Abstract
Background
DYT6 dystonia can have an unpredictable clinical course and the result of deep brain stimulation (DBS) of the internal part of the globus pallidus (GPi) is known to be less robust than in other forms of autosomal dominant dystonia. Patients who had previous stereotactic surgery with insufficient clinical benefit form a particular challenge with very limited other treatment options available.
Case Report
A pediatric DYT6 patient unexpectedly deteriorated to status dystonicus 1 year after GPi DBS implantation with good initial clinical response. After repositioning the DBS electrodes the status dystonicus resolved.
Discussion
This case study demonstrates that medication‐resistant status dystonicus in DYT6 dystonia can be reversed by relocation of pallidal electrodes. This case highlights that repositioning of DBS electrodes may be considered in patients with status dystonicus, especially when the electrode position is not optimal, even after an initial clinical response to DBS.
Keywords: Status dystonicus, deep brain stimulation, DYT6
Introduction
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions, causing abnormal, often repetitive, movements, postures, or both. Childhood dystonia is often genetic,1 and DYT6 is one of the autosomal dominant forms, caused by mutations in the thanatos-associated domain-containing apoptosis-associated protein 1 (THAP1) gene.2,3 Clinically, DYT6 is characterized by an early age of onset, with symptoms that frequently start in the craniocervical region and spread to the extremities.3,4 Case series on deep brain stimulation (DBS) of the globus pallidus internus (GPi) for DYT6 suggest that improvement is to be expected, but less robust and less predictable than DYT1 dystonia.4–6 One potential reason for this is that there is often prominent oromandibular dystonia, which is less responsive to DBS.7 Furthermore, deterioration of dystonic symptoms 1–3 years after implantation has been reported in DYT6 patients.4,5
Status dystonicus (SD) represents the severe end of a deteriorating spectrum of dystonia.8 Recently, SD has been defined as “a movement disorder emergency characterized by severe episodes of generalized or focal hyperkinetic movement disorders that had necessitated urgent hospital admission because of life-threatening complications regardless of the patient’s neurological condition at baseline.”9 To date, there is no consensus on the optimal treatment protocol for SD,8,10–12 but early surgical intervention may be a valuable addition to the medical armamentarium for its cessation.8,13 Here we report the case of an 11-year-old DYT6 patient with unexpected and rapid clinical deterioration to SD, after a 1-year period of good response to GPi DB.
The SD was reversed by repositioning of the DBS electrodes.
Case report
After a normal birth and development, our patient developed a disturbed walking pattern at the age of 3.5 years. At age 5 he was diagnosed with dystonia and 1 year later a p.Arg29Pro mutation in the THAP1 gene was found and the diagnosis DYT6 dystonia was made. His dystonia gradually progressed to the upper limbs at age 6 and at age 9 he developed generalized dystonia. Despite pharmacological treatment with different medications his symptoms further deteriorated and he was no longer able to attend school. He became wheelchair bound with hardly intelligible speech and developed a severely impaired hand function. The neurological assessment on the Burke–Fahn–Marsden Dystonia Rating Scale Movement (BFMDRS-M) at that time was 71 (range 0–120), and on the disability part of the scale (BFMDRS-D) the score was 21 (range 0–30); see Table 1. After multidisciplinary evaluation, DBS was performed with bilateral pallidal electrodes (model 3387; Medtronic, MN, USA) using direct magnetic resonance-guided stereotactic targeting (Figure 1). A postoperative computed tomography scan showed that the actual electrode positions were more lateral than intended (Table 2). Nevertheless, the patient responded well to the DBS and 1 year after the implantation, he could walk without support, and had a clearly improved hand function and speech (BFMDRS-M 69 and BFMDRS-D 14). However, after the first year the effect of pallidal stimulation decreased and at 15 months postoperatively (age 11 years) his clinical status progressively deteriorated to SD, requiring hospital admission. Constipation was considered as a possible trigger and was treated by laxatives without success. No other possible triggers were identified. Despite symptomatic treatment with trihexyphenidyl (6 mg/day, body weight 30 kg), gabapentin (300 mg/day), and clonazepam (1.0 mg/day) and reprogramming of the DBS settings, he developed severe episodes of generalized dystonic spasms, which progressed to continuous abnormal postures and sustained contractions. This was accompanied by metabolic derangements (creatine kinase levels up to 920 IU/L), exhaustion, pain, sleep disturbance, dysphagia, and cachexia. Based on the criteria described by Allen et al.,11 he was initially diagnosed with grade 3 SD, further deteriorating towards grade 4 SD. Since this is a potentially life-threatening situation, the patient was admitted to an intensive care unit (ICU). On the ICU, pharmacological treatment with high doses of benzodiazepines (up to intravenous midazolam 1 mg/kg/hour and enteral clonazepam 3.6 mg/day, body weight 25 kg), clonidine (intravenous 105 µg/day), chloral hydrate (1,250 mg/day), baclofen (12.5 mg/day), gabapentin (900 mg/day), and trihexyphenidyl (8 mg/day) had only limited effect. Nevertheless, he experienced less discomfort, less pain, and the metabolic derangements resolved. However, he suffered from severe adverse effects, especially drowsiness. When subsequently decreasing the dosages, the dystonic movements and the discomfort became more severe. After 4 weeks on the ICU, his condition deteriorated to a total BFMDRS score of 138 (Table 1).
Table 1. BFMDRS Scores at Different Time Points.
| BFMDRS Scores | May 2014 | June 2015 | December 2015 | January 2016 | February 2016 | October 2017 |
|---|---|---|---|---|---|---|
| Before 1st Surgery | 1 Year after 1st Surgery | Status Dystonicus | Before 2nd Surgery | After 2nd Surgery | 3 Years after 1st Surgery | |
| Disability | 26 | 14 | 29 | 30 | 27 | 15 |
| Movement | 71 | 69 | 90 | 108 | 73 | 64 |
| Total | 97 | 83 | 119 | 138 | 100 | 79 |
The first deep brain stimulation implantation was in May 2015, the second in February 2016. For privacy reasons, the patient and his parents did not give permission to provide supplemental videos.
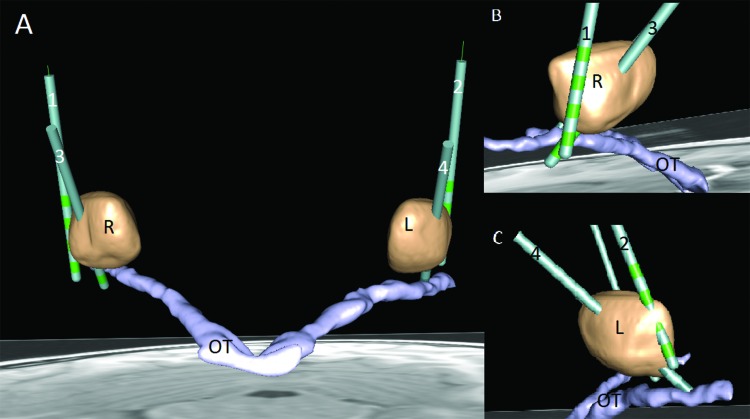
Figure 1. Schematic Depiction of the Electrode Positions. (A) Anterior coronal three-dimensional view of initial electrode positions (right 1, left 2) and electrode positions after second surgery (right 3, left 4). Note the position outside the right GPi (R) and barely inside the left GPi (L) of initial electrodes and the improved position after revision surgery. (B) Sagittal view from the right. (C) Sagittal view from the left. Note the improved position of 2 and 4 with at least two contacts within both internal parts of the globus pallidus (GPis). This is achieved by a more frontal burr hole facilitating a more oblique trajectory through the GPi. Anatomical structures and DBS electrodes were drawn into the patients using computed tomography and magnetic resonance imaging in SureTune2 software (Medtronic, MN, USA). R, GPi right; L, GPi left; OT, optic tract; 1, initial electrode right; 2, revised electrode right; 3, initial electrode left; 4, revised electrode left.
Table 2. Electrode Positions Relative to the Midcommisural Point.
| X left | Y left | Z left | X right | Y right | Z right | |
|---|---|---|---|---|---|---|
| First surgery | 22.41 | 2.7 | –2.9 | 22.61 | 3.1 | –2.8 |
| Second surgery | 20 | 3 | –4 | 20 | 3 | –4 |
Target coordinates relative to anterior commissure - posterior commissure line midpoint in millimeters.
Realized lateral coordinate left 23.1 mm and right 24.4 mm.
After extensive multidisciplinary and multicenter deliberation it was decided to reposition the pallidal electrodes to a more dorsal and more medial position. Target coordinates of the old and new electrodes are shown in Table 2 and the new target was further refined by microelectrode recording. After the repositioning of the DBS electrodes the SD ameliorated to a BFMDRS score of 100 after 1 week, and medication dosages were drastically reduced. Six months after the second surgery he was able to walk short distances unaided and attend school without medication (BFMDRS-M 64, BFMDRS-D 15). At present, the duration after the repositioning of the electrodes is 24 months, and the clinical condition of the patient is still gradually improving.
During the first surgery the goal was to place electrodes in the posteroventrolateral GPi. However, Figure 1 shows that the electrodes were actually positioned within the external segment of the globus pallidus (GPe). The new electrodes were placed more medially in the posteroventrolateral GPi. The stimulation parameters after the initial implantation were bilateral monopolar stimulation of the most ventral contacts (pulse width 90 μs, frequency 130 Hz, and voltage 2.5 V). In the first year after the initial implantation, voltages were bilaterally increased to 3.5 V. Nine months after the initial implantation stimulation parameters were switched to a big bipolar stimulation field (0–/3+ and 8–/11+), with pulse width of 90 μs, a stimulation frequency of 130 Hz, and a voltage of 4.0 V on both sides. During the SD, the stimulation frequency was changed into 180 Hz on both sides without clinical effect. After the repositioning the stimulation parameters were contacts 1–/2+ and 8–/9+, pulse width 210 μs, frequency 130 Hz, and a voltage of 5.4 V for both sites.
Discussion
This case study demonstrates that medication-resistant SD in DYT6 dystonia can be reversed by repositioning of pallidal electrodes. This is an important finding, particularly because SD can be life-threatening.8
The exact prevalence of SD in childhood is unknown.8 Two comprehensive systematic literature studies describe a total of 133 episodes of SD in 109 patients, the majority of whom were under age 16 years.8,10 Clinically, SD is characterized by the development of increasingly frequent or continuous severe episodes of generalized dystonic spasms,10,11 often complicated by one or more of the following: bulbar weakness compromising upper airway patency; exhaustion; pain; and metabolic imbalances.12 In two-thirds of cases, a precipitating factor can be identified.8,10 Important triggers include infection, pain, constipation, or a medication change.8,10,12 Addressing these factors is the first step of a recently proposed multistaged approach to childhood SD.10 Neurosurgical intervention for SD appears to have become more frequent in the management of SD, with reported percentages ranging from 40% to 66% of SD patients.8,10 In about 70% of these cases, return to pre-SD baseline or further improvements have been reported.8,10 However, prospective blinded studies on the treatment of SD with systematic follow-up are missing.
In our case, the initial DBS placement gave some clinical benefit, despite suboptimal electrode localization. Fifteen months after surgery the patient developed severe SD and repositioning of DBS electrodes led to return to the pre-SD baseline condition. The initial response to the first DBS implantation despite the lateral position of the electrodes might be explained by extension of the electrical field into the GPi. Alternatively, it could also be the effect of GPe inhibition. As proposed in the basal-ganglia-thalamic circuit (BGTC) model for dystonia,14 DBS induced increased GPe activity might disrupt the increased BGTC synchronized oscillations in dystonia.8,9,15 However, the optimal DBS target for dystonia is the posteroventrolateral GPi.7 In the literature, target coordinates vary from 18 to 22 mm lateral from the midline.16–18 In our patient, the electrodes were placed too lateral (left 23.1 mm/right 24.4 mm). The reversal of SD by repositioning of the electrodes highlights the importance of optimal electrode placement.
The case also illustrates the unpredictable clinical effect of DBS in DYT6. This is in line with previous studies focusing on the response of DYT6 patients to pallidal DBS.4–6,19 Two of these studies describe DYT6 patients who initially responded well to pallidal stimulation, but after 1–3 years of stimulation regression occurred requiring lead reposition.4,5
Noteworthy, in this case study, changes in clinical condition of the patient seem to be reflected better by the BFMDRS-D scores than by the BFMDRS-M scores. For example, the BFMDRS-M score 1 year after the first implementation (69) hardly differs from the preoperative BFMDRS-M score (71), while daily functioning was clearly improved, as is shown by a decrease in BFMDRS-D scores from 26 to 14. A possible explanation is that BFMDRS-M measures the intensity of dystonic movements, which usually fluctuate over minutes, hours, or days,8 while BFMDRS-D scores reflect disability for a longer period of time. This observation is paralleled by the results of a previous report showing that DBS may lead to a meaningful change across multiple domains of functioning and disability, even in the absence of a significant change in BFMDRS-M scores.20
In conclusion, this case study demonstrates that severe SD in DYT6 dystonia can be reversed by relocation of pallidal DBS electrodes, highlighting the importance of optimal electrode placement. Prospective multicenter studies with systematic follow-up are needed to investigate the optimal timing and patient selection for pallidal DBS in SD.
Footnotes
Funding: None.
Financial Disclosures: M.E. van Egmond received a travel grant from Medtronic. A. Saryyeva and M. Abdallat received travel grants from Medtronic and educational support. T.J. de Koning has received research grants from Metabolic Power Foundation (non-profit) and a research grant from Actelion (for profit), and honoraria for presentations to sponsored meetings from Actelion (for profit) and for contributing to a medical advisory board meeting for Nutricia medical nutrition on the ketogenic diet. M.A.J. Tijssen received research grants from Dystonia Medical research Foundation (DMRF), Science Foundation Dystonia Society, Funds Mental Health, Phelps Foundation. In addition, she received unrestricted grants from Actelion, Merz, Ipsen, Allergan Pharmaceutics & Medtronic. J.K. Krauss is a consultant to Medtronic and to Boston Scientific. Received fees for speaking from AbbVie/St. Jude. The other authors have indicated they have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors report no conflict of interest.
Ethics Statement: This study was reviewed by the authors' institutional ethics committee and was considered exempted from further review.
References
- 1.van Egmond ME, Kuiper A, Eggink H, Sinke RJ, Brouwer OF, Verschuuren-Bemelmans C, et al. Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry. 2015;86:774–781. doi: 10.1136/jnnp-2014-309106. doi: 10.1136/jnnp-2014-309106. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 3.Djarmati A, Schneider SA, Lohmann K, Winkler S, Pawlack H, Hagenah J, et al. Mutations in THAP1 (DYT6) and generalized dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- 4.Panov F, Tagliati M, Ozelius LJ, Fuchs T, Gologorsky Y, Cheung T, et al. Pallidal deep brain stimulation for DYT6 dystonia. J Neurol Neurosurg Psychiatry. 2012;83:182–187. doi: 10.1136/jnnp-2011-300979. doi: 10.1136/jnnp-2011-300979. [DOI] [PubMed] [Google Scholar]
- 5.Groen JL, Ritz K, Contarino MF, van de Warrenburg BP, Aramideh M, Foncke EM, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;14:2420–2427. doi: 10.1002/mds.23285. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- 6.Zittel S, Moll CK, Bruggemann N, Tadic V, Hamel W, Kasten M, et al. Clinical neuroimaging and electrophysiological assessment of three DYT6 dystonia families. Mov Disord. 2010;25:2405–2412. doi: 10.1002/mds.23279. doi: 10.1002/mds.23279. [DOI] [PubMed] [Google Scholar]
- 7.Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry. 2013;84:1029–1042. doi: 10.1136/jnnp-2011-301714. doi: 10.1136/jnnp-2011-301714. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden DE, King MD, Allen NM. Status dystonicus in childhood. Curr Opin Pediatr. 2017;29:674–682. doi: 10.1097/MOP.0000000000000556. doi: 10.1097/MOP.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Lopez M, Fasano A. Rethinking status dystonicus. Mov Disord. 2017;32:1667–1676. doi: 10.1002/mds.27207. doi: 10.1002/mds.27207. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Ricciardi L, Bentivoglio AR, Canavese C, Zorzi G, Petrovic I, et al. Status dystonicus: predictors of outcome and progression patterns of underlying disease. Mov Disord. 2012;27:783–788. doi: 10.1002/mds.24981. doi: 10.1002/mds.24981. [DOI] [PubMed] [Google Scholar]
- 11.Allen NM, Lin JP, Lynch T, King MD. Status dystonicus: a practical guide. Dev Med Child Neurol. 2014;56:105–112. doi: 10.1111/dmcn.12339. doi: 10.1111/dmcn.12339. [DOI] [PubMed] [Google Scholar]
- 12.Manji H, Howard RS, Miller DH, Hirsch NP, Carr L, Bhatia K, et al. Status dystonicus: the syndrome and its management. Brain. 1998;121:243–252. doi: 10.1093/brain/121.2.243. doi: 10.1093/brain/121.2.243. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Haim S, Flatow V, Cheung T, Cho C, Tagliati M, Alterman RL. Deep brain stimulation for status dystonicus: a case series and a review of the literature. Stereotact Funct Neurosurg. 2016;94:207–215. doi: 10.1159/000446191. doi: 10.1159/000446191. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix CM, Vitek JL. Toward a network model of dystonia. Ann NY Acad Sci. 2012;1265:46–55. doi: 10.1111/j.1749-6632.2012.06692.x. doi: 10.1111/j.1749-6632.2012.06692.x. [DOI] [PubMed] [Google Scholar]
- 15.Chiken S, Nambu A. Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist. 2016;22:313–322. doi: 10.1177/1073858415581986. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53–61. doi: 10.3171/jns.1992.76.1.0053. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- 17.Starr PA, Vitek JL, DeLong M, Bakay RAE. Magnetic resonance imaging-based stereotactic localization of the globus pallidus and subthalamic nucleus. Neurosurg. 1999;44:303–313. doi: 10.1097/00006123-199902000-00031. doi: 10.1097/00006123-199902000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Krauss JK, Yianni J, Loher TJ, Aziz TZ. Deep brain stimulation for dystonia. J Clin Neurophysiol. 2004;21:18–30. doi: 10.1097/00004691-200401000-00004. doi: 10.1097/00004691-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto R, Koizumi H, Morino H, Kawarai T, Maruyama H, Mukai Y, et al. DYT6 in Japan-genetic screening and clinical characteristics of the patients. Mov Disord. 2014;29:278–280. doi: 10.1002/mds.25745. doi: 10.1002/mds.25745. [DOI] [PubMed] [Google Scholar]
- 20.Gimeno H, Tustin K, Selway R, Lin JP. Beyond the Burke-Fahn-Marsden Dystonia Rating Scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol. 2012;16:501–508. doi: 10.1016/j.ejpn.2011.12.014. doi: 10.1016/j.ejpn.2011.12.014. [DOI] [PubMed] [Google Scholar]