Abstract
Background: The immune response to critical injury, including thermal injury, can heavily influence the recovery and long term prognosis for patients suffering such insults. A growing body of evidence supports that a suppressed immunologic state following critical injury can lead to adverse outcomes for adult and pediatric patients. Methods: A Pubmed literature search was conducted to review areas of the immune system that are impaired after thermal injury and identify key immune players that are potential targets for therapeutic intervention. The focus was pediatric thermal injury; however, where pediatric studies were lacking adult studies were used as reference. Results: Changes in cytokine profiles and immune cell phenotypes have been observed following thermal injury. Treatment with immunomodulatory stimulants, including IL-7 and GM-CSF, lead to improved outcomes in critically ill patients and may also be useful tools to improve immune function in pediatric burn patients. Conclusions: The innate and adaptive branches of the systemic immune system are impaired following thermal injury in adult and pediatric patients. Immunomodulatory therapies currently being used in areas outside of thermal injury may be useful tools to help improve outcomes following pediatric thermal injury.
Keywords: Burn, immune function, immunomodulation, immunoparalysis, thermal injury
Introduction
Every three hours one child dies from a burn related accident which the World Health Organization calls a preventable injury (www.who.int/mediacenter/factsheets/fs365/en). Burn injury represents the third most common cause of death in children from the ages of 5 to 9 [1]. Advances in health care have led to an increase in survivability of a major burn injury however an ever increasing disability creates a large economic burden [2].
According to the American Burn Association, seven out of the top ten complications in burn patients are infection related [3]. Infections have been reported to occur in as high as 60% of both adult and pediatric patients with thermal injury [4-8]. The most common infections in both adult and pediatric burn patients are pneumonia, followed by cellulitis and urinary tract infections [3]. Given impairment of the skin’s barrier function after thermal injury, these patients’ defense against infectious complications is primarily the cellular elements of the immune system. Unfortunately the immune response to pediatric burn injury is an area that has been understudied. The Inflammation and the Host Response to Injury collaborative network has provided much data and insight regarding immunologic alterations after burn injury. Most of the studies, however, focus on adults and have been limited to evaluation of plasma cytokine profiles and the hyper-inflammatory response. There is a paucity of data regarding the pediatric functional immune response after thermal injury. The limited data in children have demonstrated a difference in the inflammatory profile of adult and pediatric burn patients, which suggests that different therapeutic interventions may be warranted to achieve attenuation of the post-burn inflammatory response in these patients [9].
The purpose of this mini review is to provide a better understanding of the current literature regarding the systemic immune response to pediatric burn injury and to explore potential targets of immune directed therapy. While we understand that communication exists between both the local and systemic immune response to burn injury we elected to focus on the latter for the purpose of this review.
Background
Critical injuries cause alterations of the immune response typically characterized by a pro-inflammatory phase, termed the systemic inflammatory response syndrome (SIRS), as well as a concurrent anti-inflammatory cascade, named the compensatory anti-inflammatory response syndrome (CARS) (Figure 1). It is important to note while SIRS/CARS have divergent effects they occur simultaneously not as discrete entities with separate time points. At the same time both SIRS and CARS can lead to dysregulation of immune function. Upregulation of pro-inflammatory and anti-inflammatory genes are noted to occur as early as four hours after injury and can persist for up to 90 days following thermal injury [10]. Many clinical features of acute burn injury including fever, capillary leak, and poor perfusion result from an initial pro-inflammatory response, while the concurrent CARS response has no specific clinical phenotype [10-12]. A dysregulated immune response leads to suboptimal immune function. Patients with thermal injury are at high risk of infectious complications including sepsis. Infection prevention and control are crucial to burn care survival and for this reason understanding the immune response following burn injury should be of the upmost importance.
Figure 1.
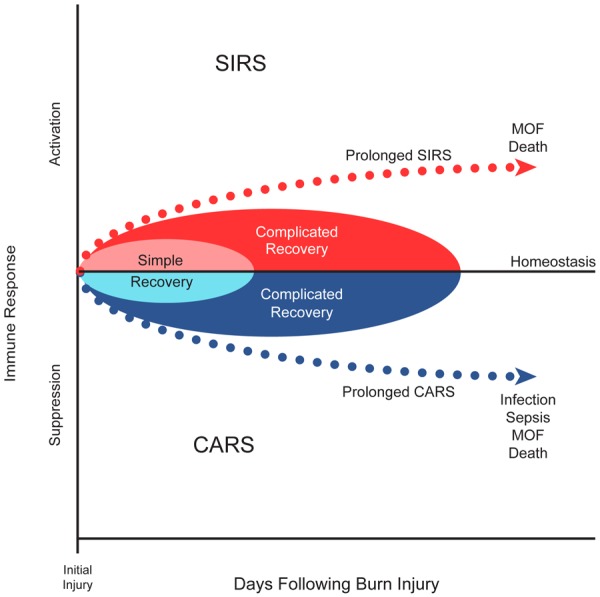
SIRS vs CARS: Critical illness results in an alteration of the immune response typically characterized by a pro-inflammatory phase, termed the systemic inflammatory response syndrome (SIRS), as well as a concurrent anti-inflammatory cascade, named the compensatory anti-inflammatory response syndrome (CARS). A balanced response typically leads to recovery as opposed to either a prolonged SIRS or CARS in which poor outcomes are attributed.
A growing body of evidence supports the use of certain immunologic biomarkers to predict clinical outcomes in adult and pediatric patients with critical illness. Impairment of innate immune function, as measured by reduced capacity of whole blood to produce the cytokine tumor necrosis factor (TNF)-α upon ex-vivo stimulation with lipopolysaccharide (LPS), has been shown to predict the development of secondary infections in children who suffered critical injury, although it has not been evaluated in pediatric burn injury [13]. Similarly, immunosuppression measured using the same method in children suffering from multiple organ dysfunction syndrome (MODS) has been associated with higher rates of mortality and nosocomial infection [14]. A reduction in antigen presenting capacity is another independent indicator of mortality risk in patients with septic shock [15]. In particular, reduced monocyte HLA-DR expression has been associated with increased post-trauma infection risk [16-20]. Limited studies have shown improvement of immune function in this setting with administration of immunostimulatory molecules such as interferon (INF)-ɣ and granulocyte monocyte colony stimulating factor (GM-CSF) [14,21-23]. Although immune monitoring and modulation studies have been conducted in adult and pediatric critical illness, studies specific to pediatric thermal injury are lacking (Figure 1).
Cytokines
The systemic inflammatory response following burn injury encompasses the release of pro-inflammatory and anti-inflammatory cytokines that modulate the innate and adaptive arms of the immune system. Specifically, in the first week post-burn, there are elevations in the pro-inflammatory cytokines TNFα, interleukin (IL)-6, IL-8, IL-1β, and IFNɣ along with anti-inflammatory cytokines such as IL-10 [9,24,25]. Notably, endogenous production of the immunostimulant GM-CSF is not significantly increased until the second week post-burn. A direct comparison of cytokine profiles in both adult and pediatric patients in the first week post-burn showed similar trends [9]. However, IL-17 and GM-CSF levels were significantly lower in the pediatric burn patients when compared to adults for the first week post-burn. These differences suggest that both populations have a unique immune response that need to be evaluated independently.
These cytokine profiles are also useful correlates of immune function as opposed to solely being markers of inflammation. A hyper-inflammatory response, as indicated by high circulating levels of IL-6, IL-8, and MCP-1, was associated with a greater number of infections including a higher rate of sepsis in pediatric burn patients [26]. A systemic elevation of the anti-inflammatory cytokine IL-10 has also been shown to be a sensitive and specific screening marker of ICU mortality in adult burn patients [24]. In addition to individual cytokines, the ratio of pro- and anti-inflammatory cytokines may prove to be a valuable tool to measure immune function and predict outcomes [24,27]. Intervening with immune therapies targeting cytokines or their production in these at-risk patients could improve outcomes.
Innate immune function
Neutrophils
Neutrophils are an important part of the innate immune response as they migrate to the site of inflammation/infection and act as an early defense mechanism. Following burn injury, neutrophils display reduced chemotaxis, phagocytosis, and generation of ROS, thus reducing innate immune function [28,29] (Figure 2).
Figure 2.
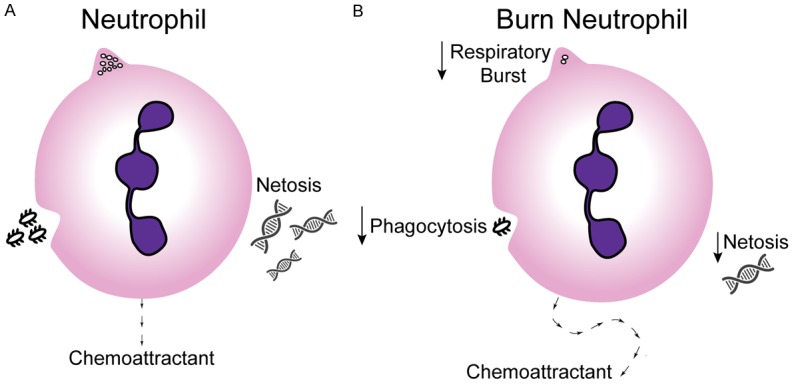
Burn Neutrophil: A. Neutrophils have several functions which are critical to innate immune defense. B. Neutrophils from burn patients display reduced chemotaxis, phagocytosis, and generation of ROS, thus reducing innate immune function.
An active area of investigation has been the ability of these cells to generate neutrophil extracellular traps (NETs). These structures are made of DNA, granule derived peptides, enzymes, and modified histones, which work to trap and kill microbial pathogens in the blood and tissues [30,31]. In a prospective study conducted on patients with severe thermal injury oxidative burst, phagocytosis index and ex-vivo NET formation were reduced as compared to healthy controls [32].
Quick and targeted migration of neutrophils is required for an effective innate immune response. Healthy neutrophils are able to respond and migrate along increasing gradients of chemoattractants, which include C5a, IL-8, LTB4, and bacterial products, in a direct path. Following burn injury neutrophils are still drawn toward these same chemoattractants, however it is along a random oscillating path. This random migration pattern is reversible with administration of antibiotic therapy and can predict the development of sepsis 48 hours prior to onset [33]. These neutrophil studies were exclusively performed in adult burn patients, underscoring the need for pediatric specific studies (Figure 2).
Monocytes
Circulating monocytes play a central role in the innate immune system of patients with thermal injury. Their key functions include cytokine production, phagocytosis and antigen presentation.
Recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) activates monocytes leading to production of chemokines and cytokines [34]. These chemokines attract neutrophils and other monocytes into the tissues where the latter become tissue macrophages. Pro-inflammatory cytokines produced by monocytes include TNFα, IL-6 and IL-1β [35]. These monocytes also produce the anti-inflammatory cytokine, IL-10 [35]. Activated monocytes phagocytose and process bacteria and other foreign particles for antigen presentation through loading and expression on major histocompatibility (MHC) class II molecules including human leukocyte antigen (HLA)-DR. These cell surface markers can then bind to receptors on T cells to participate in lymphocyte activation [34]. This bi-directional interaction can promote more monocyte activation or can blunt innate and adaptive immune function through the ligation of co-inhibitory molecules such as programmed death (PD)-1 on lymphocytes and its ligand PD-L1 on antigen presenting cells [34].
Expression of HLA-DR has been used as a marker for monitoring innate immune function in burn patients [17,20]. Studies have shown that the percentage of HLA-DR-expressing monocytes is lower in post-burn patients versus controls and is lowest in post-burn patients who go on to develop sepsis. This reduction in HLA-DR expression tends to recover in patients without sepsis, but can persist in septic patients [36,37]. Treatment with GM-CSF, IFNγ, and carbachol (a nicotinic and muscarinic receptor activator) has been shown to restore monocyte HLA-DR expression in critically ill patients, but data in burn patients are limited. These results suggest that HLA-DR expression is potentially useful as a predictor of adverse outcomes in post-burn patients and a potential immunomodulatory target (Figure 3).
Figure 3.
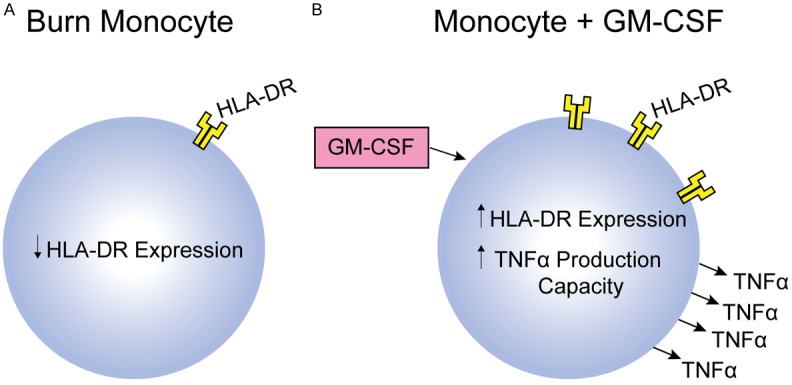
Burn Monocyte: A. Monocytes from patients with burn injury have been shown to have decreased HLA-DR expression. B. Immunomodulatory therapy targeting monocytes with the use of GM-CSF is being actively investigated in severe forms of pediatric illness/injury.
Recruitment and activation of macrophages are pivotal to recovery, local infection control and tissue generation within the burn wound bed. This review focuses on key mediators of the systemic immune response, therefore we do not discuss macrophages as markers of immune function following burn injury.
Natural killer cells
Natural killer (NK) cells are a subpopulation of innate immune cells derived from the lymphoid progenitor cell line. They represent one of the first lines of defense against neoplastic cells [38] and infectious insults, particularly viral, due to their ability to kill without histocompatibility complex recognition [39]. Strong defense against viral infections is particularly important in burn patients as these types of infections have been shown to be associated with increased mortality and morbidity following major thermal injuries [40,41].
Following burn injury, there is often no reduction in the number of NK cells, but there can be a decrease in NK cell function. This loss of NK cell activity is greater and longer lasting in adults with greater than 20% total body surface area (TBSA) burn as compared to those with smaller burn size [42]. This NK cell deficit is most significant in days 3-6 post-burn injury indicating a possible therapeutic window to modulate these cells. A potential mechanism for post-burn injury NK cell dysfunction relates to an impairment of IL-2 production capacity. Produced by CD4+ T cells, IL-2 is an important stimulator of NK cell activity [43]. Lower IL-2 production has been correlated with decreased NK activity following burn injury and NK cells have been shown to be hypo-responsive to IL-2 in post-burn patients [42,44]. This phenomenon may be unique to burn injury, as no significant decrease in NK activity or IL-2 production was found in general trauma patients.
Adaptive immune function
Lymphocytes
Lymphocytes represent the cellular elements of the adaptive immune response. Alterations of the lymphocyte response following thermal injury have been associated with an increased risk of complications in burn patients [45-49].
Lymphocyte suppression is a well-documented characteristic of adult burn injury non-survivors [46,48,49]. This has been demonstrated as early as 48 hours post-injury and has been associated with complications including infection and death [46]. This suppression tends to recover after 2-4 weeks in survivors as compared to more persistent suppression in non-survivors [47]. It has also been shown that serum immunoglobulins are reduced early after thermal injury which could relate to the lower activity of B lymphocytes and plasma cells [50,51].
CD4+ T cells can be subdivided into T helper cells (Th cells) and regulatory T cells (Tregs). There are many subtypes of T helper cells, including Th1 cells and Th2 cells. Th1 cells are generally associated with a pro-inflammatory state and their formation is induced by cytokines such as IL-12. Once differentiated and activated, they secrete IL-2 and IFNγ. The Th2 cell phenotype is induced by IL-4 and these cells secrete cytokines that promote apoptosis and anti-inflammatory responses including IL-4, IL-5, and IL-10. Both Th1 and Th2 cells promote the continued differentiation of local naïve T cells to their same type while inhibiting differentiation into the other type [52,53]. Following thermal injury, the Th2 phenotype predominates with increased IL-4 concentrations and diminished IFNγ production. Animal models have shown reversibility of this polarization after treatment with IL-12, an inducer of the Th1 phenotype, indicating a potential therapeutic option [53].
Tregs are potent blockers of T cell proliferation and play a critical role in controlling autoimmune diseases, inducing transplant tolerance, and mitigating the inflammatory response [54,55]. They are also central to the immune response following major traumatic and thermal injuries. Animal models have shown enhanced Treg function in lymph nodes following burn injury along with a concomitant depressed Th1 response. Depletion of Tregs in these models restored Th1 responses suggesting that burn injury may amplify Treg function which may in turn contribute to post-injury immunosuppression [51,55]. Similar trends of enhanced Treg potency and Th2 responses have been observed in rats and human septic patients following thermal injury [50,52,56]. Tregs represent another therapeutic target, however further analysis is needed to understand these populations and their function in pediatric burn patients.
Th17 cells function in neutrophil recruitment and activation and also have an important role in mucosal immunity. IL-17 and IL-22 are both produced by Th17 cells and are potently pro-inflammatory. Th17 and Treg cells balance each other in a similar fashion to the Th1/Th2 relationship described above. Following thermal injury, IL-17 and IL-22 levels increase very early after injury, followed by weakened Th17 responses thereafter [56]. This can lead to increased Treg function, an increased anti-inflammatory profile, and thus a higher infection rate in burn patients. Children appear to have a different expression profile of IL-17 over time as compared to adults, with younger subjects having a greater elevation in IL-17 levels at early time points post-burn. More research related to IL-17 and Treg populations/regulation post burn injury is warranted.
Gamma-delta (ɣδ) T cells are considered part of both the innate and adaptive immune system given their ability to respond to antigens without processing [57]. Although they represent a small proportion of circulating T cells, they increase in numbers following burn injury and are an important source of chemokines to recruit other immune effector cells [58]. ɣδ T cells also recruit myeloid cells to the burn wound to regulate local inflammation [58]. Limited data exists regarding the role of ɣδ T cells in pediatric burn patients. Given their unique properties and ability to control systemic and local inflammation this would be a novel source of immunomodulation in thermal injury.
Co-inhibitory molecules
Programmed death 1 (PD-1) is a co-inhibitory molecule whose primary role is downregulation of the immune response. When bound to its ligands on antigen presenting cells, programmed death ligand 1 (PD-L1) and 2 (PD-L2), transcription factors are activated to inhibit the pro-inflammatory response and promote lymphocyte apoptosis.
PD-1 is upregulated following traumatic injury in humans and may have a role in post-burn immunosuppression through downregulation of T cells. Using APACHE score as a measure of the severity of illness, those with a score greater than 20 had upregulated PD-1 on both granulocytes and monocytes as compared to those with lower scores [59]. Another study conducted on adult trauma patients with acute lung injury found that those who died had higher levels of PD-1 expression on circulating lymphocytes [60]. B- and T-lymphocyte attenuator (BTLA and CTLA-4) are other co-inhibitory molecules that may have a role in post-burn immunosuppression [61]. Clinical trials in cancer patients and studies of viral infections have used antibodies to block these co-inhibitory molecules with suggestion of improved outcomes [61]. The role of these molecules as therapeutic targets in pediatric burn injury is unknown (Figure 4).
Figure 4.
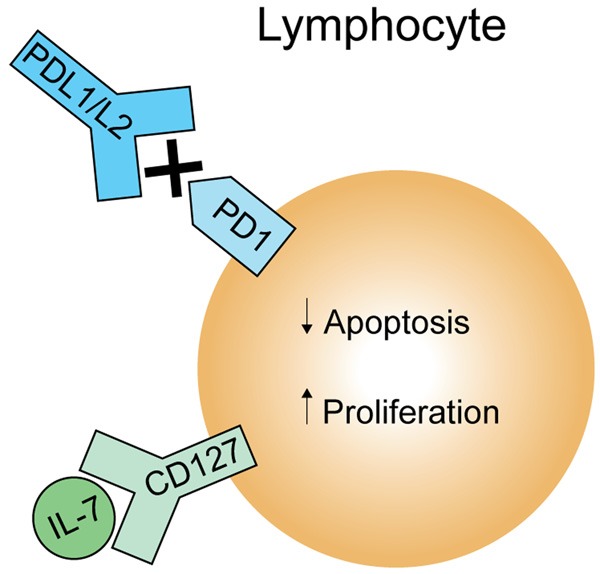
Lymphocyte Immunomodulation: Targeted therapy aimed at increasing lymphocyte proliferation and decreasing apoptosis through stimulation with IL-7 or inhibition of PD-1, respectively, is a potential avenue for immunomodulation.
Immunomodulatory therapies
There has been a plethora of clinical trials for drugs that directly or indirectly modify immune function in critically ill patients. Prior research has largely focused on reducing inflammation through the use of corticosteroids and neutralization of microbial products such as endotoxin, pro-inflammatory mediators such as TNFα, IL-1, and platelet-activating factor [62,63]. In addition, proteins that stimulate various aspects of immune function (e.g. granulocyte colony-stimulating factor [G-CSF] and IFNγ), and the administration of anti-coagulants (e.g. activated protein C [APC] and heparin) have been evaluated with minimal success in human trials [64-66]. These therapies have not been pursued on pediatric burn patients. New research into immunomodulatory therapies has begun to explore routes to prevent and reverse the immune suppression associated with critical illness, with hopes of improving outcomes for patients with thermal injury (Figures 3B and 4).
One potential target is IL-7, which is required for proper T cell development and homeostasis. It also induces proliferation of T cells during times of lymphopenia and has anti-apoptotic properties [61]. Lymphocyte proliferation and function are decreased in patients with septic shock, however improvement in both was observed following ex-vivo IL-7 treatment of isolated lymphocytes from these patients [67]. Lymphocyte suppression has also been demonstrated in thermal injury patients as discussed earlier in this review, therefore IL-7 represents an avenue for future research as a therapy for burn-induced lymphocyte suppression (Figure 4).
G-CSF is a known stimulant of the innate immune system. Its role in burn injury has been evaluated previously in which G-CSF was given to septic burn patients, including some pediatric patients, and was noted to be associated with improved survival [64]. More investigations are needed regarding this therapy, but these results suggest that it has potential to improve outcomes in burn patients.
GM-CSF is used in oncology patients following chemotherapy to restore immune function and is currently being studied for use in sepsis- and trauma-induced immune suppression [61]. GM-CSF administration has the potential to reverse macrophage dysfunction, enhance neutrophil and monocyte numbers, and improve monocyte function following critical injury (Figure 3B). Ex-vivo GM-CSF stimulation of monocytes isolated from critically injured children resulted in restoration of TNFα production capacity though these monocytes were not from children with thermal injury [13]. GM-CSF, an FDA-approved drug with a low side effect profile, appears to be a promising agent for immunostimulatory research specific to pediatric burn patients.
IFNγ has shown promise in the recovery of monocyte function in a preliminary study with septic patients [65]. Administration of this immune-stimulating cytokine restored monocyte HLA-DR expression and in vitro LPS-induced TNFα secretion in patients who had low levels of both [65]. These data suggest that IFNγ treatment may be a useful immunomodulatory strategy in this patient population and warrant further evaluation for effectiveness in pediatric burn patients.
Genome-wide analyses of enriched cellular populations from peripheral blood mononuclear cells isolated from critically injured patients have provided scientists a means to better understand regulatory networks of immune cells [68]. In addition, comparative global proteome analyses of plasma samples may provide a means to identify changes in protein concentrations and expression profiles after an immunologic insult [69]. Such analyses may be used to identify perturbations in immune cell networks specific to pediatric burn patients, which can guide immunomodulatory strategies (Figure 4).
Conclusions
Burn injury produces a profound inflammatory response which can lead to impaired immune function. A balance of the pro-inflammatory and anti-inflammatory response is needed in order to achieve best outcomes. Throughout the discussion above we have demonstrated that various cell populations are involved in the alteration of the immune response following burn injury. Novel targets and strategies are needed in order to reduce the morbidity associated with burn injury. Most literature with regard to immune function after thermal injury is derived from adult studies. More research in pediatric thermal injury is needed in order to fully evaluate the function of the immune system in this vulnerable and understudied population. From neonates to adolescents, the developmental differences in inflammation and immune response to burn injury represent an important unknown in the field [24]. There are also many other potential confounders of the immune system in the setting of pediatric burn injury that must be studied, including the effects of sex, hormonal influences, and nutrition. We must generate this new knowledge which can then be used for the development of targeted immunomodulatory therapy to improve outcomes for children with severe thermal injury.
Acknowledgements
This research is funded by internal departmental awards through The Research Institute at Nationwide Children’s Hospital and the Department of Pediatric Surgery at Nationwide Children’s Hospital.
Disclosure of conflict of interest
None.
References
- 1.Lee CJ, Mahendraraj K, Houng A, Marano M, Petrone S, Lee R, Chamberlain RS. Pediatric burns: a single institution retrospective review of incidence, etiology, and outcomes in 2273 burn patients (1995-2013) J Burn Care Res. 2016;37:e579–e585. doi: 10.1097/BCR.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez R, Shanti CM. Overview of current pediatric burn care. Semin Pediatr Surg. 2015;24:47–49. doi: 10.1053/j.sempedsurg.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 3.American Burn Association. National burn repository 2016 report: report of data from 2006-2015. 2016 [Google Scholar]
- 4.Oncul O, Oksuz S, Acar A, Ulkur E, Turhan V, Uygur F, Ulcay A, Erdem H, Ozyurt M, Gorenek L. Nosocomial infection characteristics in a burn intensive care unit: analysis of an eleven-year active surveillance. Burns. 2014;40:835–841. doi: 10.1016/j.burns.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Gastmeier P, Weigt O, Sohr D, Ruden H. Comparison of hospital-acquired infection rates in paediatric burn patients. J Hosp Infect. 2002;52:161–165. doi: 10.1053/jhin.2002.1292. [DOI] [PubMed] [Google Scholar]
- 6.Schlager T, Sadler J, Weber D, Donowitz L, Lohr J. Hospital-acquired infections in pediatric burn patients. South Med J. 1994;87:481–484. doi: 10.1097/00007611-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Wurtz R, Karajovic M, Dacumos E, Jovanovic B, Hanumadass M. Nosocomial infections in a burn intensive care unit. Burns. 1995;21:181–184. doi: 10.1016/0305-4179(95)80005-9. [DOI] [PubMed] [Google Scholar]
- 8.Weber JM, Sheridan RL, Pasternack MS, Tompkins RG. Nosocomial infections in pediatric patients with burns. Am J Infect Control. 1997;25:195–201. doi: 10.1016/s0196-6553(97)90004-3. [DOI] [PubMed] [Google Scholar]
- 9.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muszynski JA, Thakkar R, Hall MW. Inflammation and innate immune function in critical illness. Curr Opin Pediatr. 2016;28:267–273. doi: 10.1097/MOP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 12.Jewo PI, Fadeyibi IO. Progress in burns research: a review of advances in burn pathophysiology. Ann Burns Fire Disasters. 2015;28:105–115. [PMC free article] [PubMed] [Google Scholar]
- 13.Muszynski JA, Nofziger R, Greathouse K, Nateri J, Hanson-Huber L, Steele L, Nicol K, Groner JI, Besner GE, Raffel C, Geyer S, El-Assal O, Hall MW. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock. 2014;42:313–321. doi: 10.1097/SHK.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 16.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC Jr. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 17.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC Jr. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–1312. doi: 10.1001/archsurg.1988.01400350023002. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg. 1993;80:205–209. doi: 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 19.Cheadle WG, Hershman MJ, Wellhausen SR, Polk HC Jr. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am J Surg. 1991;161:639–645. doi: 10.1016/0002-9610(91)91247-g. [DOI] [PubMed] [Google Scholar]
- 20.Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, Obertacke U, Hirche H, Schade UF, Grosse-Wilde H. HLA-DR expression and soluble HLADR levels in septic patients after trauma. Ann Surg. 1999;229:246–254. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakos G, Malamou-Mitsi VD, Lachana A, Karassavoglou A, Kitsiouli E, Agnandi N, Lekka ME. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30:1488–1494. doi: 10.1097/00003246-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR. Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest. 2005;127:2139–2150. doi: 10.1378/chest.127.6.2139. [DOI] [PubMed] [Google Scholar]
- 23.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 24.Csontos C, Foldi V, Palinkas L, Bogar L, Roth E, Weber G, Lantos J. Time course of pro- and anti-inflammatory cytokine levels in patients with burns--prognostic value of interleukin-10. Burns. 2010;36:483–494. doi: 10.1016/j.burns.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 26.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsurumi A, Que YA, Ryan CM, Tompkins RG, Rahme LG. TNF-alpha/IL-10 ratio correlates with burn severity and may serve as a risk predictor of increased susceptibility to infections. Front Public Health. 2016;4:216. doi: 10.3389/fpubh.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fikrig SM, Karl SC, Suntharalingam K. Neutrophil chemotaxis in patients with burns. Ann Surg. 1977;186:746–748. doi: 10.1097/00000658-197712000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjornson AB, Bjornson HS, Altemeier WA. Serum-mediated inhibition of polymorphonuclear leukocyte function following burn injury. Ann Surg. 1981;194:568–575. doi: 10.1097/00000658-198111000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 31.Logters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, Scholz M. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol. 2009;198:211–219. doi: 10.1007/s00430-009-0121-x. [DOI] [PubMed] [Google Scholar]
- 32.Hampson P, Dinsdale RJ, Wearn CM, Bamford AL, Bishop JR, Hazeldine J, Moiemen NS, Harrison P, Lord JM. Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: a prospective observational cohort study. Ann Surg. 2017;265:1241–1249. doi: 10.1097/SLA.0000000000001807. [DOI] [PubMed] [Google Scholar]
- 33.Jones CN, Moore M, Dimisko L, Alexander A, Ibrahim A, Hassell BA, Warren HS, Tompkins RG, Fagan SP, Irimia D. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One. 2014;9:e114509. doi: 10.1371/journal.pone.0114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galbraith N, Walker S, Galandiuk S, Gardner S, Polk HC Jr. The significance and challenges of monocyte impairment: for the ill patient and the surgeon. Surg Infect (Larchmt) 2016;17:303–312. doi: 10.1089/sur.2015.245. [DOI] [PubMed] [Google Scholar]
- 35.Galbraith N, Walker S, Carter J, Polk HC Jr. Past, present, and future of augmentation of monocyte function in the surgical patient. Surg Infect (Larchmt) 2016;17:563–569. doi: 10.1089/sur.2016.014. [DOI] [PubMed] [Google Scholar]
- 36.Yang HM, Yu Y, Chai JK, Hu S, Sheng ZY, Yao YM. Low HLA-DR expression on CD14+ monocytes of burn victims with sepsis, and the effect of carbachol in vitro. Burns. 2008;34:1158–1162. doi: 10.1016/j.burns.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Venet F, Tissot S, Debard AL, Faudot C, Crampe C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- 38.Skurzak H, Steiner L, Klein E, Lamon E. Cytotoxicity of human peripheral lymphocytes for glioma, osteosarcoma, and glia cell lines. Natl Cancer Inst Monogr. 1973;37:93–102. [PubMed] [Google Scholar]
- 39.Santoli D, Trinchieri G, Lief FS. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978;121:526–531. [PubMed] [Google Scholar]
- 40.Linnemann CC Jr, MacMillan BG. Viral infections in pediatric burn patients. Am J Dis Child. 1981;135:750–753. doi: 10.1001/archpedi.1981.02130320064021. [DOI] [PubMed] [Google Scholar]
- 41.Seeman J, Konigova R. Cytomegalovirus infection in severely burned patients. Acta Chir Plast. 1976;18:142–151. [PubMed] [Google Scholar]
- 42.Blazar BA, Rodrick ML, O’Mahony JB, Wood JJ, Bessey PQ, Wilmore DW, Mannick JA. Suppression of natural killer-cell function in humans following thermal and traumatic injury. J Clin Immunol. 1986;6:26–36. doi: 10.1007/BF00915361. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann C, Zeis M, Uharek L. Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J Haematol. 2001;114:660–665. doi: 10.1046/j.1365-2141.2001.02995.x. [DOI] [PubMed] [Google Scholar]
- 44.Bender BS, Winchurch RA, Thupari JN, Proust JJ, Adler WH, Munster AM. Depressed natural killer cell function in thermally injured adults: successful in vivo and in vitro immunomodulation and the role of endotoxin. Clin Exp Immunol. 1988;71:120–125. [PMC free article] [PubMed] [Google Scholar]
- 45.Deitch EA, Landry KN, McDonald JC. Postburn impaired cell-mediated immunity may not be due to lazy lymphocytes but to overwork. Ann Surg. 1985;201:793–802. doi: 10.1097/00000658-198506000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munster AM, Winchurch RA, Birmingham WJ, Keeling P. Longitudinal assay of lymphocyte responsiveness in patients with major burns. Ann Surg. 1980;192:772–775. doi: 10.1097/00000658-198012000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schluter B, Konig W, Koller M, Erbs G, Muller FE. Studies on B-lymphocyte dysfunctions in severely burned patients. J Trauma. 1990;30:1380–1389. doi: 10.1097/00005373-199011000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Dong N, Jin BQ, Yao YM, Yu Y, Cao YJ, He LX, Chai JK, Sheng ZY. [Change in T cell-mediated immunity and its relationship with high mobility group box-1 protein levels in extensively burned patients] . Zhonghua Wai Ke Za Zhi. 2008;46:759–762. [PubMed] [Google Scholar]
- 49.Daniels JC, Sakai H, Cobb EK, Lewis SR, Larson DL, Ritzmann SE. Evaluation of lymphocyte reactivity studies in patients with thermal burns. J Trauma. 1971;11:595–601. doi: 10.1097/00005373-197107000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Arturson G, Hogman CF, Johansson SG, Killander J. Changes in immunoglobulin levels in severely burned patients. Lancet. 1969;1:546–548. doi: 10.1016/s0140-6736(69)91957-6. [DOI] [PubMed] [Google Scholar]
- 51.Munster AM, Hoagland HC, Pruitt BA Jr. The effect of thermal injury on serum immunoglobulins. Ann Surg. 1970;172:965–969. doi: 10.1097/00000658-197012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 53.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. discussion 490-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14:R3. doi: 10.1186/cc8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 56.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol. 2012;92:529–538. doi: 10.1189/jlb.0212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35:318–326. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rani M, Zhang Q, Schwacha MG. Gamma delta T cells regulate wound myeloid cell activity after burn. Shock. 2014;42:133–141. doi: 10.1097/SHK.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monaghan SF, Thakkar RK, Tran ML, Huang X, Cioffi WG, Ayala A, Heffernan DS. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock. 2012;38:117–122. doi: 10.1097/SHK.0b013e31825de6a3. [DOI] [PubMed] [Google Scholar]
- 60.Monaghan SF, Thakkar RK, Heffernan DS, Huang X, Chung CS, Lomas-Neira J, Cioffi WG, Ayala A. Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor, programmed death-1. Ann Surg. 2012;255:158–164. doi: 10.1097/SLA.0b013e31823433ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–233. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International sepsis trial study group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M. Randomized, placebo-controlled trial of the antitumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Arslan E, Yavuz M, Dalay C. The relationship between tumor necrosis factor (TNF)-alpha and survival following granulocyte-colony stimulating factor (G-CSF) administration in burn sepsis. Burns. 2000;26:521–524. doi: 10.1016/s0305-4179(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 65.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 66.Finfer S, Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Gardlund B, Marshall JC, Rhodes A. Design, conduct, analysis and reporting of a multi-national placebo-controlled trial of activated protein C for persistent septic shock. Intensive Care Med. 2008;34:1935–1947. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venet F, Foray AP, Villars-Mechin A, Malcus C, Poitevin-Later F, Lepape A, Monneret G. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 68.Laudanski K, Miller-Graziano C, Xiao W, Mindrinos MN, Richards DR, De A, Moldawer LL, Maier RV, Bankey P, Baker HV, Brownstein BH, Cobb JP, Calvano SE, Davis RW, Tompkins RG. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci U S A. 2006;103:15564–15569. doi: 10.1073/pnas.0607028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian WJ, Jacobs JM, Camp DG 2nd, Monroe ME, Moore RJ, Gritsenko MA, Calvano SE, Lowry SF, Xiao W, Moldawer LL, Davis RW, Tompkins RG, Smith RD. Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry. Proteomics. 2005;5:572–584. doi: 10.1002/pmic.200400942. [DOI] [PMC free article] [PubMed] [Google Scholar]


