Abstract
The Sigma-1 receptor (Sig-1R) has been described as a pluripotent modulator of distinct physiological functions and its involvement in various central and peripheral pathological disorders has been demonstrated. However, further investigations are required to understand the complex role of the Sig-1R as a molecular chaperon. A specific PET radioligand would provide a powerful tool in Sig-1R related studies. As part of our efforts to develop a Sig-1R PET radioligand that shows antagonistic properties, we investigated the suitability of 1-(4-(6-methoxynaphthalen-1-yl)butyl)-4-methylpiperidine (designated PB212) for imaging Sig-1R. PB212 is a Sig-1R antagonist and exhibits subnanomolar affinity (K i = 0.030 nM) towards Sig-1R as well as good to excellent selectivity over Sig-2R. The radiolabelling of [11C]PB212 was accomplished by O-methylation of the phenolic precursor using [11C]MeI. In vitro autoradiography with [11C]PB212 on WT and Sig-1R KO mouse brain tissues revealed high non-specific binding, however using rat spleen tissues from CD1 mice and Wistar rats, high specific binding was observed. The spleen is known to have a high expression of Sig-1R. In vivo PET experiments in Wistar rats also showed high accumulation of [11C]PB212 in the spleen. Injection of Sig-1R binding compounds, haloperidol (1 mg/kg) or fluspidine (1 mg/kg) shortly before [11C]PB212 administration induced a drastic reduction of radiotracer accumulation, confirming the specificity of [11C]PB212 towards Sig-1R in the spleen. The results obtained herein indicate that although [11C]PB212 is not suitable for imaging Sig-1R in the brain, it is a promising candidate for the detection and quantification of Sig-1Rs in the periphery.
Keywords: PB212, sigma-1 receptor, PET imaging
Introduction
The term sigma receptor was initially introduced by Martin and colleagues in 1976 to describe the target of the psychotomimetic benzomorphan SKF-10,047 (N-allylnormetazocine) [1]. This receptor was first included in the opioid system (sigma opioid receptor) since the effects of SKF-10,047 were blocked by the opioid antagonist naltrexone [1]. However, subsequent studies revealed that the SKF-10,047 binding site was poorly inhibited by naltrexone and that the psychotomimetic effects of this drug in dogs were not antagonized by naltrexone pretreatment [2,3]. Hence, the sigma opioid receptor introduced by Martin emerged as a non-opioid, non-phencyclidine, and non-dopamine receptor so that the term ‘opioid’ was removed from the definition of the sigma receptor (Sig-R) [4]. To date, we know that the sigma system consists of at least two different receptor subtypes, Sigma-1 (Sig-1R) and Sigma-2 (Sig-2R), differing in molecular weight, localization, ligand engagement as well as biological functions [5,6].
The Sig-1R was cloned in 1996, yielding a protein of 223 amino acids which exhibits no homology with any other known mammalian protein [7,8]. Several non-related endogenous compounds, such as steroids (e.g., progesterone, dehydroepiandrosterone sulfate, etc.), the hallucinogen N,N-dimethyltryptamine, and sphingosine have been proposed as endogenous ligands of Sig-1R. However, their affinities are not sufficient to explicitly define their role as endogenous ligands [4,9-12]. Furthermore, Sig-1R shows moderate to high affinity towards a wide spectrum of exogenous ligands, thereby covering several different structural classes and with distinct pharmacological effects (neuroleptics, antidepressants, antitussives, drugs for the treatment of neurodegenerative disorders, drugs of abuse, etc.) [9]. Sig-1Rs are widely distributed in the human body, both in the central nervous system (CNS) [13,14] and in the periphery (mainly in spleen, liver, lung, kidney, and adrenal gland) [6,7,14-17]. At the subcellular level, the Sig-1R is mainly localized at the endoplasmic reticulum, specifically at the interface with the mitochondrion, but it is also found in the plasma membrane and in the nuclear envelope [9,18]. The information available on the physiology and the pharmacology of Sig-1Rs supports their classification as molecular chaperons, regulating the activity of several cellular proteins, such as receptors, ion channels, kinases, etc. [9,18,19].
Taken together, the wide anatomical and subcellular distribution, the ability to interact with several different exogenous ligands, as well as the chaperon activity towards different cellular targets render the Sig-1R a pluripotent modulator [18] involved in many physiological pathways and, consequently, in many central and peripheral pathological disorders (comprehensive reviews: Cobos et al. [9], Su et al. [18], Maurice and Su [19], Nguyen et al. [20]). However, further investigations are crucial to understand the complex role of Sig-1Rs both in physiological and pathological conditions. Hence, the development of specific radiotracers for PET imaging provides a powerful tool in this direction. In this study, we aim to evaluate PB212 (1-(4-(6-methoxynaphthalen-1-yl)butyl)-4-methylpiperidine) [21] as a potential PET imaging agent for Sig-1R. PB212 exhibits subnanomolar affinity towards Sig-1R (K i = 0.030 nM) [21], good to excellent selectivities against Sig-2R (K i = 17.9 nM) [21], emopamil binding protein (EBP, K i = 8.04 nM) [21], serotonin (K i 5-HT1A > 1000 nM, K i 5-HT7 > 1000 nM), dopamine (K i D2R > 1000 nM, K i D3R > 1000 nM) and adrenergic receptors (IC50 α1 > 100 nM) [22]. In addition, PB212 has shown antagonistic properties both in in vitro [23] and in vivo [22] assays. Almost all Sig-1R radiotracers developed to date are agonists and as such comparison of the data obtained from [11C]PB212 with Sig-1R agonists, such as [11C]SA4503, could potentially provide important information about Sig-1R physiology, given that agonists and antagonists may bind to different states of the receptor. With this in mind, we radiolabelled PB212 with carbon-11 and assessed its suitability as a PET radioligand for imaging Sig-1R [24].
Materials and methods
Animal experiments were conducted in accordance with the Swiss Animal Welfare legislation and were approved by the Veterinary Office of the Canton Zurich. Male Wistar rats were purchased from Charles River (Sulzfeld, Germany) and kept under standard conditions.
All chemicals, unless otherwise stated, were purchased from Sigma Aldrich GmbH (Taufkirchen, Germany), ABCR GmbH (Karlsruhe, Germany), Merck (Darmstadt, Germany), or Fluka (Buchs, Switzerland) and were used without further purification. Fluspidine was kindly provided by Prof. Bernand Wünsch (Westfälische Wilhelms-Universität Münster, Germany).
Analytical radio-HPLC was performed with a flow rate of 1 mL/min on an Agilent 1100 series system equipped with a Raytest Gabi Star radiodetector (Agilent Technologies, Morges, Switzerland). Semi-preparative HPLC purifications were carried out using a reversed phase column (Phenomenex Luna, 5 µm, 250 × 10 mm) under the following conditions: 50 mM ammonium formate in H2O pH = 4.4 (solvent A), MeCN (solvent B); isocratic, 45% B; flow rate: 5 mL/min. Molar activity was calculated by comparing ultraviolet peak intensity of the final formulated product with calibration curve of corresponding non-radioactive standard of known concentrations.
Radiochemistry
[11C]CO2 was produced by proton bombardment of nitrogen gas fortified with 0.5% oxygen using IBA Cyclone 18/9 cyclotron (18-MeV; IBA, Ottignies-Louvain-la-Neuve, Belgium) applying the well established 14N(p,a)11C nuclear reaction. In a first step, nickel-based catalytic reduction of [11C]CO2 yielded [11C]CH4 which was subsequently iodinated to give [11C]MeI. Subsequently, [11C]MeI was bubbled into a reaction mixture containing phenolic intermediate 1 (1 mg) and cesium carbonate (6 mg) in DMF (0.4 mL) and stirred at 90°C for 3 min. After dilution of the crude product with water (1.6 mL), purification was performed by semi-preparative HPLC. The collected radiotracer was diluted with water (8 mL) and passed through a C18 cartridge (Waters, pre-conditioned with 5 mL EtOH and 10 mL water). After washing of the cartridge with water (5 mL), the product was eluted with EtOH (0.8 mL) into a sterile vial and diluted with water for injection (9.2 mL) to give a final formulation containing 8% of ethanol. For quality control, an aliquot of the final formulation was injected into the analytical HPLC system. Identity of the product was confirmed by co-injection and comparison with the retention time of the standard reference. The molar activity was calculated by linear regression using a UV-intensity based calibration curve of standard reference.
In vitro autoradiography
Autoradiography was performed on rat and mouse brain as well as spleen tissues. Sections were prepared in 10 μm thickness using a cryostat (Cryo-StarNX50; Thermo Scientific). The tissue slices were adsorbed to SuperFrost Plus (Menzel) slides and stored at -20°C until use. After thawing at room temperature (rt) for 10 min, sections were pre-incubated for 15 min at rt in the incubation buffer (50 mM TRIS·HCl and 0.01% BSA in H2O; pH 7.4). Slices were dried and incubated in a humidified chamber with [11C]PB212 (0.3-0.5 nM) in incubation buffer for 15 min at rt. To test for specificity towards Sig-1R, solutions containing the radiotracer and an excess of a different Sig-1R ligand (either haloperidol, SA4503, or fluspidine; 10 μM) were prepared and added to the tissues. The tissue slices were washed three times with the washing buffer (50 mM TRIS·HCl in H2O; pH 7.4) (each 2 min) and twice with distilled water (each 5 s) on ice, air dried, and exposed to a phosphor imager plate for a period of 30 min. The plate was scanned using a BAS5000 reader (Fujifilm, Dielsdorf, CH).
In vivo PET/CT imaging
PET and CT scans were obtained with a Super Argus PET/CT tomograph (Sedecal, Madrid, Spain) after injection of [11C]PB212 (18.1-21.4 MBq, 0.58-1.36 nmol/kg) into the tail of male Wistar rats (357-389 g, n = 3) which were kept under anesthesia using isoflurane. Under baseline conditions, radiotracer accumulation was recorded in the region of the spleen in dynamic PET acquisition mode over 90 min. During this period body temperature and respiratory rate were constantly monitored. Under blockade conditions, 1 mg/kg of either haloperidol or fluspidine were injected 30 seconds before radiotracer application in two of the three rats. Acquired PET data were reconstructed as user-defined time frames with a voxel size of 0.3875 × 0.3875 × 0.775 mm. For anatomical orientation, CT scans were acquired after each PET scan. Images were evaluated with PMOD v3.4 (PMOD Technologies Inc., Zurich, CH) software. Regions of interest (spleen and muscle) were drawn manually using the PMOD fusion tool. Time activity curves (TACs) for spleen and muscle were expressed as standardized uptake values (SUVs).
Results
Radiochemistry
Reference compound PB212 was synthesized according to a published procedure [21] and subsequently demethylated by reaction with BBr3 to provide phenolic precursor 1 [25]. The radiosynthesis of [11C]PB212 was accomplished by O-methylation of the cesium salt of phenolic precursor 1 using [11C]MeI (Scheme 1). The obtained radiochemical yields ranged from 16 to 33% (decay corrected) with molar activities ranging between 39 and 391 GBq/μmol at the end of synthesis. In all cases, a radiochemical purity ≥ 99% was obtained after semi-preparative HPLC purification. The total radiosynthesis time from the end of bombardment to the end of synthesis was approximately 30 min.
Scheme 1.
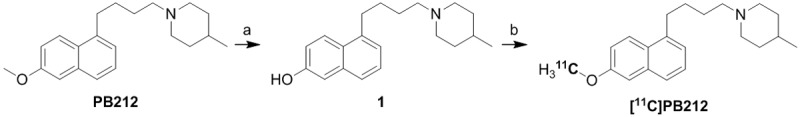
Synthesis of precursor 1 [25] and radiosynthesis of [11C]PB212. a) BBr3, dry CH2Cl2, 78°C→rt; b) [11C]CH3I, Cs2CO3, dry DMF.
In vitro characterization
In vitro autoradiography with WT and Sig-1R KO mouse brain tissues revealed high binding of [11C]PB212 in both wildtype and Sig-1R KO mouse, which could not be blocked in wild type mouse, indicating that the binding to mouse brain tissue is mainly nonspecific (Figure 1). In contrast, high and specific binding of [11C]PB212 was observed in the autoradiography experiments using spleen tissues obtained from CD1 mice (Figure 2A) and Wistar rats (Figure 2B). The high and known physiological expression of Sig-1R in the spleen prompted us to use the spleen as a target organ for the evaluation of [11C]PB212 for imaging Sig-1R expression in the periphery [14-16]. Three different blockers were selected and used to assess [11C]PB212 binding specificity towards Sig-1Rs in the spleen: 1) haloperidol, a nonselective Sig-1R ligand (K i Sig-1R = 0.9 nM, SISig-2R/Sig-1R = 8.8) [26] with high binding affinity also for dopamine receptors (e.g., K i D2R = 2.0 nM, K i D3R = 4.0 nM, K i D4R = 15 nM) [27], serotonin receptors (e.g., K i 5-HT2A = 70 nM) [27], and adrenergic receptors (e.g., K i α1 = 12 nM) [27]; 2) SA4503, a Sig-1R ligand (K i Sig-1R = 4.63 nM, SISig-2R/Sig-1R = 13.6) [26] with weak or no binding towards several receptors, ion channel, and second messenger systems [28], except for the EBP (K i = 1.7 nM) [29] and the vesicular acetylcholine transporter (VAChT, K i = 50.2 nM) [30]; 3) fluspidine, a selective Sig-1R ligand (K i Sig-1R = 0.59 nM, SISig-2R/Sig-1R = 1331) [31] with weak binding affinities towards several receptors (e.g., phencyclidine NMDA binding site, μ, δ, and κ receptors) [31], including EBP (K i = 211 nM) and VAChT (K i = 1.4 μM) [15].
Figure 1.
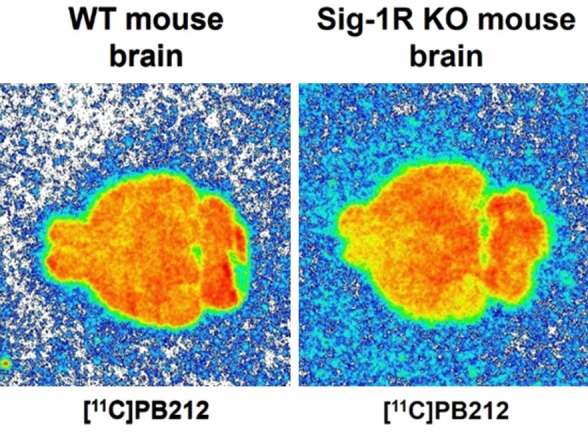
In vitro autoradiography with [11C]PB212 on WT and Sig-1R KO mouse brain tissues.
Figure 2.
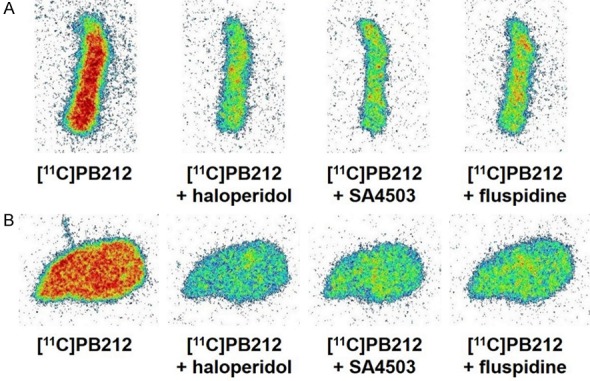
In vitro autoradiography with [11C]PB212 on mouse (A) and rat (B) spleen tissues. For blocking conditions, an excess (10 μM) of either haloperidol, SA4503, or fluspidine was used.
In vivo characterization
Time activity curves (TACs) for spleen and muscle under baseline and blockade conditions are depicted in Figure 3. Blocking experiments were performed using either haloperidol or fluspidine because of their different binding profile: the former is a nonselective Sig-1R ligand, and the latter can be considered one of the most selective Sig-1R ligands currently available. The injection of haloperidol (1 mg/kg) shortly before [11C]PB212 administration led to a drastic reduction of the radiotracer accumulation in the spleen, reaching radioactivity levels detected in the muscle (background region) 60 minutes post injection. The injection of fluspidine (1 mg/kg) induced a partial, but significant, reduction of [11C]PB212 accumulation in the spleen.
Figure 3.
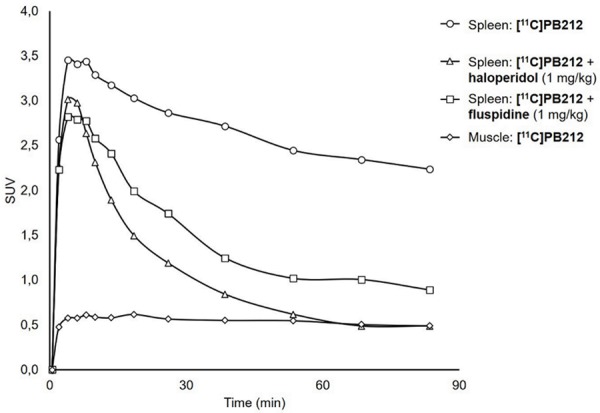
Typical time activity curves of [11C]PB212 in whole spleen and muscle under baseline or blocking conditions with 1 mg/kg of either haloperidol or fluspidine shortly prior injection of the radioligand.
Discussion
Sig-1Rs play a crucial but not yet fully understood role in physiological and pathological conditions. A connection of Sig-1R with cocaine abuse has been demonstrated [32,33], while knocking-down of Sig-1R has been associated with neurotoxic effects and neurodegeneration in amyotrophic lateral sclerosis (ALS), Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD). Additionally, some Sig-1R ligands have shown important therapeutic effects in the animal models of these neurodegenerative diseases [18,20]. Accordingly, clinical trials are currently ongoing to evaluate the diagnostic and therapeutic potentials of Sig-1R ligands [34,35]. Furthermore, Sig-1R overexpression has been found in several tumor cell lines and tumor biopsies, and anti-cancer effects have been observed for some Sig-1R ligands [36-38]. A specific Sig-1R PET tracer would help to gain deeper insight into the physiological and pathological pathways in which the Sig-1R is involved, both in the CNS and in the periphery. In addition, such a tracer could be a useful tool not only for detecting the down- or upregulation of Sig-1Rs associated with different disorders, but also for the monitoring of disease progression and therapeutic outcome in the clinic.
Several radioligands have been developed for the PET imaging of Sig-1R and a summary of existing ligands can be found in [39-42]. [11C]SA4503 [16], [18F]fluspidine [15] and [18F]FTC-146 [43] are three examples of the most assessed radioligands for PET imaging of Sig-1R in the CNS. [11C]SA4503 is one of the first valuable radiotracers developed for brain PET imaging of Sig-1R and it has been studied in healthy human volunteers as well as in PD and AD patients [44,45]. The radioligands with longer half-lives [18F]fluspidine and [18F]FTC-146 have been tested in human volunteers and have emerged as two promising 18F-radiolabelled Sig-1R radiotracers for brain PET imaging [46,47]. Much more limited is the development of radioligands for PET detection of Sig-1R in cancer, with [11C]SA4503 showing specific uptake in tumor-bearing rodents [48-52]. Nevertheless, the clinical utility of these radiotracers has not yet been established. In this study, we therefore aimed to evaluate [11C]PB212, which is an antagonist and shows high affinity for Sig-1R and selectivity towards other receptors. In particular, the selectivity of PB212 over Sig-2R receptor subtype is much higher than the selectivity reported for SA4503 [21,22,26].
The 11C-radiolabelling was successfully achieved using [11C]MeI as methylating agent leading to high molar activity and ≥ 99% radiochemical purity of the final product. In vitro autoradiography experiments showed that [11C]PB212 binds homogenously to WT and Sig-1R knock-out mouse brain, demonstrating a lack of tissue specificity (Figure 1). This result is in agreement with previous reports, which showed that picomolar affinities lead to low specific binding of Sig-1R radiotracers to brain tissue [40]. In addition, we speculate that the lipophilicity of [11C]PB212 (clogD7.4 = 2.38, calculated through https://chemicalize.com/) could be a reason for the lack of CNS specificity. Nevertheless, in vitro autoradiography in rodent spleen tissue demonstrated a complete displacement of [11C]PB212 in the presence of three Sig-1R blockers (i.e. haloperidol, SA4503 and fluspidine, Figure 2A, 2B). These results were further confirmed by in vivo PET imaging in Wistar rats. Figure 3 shows the corresponding TACs comparing [11C]PB212 uptake in the spleen and background (muscle) under baseline conditions with blockade conditions using either haloperidol or fluspidine. PET images are presented in Figure 4 and show the tracer accumulation in the spleen under baseline (panel A) as well as blockade conditions with haloperidol (panel B) and fluspidine (panel C). Both haloperidol and fluspidine administered at a concentration of 1 mg/kg significantly reduced radioactivity uptake in the spleen, suggesting reversible and specific binding of [11C]PB212 to Sig-1R. The reduction of radioactivity in the spleen was more pronounced with haloperidol and reached background (muscle) levels towards the end of the studies.
Figure 4.
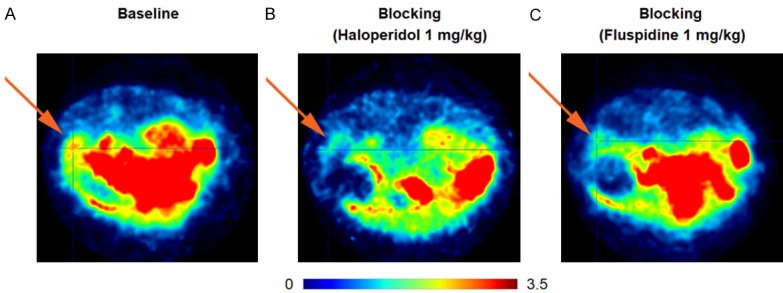
Transaxial PET images of the abdominal region, averaged from 0 to 60 minutes post injection. The spleen is indicated by an arrow. After injection of the tracer (18-21 MBq), the animals were maintained at 2% isoflurane for anesthesia. Color bar indicates SUV. A: Baseline conditions, B: Blockade conditions using haloperidol (1 mg/kg), C: Blockade conditions using fluspidine (1 mg/kg).
The results of the PET imaging studies in the spleen clearly demonstrate that [11C]PB212 can be used to assess the peripheral expression of Sig-1R in vivo and support the further evaluation of [11C]PB212 in the periphery.
Conclusion
Here, we describe the radiolabelling and the biological evaluation of [11C]PB212, a well described Sig-1R antagonist with subnanomolar affinity and excellent selectivities against a number of receptors. [11C]PB212 was synthesized in good radiochemical yields (16-33%) and excellent radiochemical purity as well as good molar activity (39-391 GBq/μmol). Although [11C]PB212 is not suitable for imaging Sig-1R in the brain, the high in vitro and in vivo specificity observed in the spleen suggests that [11C]PB212 can be used to image Sig-1R expression in the periphery. These promising results warrant further studies in Sig-1R-positive tumor bearing mice in order to shed more light on the utility of [11C]PB212 for peripheral Sig-1R PET detection in healthy and diseased organs. Furthermore, being a Sig-1R antagonist, [11C]PB212 could provide additional information on Sig-1R physiology which cannot otherwise be obtained with Sig-1R agonists such as [11C]SA4503 and [18F]fluspidine.
Acknowledgements
We thank Mr. Bruno Mancosu for his support with carbon-11 radiolabelling, and Prof. Bernard Wünsch (Department of Pharmaceutical and Medicinal Chemistry, Westfälische Wilhelms-Universität Münster, Germany) for kindly providing fluspidine.
Disclosure of conflict of interest
None.
References
- 1.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 2.Su TP. Evidence for sigma opioid receptor: binding of [3H] SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- 3.Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur J Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- 4.Su TP. Sigma receptors. Putative links between nervous, endocrine and immune systems. Eur J Biochem. 1991;200:633–642. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- 5.Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 6.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of σ1 and σ2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 7.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 9.Cobos EJ, Entrena JM, Nieto FR, Cendan CM, Del Pozo E. Pharmacology and therapeutic potential of sigma1 receptor ligands. Curr Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011;300:C328–337. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruoho AE, Chu UB, Ramachandran S, Fontanilla D, Mavlyutov T, Hajipour AR. The ligand binding region of the sigma-1 receptor: studies utilizing photoaffinity probes, sphingosine and N-alkylamines. Curr Pharm Des. 2012;18:920–929. doi: 10.2174/138161212799436584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 14.Kitaichi K, Chabot JG, Moebius FF, Flandorfer A, Glossmann H, Quirion R. Expression of the purported sigma1 (σ1) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat. 2000;20:375–387. doi: 10.1016/s0891-0618(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer S, Wiese C, Maestrup EG, Hiller A, Deuther-Conrad W, Scheunemann M, Schepmann D, Steinbach J, Wunsch B, Brust P. Molecular imaging of sigma receptors: synthesis and evaluation of the potent σ1 selective radioligand [18F] fluspidine. Eur J Nucl Med Mol Imaging. 2011;38:540–551. doi: 10.1007/s00259-010-1658-z. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura K, Ishiwata K, Tajima H, Ishii S, Matsuno K, Homma Y, Senda M. In vivo evaluation of [11C] SA4503 as a PET ligand for mapping CNS sigma1 receptors. Nucl Med Biol. 2000;27:255–261. doi: 10.1016/s0969-8051(00)00081-0. [DOI] [PubMed] [Google Scholar]
- 17.Lever JR, Litton TP, Fergason-Cantrell EA. Characterization of pulmonary sigma receptors by radioligand binding. Eur J Pharmacol. 2015;762:118–126. doi: 10.1016/j.ejphar.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su TP, Su TC, Nakamura Y, Tsai SY. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol Sci. 2016;37:262–278. doi: 10.1016/j.tips.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen L, Lucke-Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, Matsumoto RR. Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci. 2015;127:17–29. doi: 10.1016/j.jphs.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Berardi F, Ferorelli S, Abate C, Pedone MP, Colabufo NA, Contino M, Perrone R. Methyl substitution on the piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives as a probe for selective binding and activity at the σ1 receptor. J Med Chem. 2005;48:8237–8244. doi: 10.1021/jm050654o. [DOI] [PubMed] [Google Scholar]
- 22.Skuza G, Sadaj W, Kabzinski M, Cassano G, Gasparre G, Abate C, Berardi F. The effects of new sigma (σ) receptor ligands, PB190 and PB212, in the models predictive of antidepressant activity. Pharmacol Rep. 2014;66:320–324. doi: 10.1016/j.pharep.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Gasparre G, Abate C, Berardi F, Cassano G. The sigma-1 receptor antagonist PB212 reduces the Ca2+-release through the inositol (1,4,5)-trisphosphate receptor in SK-N-SH cells. Eur J Pharmacol. 2012;684:59–63. doi: 10.1016/j.ejphar.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Wang M, Hutchins GD, Zheng QH. Synthesis of carbon-11-labeled piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives as new selective PET sigma1 receptor probes. Appl Radiat Isot. 2010;68:459–465. doi: 10.1016/j.apradiso.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Abate C, Riganti C, Pati ML, Ghigo D, Berardi F, Mavlyutov T, Guo LW, Ruoho A. Development of sigma-1 (σ1) receptor fluorescent ligands as versatile tools to study sigma1 receptors. Eur J Med Chem. 2016;108:577–585. doi: 10.1016/j.ejmech.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. σ1 and σ2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16:3385–3403. doi: 10.2174/1568026616666160608084834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S. Binding properties of SA4503, a novel and selective σ1 receptor agonist. Eur J Pharmacol. 1996;306:271–279. doi: 10.1016/0014-2999(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 29.Berardi F, Ferorelli S, Colabufo NA, Leopoldo M, Perrone R, Tortorella V. A multireceptorial binding reinvestigation on an extended class of sigma ligands: N-[omega-(indan-1-yl and tetralin-1-yl)alkyl] derivatives of 3,3-dimethylpiperidine reveal high affinities towards sigma1 and EBP sites. Bioorg Med Chem. 2001;9:1325–1335. doi: 10.1016/s0968-0896(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 30.Shiba K, Ogawa K, Ishiwata K, Yajima K, Mori H. Synthesis and binding affinities of methylvesamicol analogs for the acetylcholine transporter and sigma receptor. Bioorg Med Chem. 2006;14:2620–2626. doi: 10.1016/j.bmc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 31.Maestrup EG, Wiese C, Schepmann D, Brust P, Wunsch B. Synthesis, pharmacological activity and structure affinity relationships of spirocyclic σ1 receptor ligands with a (2-fluoroethyl) residue in 3-position. Bioorg Med Chem. 2011;19:393–405. doi: 10.1016/j.bmc.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farre D, Aguinaga D, Mallol J, Cortes A, Casado V, Lluis C, Ferre S, Franco R, Canela E, McCormick PJ. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8:e61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr Pharm Des. 2012;18:902–919. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- 34.A study to evaluate sigma-1 and dopamine-2 receptor occupancy by pridopidine in the human brain of healthy volunteers and in patients with Huntington’s disease. https://ClinicalTrials. gov/show/ NCT03019289.
- 35.Double blind placebo control opipramol-baclofen treatment for addiction. https://ClinicalTrials. gov/show/ NCT03065998.
- 36.Crottes D, Guizouarn H, Martin P, Borgese F, Soriani O. The sigma-1 receptor: a regulator of cancer cell electrical plasticity? Front Physiol. 2013;4:175. doi: 10.3389/fphys.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim FJ, Maher CM. Sigma1 pharmacology in the context of cancer. Handb Exp Pharmacol. 2017;244:237–308. doi: 10.1007/164_2017_38. [DOI] [PubMed] [Google Scholar]
- 38.Soriani O, Rapetti-Mauss R. Sigma 1 receptor and ion channel dynamics in cancer. Adv Exp Med Biol. 2017;964:63–77. doi: 10.1007/978-3-319-50174-1_6. [DOI] [PubMed] [Google Scholar]
- 39.Toyohara J, Sakata M, Ishiwata K. PET imaging of sigma1 receptors. In: Dierckx RAJO, Otte A, de Vries EFJ, van Waarde A, Luiten PGM, editors. PET and SPECT of neurobiological systems. Berlin Heidelberg: Springer; 2014. pp. 741–763. [Google Scholar]
- 40.Brust P, Deuther-Conrad W, Lehmkuhl K, Jia H, Wünsch B. Molecular imaging of σ1 receptors in vivo: current status and perspectives. Curr Med Chem. 2014;21:35–69. doi: 10.2174/09298673113209990214. [DOI] [PubMed] [Google Scholar]
- 41.van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RA. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim Biophys Acta. 2015;1848:2703–2714. doi: 10.1016/j.bbamem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Weber F, Brust P, Laurini E, Pricl S, Wünsch B. Fluorinated PET tracers for molecular imaging of σ1 receptors in the central nervous system. In: Smith SB, Su TP, editors. Sigma receptors: their role in disease and as therapeutic targets. Cham: Springer International Publishing; 2017. pp. 31–48. [Google Scholar]
- 43.James ML, Shen B, Zavaleta CL, Nielsen CH, Mesangeau C, Vuppala PK, Chan C, Avery BA, Fishback JA, Matsumoto RR, Gambhir SS, McCurdy CR, Chin FT. New positron emission tomography (PET) radioligand for imaging σ-1 receptors in living subjects. J Med Chem. 2012;55:8272–8282. doi: 10.1021/jm300371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, Oda K, Sasaki T, Sakayori O, Hamamoto M, Kobayashi S, Katayama Y. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol Scand. 2005;112:103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 45.Mishina M, Ohyama M, Ishii K, Kitamura S, Kimura Y, Oda K, Kawamura K, Sasaki T, Kobayashi S, Katayama Y, Ishiwata K. Low density of sigma1 receptors in early Alzheimer’s disease. Ann Nucl Med. 2008;22:151–156. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- 46.Kranz M, Sattler B, Wüst N, Deuther-Conrad W, Patt M, Meyer P, Fischer S, Donat C, Wünsch B, Hesse S, Steinbach J, Brust P, Sabri O. Evaluation of the enantiomer specific biokinetics and radiation doses of [18F] fluspidine-A new tracer in clinical translation for imaging of σ1 receptors. Molecules. 2016;21 doi: 10.3390/molecules21091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hjørnevik T, Cipriano PW, Shen B, Hyung Park J, Gulaka P, Holley D, Gandhi H, Yoon D, Mittra ES, Zaharchuk G, Gambhir SS, McCurdy CR, Chin FT, Biswal S. Biodistribution and radiation dosimetry of 18F-FTC-146 in humans. J Nucl Med. 2017;58:2004–2009. doi: 10.2967/jnumed.117.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiwata K, Kawamura K, Kubota K, Kobayashi T, Elsinga PH, Ono M, Maeda M. Evaluation of [11C] SA5845 and [11C] SA4503 for imaging of sigma receptors in tumors by animal PET. Ann Nucl Med. 2005;19:701–9. doi: 10.1007/BF02985120. [DOI] [PubMed] [Google Scholar]
- 49.van Waarde A, Buursma AR, Hospers GAP, Kawamura K, Kobayashi T, Ishii K, Oda K, Ishiwata K, Vaalburg W, Elsinga PH. Tumor imagirng with 2 σ-receptor ligands, 18F-FE-SA5845 and 11C-SA4503: a feasibility study. J Nucl Med. 2004;45:1939–1945. [PubMed] [Google Scholar]
- 50.van Waarde A, Jager PL, Ishiwata K, Dierckx RA, Elsinga PH. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Nucl Med. 2006;47:150–154. [PubMed] [Google Scholar]
- 51.van Waarde A, Shiba K, de Jong JR, Ishiwata K, Dierckx RA, Elsinga PH. Rapid reduction of σ1-receptor binding and 18F-FDG uptake in rat gliomas after in vivo treatment with doxorubicin. J Nucl Med. 2007;48:1320–1326. doi: 10.2967/jnumed.107.042085. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa K, Kanbara H, Kiyono Y, Kitamura Y, Kiwada T, Kozaka T, Kitamura M, Mori T, Shiba K, Odani A. Development and evaluation of a radiobromine-labeled sigma ligand for tumor Imaging. Nucl Med Biol. 2013;40:445–450. doi: 10.1016/j.nucmedbio.2013.02.008. [DOI] [PubMed] [Google Scholar]


