using FlowJo software version 10 (Ashland, OR). Antibodies used for cytometric analysis include: mouse IgG anti-Isl1 (1H9; Abcam, Cambridge, MA); rat IgG2b Kappa anti-c-Kit (2B8) conjugated to Dylight 650 (Novus Biologicals, Littleton CO); rabbit IgG anti-Akt (C67E7; Cell Signaling Technology, Danvers, MA); rabbit anti-human S473 P-Akt (Cell Signaling Technology, Danvers, MA); rabbit IgG anti-human HSP40 (Thermo Fisher, Waltham, MA); mouse IgG1 anti-human HSP90 (5G5; Thermo Fisher, Waltham, MA); and fluorescein-anti-BrdU (PRB-1; Phoenix Flow Systems, San Diego, CA). Secondary antibodies included FITC goat anti-mouse IgG polyclonal Ab (Southern Biotech, Birmingham, AL), PE goat anti-mouse IgG polyclonal Ab (Southern Biotech, Birmingham, AL), and FITC goat anti-rabbit IgG polyclonal Ab (BD Biosciences, San Jose, CA). Negative and positive populations were defined using Alexa Fluor 647-conjugated isotype control (R&D Systems, Minneapolis, MN) for c-Kit analysis, Alexa Fluor 488-conjugated isotype control (MOPC-173; BioLegend, San Diego, CA) for HSP70 analysis, or secondary antibody alone for all other proteins.
Protein simple western blotting
Cells were detached following the protocol outlined in Abrahamsen, et al [23]. Following detachment, neonatal and adult CPCs were homogenized using RIPA buffer containing phosphatase inhibitor cocktail (Millipore, Temecula, CA), followed by centrifugation at 14,000 g for 15 minutes at 4°C and collection of the supernatant for analysis. Total protein concentrations were determined using the Pierce Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Mouse IgG2b anti-human β-Actin (1:50; 8H10D10; Cell Signaling Technology, Danvers, MA), rabbit IgG anti-Akt (1:50; C67E7; Cell Signaling Technology, Danvers, MA), rabbit anti-human S473 P-Akt (1:10; Cell Signaling Technology, Danvers, MA), rabbit IgG anti-human HSP40 (1:50; Thermo Fisher, Waltham, MA), and mouse IgG1 anti-human HSP90 (1:50; 5G5; Thermo Fisher, Waltham, MA) were used with a capillary-based western blotting system (ProteinSimple Wes, San Jose, CA) to assess protein expression. All procedures were completed according to the manufacturer’s instructions and default settings. The anti-rabbit and anti-mouse secondary antibodies included in the Wes Detection Module kit (ProteinSimple, San Jose, CA) were used. All data were analyzed with the Compass Software associated with the Wes instrument (ProteinSimple, San Jose, CA). Using peak heights of fluorescence signal, P-Akt, HSP40, and HSP90 values were normalized to β-Actin for each neonatal and adult clone sample.
Stromal cell-derived factor-1α (SDF-1α) P-Akt western blotting
A protein immunoblot was performed for β-Actin and P-Akt using samples obtained from untreated normoxic controls, normoxic CPCs treated with stromal cell-derived factor-1α (SDF-1α), and CPCs treated both with SDF-1α and hypoxia. Following 18 hours of serum deprivation, CPCs were stimulated using 10 µg/mL of SDF-1α (Biolegend, San Diego, CA). Hypoxic groups were exposed to low oxygen conditions during the final six hours of the 18-hour starvation period. Protein lysates were loaded into a 12% Tris-glycine pre-cast gel (Thermo Scientific, Waltham, MA), separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA). After blocking with 5% bovine serum albumin in Tris-buffered saline with Tween-20, membranes were labeled with mouse anti-human β-Actin antibody (1:500 dilution, Biolegend, San Diego, CA) or rabbit anti-human P-Akt antibody (1:300 dilution, Cell Signaling Technology, Danvers, MA) overnight at 4°C with agitation. The following day, membranes were washed and labeled using either an IRDye® 680RD-conjugated goat anti-mouse antibody (LI-COR, Lincoln, NE) or an IRDye® 800CW-conjugated goat anti-rabbit antibody (LI-COR, Lincoln, NE) for 60 minutes at room temperature. Final protein levels were visualized using an Odyssey® infrared imaging system (LI-COR, Lincoln, NE) model 9120. Resulting protein bands were analyzed using ImageJ software.
Terminal deoxynucleotidyl transferase dUTP nick end labeling
CPCs were treated with a solution of 0.5 mM hydrogen peroxide for 21 hours [24]. Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) assay was performed following the manufacturer’s recommendations. Briefly, 106 cells were fixed in 1% paraformaldehyde on ice for 30 minutes and placed in 70% ethanol for overnight incubation at -20°C. The next day, the cells were labeled with Br-dUTP (Phoenix Flow Systems, San Diego, CA) and re-suspended in antibody solution containing Fluorescein anti-BrdU antibody (Phoenix Flow Systems, San Diego, CA). Population analysis was performed using flow cytometry.
Transwell® invasion assay
Cultrex® basement membrane extract (Trevigen, Gaithersburg, MD) was applied to the upper chamber of a Corning HTS Transwell® plate (8.0-µm pore size, Venlo, Limburg). CPCs were suspended in starvation media composed of 98.5% Iscove’s Modified Dulbecco’s Medium with GlutaMAX™ (Life Technologies, Carlsbad, CA), 1.0% insulin-transferrin-selenium (Life Technologies, Carlsbad, CA), and 0.5% fetal bovine serum (Thermo Scientific, Waltham, MA), and then plated onto the coated wells at a density of 50,000 cells per well. Stromal cell-derived factor-1α (SDF-1α, Life Technologies, Carlsbad, CA), a chemoattractant, was diluted with growth media to a final concentration of 100 ng/mL and administered to the lower chamber. After 48 hours of incubation at 37°C, the cells in the lower chamber were dissociated, stained with calcein AM (BD Biosciences, San Jose, CA), and analyzed using an FLx800™ microplate fluorescence reader (BioTek Instruments, Winooski, VT).
Cell cycle analysis
CPCs were fixed in ice-cold 70% ethanol and stored at -20°C overnight. The following day, cells were incubated at 37°C for 1 hour with RNase A (0.5 mg/mL, Life Technologies, Carlsbad, CA). Propidium Iodide solution (0.5 mg/mL) was added, and the resulting cell solution was immediately analyzed using a MACSQuant® analyzer (Miltenyi Biotec, Auburn, CA). Cytometer data was quantified using FlowJo software (Ashland, OR).
Statistical analysis
All data were assumed to be normally distributed. Using Microsoft Excel or Prism 7 version 7.02 (GraphPad, La Jolla, CA), we performed a two-tailed, paired Student’s t-test to calculate the mean and standard error of the mean. Error bars were designed using either Prism 7 software or with the Cousineau method [25]. P values < 0.05 were assumed to be significant.
Results
Gene expression changes are predicted to impact PI3K/AKT pathway
Neonatal CPCs expressing markers of early cardiovascular development (Figure 1) were exposed to hypoxia for six hours. Gene expression changes were measured using an EGF/PDGF array plate. Fold changes that were conserved across clones were then analyzed using Ingenuity Pathway Analysis (Figure 2A), which predicted that the PI3K/AKT pathway was significantly altered by hypoxia pretreatment. Akt functions along several pathways (Figure 2B), such as the AKT/PI3K pathway that enhances the stability of Cyclin D1 (CCND1), c-Jun, and c-Myc, which promote the G1-to-S cell cycle phase transition, while also targeting the NFκB sub-unit RelA during anti-apoptotic signaling [21].
Figure 1.
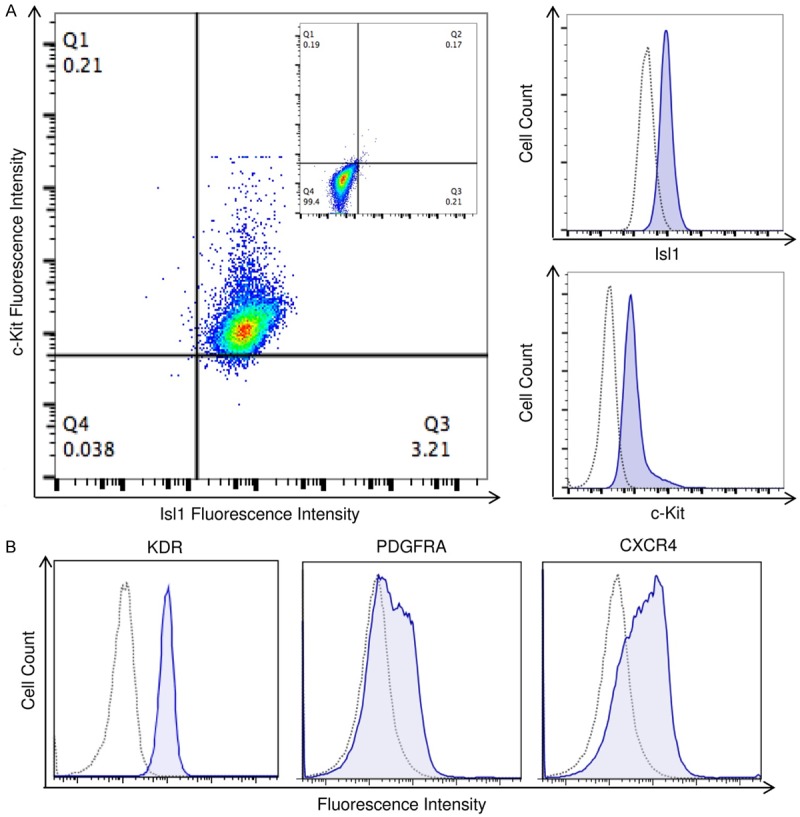
Expression of early progenitor markers within a representative clonal CPC population. CPCs used in experiments were screened for the co-expression of Isl1 and c-kit (A), as indicated by flow cytometry. These Isl1+c-kit+ CPCs also exhibit other markers of early cardiac development, including KDR and PDGFRA, as well as the chemokine receptor CXCR4 (B). Dotted lines indicate isotype controls and solid lines indicate labeled CPCs. Double-labeled cells are shown as a dot-plot.
Figure 2.
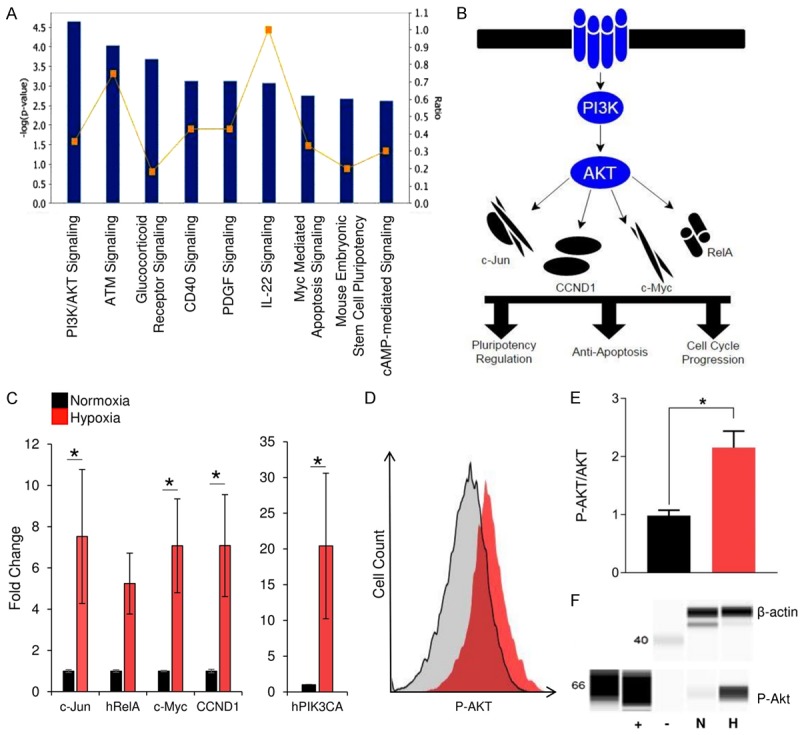
Short-term hypoxia upregulates Akt pathway activity in neonatal CPCs. Changes to gene expression following exposure to short-term hypoxia were analyzed using Ingenuity Pathway Analysis (A). The bar chart represents significance of gene enrichment, (i.e., -log (p-value) for that pathway) while the yellow line indicates the ratio of genes from the dataset that map to the pathway and the total number of genes in that pathway. Pathway analysis indicated that the PI3K/AKT pathway (B) was among the most statistically affected pathways. RT-PCR experiments (C) confirmed that hypoxia preconditioning resulted in a significant increase in expression of Akt-related genes (n=9). Akt phosphorylation was then measured using flow cytometry (D, E). Representative histogram (D) of the measured increase in P-Akt (E; n=3). Western blot analysis was used to confirm the increase in Akt phosphorylation (F) from normoxia (N) to hypoxia (H). Positive (+) and negative (-) controls are shown. *P < 0.05. Values reported as the average ± S.E.M.
Hypoxic preconditioning promotes Akt pathway constituent gene expression and Akt phosphorylation in CPCs
To determine the effect of short-term hypoxia on the Akt pathway within neonatal CPCs, we exposed CPCs to hypoxic preconditioning. Subsequent expression of Akt pathway constituents in both control and experimental cells was measured via qRT-PCR. Data analysis revealed that pretreated cells expressed significantly higher levels of nearly all Akt-related genes (Figure 2C). The activation of Akt has an important role in the survival and recruitment [21] of transplanted cells to the damaged myocardium. To validate this increase in Akt activity following exposure to hypoxia, CPCs were labeled with an antibody specific for Ser473-phosphorylated Akt and analyzed using flow cytometry and immunoblotting. In doing so, we measured a significant increase in Akt phosphorylation in CPCs that received hypoxic pretreatment as compared to CPCs kept under control conditions (n=3, p < 0.05; Figure 2D-F).
Hypoxia-mediated Akt activation is age-dependent
Enhanced Akt phosphorylation is of main interest for its potential benefit in autologous CPC-based therapy for myocardial infarction in older adults. To determine whether the hypoxia-mediated activation of Akt is significantly influenced by the age of the cell donor, adult CPC clones were exposed to hypoxia and assessed for Akt pathway activation. Similar to neonatal CPCs, pretreated adult CPCs expressed significantly higher levels of several Akt-related genes (Figure 3A). Flow cytometry indicated that Akt phosphorylation indeed trended upwards in preconditioned adult clones; however, it was not significant (Figure 3B). Western blot analysis of protein lysates obtained from hypoxia pre-treated adult CPCs confirmed this increase in Akt phosphorylation (Figure 3C). We then assessed if a combinatory treatment of SDF-1α, an important paracrine factor in myocardial regeneration, and hypoxia could improve Akt phosphorylation in adult CPCs. Western blot analysis demonstrated that SDF-1α treatment alone did not induce Akt phosphorylation, while the combinatory treatment promoted such activity.
Figure 3.
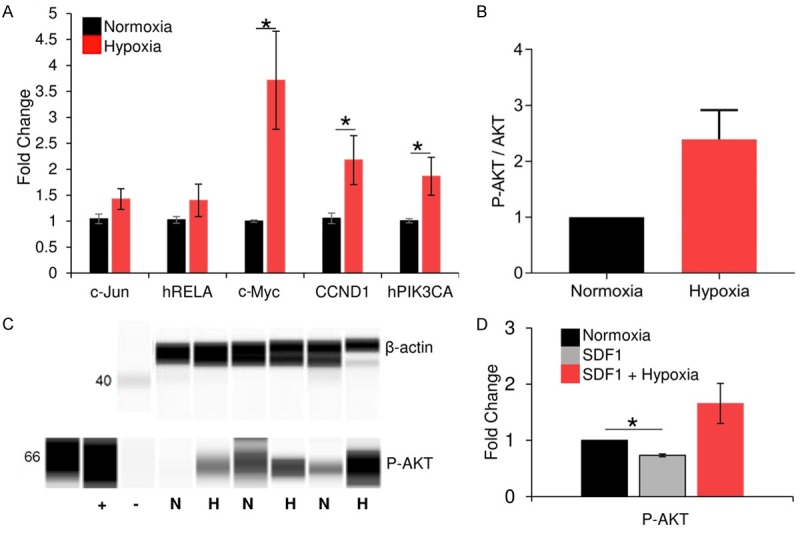
Short-term hypoxia upregulates Akt pathway activity in adult CPCs. RT-PCR experiments (A) demonstrated that hypoxia preconditioning resulted in a significant increase in expression of Akt-related genes in adult CPCs (n=5). Akt phosphorylation was then measured using flow cytometry (B; n=3). Western blot was used to confirm the increase in P-Akt (C; n=3) from normoxia (N) to hypoxia (H). Positive (+) and negative (-) controls are shown. (D) Adult CPCs were treated with SDF-1α alone or in combination with hypoxia. P-AKT expression was resolved using western blot, and fold changes in phosphorylated Akt, relative to β-actin, were quantified using ImageJ. *P < 0.05. Values reported as the average ± S.E.M.
Short-term hypoxia promotes a pro-survival response in CPCs
In other cell types, hypoxic pre-treatment has been shown to improve survival [17,18]. To determine the effect of short-term hypoxia on CPCs, the expression profiles of several heat-shock proteins in control and experimental populations were examined using qRT-PCR, flow cytometry, and immunoblotting. Data analysis confirmed that pretreated neonatal CPCs expressed a significantly increased level of HSP70, while both neonatal and adult CPCs generally express a higher level of HSP40 and HSP90 (Figure 4A, 4B). As indicated by flow cytometry, following hypoxic pre-treatment, HSP70 was more highly expressed in neonatal CPCs, while HSP40 was induced in both adult and neonatal CPCs (Figure 4C). Adult CPCs were found to endogenously express lower levels of HSP70 compared to neonatal CPCs (Figure 4D). To confirm HSP40 induction in the absence of significant changes to gene expression, immunoblotting was performed on HSP40 (Figure 4E), which indicated an increase in HSP40 protein levels. In this context, HSP90 immunoblotting was also performed (Figure 4F).
Figure 4.
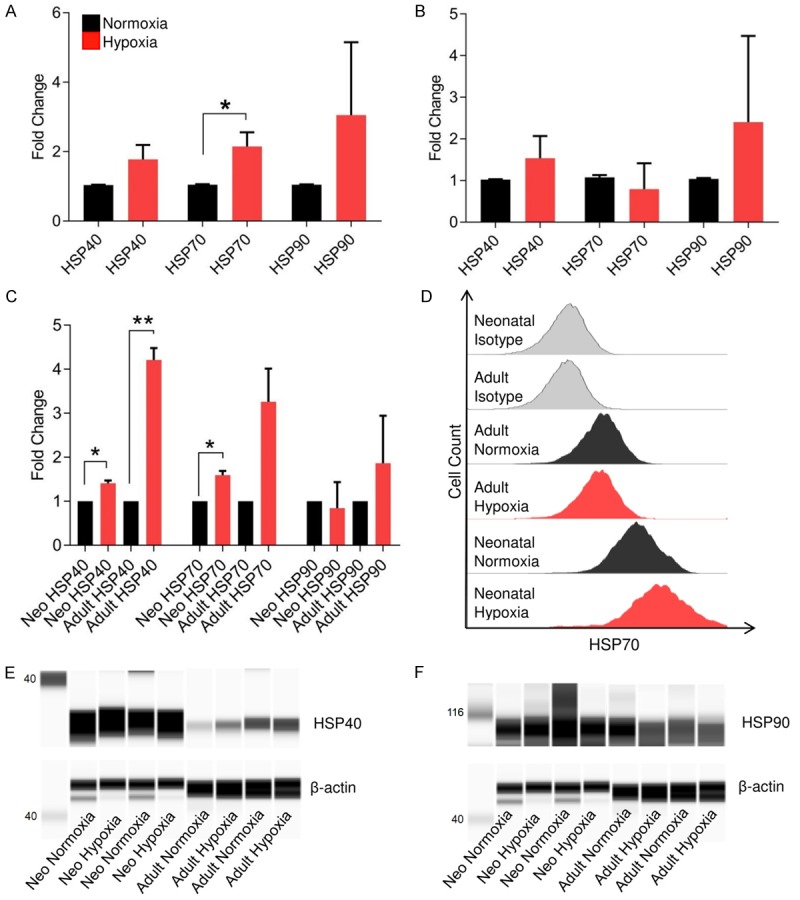
HSP expression in response to short-term hypoxia. RT-PCR indicated a significant induction of HSP70 expression and a general increase in HSP40 and HSP90 expression in neonatal (A) and adult (B) CPCs following pretreatment with short-term hypoxia (n=6 neonatal clones, n=4 adult clones). Flow cytometry-based analysis of HSP40, HSP70, and HSP90 (C), indicating a significant increase in expression of HSP40 in neonatal and adult CPCs and a significant increase in expression of HSP70 in neonatal CPCs (n=3 for each age group). Adult CPCs endogenously express lower levels of HSP70 compared to neonatal CPCs (D). Since HSP40 induction was found to be significant by flow cytometry, but not by RT-PCR, western blot analysis was used to assess the induction of HSP40 (E) as well as HSP90 (F) in both neonatal and adult CPCs. *P < 0.05; **P < 0.01. Values reported as the average ± S.E.M.
Further gene expression analysis indicated that short-term hypoxia not only induced a stress response, but also upregulated BCL2, HMOX1, and RELA, which are cell survival-associated genes (Figure 5A, 5B). To assess cell survival, apoptosis was induced using 0.5 mM hydrogen peroxide in both control and hypoxia-treated CPCs and relative DNA fragmentation was measured using Brd-U. Within a six-hour timeframe, we found that the hypoxia-induced modifications to the pro-survival gene program resulted in fewer apoptotic cells in response to oxidative stress (Figure 5C). TUNEL assay results from neonatal (Figure 5D) and adult (Figure 5E) CPCs revealed a downward trend in the number of apoptotic cells in response to hydrogen peroxide.
Figure 5.
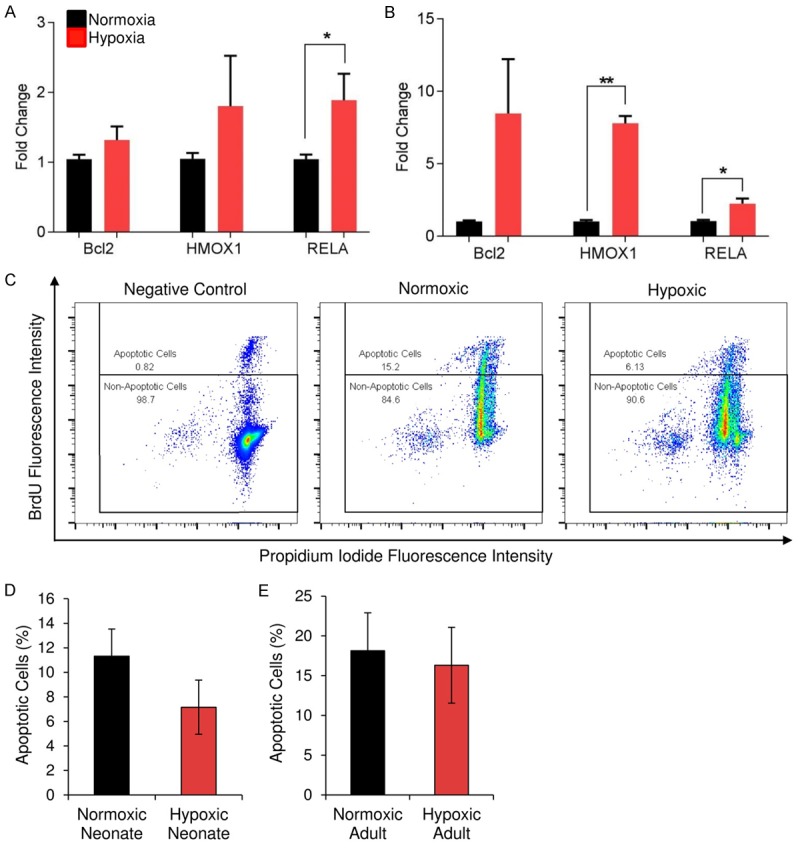
Cell survival improves following CPC treatment with short-term hypoxia. Expression of genes associated with cell survival increased after hypoxia pre conditioning in both neonatal (A; n=3) and adult (B; n=3) CPCs. To assess cell survival, apoptosis was induced using 0.5 mM H2O2 in both control and hypoxia-treated CPCs and relative DNA fragmentation was measured using Brd-U. Flow cytometry (C) of BrdU-DNA binding within a representative clone, with and without hypoxic pretreatment, is shown. CPCs treated with short-term hypoxia exhibited an approximate 50% decrease in apoptotic cells after induction of cellular death by 0.5 mM H2O2. Quantification of TUNEL assay results obtained using three neonatal (D) and three adult (E) adult clones is shown. Apoptosis in response to oxidative stress trends downward in the hypoxia-pre-conditioned group. *P < 0.05, **P < 0.01. Values reported as the average ± S.E.M.
CPCs invade more readily after exposure to short-term hypoxia
To determine if short-term hypoxia has any effect on CPC motility, a representative clone was pretreated with hypoxia for 3 hours, 6 hours, and 18 hours, and then assessed for invasiveness in response to SDF-1α using a Transwell® invasion assay. The six-hour hypoxia-pretreated group most efficiently invaded through the basement membrane layer (Figure 6A). Neonatal and adult CPCs were then pretreated with six hours of hypoxia and assessed using the same invasion assay. While both age groups benefited from hypoxia pretreatment, only neonatal CPCs exhibited a statistically significant improvement in chemotactic response to SDF-1α (Figure 6B).
Figure 6.
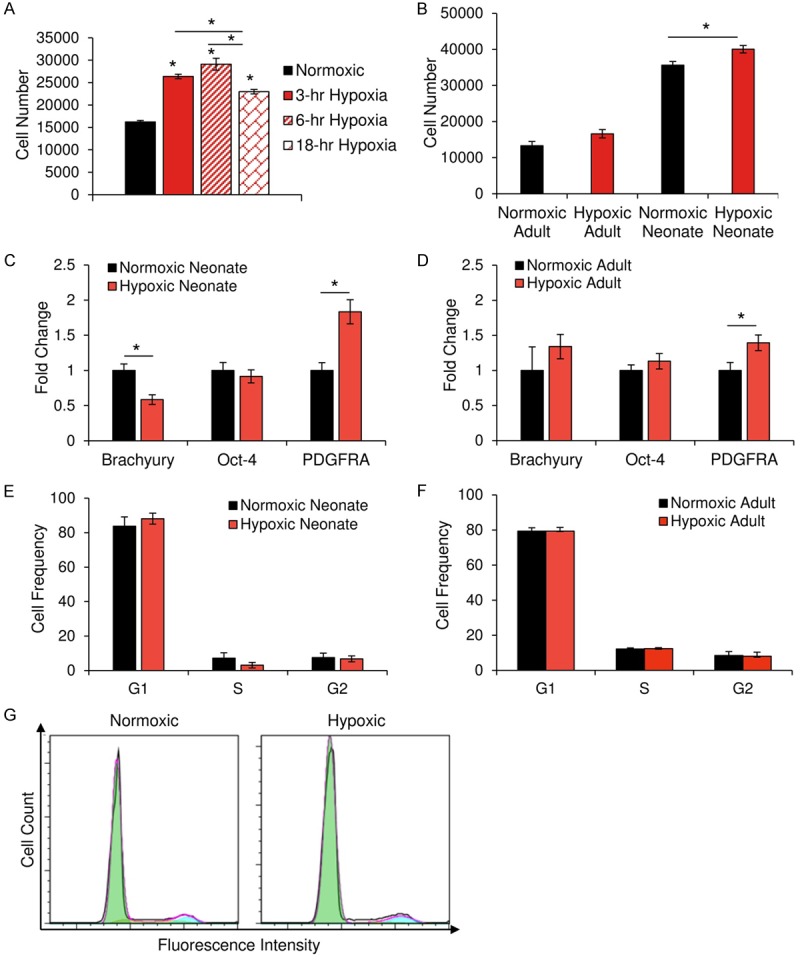
CPCs experience improvements in invasive ability without a change in developmental status. Invasiveness in response to SDF-1α, a chemoattractant, was assessed using the Transwell® invasion assay. In response to SDF-1α, CPCs invade through matrigel into a reservoir, where they are then stained with calcein AM and measured by spectrophotometry in quadruplicate. (A) Quantification of the invasiveness of a representative CPC clone following exposure to hypoxia for various lengths of time. A significant increase in cell invasion was noted for each time point, with six hours of hypoxia inducing the greatest level of invasive activity. While both neonatal and adult CPCs that are pretreated with hypoxia exhibit improved invasiveness (B; n=10), only neonatal CPCs experienced significant improvements. RT-PCR was used to examine the effects of short-term hypoxia on the expression of differentiation markers present in neonatal (C; n=4) and adult (D; n=3) CPCs. Flow cytometry of propidium iodine-stained CPCs revealed no significant change in the cell cycle profile of neonatal (E; n=6) or adult (F; n=9) CPCs following treatment with hypoxia. A representative histogram (G) of flow cytometry-based cell cycle analysis. *P < 0.05. Values reported as the average ± S.E.M.
Hypoxia-pretreated CPCs retain an early cardiac phenotype and exhibit no alterations in cell cycling
We measured changes in the expression of Brachyury, OCT4, and PDGFRA, as well as in cell cycling. In neonatal clones, a significant downregulation of Brachyury and upregulation of PDGFRA were noted (Figure 6C), while only PDGFRA expression increased between pre-treated and non-treated adult CPCs (Figure 6D). Since PDGFRA is involved in the PI3K/AKT pathway, we performed cell cycle analysis to assess if this increase in PDGFRA expression was relevant to developmental status changes or instead a response to PI3K/AKT pathway activation. Cell cycle analysis was performed on non-treated and pre-treated neonatal and adult CPCs. No significant differences were identified between control and experimental groups (Figure 6E, 6F). Upon inspection of a representative cell cycle profile, as determined by flow cytometry, no apparent differences between normoxic and hypoxic groups, whether adult and neonatal, were observed in cell cycle progression (Figure 6G).
Prostaglandin receptors 1 and 4 are induced in neonatal CPCs after short-term hypoxia
Previous reports have demonstrated that AKT induces prostaglandin receptor (PTGER) expression [26], whose ligand, prostaglandin E2, has been studied for its ability to promote cardiomyocyte replenishment [27]. For this reason, we assessed the effect of hypoxia on prostaglandin receptor expression. After six hours of hypoxia pre-treatment, neonatal CPCs expressed significantly higher levels of PTGER1 and PTGER4 (n=9, p<0.05; Figure 7A). This was not observed in adult CPCs (Figure 7B). Gel electrophoresis confirmed the increased expression in neonatal CPCs (Figure 7C).
Figure 7.

PTGER induced following short-term hypoxia. Following hypoxia pre-treatment, neonatal (A) and adult (B) CPCs were assessed for changes in PTGER1-4 gene expression. Significant changes were observed in neonatal, but not adult, PTGER1 and PTGER4 expression, which was confirmed using gel electrophoresis (C). This is supported by previous reports and IPA modeling, wherein AKT induces PTGER4 expression, which subsequently induces HIF-1α expression (D). This prostaglandin-mediated induction of HIF-1α can then promote subsequent hypoxia-like cell responses. Data are presented as the average ± S.E.M. *p<0.05; n=9 for each neonatal and adult experiments.
Discussion
In this study, early CPCs were expanded as clonal populations and used as a model to test the hypothesis that short-term hypoxia exposure enhances CPC function in vitro. The results presented here show that short-term hypoxia is a feasible and practical pretreatment that benefits CPC invasive capabilities in vitro.
Recent studies lend credence to the efficacy of cardiac stem cell transplants for the amelioration of cardiac dysfunction in mouse models [7,28]. However, improvements in cardiac function - typically measured using left ventricular ejection fraction - are thought to be a result of paracrine signaling stemming from the growth factors secreted by newly transplanted cells [7,29]. Additionally, the functional capabilities of CPCs are known to vary greatly between age groups with neonatal CPCs consistently outperforming their adult counterparts [8]. Hence, the administration of autologous CPCs for treatment of the majority of cardiac injuries is limited and appears to stimulate repair indirectly. Cell-based therapies for the human heart - of all ages - may be augmented via the use of a novel cell type or pre-treatment method, such as hypoxic exposure. If pre-conditioning indeed improves CPC function, behavior of adult-derived clones could be improved to reflect that of the functionally superior neonatal counterparts. Additionally, the supplemental implementation of novel early CPC populations may also aid in the effort to optimize autologous cell-based therapies for regeneration of the heart.
Once believed to be found mainly within fetal progenitor populations [30,31], early CPCs that co-express Isl1 and c-Kit were recently described within endogenous cardiac progenitor populations in the adult human heart [8]. Isl1 is a transcription factor that is required early in development for the survival, proliferation, and migration of cardiac progenitors into the primordial heart and, as a result, the developed heart is largely a product of Isl1+ cells [12]. One of the most widely studied progenitor cell types, however, is the c-Kit+ cardiac progenitor. While current clinical trials using c-Kit+ cells show promise, the role of c-Kit+ cells in the development and regeneration of the heart remains controversial [32-36]. The combined benefits of using other progenitor cell types, such as those characterized by Isl1, cKit, PDGFRA, and KDR, and pre-treatment to prepare these transplanted cells for the hypoxic environment of the damaged heart, may allow for improved cardiac regeneration in vivo.
The idea that preconditioning cells prior to transplantation may benefit donor cell function in vivo, has gained a significant following in recent years [16-18,37-39]. However, although this research is gaining momentum, the genetic analysis and functional assays presented in this study have never been performed on early CPCs co-expressing Isl1 and c-Kit. Using other models, previous studies have established that hypoxia induces a persistent increase in phosphorylation of Akt for up to 24 hours, with the effect peaking at six hours [20]. Activation of Akt, a versatile kinase that regulates several cellular functions, may result in CPC functional improvements and enhanced cardiac repair. In the present study, enhanced Akt phosphorylation was indeed observed after only six hours of hypoxia. While both age groups displayed increased phosphorylation of Akt after the six-hour time point, only neonatal CPCs exhibited a statistically significant increase. Notably, adult clones are notoriously difficult to stimulate and, in response to SDF-1α after starvation, Akt is not phosphorylated. Short-term hypoxic preconditioning, on the other hand, elevated levels of phosphorylated Akt in adult CPCs. Hypoxia pre-conditioning induces the expression of CXCR4, the receptor of SDF-1α [40]. In this way, hypoxia pre-conditioning may enhance both regenerative potential via Akt pathway activation while also enhancing its ability to respond to paracrine factors secreted by the damaged myocardium.
Nonetheless, hypoxia is a stressor that, as demonstrated here, triggers a physiological response in CPCs. As oxygen levels decline, the mitochondria within a cell increase the production of reactive oxygen species [41,42]. The accumulation of such oxygen species after short periods of hypoxia has been shown to confer resistance against future oxygen shortages [43]. However, longer periods of hypoxia coincide with excessive accumulation of these reactive oxygen species that are known to promote cell death via caspase activation and DNA damage [44-47]. Altogether, chronic oxidative stress has the potential to impair the functional capacity and overall health of a population of cells [16]. The extent of this stress response in CPCs was evaluated by measuring the impact of short-term hypoxia on the expression of several HSPs. The induction of the heat-shock pathway during hypoxia has been well documented in other cell types [48]. Both neonatal and adult CPCs exhibited a trend towards hypoxia-mediated activation of several heat shock proteins. HSP40 is known to repair proteins denatured by hypoxic stress and supports HSP70 function [49,50]. A deep proteomic analysis of the secretome of neonatal and adult CPCs found several HSPs, including HSP70 and HSP90, to be highly represented in the secretome of neonatal CPCs [51]. They found that this may be integral to the high proliferative capacity of neonatal CPCs. Indeed, other studies have found that HSP70 has been shown to stimulate growth in both adult mesenchymal stem [52] and mouse mesoangioblast stem cells [53]. Meanwhile, HSP90 directly interacts with Akt to help maintain its phosphorylated state [54-56] and associates with the alpha subunit of HIF-1 to protect it from degradation [57]. HIF-1 is a transcription factor that is induced by hypoxia-mediated shifts in HIF-1α expression, is stabilized by ROS, and is ultimately responsible for adaptation to hypoxic stress [58,59]. Interestingly, the activation of prostaglandin receptor 4, whose expression is induced under hypoxic conditions, promotes HIF-1 translocation to the nucleus, thereby further augmenting the AKT-mediated effects of hypoxia [60,61]. Future studies should consider the effects of co-treating CPCs with prostaglandin E2, the ligand of prostaglandin receptor 4, and hypoxia. It is possible that the AKT-mediated effects of hypoxia are augmented by prostaglandin E2 treatment, which may be an avenue to further pre-treat adult CPCs for use in myocardial repair.
Accordingly, pro-survival gene expression was found to be significantly upregulated in hypoxic CPCs. Bcl-2 encodes its mitochondrial protein counterpart that inhibits apoptosis and extends the survival of cells [62,63]. Preconditioned CPCs displayed a dramatic increase in transcripts encoding Hmox1, a pro-survival gene that becomes upregulated in response to hypoxia and affords protection against future oxidative damage [64]. Although a significant difference in apoptosis was not observed, hypoxia-preconditioned CPCs indeed exhibited significantly enhanced invasion capabilities when compared to their normoxic counterparts. Similarly, pretreated CPCs displayed significantly improved chemotaxis in response to SDF-1α [15,16,18,40].
Depending on the cell type, however, hypoxic exposure may either enhance proliferation [16,19,65,66], lead to G1 arrest [67,68], or influence the differentiation process [19,68,69]. After treatment with hypoxia, human mesenchymal stem cells acquire enhanced proliferative abilities [66] while, on the other hand, murine embryonic fibroblasts encounter G1 arrest [70]. Since the length of exposure to hypoxia may influence cell cycle progression, the timeframe for enhancing cellular function using hypoxia must be optimized to achieve the desired effect. Ideally, in the early stages after transplantation, donor cells must survive, continue to divide, migrate to the damaged myocardium, and remain multipotent as they engraft. CPCs indeed migrate more readily, progress normally through the cell cycle, and retain expression of markers of an early cardiac phenotype after short-term hypoxic treatment. While PDGFRA expression increased, it likely is involved in Akt activation [71] rather than in developmental changes in the context of hypoxia pre-treatment since the cell cycle profile remained unchanged. Furthermore, the fate of other cell types appears to be influenced, at least in part, by PTGER4 receptor activation [72,73]. Similarly, the increased expression of PTGER4 by hypoxia may contribute to the maintenance of pluripotency in neonatal CPCs. The results reported herein using CPC clones suggest that pretreatment with short-term hypoxia can enhance the functional efficacy of cardiovascular stem cell transplantation for the repair of the heart.
In conclusion, short-term hypoxia, as a pretreatment, is a viable approach for supporting cell survival and enhancing migratory capabilities in CPCs, possibly through enhanced Akt signaling. These findings, if implemented in vivo, may improve cardiac repair after infarction. Therefore, hypoxia-preconditioned CPCs warrant further investigation in animal models. These positive effects of short-term hypoxia include: 1) increased Akt phosphorylation and signaling, which supports several pro-survival and developmental processes; and 2) enhanced chemotaxis, which would render the cells more likely to reach damaged tissues and successfully engraft. Future experiments, comparing in vivo cellular performance and additional pre-treatment methods for CPCs, are planned to validate the efficacy of short-term hypoxia as a part of an effective pretreatment strategy to optimize cell-based repair.
Acknowledgements
This work was supported by CIRM Bridges to Stem Cell Research to IH [grant number TB1-01185, 2016].
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Deaton C, Froelicher ES, Wu LH, Ho C, Shishani K, Jaarsma T. The global burden of cardiovascular disease. European Journal of Cardiovascular Nursing. 2011;10(Suppl):S5–S13. doi: 10.1016/S1474-5151(11)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five~year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbin KA, Murry CE. The winding road to regenerating the human heart. Cardiovasc Pathol. 2015;24:133–40. doi: 10.1016/j.carpath.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landin AM, Hare JM. The quest for a successful cell-based therapeutic approach for heart failure. Eur Heart J. 2017;38:661–664. doi: 10.1093/eurheartj/ehw626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuentes TI, Appleby N, Tsay E, Martinez JJ, Bailey L, Hasaniya N, Kearns-Jonker M. Human neonatal cardiovascular progenitors: unlocking the secret to regenerative ability. PLoS One. 2013;8:e77464. doi: 10.1371/journal.pone.0077464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuentes T, Kearns-Jonker M. Endogenous cardiac stem cells for the treatment of heart failure. Stem Cells Cloning. 2013;6:1–12. doi: 10.2147/SCCAA.S29221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 12.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YP, Li HR, Cao XM, Wang QX, Qiao CJ, Ya J. Second heart field and the development of the outflow tract in human embryonic heart. Dev Growth Differ. 2013;55:359–67. doi: 10.1111/dgd.12050. [DOI] [PubMed] [Google Scholar]
- 14.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–42. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 15.Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S, Naldini A. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1α and PI3K/Akt pathway. J Cell Physiol. 2014;229:2067–76. doi: 10.1002/jcp.24666. [DOI] [PubMed] [Google Scholar]
- 16.van Oorschot AA, Smits AM, Pardali E, Doevendans PA, Goumans MJ. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J Cell Mol Med. 2011;15:2723–34. doi: 10.1111/j.1582-4934.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Yan G, Xu H, He W, Liu Z, Ma G. Hypoxic preconditioning increases survival of cardiac progenitor cells via the pim-1 kinase-mediated anti-apoptotic effect. Circulation Journal. 2014;78:724–31. doi: 10.1253/circj.cj-13-0841. [DOI] [PubMed] [Google Scholar]
- 18.Yan F, Yao Y, Chen L, Li Y, Sheng Z, Ma G. Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1α-CXCR4 axis. PLoS One. 2012;7:e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–83. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beitner-Johnson D, Rust RT, Hsieh TC, Millhorn DE. Hypoxia activates Akt and induces phosphorylation of GSK-3 in PC12 cells. Cell Signal. 2001;13:23–7. doi: 10.1016/s0898-6568(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsen I, Lorens JB. Evaluating extracellular matrix influence on adherent cell signaling by cold trypsin phosphorylation-specific flow cytometry. BMC Cell Biol. 2013;14:36. doi: 10.1186/1471-2121-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clément MV, Ponton A, Pervaiz S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Letters. 1998;440:13–8. doi: 10.1016/s0014-5793(98)01410-0. [DOI] [PubMed] [Google Scholar]
- 25.Morey RD. Confidence intervals from normalized data: a correction to Cousineau (2005) The Quantitative Methods for Psychology. 2008;4:61–4. [Google Scholar]
- 26.Lee J, Banu SK, Nithy TK, Stanley JA, Arosh JA. Early pregnancy induced expression of prostaglandin E2 receptors EP2 and EP4 in the ovine endometrium and regulated by interferon tau through multiple cell signaling pathways. Mol Cell Endocrinol. 2012;348:211–23. doi: 10.1016/j.mce.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh YC, Wu JM, Yu CK, Wu KK, Hsieh PC. Prostaglandin E2 promotes post-infarction cardiomyocyte replenishment by endogenous stem cells. EMBO Mol Med. 2014;6:496–503. doi: 10.1002/emmm.201303687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–17. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–41. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 30.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126(Suppl 1):S46–S53. doi: 10.1161/CIRCULATIONAHA.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou X, Appleby N, Fuentes T, Longo LD, Bailey LL, Hasaniya N, Kearns-Jonker M. Isolation, characterization, and spatial distribution of cardiac progenitor cells in the sheep heart. J Clin Exp Cardiolog. 2012:S6. [PMC free article] [PubMed] [Google Scholar]
- 32.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–57. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira-Martins J, Ogorek B, Cappetta D, Matsuda A, Signore S, D’Amario Kostyla J, Steadman E, Ide-Iwata N, Sanada F, Iaffaldano G, Ottolenghi S, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110:701–15. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, Liu W, Smith RR, Marbán E. Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc. 2014;3:e001260. doi: 10.1161/JAHA.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo J, Weaver MS, Cao B, Dennis JE, Van Biber B, Laflamme MA, Allen MD. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Transl Med. 2014;3:734–44. doi: 10.5966/sctm.2013-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–43. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblum S, Smith TN, Wang N, Chua JY, Westbroek E, Wang K, Guzman R. BDNF pretreatment of human embryonic-derived neural stem cells improves cell survival and functional recovery after transplantation in hypoxicischemic stroke. Cell Transplant. 2014;24:2499–61. doi: 10.3727/096368914X679354. [DOI] [PubMed] [Google Scholar]
- 40.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxia preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 42.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, Park JH. ROS-dependent caspase-9 activation in hypoxic cell death. FEBS Lett. 2003;549:94–8. doi: 10.1016/s0014-5793(03)00795-6. [DOI] [PubMed] [Google Scholar]
- 45.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 46.Moungjaroen J, Nimmannit U, Callery PS, Wang L, Azad N, Lipipun V, Chanvorachote P, Rojanasakul Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. J Pharmacol Exp Ther. 2006;319:1062–9. doi: 10.1124/jpet.106.110965. [DOI] [PubMed] [Google Scholar]
- 47.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–88. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird NA, Turnbull DW, Johnson EA. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem. 2006;281:38675–81. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- 49.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Hwang NR, Lee K. Heat shock responses for understanding diseases of protein denaturation. Molecules and Cells. 2007;23:123. [PubMed] [Google Scholar]
- 51.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR, Kaushal S. A deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res. 2017;120:816–834. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreeva NV, Zatsepina OG, Garbuz DG, Evgen’ev MB, Belyavsky AV. Recombinant HSP70 and mild heat shock stimulate growth of aged mesenchymal stem cells. Cell Stress Chaperones. 2016;21:727–33. doi: 10.1007/s12192-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turturici G, Geraci F, Candela ME, Cossu G, Ciudice G, Sconzo G. Hsp70 is required for optimal cell proliferation in mouse A6 mesangioblast stem cells. Biochem J. 2009;421:193–200. doi: 10.1042/BJ20082309. [DOI] [PubMed] [Google Scholar]
- 54.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–7. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yun BG, Matts RL. Hsp90 functions to balance the phosphorylation state of Akt during C2C12 myoblast differentiation. Cell Signal. 2005;17:1477–85. doi: 10.1016/j.cellsig.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Schmid T, Frank R, Brüne B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1α from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- 58.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 60.Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–6. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 61.Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev. 2013;65:1010–52. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 62.Hockenbery D, Nuñez G, Milliman C, Schreiber R, Korsmeyer S. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 63.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–7. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 64.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kook SH, Son YO, Lee KY, Lee HJ, Chung WT, Choi KC, Lee JC. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol Int. 2008;32:871–8. doi: 10.1016/j.cellbi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 67.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–56. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett T. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–83. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 70.Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–26. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 71.Ho AL, Vasudeva SD, Laé M, Saito T, Barbashina V, Antonescu CR, Ladanyi M, Schwartz GK. PDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: implications for combination targeted therapy of synovial sarcoma. Cancer Res. 2012;72:4515–25. doi: 10.1158/0008-5472.CAN-12-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin MC, Chen SY, Tsai HM, He PL, Lin YC, Herschman H, Li HJ. PGE2/EP4 signaling controls the transfer of the mammary stem cell state by lipid rafts in extracellular vesicles. Stem Cells. 2017;35:425–444. doi: 10.1002/stem.2476. [DOI] [PubMed] [Google Scholar]
- 73.Ikushima YM, Arai F, Hosokawa K, Toyama H, Takubo K, Furuyashiki T, Narumiya S, Suda T. Prostaglandin E2 regulates murine hematopoietic stem/progenitor cells directly via EP4 receptor and indirectly through mesenchymal progenitor cells. Blood. 2013;121:1995–2007. doi: 10.1182/blood-2012-06-437889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


