Abstract
Surgery, radiation therapy and chemotherapy are the oldest modalities for cancer treatment. However, as part of a continuous research in medicine, in order to improve therapeutic precision and biological effectiveness, there is an increasing interest into the use of heavy particle (e.g. protons or heavy ions) in the treatment of solid tumors. However, the restricted availability of the technology has concentrated the expertise in highly specialized centers that take care and treat extreme cases and rare pathologies. One of the tumors that has mostly beneficiated from heavy ion therapy is represented by adenoid cystic carcinoma (ACC) of the head and neck. In the current review we will focus our attention on the role of heavy particle therapy in general, with particular interest on ACC. The article will also summarize recent clinical evidence comparing traditional radiotherapy with the new heavy particles. Moreover, molecular imaging features of this uncommon tumor with 18F-FDG and 11C-MET will be discussed and illustrated.
Keywords: Adenoid cystic carcinoma, heavy particle therapy, CIRT, neutron therapy, proton therapy, molecular imaging, positron emission tomography
Introduction
Adenoid cystic carcinoma (ACC) is a rare tumor that arises from the secretory epithelial cells of the salivary glands; it is regarded to affect 1% of head and neck cancers and approximately 10% of all salivary gland tumors. Sporadically, ACC can occur in other exocrine glands of the body, e.g. breast, lacrimal glands, nasal passages, tracheobronchial tree, prostate, cervix and vulva. The tumor occurs in all age groups, children included, but it has a slightly higher frequency in middle age patients (50-60 s) [1-4]. Histologically, ACC is composed by two kinds of cells, either myoepithelial or ductal cells, in different combinations providing three different growth patterns, namely cribriform, tubular and solid form, and consequently three grades classification based on the percent of solid component. Small set of genes, differentially expressed between normal salivary tissues and ACC specimens, have been discovered. For instance, up to 86% of ACCs are characterized by translocation t(6;9), which results in a fusion of the MYB oncogene to the transcription factor gene and leads to promote tumorigenesis by deregulation of MYB target genes expression [5]. Clinical symptoms vary according to the site of disease. The most common sign reported in 98% of the cases is a slowly growing mass, followed by pain (48%), attributed to its tendency for perineural invasion, ulceration and facial nerve paralysis, although less frequently. Distant haematogenous metastases (lung mostly, bone, liver and brain) and high propensity to perineural invasios are far more common than regional lymph node metastases [6-10]. The main factors related to loco-regional recurrence or distant metastases are tumor grade, site of presentation, tumor size, TNM stage, perineural invasion, invasion of large trunk, incomplete resection and post-operative RT dose less than 60 Gy [11]. Histopathological diagnosis remains the ‘gold standard’, especially when the planned therapeutic intervention involves radical surgery and possible sacrifice of the facial nerve. Magnetic resonance imaging (MRI) is the ideal imaging modality for staging and for treatment response evaluation in ACC, whereas the role of molecular imaging, Positron Emission Tomography/Computed Tomography (PET/CT) with 18F-Fluorodeoxyglucose (18F-FDG) in ACC, is still controversial [12-14].
The best treatment for ACC depends on tumor localization, on stage and on its biological features. The combination of surgery and radiotherapy, rather than single approach, has shown better results and confirmed as standard of care [15,16]. Heavy particle therapy, such as proton beam, neutron and carbon ions, seems show interesting results against ACC. Instead, systemic cytotoxic chemotherapy and targeted molecular therapies do not yet result in patient cure with advanced ACC [17-19].
In this review, we summarize the current status of traditional imaging and treatment in ACC and focus on possible implications stemming from new molecular imaging techniques and heavy particle therapy.
Diagnostic imaging
As cancer localization affects the treatment option, imaging technique depends by the site as well. Ultrasound (US), often combined with fine needle aspiration citology (FNAC), is the preferred imaging when the malignancy is superficial, e.g. submandibular or parotid mass. US can provides a well tissue representation and the vascular pattern. However, it is strictly operator-dependent and unable to detect lesions in the deep compartments, retropharyngeal lymphadenopathy or perineural spread. Indeed, its sensitivity, specificity and accuracy vary widely, ranging from 62% to 84%, 88% to 96%, and from 57% to 96%, respectively [20].
As mentioned above, MRI characteristics make it the preferred imaging for staging and follow-up of malignancy of the head and neck district/ACC (Figure 1), due to its high soft-tissue contrast, multi-planar display and optimal anatomic representation. As salivary glands have a fatty degeneration, normal fat has a high signal on turbo spin-echo (TSE) T1 and T2 sequences, whereas tumor presents a low T1 signal. Hence, using gadolinium that shortens relaxation time, the contrast between the enhanced lesions and normal fat is amplified. Typically, the lesions appear with ill-defined borders, invasion into adjacent tissues, low T2 signal, heterogeneous enhancement, cystic changes and necrosis. Additionally, MRI can be useful to better identify perineural spread, which appears as a replacement of fat in neural foramina and pathologic enhancement and thickening of the cranial nerves involved [13,21,22]. Dynamic contrast enhancement (DCE) MRI allows distinguishing malignant tumors, which usually show rapid enhancement and slow washout of the contrast agent. In diffusion-weighted imaging (DWI) MRI, instead, a technique that expresses freedom of motion of water molecules in intercellular spaces and relates to cellular density of tissues, the apparent diffusion coefficient of malignant tumors is generally lower than that of benign tumors.
Figure 1.
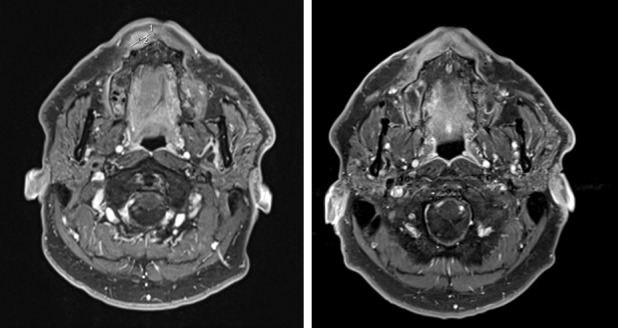
Gadolium-enhanced MRI in a patient with a right labial localization of ACC visualized before (left panel) and 3 months after CIRT (right panel). The two measurements in the first MRI correspond respectively to the longitudinal (line 1:11 mm) and transversal diameters (line 2:15 mm) of the lesion. The tumor is no longer visible after therapy.
Computed Tomography (CT) is complementary to MRI for delineating bone invasion of the skull base, orbit or mandible, or when MRI is contraindicated (i.e. cardiac pacemaker, claustrophobia), although soft tissue contrast is lower compared to MRI. Thinner slices, permitting a better multiplane reconstruction, and a shorter time of the procedure are the main advantages of CT over MRI. On the other side, patients should be screened for contrast-allergy and renal function [23].
Treatment strategies
The “gold-standard” treatment for ACCs is radical surgical resection, ensuring free margins, and postoperative radiotherapy. Many authors have demonstrated that adjuvant radiotherapy (RT) improves loco-regional control and further improve survival in ACC of head and neck [24-29]. The Dutch Head and Neck Oncology Cooperative Group [30] analyzed 565 patients (148 ACCs) and found that local and regional control were significantly higher for the group receiving combined treatment (surgery and post-operative RT) compared with those receiving surgery alone. Balamucki et al [19] confirmed furtherly the importance of adjuvant RT in primary head and neck ACC in terms of local disease control, distant metastases free survival, and overall survival. In fact, local control rates at 5 and 10 years ranged from 43% and 80%, in patients treated exclusively with RT, to 80% and 60% respectively in patients that had adjuvant RT. Moreover, the authors suggested that a low dose elective radiation of clinically negative regional nodes in ACCs arising from lymphatic-rich area could improve clinical outcome. Conversely, the recent Surveillance, Epidemiology and End Results Database (SEER) retrospective study [31] and Meyers et al [32] have shown conflicting data from the previous reports: in fact, the authors argued that ACC patients treated with adjuvant RT have no benefit in terms of cause specific survival, while they have an increased risk of cause specific death, suggesting that radiotherapy treatment should be avoid. These contradictory data could be explained by the fact that SEER analysis evaluated not only head and neck ACC but also breast, soft tissue and aero-digestive tract, which all have a different prognosis. SEER database has no information on tumor grade and subtype, perineural invasion, post-surgical margins and radiation doses used, which are all known to have an important prognostic role. Also Kokemueller et al [33], previously, were not able to demonstrate a positive effect for postoperative radiation.
Nowadays, local control rate has certainly increased thanks to the new protocols of RT. Compared to conventional photon therapy, dose escalation, intensity modulated radiation therapy (IMRT), stereotactic radiotherapy can improve local control, and disease free rate can reach 38% of cases, even in large primary tumors. At first, Münter et al [34] showed a high control rates and low side effects by using IMRT. In a recent study on skull base ACCs [35], it has been demonstrated that the combination treatments can provided local control in more than 70% of the patients. Additionally, surgery and radiotherapy were associated with overall survival advantage and improved progressive-free survival of more than three times compared to patients treated with surgery alone or with RT alone. On the other hand, recurrence radiotherapy treatment in patients that had a relapse after a previous irradiation show no benefits and significant survival disadvantage. Also Askoxylakis et al [36] report supported the results of previous studies, indicating that IMRT represents an effective and safe treatment approach for patients with sinonasal carcinomas (47 ACCs).
To summarize adjuvant RT is the standard of care after surgery in case of perineural invasion, primary tumor size above 4 cm and in presence of clinical positive lymph nodes, regardless surgical margin features (R0-R1-R2), and in locally advanced ACC. New RT techniques, such as IMRT, have a more precise target definition and dose-escalation schemes could help to administer sufficient therapeutic radiation doses even in the areas near the critical organs. However, dose restriction for the critical organs, such as brain stem, spinal cord, eyeballs and optic nerves located in the proximity of target lesion limit the administration of sufficient therapeutic radiation doses. Table 1 displays the main reports on RT in ACC.
Table 1.
Summary of the available studies regarding radiation therapy in ACC
| Number of patients with ACC | Therapy | Results/Comments | |
|---|---|---|---|
| Chen et al [24] | 140 | Surgery alone vs Surgery + RT | 5 y OS = 88%, |
| 10 y OS = 77% | |||
| Gandhi et al [25] | 90 | Surgery + RT | 2 y DFS = 75%, |
| 4 y DFS = 71% | |||
| Garden et al [26] | 198 | Surgery + RT | Excellent local control rates were obtained using surgery and postoperative RT |
| Mendenhall et al [27] | 101 | Surgery alone vs Surgery + RT | 5 y LC = 56%, OS = 57% for RT alone |
| 10 y LC = 43%, OS = 42% for RT alone | |||
| 5 y LC = 94%, OS = 77% for for combined procedures | |||
| 10 y LC = 91%, OS = 55% for for combined procedures | |||
| Zeidan et al [28] | 252 | With or without adjuvant RT | Adjuvant RT is associated with improved survival |
| Shen et al [29] | 133 | Surgery alone vs Surgery + RT | 5 y LC = 53.4%, DFS = 50% for surgery alone |
| 5 y LC = 81%, DFS = 71.3% for combined procedures | |||
| Terhaard et al [30] | 148 | Surgery and/or RT | 10 y LC = 78%, MFS = 67%, OS = 50% |
| Balamucki et al [19] | 120 | Surgery alone vs Surgery + RT | 10 y LC = 36%, DFS = 46%, MFS = 76%, OS = 37% for RT alone’ |
| 10 y LC = 84%, DFS = 71%, MFS = 62%, OS = 57% for combined procedures | |||
| Joshi et al [31] | 3427 | With or without RT | No evidence of survival benefit for adjuvant RT for local ACC |
| Meyers et al [32] | 95 | Surgery + RT | Adjuvant radiation therapy did not emerge as a prognostic factor |
| Kokemueller et al [33] | 74 | Surgery + RT | No evidence of survival benefit for adjuvant RT |
| Münter et al [34] | 25 | IMRT | 3 y OS = 72% |
| Mucositis grade 2 = 20% | |||
| Ramakrishna et al [35] | 51 | Surgery + RT vs no RT | DFS = 7.8 years, OS = 16.2 years for adjuvant RT |
| DFS = 2.1 years, OS = 5.5 years no RT | |||
| Askoxylakis et al [36] | 122 (47 ACC) | IMRT | 1 y OS = 91% |
| 3 y OS = 81% | |||
| 5 y OS = 64% | |||
| Spratt et al [41] | 27 (10 ACC) | RT | 2 y LC = 69%, MFS = 71%, OS = 50%, |
| 5 y LC = 55%, MFS = 51%, OS = 29% | |||
| Grade 3 acute toxicity = 48% | |||
| Grade 3 late toxicity = 11% |
Abbreviations: LC, local control; DFS, disease free survival; MFS, metastases free survival; DFS, disease free survival; OS, overall survival; RT, radiotherapy; IMRT, intensity-modulated radiotherapy.
Heavy particles
Conventional radiotherapy, comprising X-Rays (photons) and electrons produced both by linear accelerators, is used to treat more than 50% of all cancer patients. Particle therapy or, its synonym hadron therapy, is a form of external beam radiation therapy that uses heavier particles, which may be neutral (neutrons) or charged (protons, pions, or helium, neon, silicon, argon, and carbon ions).
Heavier particles, conversely to photons, have a peak of energy deposition at the end of the track (“Bragg peak” phenomenon), so that few energy is deposited in the healthy tissue before and beyond the tumor [37]. For these reasons, treatment with heavy particles may be of particular benefit in malignancies that are difficult or hazardous to treat with surgery, or in tumors anatomically close to relevant structure (e.g. optic nerve, spinal cord, central nervous system, and structures of the head and neck) that could be damaged by classic radiotherapy. The main limit to developing heavy particle facilities is the high initial capital cost, which requires a particular system to accelerate the particles. However, capital costs are reducing and, since 2002, the number of patients treated has increased notably. To date, there are 19 proton facilities in the United States, 19 in Europe, 13 in Japan and 4 in China, only 10 carbon ion facilities (Figure 2), but more than 30 particle therapy centers, with about 80 treatment rooms, are under construction worldwide.
Figure 2.
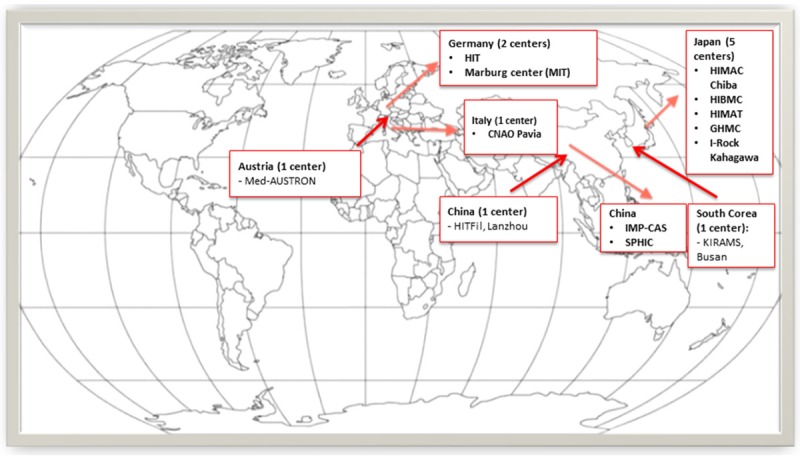
Distribution of the heavy particle centers worldwide already in use or under construction.
Neutrons
Neutrons are high LET particles with no charge. They are differentiated by X-rays for their high biological relative effectiveness (RBE), but not brilliant physics dose distribution as well. Douglas et al [38] have evaluated 151 patients with locally advanced-recurrent ACC treated with neutrons, concluding that fast neutron radiotherapy is an effective treatment for locally advanced ACC with an acceptable toxicity. Huber et al [39] have demonstrated that, exclusive irradiation with neutrons has higher local control compared to irradiation with photons, although with more prevalence of grade 3 and 4 late toxicity in the neutron treatment group. Thus, they suggested neutron therapy in patients with inoperable or incompletely resettable lesions, although no significant difference in terms of survival among the groups has been observed. A randomized study by Laramore et al [40], sponsored by Radiation Therapy Oncology Group and Medical Research Council, have compared neutrons and photons treatment in patients with inoperable or recurrent cancer of the salivary glands. The loco-regional control and overall survival rates at 2 and 10 years were significantly higher in the neutron arm than photon group, but with an increased late toxicity. Spratt et al [41], based on the abovementioned study, showed similar results applying 3D-CRT and IMRT radiotherapy with low rate of late complications. So that, they concluded that photon based radiotherapy might be an acceptable alternative to neutron radiotherapy in patients who present with unresectable salivary gland tumors.
Protons
Protons are low LET charged particle accelerated by either cyclotron or synclotron up to 250 MeV. The higher the energies, the larger Bragg peaks. A small study by Zenda et al [42], based on 39 patients with unresettable nasal cavity and para-nasal sinus tumor (only five ACCs) treated with proton beam therapy, has shown promising results with a good local control rate, progression free survival and overall survival. The most common acute toxicities were only mild dermatitis, while no grade 3 toxicity occurred. Pommier et al [43] have demonstrated that ACC arising from nasopharynx and paranasal sites could be excellently treated with passive scatter proton therapy combined with five-field 3D conformal photon therapy. No patients had neck recurrences. Linton et al [44] have evaluated proton beams in 26 ACC patients as adjuvant treatment. This retrospective analysis has shown encouraging results with a 2-years local disease control of 95% in patients treated for initial disease and 86% for those treated for recurrent disease. Also in Fukumitsu et al report [45], the previous good results of proton beams were confirmed, demonstrating reduction of irradiation to the optic chiasm and brainstem. In a small cohort of 16 patients, Lin et al [46] showed a better progression free survival in patients with recurrent nasopharyngeal carcinoma receiving an optimal proton dose coverage compared to those receiving suboptimal dose.
In the literature, there is only one paper investigating a possible role of multifield optimization (MFO) intensity modulated proton therapy (IMPT) in head and neck cancer. This small prospective study, by Frank et al [47], has evaluated 10 patients with squamous cell carcinoma and 5 with ACC. The overall clinical complete response was 93% with no treatment related death. A single patient had grade 3 xerostomia during the treatment and six patients developed grade 3 mucositis within the planning target volumes. The early results were challenging but longer outcome are needed to evaluate late toxicity and to correlate MPO-IMPT to the prognosis. On the other hand, temporal lobe necrosis after skull base proton therapy is a possible rare side effect, related to dose volume. Hence, McDonald et al [48] suggest that treatment-planning goals should include constraints on the volume of temporal lobes receiving higher dose.
In summary, protons have a good local control disease and a better toxicity profile compared to neutrons, so that they might be used alone or associated with conventional radiotherapy.
Carbon-ion radiotherapy (CIRT)
Carbon ions with their high LET and RBE may be a more effective treatment option than conventional radiotherapy or protons. Heavier than protons, carbon ions undergo a two-stage acceleration process: initial acceleration in a linear accelerator up to 10 per cent of light speed, followed by further acceleration in a synchrotron up to 75 per cent of light speed. Due to their denser deposition of destructive energy, a little effect of fraction size is determined, so that the ability to deliver shorter courses of treatment (hypofractionation) makes carbon ions an attractive treatment option [49,50].
A recent retrospective study, by Takagi et al [51], has compared clinical outcomes and late complications in ACC patients treated with proton therapy or CIRT. No significant difference between these two charged particles with respect to survival and local control was demonstrated. The authors have hypothesized that the reason for the similarity in treatment efficacy was related to the range of equivalent dose as 2-Gy fractions for α/β = 10 (EQD10/2), almost the same in both groups. Additionally, no statistical difference in late toxicity was found. A review of Mizoe et al [52], based on 236 patients with head and neck cancer (including 69 ACCs) has demonstrated that CIRT has similar results in terms of local control and survival compared to neutron based therapy but no late grade 3 and 4 toxicity. Previously, the same group [53] demonstrated that reduction of fractions during CIRT therapy had a positive impact on clinical outcome in terms of morbidity and local control. Another retrospective Japanese study [54], has confirmed that CIRT could be a useful approach with an acceptable toxicity based in 18 patients with locally advanced tongue ACC (T4a). No cases of grade 4 toxicity were reported, and only few cases had grade 3 toxicity. Compared to surgical treatment, CIRT preserved speech and the swallowing function in all the patients. In Germany, Schultz Ertner et al [55] have retrospectively evaluated 65 patients with locally advanced ACC of head and neck treated with stereotactic radiotherapy/IMRT and CIRT boost or radiotherapy alone. Although no statistical differences were found, the graphs showed that a combination of photon RT and CIRT being more advantageous, with a trend toward higher loco regional control rates compared with conventional radiotherapy alone. Another recent German retrospective study, by Jensen et al [56], has compared 95 patients with locally advanced ACC treated combining IMRT and CIRT or photon radiotherapy separately. Even in this report, combined approach showed better results in terms of local control, progression free survival, local recurrent rate and overall survival at 5 and 10 years than photon radiotherapy alone. Acute and late toxicity related to CIRT occurred only in few patients. Based on the challenging results of the previous study, the Heidelberg Ion-Beam Therapy Centre have designed a prospective phase II clinical trial (COSMIC) [57], whose aim was to evaluate toxicity profile and survival of CIRT and IMRT in malignant tumors of the salivary glands (89% ACCs). Moderate toxicity without difference among groups was found and local-regional control showed no difference regarding resection status, so that the authors advised to avoid mutilating surgical procedure. The prospective mono-centric phase I/II clinical trial (ACCEPT trial) [58], evaluating the possible role of the combined chemotherapy (Erbitux) with radiotherapy (IMRT and CIRT) in the ACCS, is still on going. Representative examples of CIRT therapy pre and post treatment are shown in Figure 3.
Figure 3.
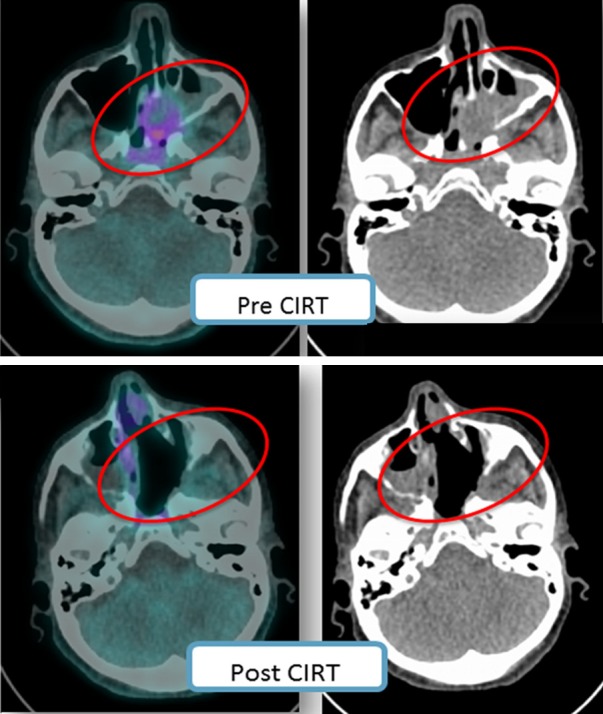
Pictorial example of a patient with nasal ACC investigated with MET PET before (upper panels) and after CIRT (lower panels); in both cases fused MET PET/CT (left side image) and low dose CT (right side image) are shown. The patient obtained a complete response to therapy.
In summary, local tumor control has a significant impact on quality of life and it can improve survival even in the presence of distant metastases. Particle therapy shows better local control than external beam radiotherapy and CIRT shows a lower rate of toxicities, compared to neutron and photon radiotherapy, with the same good results. CIRT might be also an effective treatment in loco-regional relapse occurring after photon radiotherapy. Table 2 summarizes the studies found in the literature regarding heavy particle therapy in ACC.
Table 2.
Summary of the available studies regarding heavy particle therapy in ACC
| Number of patients with ACC | Therapy | Results/Comments | |
|---|---|---|---|
| Douglas et al [38] | 151 | Neutrons | 5 y LC = 100%, DFS = 67%, MFS = 83.3%, OS = 72%, grade 3-4 toxicity 10% |
| Huber et al [39] | 75 | Neutrons, photons or both | 5 y LC = 75% for neutrons and 32% for mixed beam and photons. |
| No significant difference in survival. | |||
| Grade 3-4 toxicity more prevalent in neutrons (19%). | |||
| Laramore et al [40] | 32 | Neutron vs photon irradiation | 2 y LC = 67% for neutron and 17% for photons. |
| 10 y LC = 56% for neutron and 17% for photons. | |||
| No significant difference in survival. | |||
| Grade 3 toxicity more prevalent in neutron arm (69%). | |||
| Zenda et al [42] | 39 (5 ACC) | Protons | 6 mo LC = 84.6%, 1 y LC = 77%. |
| 3 y PFS = 49 %, OS = 59%. No grade 3 toxicity | |||
| Pommier et al [43] | 23 | Protons + photons | 5 y LC = 93%, DFS = 56%, MFS = 62%, OS = 77% |
| Linton et al [44] | 26 | Protons | 2 y LC = 86% (recurrent disease) 95% (primary disease), |
| MFS = 25% (both groups) | |||
| OS = 57% (recurrent disease), 93% (primary disease). | |||
| Grade 3 toxicity in 1 patient. | |||
| Fukumitsu et [45] | 102 head-neck (2 ACC) | Protons | 2 y LC = 35%, OS = 47.1%, |
| 5 y LC = 17%, OS = 15.7% | |||
| ≥ Grade 3 late toxicity in 2 patients | |||
| Lin et al [46] | 16 nasopharyngeal carcinoma | Protons | 2 y OS = 83% (optimal dose group) vs 17% (suboptimal dose) |
| Frank et al [47] | 15 (5 ACC) | MFO-IMPT alone or combined with chemotherapy | 28 mo OS = 93.3%. |
| Grade 3 toxicity in 7 patients | |||
| Takagi et al [51] | 80 | Protons vs CIRT | No significant difference in survival |
| Mizoe et al [52] | 236 (69 ACC) | CIRT | 5 y LC = 73%, OS = 68% for ACC |
| Mizoe et al [53] | 36 (9 ACC) | CIRT with dose escalation | 5 y LC = 90% for 9 patients with ACC |
| Koto et al [54] | 18 | CIRT | 5 y LC = 92%, DFS = 44%, OS = 72% |
| Grade 3 late toxicity in 3 patients | |||
| Schultz-Ertner [55] | 63 | Photons + CIRT boost. (29) vs Photons alone (34) | 2 y LC = 77.5%, DFS = 71.5%, OS = 86.6%, |
| 4 y LC = 77.5%, DFS = 53%, OS = 75.8%, | |||
| 2 y LC = 72.2%, DFS = 69.2%, OS = 77.9%, | |||
| 4 y LC = 24.6%, DFS = 23%, OS = 77.9%, | |||
| Grade 3 late toxicity in < 5% for both groups | |||
| Jensen et al [56] | 95 | CIRT boost + IMRT (58) vs Photons (37) | 5 y LC = 59.6%, DFS = 48.4%, OS = 76.5% |
| 5 y LC = 39.9%, DFS = 27%, OS = 58.7% | |||
| Jensen et al [57] | 53 | CIRT boost + IMRT | 3 y LC = 81.9%, DFS = 57.9%, OS = 78.4%. |
| No significant difference regarding resection status. |
Abbreviations: LC, local control; DFS, disease free survival; MFS, metastases free survival; OS, overall survival.
Molecular imaging
FDG PET
The 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is an imaging mo-dality that provides functional and anatomical information based on glucose metabolism. It is considered to be of additional value in the assessment of disease extent and in the treatment planning of head and neck cancer patients [59]. In particular, FDG PET has a well-known predictive value in squamous cell carcinoma (SCC), while since ACC is characterized by low FDG uptake, caution is needed to evaluate the usefulness of FDG PET in this context [60]. In the literature, some case reports [61,62] and few studies have assessed the clinical impact of FDG PET in ACC. Lee et al [63] and Kim et al [64] have evaluated whether SUVmax on pretreatment FDG-PET/CT was a significant predictor of survival. They found that SUVmax was higher in aggressive ACC, namely patients who developed distant metastases. In addition, multivariate analysis showed that histological grade and SUVmax were significant prognostic factors for distant metastases free survival and disease free survival. However, SUVmax is measured using the uptake in the pixel with the highest value within a region of interest, without taking in account the metabolic activity of the whole tumor. Compared to SCC, SUVmax was lower in ACC patients, probably due to the lower expression of GLUT-1. Indeed, Tomura et al [65] have revealed that the low GLUT-1 expression in ACC can consequently lead to false negative results and low FDG uptake might be obscured by the normal physiologic FDG uptake of the salivary glands. Moreover, normal brain tissue recognizes glucose as a metabolic substrate and increased FDG uptake in the brain interferes with PET visualization of tumors with skull base involvement. Recently, Jung et al [66] have demonstrated that FDG-PET has comparable sensitivity (92.3%) to conventional CT for primary lesion detection, but it changes the N and M status in part of ACC patients. Also, they confirmed SUVmax, together with total lesion glycolysis (TLG), as prognostic factors of progression free survival, though the cutoff value for SUVmax did not show uniformity with previous studies.
MET PET
Methionine is a neutral essential amino acid involved in ribosomal protein synthesis and in citric acid cycle, which provides energy in the cell, and as a cofactor for transferring mono-carbon units. Methionine is transported across the cell membrane by an amino-acid symport LAT-1 with a facilitated diffusion. Altered MET metabolism plays a central role in the tumor growth, leading to an over uptake of methionine in the cancer cells [67-69]. This metabolic network can be evaluated by carbon-11 labeled methionine (11C-MET) PET/CT. However, as 11C has a short half live (20 min), its use is limited to PET centers with own cyclotron facility.
11C-methionine (MET) is a PET tracer mainly employed in the evaluation of central nervous system tumors, where it shows not uptake/very low uptake in the healthy brain. Tomura et al [65] have demonstrated that MET PET has a superior reviewers’ inter-rater agreement compared to FDG PET in the evaluation of skull base involvement. In this study MET had a significantly higher tumor-to-normal brain uptake ratio than FDG. Shishido et al [70], additionally, have highlighted the ability of MET PET/CT in distinguishing treatment induced tissue necrosis, such as fibrosis and edema, from brain tumor recurrences and in improving survival rates by early identification of disease relapse. In fact, MET PET has a lower uptake in inflammatory and post-treatment alterations compared to FDG. Also Minamoto et al [71] have demonstrated that recurrent tumors and radio-induced necrosis have statistically different tumor-to-normal tissue ratio cut-off. The rate of MET uptake seems to be correlated to Ki67 expression, proliferation cell nuclear antigen, and micro-vessel density in brain tumors. Moreover, some authors suggest that MET can evaluate not only tumor proliferating potential but also its angiogenic capability [72].
While MET PET advantages compared to FDG are widely established in brain tumor management, only few reports, at the present, have evaluated it role in head and neck tumors. Lindoholm et al [73] underscored MET uptake is a better reflection of tumor proliferative activity in squamous head and neck cancer cell lines when compared with FDG uptake. In the 1999, Nuutinent et al [74] have investigated the potential role of MET PET in the head neck cancer after radiotherapy, showing no difference of SUV among patients with local control and those without local control. On the other hand, Hasebe et al [75] have shown that changes of tumor-to-normal tissue ratio before and after CIRT were independent prognostic factors of local and distant recurrences (Figure 4). Also Toubaru et al [76], in a recent paper, suggest MET PET as useful tool for treatment strategy in patients affected by ACC. They have demonstrated that high residual tumor after CIRT was a significant predictor of recurrence, suggesting that this information might be used for a better patient’ selection to individualize those who are eligible to a strict follow-up and/or active combination of other treatments.
Figure 4.
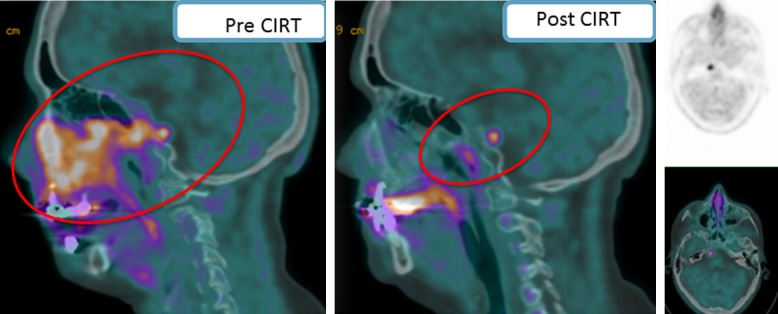
Pictorial example of an ACC cases with extensive clivus erosion, mandibular and etmoid invasion, as well as bilateral maxillary sinus involvement investigated with MET PET/CT before and after CIRT. Despite an almost complete response to therapy (red circles), at post-treatment restaging the scan showed the presence of a focal uptake in the right side of the Pons consistent with residual disease.
The same group of Hasebe et al [77], recently, further confirmed these encouraging results on MET-PET in patients with head and neck mucosal malignant melanoma after CIRT, where they found tumor-to-normal tissue ratio before therapy as a factor influencing local recurrence, metastasis, and outcomes.
New perspectives
Prostate Specific Membrane Antigen (PSMA) is a type II transmembrane glycoprotein of the prostate secretory acinar epithelium. Functional imaging of cells expressing PSMA using radiolabeled ligands with PET/CT is primarily used for the detection and (re)staging of prostate cancer. PSMA expression has been frequently investigated by immunohistochemistry (IHC) and was found to be associated with endothelial cells or tumor neovasculature in malignant disease. PMSA-ligand uptake in ACCs on PET/CT has been described previously in two case reports [78,79] and, recently, in a retrospective analysis by Klein Nulent et al [80]. The authors evaluated 9 patients with ACC by PSMA PET/CT referred either because of suspected local recurrence or distant metastases. In the study, PSMA PET/CT detected and visualized either local recurrent or distant metastatic ACCs. Additionally, PSMA-specific targeting was supported by high PSMA expression on IHC. When compared to FDG PET, the authors found concordant tracer uptake without under diagnosing clinically relevant disease progression of both local recurrence and distant metastasis.
A summary of all studies with PET tracers in ACC are illustrated in Table 3.
Table 3.
Summary of available studies with PET tracers in ACC
| Number of patients with ACC | PET Tracer | Results | |
|---|---|---|---|
| Kim et al [64] | 34 | 18F-FDG | Pre-treatment SUVmax of the primary tumor is an independent prognostic factor in patients with ACC of the head and neck |
| Jung JH et al [66] | 40 | 18F-FDG | 18F-FDG PET/CT showed comparable sensitivity to conventional CT and SUVmax and TLG were significant prognostic factors |
| Tomura et al [65] | 27 head-neck (5 ACC) | 18F-FDG vs 11C MET | MET-PET/CT showed superior inter-rater agreement and had higher uptake for tumors at the skull base than FDG-PET/CT |
| Lindoholm et al [73] | 39 SCC | 11C MET | MET PET/CT imaging is effective in SCC in head and necK |
| Nuutient et al [74] | 15 SCC | 11C MET | No significance to predict the response to RT |
| Hasebe et al [75] | 26 | 11C MET | TNR in CIRT is an independent factor for predicting local recurrence, the incidence of metastasis, and the prognosis |
| Toubaru et al [76] | 67 | 11C MET | The TNRpre was significantly related to the occurrence of metastasis and the disease-specific survival after CIRT TNRpost was a factor that was significantly related to the development of local recurrence as well |
| Hasabe et al [77] | 85 head-neck malignant melanoma | 11C MET | MET-PET/CT is useful for predicting the outcomes of patients with head and neck mucosal malignant melanoma treated with CIRT |
| Klein Nulent et al [80] | 9 | 68Ga PMSA | PSMA PET/CT is able to detect and visualize local recurrent and distant metastatic disease |
Abbreviations: SCC, squamous cell carcinoma. TNR, tumor-to-normal ratio.
Finally, PET/MRI is a new imaging technique combining functional imaging with anatomic information. This is a highly sophisticated and complex technique that can overcome some of the limitations related to ACC localization in head and neck district [81]. However, until now, no clinically applicable result have been published for patients with ACC.
Acknowledgements
AIRC (Associazione Italiana per la Ricerca sul Cancro) for the support on research.
Disclosure of conflict of interest
None.
References
- 1.Moskaluk CA. Adenoid cystic carcinoma: clinical and molecular features. Head Neck Pathol. 2013;7:17–22. doi: 10.1007/s12105-013-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38:620–627. doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Xu L, Zhao H, El-Naggar AK, Sturgis EM. A comparison of the demographics, clinical features, and survival of patients with adenoid cysticcarcinoma of major and minor salivary glands versus less common sites within the surveillance, epidemiology and end results registry. Cancer. 2012;118:3945–3953. doi: 10.1002/cncr.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med. 2011;135:511–515. doi: 10.5858/2009-0527-RS.1. [DOI] [PubMed] [Google Scholar]
- 5.Quattlebaum FW, Dockerty MB, Mayo CW. Adenocarcinoma, cylindroma type, of the parotid gland. Surg Gynecol Obstet. 1946;82:342–7. [PubMed] [Google Scholar]
- 6.Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127–132. doi: 10.1097/00020840-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Bell D, Hanna EY. Head and neck adenoid cystic carcinoma: what is new in biological markers and treatment? Curr Opin Otolaryngol Head Neck Surg. 2013;21:124–129. doi: 10.1097/MOO.0b013e32835c05fd. [DOI] [PubMed] [Google Scholar]
- 8.Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062–9. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Maruya S, Kim HW, Weber RS, Lee JJ, Kies M, Luna MA, Batsakis JG, El-Naggar AK. Gene expression screening of salivary gland neoplasms: molecular markers of potential histogenetic and clinical significance. J Mol Diagn. 2004;6:180–90. doi: 10.1016/s1525-1578(10)60508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimento AG, Amaral AL, Prado LA, Kligerman J, Silveira TR. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312–9. doi: 10.1002/1097-0142(19860115)57:2<312::aid-cncr2820570220>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Min R, Siyi L, Wenjun Y, Ow A, Lizheng W, Minjun D, Chenping Z. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: a preliminary study of 62 cases. Int J Oral Maxillofac Surg. 2012;41:952–7. doi: 10.1016/j.ijom.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Husain Q, Kanumuri VV, Svider PF, Radvansky BM, Boghani Z, Liu JK, Eloy JA. Sinonasal adenoid cystic carcinoma: systematic review of survival and treatment strategies. Otolaryngol Head Neck Surg. 2013;148:29–39. doi: 10.1177/0194599812464020. [DOI] [PubMed] [Google Scholar]
- 13.Maroldi R, Farina D, Borghesi A, Marconi A, Gatti E. Perineural tumor spread. Neuroimag Clin N Am. 2008;18:413–29. doi: 10.1016/j.nic.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Osmolski A. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions and pitfalls. Radiographics. 2006;26:745–63. doi: 10.1148/rg.263055024. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki H, Yanagi Y, Hara M, Katase N, Asaumi J, Hisatomi M, Unetsubo T, Konouchi H, Takenobu T, Nagatsuka H. Minor salivary gland tumors in the oral cavity: diagnostic value of dynamic contrast-enhanced MRI. Eur J Radiol. 2012;81:2684–91. doi: 10.1016/j.ejrad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A, Haigentz M Jr, Takes RP, Mondin V, Teymoortash A, Thompson LD, Ferlito A. Adenoid cystic carcinoma of the head and neck--an update. Oral Oncol. 2015;51:652–61. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Roh JL, Ryu CH, Choi SH, Kim JS, Lee JH, Cho KJ, Nam SY, Kim SY. Clinical utility of 18F-FDG PET for patients with salivary gland malignancies. J Nucl Med. 2007;48:240–6. [PubMed] [Google Scholar]
- 18.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 19.Balamucki CJ, Amdur RJ, Werning JW, Vaysberg M, Morris CG, Kirwan JM, Mendenhall WM. Adenoid cystic carcinoma of the head and neck. Am J Otolaryngol. 2012;33:510–8. doi: 10.1016/j.amjoto.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Bozzato A, Zenk J, Greess H, Hornung J, Gottwald F, Rabe C, Iro H. Potential of ultrasound diagnosis for parotid tumors: analysis of qualitative and quantitative parameters. Otolaryngol Head Neck Surg. 2007;137:642–6. doi: 10.1016/j.otohns.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 21.Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol. 2008;66:419–36. doi: 10.1016/j.ejrad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Weon YC, Park SW, Kim HJ, Jeong HS, Ko YH, Park IS, Kim ST, Baek CH, Son YI. Salivary duct carcinomas: clinical and CT and MR imaging features in 20 patients. Neuroradiology. 2012;54:631–40. doi: 10.1007/s00234-012-1014-z. [DOI] [PubMed] [Google Scholar]
- 23.Hong SI, Ahn S, Lee YS, Kim WY, Lim KS, Lee JH, Lee JL. Contrast-induced nephropathy in patients with active cancer undergoing contrast-enhanced computed tomography. Support Care Cancer. 2016;24:1011–7. doi: 10.1007/s00520-015-2875-6. [DOI] [PubMed] [Google Scholar]
- 24.Chen AM, Bucci MK, Weinberg V, Garcia J, Quivey JM, Schechter NR, Phillips TL, Fu KK, Eisele DW. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66:152–9. doi: 10.1016/j.ijrobp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi AK, Roy S, Biswas A, Bhasker S, Sharma A, Thakar A, Mohanti BK. Adenoid cystic carcinoma of head and neck: a single institutional analysis of 66 patients treated with multi-modality approach. Indian J Med Paediatr Oncol. 2015;36:166–71. doi: 10.4103/0971-5851.166729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–26. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 27.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 28.Zeidan YH, Pekelis L, An Y, Holsinger FC, Kong CS, Chang DT, Le QT. Survival benefit for adjuvant radiation therapy in minor salivary gland cancers. Oral Oncol. 2015;51:438–45. doi: 10.1016/j.oraloncology.2015.02.096. [DOI] [PubMed] [Google Scholar]
- 29.Shen C, Xu T, Huang C, Hu C, He S. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol. 2012;48:445–9. doi: 10.1016/j.oraloncology.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Terhaard CH, Lubsen H, Van der Tweel I, Hilgers FJ, Eijkenboom WM, Marres HA, Tjho-Heslinga RE, de Jong JM, Roodenburg JL Dutch Head and Neck Oncology Cooperative Group. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26:681–92. doi: 10.1002/hed.10400. [DOI] [PubMed] [Google Scholar]
- 31.Joshi P, Smolkin ME, Dillon PM. Role of postoperative radiation therapy in patients with adenoid cystic carcinoma: a SEER analysis. J Radiat Oncol. 2016;5:153–159. [Google Scholar]
- 32.Meyers M, Granger B, Herman P, Janot F, Garrel R, Fakhry N, Poissonnet G, Baglin AC, Lefèvre M, Baujat B REFCOR members. Head and neck adenoid cystic carcinoma: a prospective multicenter REFCOR study of 95 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:13–7. doi: 10.1016/j.anorl.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck--a 20 years experience. Int J Oral Maxillofac Surg. 2004;33:25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 34.Münter MW, Schulz-Ertner D, Hof H, Nikoghosyan A, Jensen A, Nill S, Huber P, Debus J. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the treatment of incompletely and completely resected adenoid cystic carcinomas of the head and neck: initial clinical results and toxicity of treatment. Radiat Oncol. 2006;1:17. doi: 10.1186/1748-717X-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramakrishna R, Raza SM, Kupferman M, Hanna E, DeMonte F. Adenoid cystic carcinoma of the skull base: results with an aggressive multidisciplinary approach. J Neurosurg. 2016;124:115–21. doi: 10.3171/2015.1.JNS142462. [DOI] [PubMed] [Google Scholar]
- 36.Askoxylakis V, Hegenbarth P, Timke C, Saleh-Ebrahimi L, Debus J, Röder F, Huber PE. Intensity modulated radiation therapy (IMRT) for sinonasal tumors: a single center long-term clinical analysis. Radiat Oncol. 2016;11:17. doi: 10.1186/s13014-016-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fokas E, Kraft G, An H, Engenhart-Cabillic R. Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta. 2009;1796:216–29. doi: 10.1016/j.bbcan.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Douglas JG, Laramore GE, Austin-Seymour M, Koh W, Stelzer K, Griffin TW. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:551–7. doi: 10.1016/s0360-3016(99)00445-9. [DOI] [PubMed] [Google Scholar]
- 39.Huber PE, Debus J, Latz D, Zierhut D, Bischof M, Wannenmacher M, Engenhart-Cabillic R. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photos or mixed beams? Radiother Oncol. 2001;59:161–7. doi: 10.1016/s0167-8140(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 40.Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, Maor MH, Davis LW. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical research council. Int J Radiat Oncol Biol Phys. 1993;27:235–40. doi: 10.1016/0360-3016(93)90233-l. [DOI] [PubMed] [Google Scholar]
- 41.Spratt DE, Salgado LR, Riaz N, Doran MG, Tam M, Wolden S, Katsoulakis E, Rao S, Ho A, Wong R, Lee NY. Results of photon radiotherapy for unresectable salivary gland tumors: is neutron radiotherapy’s local control superior? Radiol Oncol. 2014;48:56–61. doi: 10.2478/raon-2013-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zenda S, Kohno R, Kawashima M, Arahira S, Nishio T, Tahara M, Hayashi R, Kishimoto S, Ogino T. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2011;81:1473–8. doi: 10.1016/j.ijrobp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Pommier P, Liebsch NJ, Deschler DG, Lin DT, McIntyre JF, Barker FG 2nd, Adams JA, Lopes VV, Varvares M, Loeffler JS, Chan AW. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242–9. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 44.Linton OR, Moore MG, Brigance JS, Summerlin DJ, McDonald MW. Proton therapy for head and neck adenoid cystic carcinoma: initial clinical outcomes. Head Neck. 2015;37:117–24. doi: 10.1002/hed.23573. [DOI] [PubMed] [Google Scholar]
- 45.Fukumitsu N, Okumura T, Mizumoto M, Oshiro Y, Hashimoto T, Kanemoto A, Hashii H, Ohkawa A, Moritake T, Tsuboi K, Tabuchi K, Wada T, Hara A, Sakurai H. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys. 2012;83:704–11. doi: 10.1016/j.ijrobp.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Lin R, Slater JD, Yonemoto LT, Grove RI, Teichman SL, Watt DK, Slater JM. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy--dose-volume histogram analysis. Radiology. 1999;213:489–94. doi: 10.1148/radiology.213.2.r99nv29489. [DOI] [PubMed] [Google Scholar]
- 47.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald MW, Linton OR, Calley CS. Dose-volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int J Radiat Oncol Biol Phys. 2015;91:261–7. doi: 10.1016/j.ijrobp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Kagawa K, Murakami M, Hishikawa Y, Abe M, Akagi T, Yanou T, Kagiya G, Furusawa Y, Ando K, Nojima K, Aoki M, Kanai T. Preclinical biological assessment of proton and carbon ion beams at Hyogo Ion beam medical center. Int J Radiat Oncol Biol Phys. 2002;54:928–38. doi: 10.1016/s0360-3016(02)02949-8. [DOI] [PubMed] [Google Scholar]
- 50.Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J, Trofimov A. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Takagi M, Demizu Y, Hashimoto N, Mima M, Terashima K, Fujii O, Jin D, Niwa Y, Morimoto K, Akagi T, Daimon T, Sasaki R, Hishikawa Y, Abe M, Murakami M, Fuwa N. Treatment outcomes of particle radiotherapy using protons or carbon ions as a single-modality therapy for adenoid cystic carcinoma of the head and neck. Radiother Oncol. 2014;113:364–70. doi: 10.1016/j.radonc.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Mizoe JE, Hasegawa A, Jingu K, Takagi R, Bessyo H, Morikawa T, Tonoki M, Tsuji H, Kamada T, Tsujii H, Okamoto Y Organizing Committee for the Working Group for Head Neck Cancer. Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol. 2012;103:32–7. doi: 10.1016/j.radonc.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Mizoe JE, Tsujii H, Kamada T, Matsuoka Y, Tsuji H, Osaka Y, Hasegawa A, Yamamoto N, Ebihara S, Konno A Organizing Committee for the Working Group for Head-And-Neck Cancer. Dose escalation study of carbon ion radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60:358–64. doi: 10.1016/j.ijrobp.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 54.Koto M, Hasegawa A, Takagi R, Ikawa H, Naganawa K, Mizoe JE, Jingu K, Tsujii H, Tsuji H, Kamada T, Okamoto Y Organizing Committee for the Working Group for Head and Neck Cancer. Evaluation of the safety and efficacy of carbon ion radiotherapy for locally advanced adenoid cystic carcinoma of the tongue base. Head Neck. 2016;38(Suppl 1):E2122–6. doi: 10.1002/hed.24397. [DOI] [PubMed] [Google Scholar]
- 55.Schulz-Ertner D, Nikoghosyan A, Didinger B, Münter M, Jäkel O, Karger CP, Debus J. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer. 2005;104:338–44. doi: 10.1002/cncr.21158. [DOI] [PubMed] [Google Scholar]
- 56.Jensen AD, Nikoghosyan AV, Poulakis M, Höss A, Haberer T, Jäkel O, Münter MW, Schulz-Ertner D, Huber PE, Debus J. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer. 2015;121:3001–9. doi: 10.1002/cncr.29443. [DOI] [PubMed] [Google Scholar]
- 57.Jensen AD, Nikoghosyan AV, Lossner K, Haberer T, Jäkel O, Münter MW, Debus J. COSMIC: a regimen of intensity modulated radiation therapy plus dose-escalated, raster-scanned carbon ion boost for malignant salivary gland tumors: results of the prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2015;93:37–46. doi: 10.1016/j.ijrobp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Jensen AD, Nikoghosyan A, Hinke A, Debus J, Münter MW. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT [ACC, Erbitux® and particle therapy] . BMC Cancer. 2011;11:70. doi: 10.1186/1471-2407-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freling N, Crippa F, Maroldi R. Staging and follow-up of high grade malignant salivary gland tumours: the role of traditional versus functional imaging approaches-a review. Oral Oncol. 2016;60:157–66. doi: 10.1016/j.oraloncology.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, Bridji B, Digue L, Toubeau M, Grégoire V. Positron emission tomography with [18F] fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J. Clin. Oncol. 2010;28:1190–1195. doi: 10.1200/JCO.2009.24.6298. [DOI] [PubMed] [Google Scholar]
- 61.Deshpande PS, Chintamaneni RL, Sujanamulk B, Prabhat MP, Gummadapu S. Intraosseous adenoid cystic carcinoma of maxilla: a rare case report. Contemp Clin Dent. 2013;4:239–242. doi: 10.4103/0976-237X.114872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhagat N, Zuckier LS, Hameed M, Cathcart C, Baredes S, Ghesani NV. Detection of recurrent adenoid cystic carcinoma with PET-CT. Clin Nucl Med. 2007;32:574–577. doi: 10.1097/RLU.0b013e3180646b0f. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Choi JY, Ko YH, et al. 18F-FDG PET/CT in patients with initially diagnosed adenoid cystic carcinoma of the head and neck: clinicopathologic correlation. Nucl Med Mol Imaging. 2009;43:395–401. [Google Scholar]
- 64.Kim D, Kim W, Lee J, Ki Y, Lee B, Cho K, Kim S, Nam J, Lee J, Kim D. Pretreatment maximum standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography as a predictor of distant metastasis in adenoid cystic carcinoma of the head and neck. Head Neck. 2016;38:755–761. doi: 10.1002/hed.23953. [DOI] [PubMed] [Google Scholar]
- 65.Tomura N, Mizuno Y, Saginoya T. PET/CT findings for tumors in the base of the skull: comparison of 18F-FDG with 11C-methionine. Acta Radiol. 2016;57:325–32. doi: 10.1177/0284185115575342. [DOI] [PubMed] [Google Scholar]
- 66.Jung JH, Lee SW, Son SH, Kim CY, Lee CH, Jeong JH, Jeong SY, Ahn BC, Lee J. Clinical impact of 18F-FDG positron emission tomography/CT on adenoid cystic carcinoma of the head and neck. Head Neck. 2017;39:447–455. doi: 10.1002/hed.24605. [DOI] [PubMed] [Google Scholar]
- 67.Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro. 1984;20:663–70. doi: 10.1007/BF02619617. [DOI] [PubMed] [Google Scholar]
- 68.Stern PH, Wallace CD, Hoffman RM. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J Cell Physiol. 1984;119:29–34. doi: 10.1002/jcp.1041190106. [DOI] [PubMed] [Google Scholar]
- 69.Wheatley DN. On the problem of linear incorporation of amino acids into cell protein. Experientia. 1982;38:818–20. doi: 10.1007/BF01972291. [DOI] [PubMed] [Google Scholar]
- 70.Shishido H, Kawai N, Miyake K, Yamamoto Y, Nishiyama Y, Tamiya T. Diagnostic value of 11C-methionine (MET) and 18F-fluorothymidine (FLT) positron emission tomography in recurrent high-grade gliomas; differentiation from treatment-induced tissue necrosis. Cancers (Basel) 2012;4:244–56. doi: 10.3390/cancers4010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minamimoto R, Saginoya T, Kondo C, Tomura N, Ito K, Matsuo Y, Matsunaga S, Shuto T, Akabane A, Miyata Y, Sakai S, Kubota K. Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS One. 2015;10:e0132515. doi: 10.1371/journal.pone.0132515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka K, Yamamoto Y, Maeda Y, Yamamoto H, Kudomi N, Kawai N, Toyohara J, Nishiyama Y. Correlation of 4’-[methyl-(11)C] -thiothymidine uptake with Ki-67 immunohistochemistry and tumor grade in patients with newly diagnosed gliomas in comparison with 11C-methionine uptake. Ann Nucl Med. 2016;30:89–96. doi: 10.1007/s12149-015-1035-x. [DOI] [PubMed] [Google Scholar]
- 73.Lindholm P, Leskinen S, Lapela M. Carbon-11-methionine uptake in squamous cell head and neck cancer. J Nucl Med. 1998;39:1393–7. [PubMed] [Google Scholar]
- 74.Nuutinen J, Jyrkkiö S, Lehikoinen P, Lindholm P, Minn H. Evaluation of early response to radiotherapy in head and neck cancer measured with [11C] methionine-positron emission tomography. Radiother Oncol. 1999;52:225–32. doi: 10.1016/s0167-8140(99)00091-2. [DOI] [PubMed] [Google Scholar]
- 75.Hasebe M, Yoshikawa K, Ohashi S, Toubaru S, Kawaguchi K, Sato J, Mizoe J, Tsujii H. A study on the prognostic evaluation of carbon ion radiotherapy for head and neck adenocarcinoma with C-11 methionine PET. Mol Imaging Biol. 2010;12:554–62. doi: 10.1007/s11307-010-0318-9. [DOI] [PubMed] [Google Scholar]
- 76.Toubaru S, Yoshikawa K, Ohashi S, Tanimoto K, Hasegawa A, Kawaguchi K, Saga T, Kamada T. Accuracy of methionine-PET in predicting the efficacy of heavy-particle therapy on primary adenoid cystic carcinomas of the head and neck. Radiat Oncol. 2013;8:143. doi: 10.1186/1748-717X-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasebe M, Yoshikawa K, Nishii R, Kawaguchi K, Kamada T, Hamada Y. Usefulness of 11C-methionine-PET for predicting the efficacy of carbon ion radiation therapy for head and neck mucosal malignant melanoma. Int J Oral Maxillofac Surg. 2017;46:1220–1228. doi: 10.1016/j.ijom.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 78.De Keizer B, Krijger GC, Ververs FT, van Es RJJ, de Bree R, Willems S. 68Ga-PSMA PET-CT imaging of metastatic adenoid cystic carcinoma. Nucl Med Mol Imaging. 2017;51:360–361. doi: 10.1007/s13139-016-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lütje S, Sauerwein W, Lauenstein T, Bockisch A, Poeppel TD. In vivo visualization of prostate-specific membrane antigen in adenoid cystic carcinoma of the salivary gland. Clin Nucl Med. 2016;41:476–7. doi: 10.1097/RLU.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 80.Klein Nulent TJW, van Es RJJ, Krijger GC, de Bree R, Willems SM, de Keizer B. Prostate-specific membrane antigen PET imaging and immunohistochemistry in adenoid cystic carcinoma-a preliminary analysis. Eur J Nucl Med Mol Imaging. 2017;44:1614–1621. doi: 10.1007/s00259-017-3737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varoquaux A, Rager O, Dulguerov P, Burkhardt K, Ailianou A, Becker M. Diffusion-weighted and PET/MR imaging after radiation therapy for malignant head and neck tumors. Radiographics. 2015;35:1502–27. doi: 10.1148/rg.2015140029. [DOI] [PubMed] [Google Scholar]


