Abstract
We aimed to evaluate the comparative efficacy and safety of dipeptidyl peptidase-4 inhibitors (DPP4i), glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose co-transporter 2 inhibitors (SGLT2i), or thiazolidinedione (TZD) as an adjunctive treatment in patients with poorly controlled type 2 diabetes mellitus (T2DM) on insulin therapy. We searched Medline, Embase, the Cochrane Library, and ClinicalTrials.gov through April 2016. Bayesian network meta-analyses were performed with covariate adjustment. The primary outcome was the change in glycated hemoglobin A1c (HbA1c) from baseline. Fifty randomized controlled trials covering 15,494 patients were included. GLP-1RA showed the greatest HbA1c-lowering effect compared to the control (−0.84%; 95% credible interval, −1.00% to −0.69%), followed by TZD (−0.73%; −0.93 to −0.52%), SGLT2i (−0.66%; −0.84% to −0.48%), and DPP4i (−0.54%; −0.68% to −0.39%). SGLT2i showed the greatest fasting plasma glucose reduction. GLP-1RA and SGLT2i showed greater body weight reduction, whereas TZD increased body weight. TZD was ranked the highest in terms of insulin dose reduction. The risk of hypoglycemia was increased with TZD or GLP-1RA. The study provides the best available evidence on the comparative efficacy and safety of non-insulin anti-diabetic agents on top of pre-existing insulin therapy for inadequately controlled T2DM patients.
Introduction
Impaired insulin secretion in the presence of insulin resistance is the key feature of type 2 diabetes mellitus (T2DM). The progressive nature of insulin secretory failure makes T2DM patients require insulin therapy to achieve their glycemic goals1, but intensifying insulin therapy increases the risk of hypoglycemia and weight gain2. Therefore, as an alternative to more intensive insulin therapy, a non-insulin anti-diabetic drug may be used as an add-on drug to insulin therapy. In this regard, recent guidelines for T2DM management have recommended the use of dipeptidyl peptidase-4 inhibitors (DPP4i), glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose co-transporter 2 inhibitors (SGLT2i), or thiazolidinedione (TZD) on top of pre-existing insulin therapy, particularly basal insulin3,4. Each agent has both advantages and disadvantages according to the characteristics of the drug, which need to be considered when determining the most appropriate treatment for an individual patient. To the best of our knowledge, there has been no comparative efficacy or effectiveness study of adjunctive treatments to insulin therapy. Hence, we performed a systematic review with a network meta-analysis to evaluate the comparative efficacy and safety of DPP4i, GLP-1RA, SGLT2i, and TZD as an adjunctive treatment in patients with poorly controlled T2DM on insulin therapy.
Methods
We conducted a systematic review and network meta-analysis following a pre-developed protocol (Supplementary Appendix 1).
Search strategy and study selection
The following electronic databases were searched from inception to April 2016: Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov. The detailed search strategy used in Medline is available in Supplementary Appendix 2.
We included randomized controlled trials (RCTs) that investigated the effect of DPP4i, GLP-1RA, SGLT2i, or TZD as an add-on drug to pre-existing insulin therapy in patients with T2DM. Both placebo-controlled and open-label trials were included, and concurrent use of other anti-diabetic agents was allowed. Studies reporting the change of HbA1c from baseline were included when some information on the mean and its variability was available. An intervention of at least 12 weeks was required and studies in English were considered for inclusion. Duplicate publications or studies on post-hoc analysis were excluded. We also excluded studies with patients treated using an insulin pump. Two reviewers (J.-H.Y. and S.H.M.) independently screened all studies by title and abstract, and then by full text, to assess the eligibility of the studies. Any discrepancies between the authors were resolved through mutual discussions with the other authors (C.H.A., Y.M.C., and S.H.).
Data extraction
We extracted the following information from each study: study information, participant characteristics at baseline, information on interventions and values of outcome variables (as a primary outcome, the change in HbA1c from baseline; as secondary efficacy outcomes, the change in fasting plasma glucose [FPG] levels, body weight, insulin dose and the proportion of patients achieving HbA1c goals; and as a safety outcome, the risk of hypoglycemia). We attempted to classify various insulin regimens into 2 categories: a stable insulin dose group, in which the insulin doses were kept relatively constant throughout the study duration unless the dose needed to be altered for safety reasons, and an insulin dose titration group, in which the insulin doses were titrated according to study-specific predefined titration algorithms. We obtained information from the ClinicalTrials.gov website when the studies had never been published as an article.
Assessment of the study quality and risk of bias
Two independent reviewers (J.-H.Y. and S.H.M.) assessed the study quality and risk of bias according to the Cochrane Collaboration tool5, and any controversies were resolved by mutual discussion. We considered the 6 aspects of risk of bias, including the adequacy of random sequence generation, allocation concealment, blinding of participants and personnel, completeness of outcome data, selective reporting, and other sources of bias. For the completeness of outcome data, analyses based on the intention-to-treat principle or the full analysis set were considered to be low-risk. Selective reporting was assessed as high-risk when any results were regarded as missing while some outcome variables or conditions of analysis were mentioned in the articles or when the publication was not considered as in full. Other sources of bias were assessed according to the comparability of the baseline characteristics of the participants in the trial groups.
Statistical analysis
For the continuous variables, pooled weighted mean differences between treatment groups were calculated. For the dichotomous variables, we calculated the pooled relative risks (RR). Prior to conducting the network meta-analysis, homogeneity in the common comparator (insulin therapy with placebo add-on or open-label; hereafter, ‘control group’ collectively) was assessed as an indicator of study comparability6,7. This was done primarily by a qualitative review of the regimen used in each study, and by a quantitative comparison of the results from the control group in all included studies using the 2 test and I2 statistics, with a forest plot presenting the pooled weighted mean and the 95% confidence intervals (CIs). We considered that participants’ age, sex, body mass index (BMI), baseline HbA1c, duration of diabetes, and baseline insulin dose could affect within-group or between-group heterogeneity, and therefore could be potential confounders. Although we reviewed each insulin titration algorithm used in all the included studies, it was not possible to include it as a covariate for adjustment due to the diversity of algorithms. However, since the treatment results from the control group inherently reflect the effects of various insulin regimens, we used them as a modifier representing unmeasured prognostic factors related to various insulin regimens across the included trials8. We explored the potential relationship between each candidate covariate and the outcome by a scatter plot with a conventional random-effects meta-regression. The network meta-analysis was conducted using a Bayesian approach, and the posterior distribution for each parameter of interest was summarized with a median and the 95% credible interval (CrI)9,10. We selected covariates that contributed to the network meta-analysis, including each as a covariate at a time, based on the 95% CrI of the coefficient. Covariates with a strong clinical rationale were also selected. The final network meta-analysis was adjusted for the selected covariates with a random-effects model. We performed sensitivity analyses after mean imputation of the values of covariates missing in some studies, and additionally by including only placebo-controlled trials. We estimated the surface under the cumulative ranking curve11 for each treatment for each outcome, which provides the probability for each treatment to be ranked as the most effective treatment or to have the highest chance for a respective adverse event. We used STATA version 12 (Stata Corp, College Station, TX, USA), R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria), and WinBUGS version 14 (Imperial College and Medical Research Council, London, United Kingdom).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Search results and study characteristics
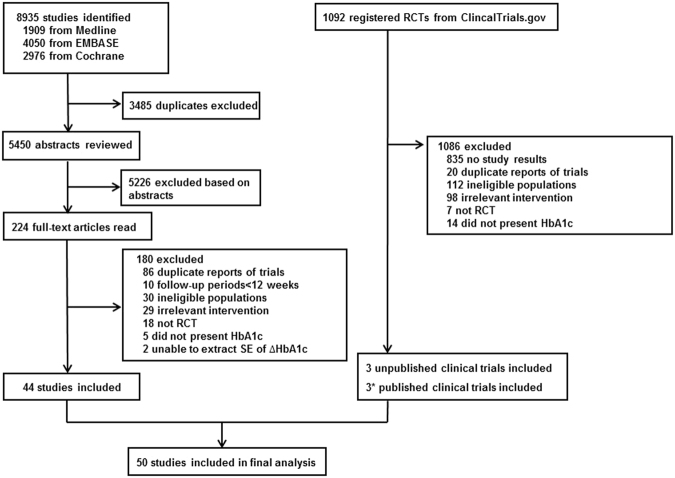
The flowchart of the literature selection is shown in Fig. 1. We retrieved 8935 potentially relevant studies, of which 44 articles were finally included in the analysis12–55. We additionally selected 6 trials56–61 among the 1092 clinical trials identified from ClinicalTrials.gov, of which 3 trials57–59 were unpublished. The network frame based on the 50 studies is depicted in Fig. 2. There were 15 DPP4i15,22,23,26,29–31,34,38,45,46,50,55,57,61, 13 GLP-1RA12,16,18,20,21,32,33,41,42,47,48,56,59, 9 SGLT2i13,19,37,43,44,51,52,58,60 and 13 TZD14,17,24,25,27,28,35,36,39,40,49,53,54 studies included in the network. No study with head-to-head comparisons between the non-insulin agents was found. The characteristics of the included trials are described in Table 1. Fifty studies with a mean study duration of 23 weeks included 15,494 randomized participants. The patients’ overall mean age was 58.7 years and the age distribution was similar in all treatment groups. The mean baseline HbA1c ranged from 7.3% to 9.8% among studies. The mean duration of diabetes was approximately 13 years, and was similar in most of the studies. The mean baseline insulin dose was 54.6 units per day over all studies. Twenty-six studies12,13,15,19,22,25,26,29–31,37–41,43–45,47,48,50–52,55,60,61 were considered as having a stable insulin dose, whereas 19 studies14,16–18,20,21,24,27,28,32–35,42,46,49,53,54,56 involved insulin dose titration. The rest23,36,57–59 of the studies could not be classified in either group because they did not describe the method of insulin dosage. Two studies33,56 with fixed ratio combination products of GLP-1RA and insulin were included.
Figure 1.
Study selection process. RCT, randomized controlled trial; SE, standard error. The asterisk indicates that 3 trials of 6 previously unpublished studies were published after literature search.
Figure 2.
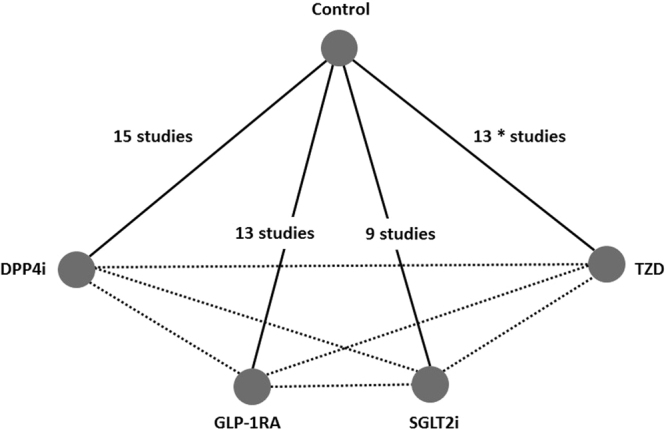
Structure of the network formed by interventions and both direct and indirect comparisons for primary outcomes. Solid lines and dashed lines indicated direct and indirect comparisons, respectively. The numbers next to each solid line joining two groups correspond to the number of studies that compared those groups. The asterisk indicates that 1 of the 13 trials was a 3-arm trial that compared 2 different kinds of TZD with the control. Control, insulin therapy with placebo add-on or open-label; GLP-1RA, glucagon-like peptide-1 receptor agonists plus insulin; DPP4i, dipeptidyl peptidase-4 inhibitor plus insulin; SGLT2i, sodium-glucose co-transporter 2 inhibitor plus insulin; TZD, thiazolidinedione plus insulin.
Table 1.
Summary of the studies included in the network meta-analysis.
| Study source | Study duration of primary phase, weeks | Insulin regimen | Study arms | Randomized participants, N | Age, years | Proportion of males, % | Baseline BMI, kg/m2 | Baseline HbA1c, % | Duration of diabetes, years | Baseline insulin dose, IU/day |
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin plus DPP4 inhibitor | ||||||||||
| Barnett et al.15 | 24 | Stable | saxagliptin 5 mg + insulin ± metformin | 304 | 57.2 | 40.0 | 32.6 | 8.7 | 11.8 | 53.6 |
| placebo + insulin ± metformin | 151 | 57.3 | 45.0 | 31.8 | 8.6 | 12.2 | 55.3 | |||
| Fonseca et al.22 | 24 | Stable | vildagliptin 50 mg bid + insulin | 144 | 59.6 | 47.9 | 33.3 | 8.4 | 14.4 | 81.2 |
| placebo + insulin | 152 | 58.9 | 54.6 | 32.9 | 8.4 | 14.9 | 81.9 | |||
| Franc et al.23 | 12 | NR | vildagliptin 50 mg bid + insulin + metformin | 31 | 59.4 | NR | 28.6 | 7.7 | 6.1 | 39.3 |
| placebo + insulin + metformin | 31 | 59.4 | NR | 28.6 | 7.7 | 6.1 | 39.3 | |||
| Hirose et al.26 | 12 | Stable | vildagliptin 50 mg bid + insulin ± metformin | 78 | 58.5 | 70.5 | 25.3 | 8.1 | 12.8 | 20.7 |
| placebo + insulin ± metformin | 78 | 60.1 | 71.8 | 26.0 | 8.1 | 12.7 | 21.1 | |||
| Kadowaki et al.29 | 16 | Stable | sitagliptin 50 mg qd + insulin | 129 | 62.3 | 58.9 | 25.2 | 8.9 | 14.1 | 24.4 |
| placebo + insulin | 137 | 60.2 | 58.4 | 25.2 | 8.9 | 14.0 | 24.3 | |||
| Kaku et al.30 | 12 | Stable | alogliptin 25 mg qd + insulin | 90 | 62.9 | 55.6 | 23.9 | 8.4 | 15.3 | 22.6 |
| placebo + insulin | 89 | 62.4 | 52.8 | 24.7 | 8.4 | 14.5 | 23.7 | |||
| Kothny et al.31 | 24 | Stable | vildagliptin 50 mg bid + insulin ± metformin | 228 | 59.3 | 47.8 | 28.9 | 8.8 | 12.9 | 39.9 |
| placebo + insulin ± metformin | 221 | 59.1 | 52.0 | 29.0 | 8.8 | 13.2 | 41.9 | |||
| Mathieu et al.34 | 24 | Titration | sitagliptin 100 mg per day + glargine ± metformin | 329 | 59.3 | 45.9 | 31.9 | 8.7 | 13.2 | 37.3 |
| placebo + glargine ± metformin | 329 | 58.3 | 49.8 | 32.3 | 8.8 | 13.7 | 36.6 | |||
| Ning et al.38 | 24 | Stable | vildagliptin 50 mg bid + insulin ± metformin | 146 | 57.8 | 41.8 | 26.2 | 8.6 | 11.2 | 33.3 |
| placebo + insulin ± metformin | 147 | 58.4 | 44.9 | 26.0 | 8.7 | 11.4 | 31.7 | |||
| Rosenstock et al.45 | 26 | Stable | alogliptin 25 mg qd + insulin ± metformin | 129 | 55.9 | 34.0 | 32.3 | 9.3 | 13.4 | 55.0 |
| placebo + insulin ± metformin | 130 | 55.0 | 48.0 | 32.4 | 9.3 | 12.2 | 57.0 | |||
| Sato et al.46 | 24 | Titration | sitagliptin 50 mg or 100 mg qd + insulin ± OADs | 25 | 66.0 | 64.0 | 24.5 | 7.9 | 19.0 | 31.8 |
| insulin ± OADs | 24 | 66.0 | 75.0 | 26.8 | 7.8 | 20.0 | 32.5 | |||
| Shankar et al.61 | 24 | Stable | sitagliptin 100 mg qd + insulin ± metformin | 234 | 58.6 | 55.6 | 25.9 | 8.7 | 11.0 | 34.5 |
| placebo + insulin ± metformin | 233 | 56.7 | 49.8 | 26.1 | 8.8 | 11.3 | 34.5 | |||
| Vilsboll et al.50 | 24 | Stable | sitagliptin 100 mg qd + insulin ± metformin | 322 | 58.3 | 49.0 | 31.0 | 8.7 | 13.0 | 44.2 |
| placebo + insulin ± metformin | 319 | 57.2 | 53.0 | 31.0 | 8.6 | 12.0 | 44.5 | |||
| Yki-Jarvinen et al.55 | 24 | Stable | linagliptin 5 mg qd + insulin ± metformin ± pioglitazone | 631 | 59.7 | 52.1 | 30.8 | 8.3 | NR | 41.5 |
| placebo + insulin ± metformin ± pioglitazone | 630 | 60.4 | 52.2 | 31.2 | 8.3 | NR | 40.1 | |||
| NCT0208159957 | 16 | NR | teneligliptin 20 mg qd + insulin | 77 | NR | 76.6 | NR | NR | NR | NR |
| placebo + insulin | 71 | NR | 74.6 | NR | NR | NR | NR | |||
| Insulin plus GLP-1 receptor agonist | ||||||||||
| Ahmann et al.12 | 26 | Stable | liraglutide 0.6–1.8 mg qd + insulin ± metformin | 225 | 59.3 | 53.3 | 32.3 | 8.2 | 12.1 | 40.5 |
| placebo + insulin ± metformin | 225 | 57.5 | 60.4 | 32.2 | 8.3 | 12.1 | 40.5 | |||
| Aroda et al.56 | 30 | Titration | fixed combination (lixisenatide + glargine) ± metformin | 367 | 59.6 | 45.0 | 31.3 | 8.1 | 12.0 | 35.0 |
| glargine ± metformin | 369 | 60.3 | 48.5 | 31.0 | 8.1 | 12.1 | 35.2 | |||
| Buse et al.16 | 30 | Titration | exenatide 10 μg bid + insulin ± metformin ± pioglitazone | 137 | 59.0 | 51.0 | 33.8 | 8.3 | 12.0 | 49.5 |
| placebo + insulin ± metformin ± pioglitazone | 122 | 59.0 | 64.0 | 33.1 | 8.5 | 12.0 | 47.4 | |||
| Buse et al.18 | 26 | Titration | liraglutide 0.6–1.8 mg qd + degludec + metformin ± SU/glinides | 199 | 57.0 | 56.0 | 33.6 | 8.7 | 10.0 | 29.0 |
| placebo + degludec + metformin ± SU/glinides | 199 | 58.0 | 53.0 | 33.8 | 8.8 | 11.0 | 29.0 | |||
| De Wit et al.20 | 26 | Titration | liraglutide 0.6–1.8 mg qd + insulin ± metformin ± SU | 26 | 57.0 | 61.5 | 34.0 | 7.2 | 8.3 | 54.0 |
| insulin ± metformin ± SU | 24 | 59.0 | 62.5 | 32.0 | 7.5 | 7.6 | 50.0 | |||
| Distiller et al.21 | 24 | Titration | exenatide 10 μg bid + insulin + metformin | 14 | 49.1 | 50.0 | 41.9 | 8.7 | 12.3 | 253.0 |
| insulin + metformin | 14 | 54.3 | 50.0 | 40.2 | 9.2 | 12.4 | 237.0 | |||
| Lind et al.32 | 24 | Titration | liraglutide 0.6–1.8 mg qd + insulin ± metformin | 64 | 63.7 | 62.5 | 33.7 | 9.0 | 17.3 | 105.3 |
| placebo + insulin ± metformin | 60 | 63.5 | 66.7 | 33.5 | 8.9 | 17.0 | 105.7 | |||
| Lingvay et al.33 | 26 | Titration | fixed combination (liraglutide 0.6–1.8 mg qd + degludec) ± metformin | 278 | 58.4 | 51.4 | 31.7 | 8.4 | 11.64 | 31.0 |
| glargine ± metformin | 279 | 59.1 | 49.1 | 31.7 | 8.2 | 11.33 | 32.0 | |||
| Riddle et al.41 (GetGoal-L) | 24 | Stable | lixisenatide 10–20 μg qd + insulin ± metformin | 328 | 57.0 | 45.0 | 31.9 | 8.4 | 12.5 | 54.0 |
| placebo + insulin ± metformin | 167 | 57.0 | 49.0 | 32.6 | 8.4 | 12.4 | 58.0 | |||
| Riddle et al.42 (GetGoal-Duo 1) | 24 | Titration | lixisenatide 10–20 μg qd + glargine + metformin ± TZD | 223 | 56.0 | 49.0 | 32.0 | 7.6 | 9.6 | 43.4 |
| placebo + glargine + metformin ± TZD | 223 | 56.0 | 51.0 | 31.7 | 7.6 | 8.7 | 44.2 | |||
| Seino et al.48 | 24 | Stable | lixisenatide 20 μg qd + insulin ± SU | 154 | 58.7 | 44.8 | 25.4 | 8.5 | 13.7 | 24.9 |
| placebo + insulin ± SU | 157 | 58.0 | 51.0 | 25.2 | 8.5 | 14.1 | 24.1 | |||
| Seino et al.47 | 16 | Stable | liraglutide 0.9 mg qd + insulin | 127 | 61.3 | 54.3 | 26.2 | 8.8 | 14.32 | 30.0 |
| placebo + insulin | 130 | 59.8 | 57.7 | 25.2 | 8.8 | 14.69 | 29.0 | |||
| NCT0215237159 | 28 | NR | dulaglutide 1.5 mg qw + glargine ± metformin | 150 | 60.2 | 56.7 | NR | NR | NR | 40.7 |
| placebo + glargine ± metformin | 150 | 60.6 | 58.7 | NR | NR | NR | 36.6 | |||
| Insulin plus SGLT2 inhibitor | ||||||||||
| Araki et al.13 | 16 | Stable | dapagliflozin 5 mg qd + insulin ± DPP4i | 122 | 58.3 | 73.0 | 26.89 | 8.3 | 15.32 | 37.9 |
| placebo + insulin ± DPP4i | 60 | 57.6 | 66.7 | 26.12 | 8.5 | 14.24 | 40.6 | |||
| Cefalu et al.19 | 24 | Stable | dapagliflozin 10 mg qd + insulin ± OADs | 234 | NR | NR | NR | 8.3 | NR | 56.8 |
| placebo + insulin ± OADs | 242 | NR | NR | NR | 8.2 | NR | 49.2 | |||
| Inagaki et al.60 | 16 | Stable | canagliflozin 100 mg qd + insulin | 76 | 59.7 | 57.9 | 26.88 | 8.9 | 15.18 | 31.1 |
| placebo + insulin | 70 | 56.1 | 70.0 | 25.99 | 8.9 | 12.34 | 28.1 | |||
| Neal et al.37 | 18 | Stable | canagliflozin 300 mg qd + insulin ± OADs | 690 | 63.0a | 65.0 | 33.3 | 8.3 | 16.3 | 60.0a |
| placebo + insulin ± OADs | 690 | 63.0a | 66.0 | 33.1 | 8.3 | 16.0 | 58.0a | |||
| Rosenstock et al.43 | 18 | Stable | empagliflozin 25 mg qd + insulin ± metformin | 189 | 58.0 | 44.0 | 35.0 | 8.3 | NR | 92.9 |
| placebo + insulin ± metformin | 188 | 55.3 | 40.0 | 34.7 | 8.3 | NR | 93.1 | |||
| Rosenstock et al.44 | 18 | Stable | empagliflozin 25 mg qd + insulin ± metformin ± SU | 155 | 59.9 | 60.0 | 32.7 | 8.3 | NR | 48.4 |
| placebo + insulin ± metformin ± SU | 170 | 58.1 | 53.0 | 31.8 | 8.2 | NR | 47.8 | |||
| Wilding et al.51 | 12 | Stable | dapagliflozin 10 mg qd + insulin ± metformin ± TZD | 24 | 55.7 | 54.2 | 35.5 | 8.4 | 11.8 | 93.0 |
| placebo + insulin ± metformin ± TZD | 23 | 58.4 | 69.6 | 34.8 | 8.4 | 13.8 | 80.0 | |||
| Wilding et al.52 | 24 | Stable | dapagliflozin 10 mg qd + insulin ± OADs | 194 | 59.3 | 44.8 | 33.4 | 8.6 | 14.2 | 78.0 |
| placebo + insulin ± OADs | 193 | 58.8 | 49.5 | 33.1 | 8.5 | 13.5 | 73.7 | |||
| NCT0209670558 | 24 | NR | dapagliflozin 10 mg qd + insulin | 139 | 56.5 | 47.5 | NR | NR | NR | NR |
| placebo + insulin | 133 | 58.6 | 48.1 | NR | NR | NR | NR | |||
| Insulin plus thiazolidinedione | ||||||||||
| Asnani et al.14 | 16 | Titration | pioglitazone 30 mg qd + insulin ± OADs | 8 | 59.0 | NR | NR | 10.0 | 17.0 | NR |
| placebo + insulin ± OADs | 8 | 57.0 | NR | NR | 8.7 | 11.0 | NR | |||
| Buse et al.17 | 26 | Titration | troglitazone 400 mg qd + insulin | 76 | 58.0 | 50.0 | 34.8 | 9.0 | NR | NR |
| placebo + insulin | 71 | 57.0 | 49.0 | 34.5 | 9.0 | NR | NR | |||
| Hanefeld et al.24 | 24 | Titration | pioglitazone 15 mg bid + glargine + metformin | 39 | 63.3 | 66.7 | 33.1 | 7.3 | 11.0 | 34.9 |
| placebo + glargine + metformin | 42 | 64.2 | 54.8 | 31.8 | 7.4 | 12.3 | 36.6 | |||
| Henriksen et al.25 | 26 | Stable | pioglitazone 45 mg qd + insulin | 102 | 60.1 | 69.0 | 33.2 | 8.7 | 13.8 | 78.4 |
| balaglitazone 20 mg qd + insulin | 97 | 60.5 | 55.0 | 34.1 | 8.5 | 14.7 | 80.2 | |||
| placebo + insulin | 106 | 60.9 | 62.0 | 33.9 | 8.5 | 12.6 | 75.2 | |||
| Hodis et al.27 | 24 | Titration | troglitazone 400 mg qd + insulin | 142 | 52.4 | 33.1 | 32.1 | 9.9 | 9.8 | 51.5 |
| placebo + insulin | 134 | 52.6 | 32.1 | 31.1 | 9.7 | 9.7 | 54.5 | |||
| Hollander et al.28 | 24 | Titration | rosiglitazone 2 mg bid + insulin | 189 | 52.6 | 48.1 | 33.7 | 9.0 | 13.0 | 73.5 |
| placebo + insulin | 186 | 53.8 | 46.2 | 33.0 | 9.1 | 12.6 | 80.3 | |||
| Mattoo et al.35 | 24 | Titration | pioglitazone 30 mg qd + insulin ± OADs | 142 | 58.8 | 43.7 | 32.5 | 8.9 | 13.62 | NR |
| placebo + insulin ± OADs | 147 | 58.9 | 42.9 | 31.8 | 8.8 | 13.41 | NR | |||
| Naka et al.36 | 24 | NR | rosiglitazone 4 mg qd + insulin | 17 | 64.7 | 17.6 | 28.8 | 8.8 | 20.1 | 48.4 |
| insulin | 14 | 67.3 | 28.6 | 29.0 | 8.8 | 17.1 | 47.1 | |||
| Raskin et al.39 | 26 | Stable | rosiglitazone 4 mg bid + insulin | 103 | 57.7 | 54.4 | 32.3 | 9.0 | 12.5 | 77.7 |
| placebo + insulin | 104 | 55.6 | 55.8 | 32.7 | 8.9 | 11.1 | 70.1 | |||
| Reynolds et al.40 | 24 | Stable | rosiglitazone 4 mg per day + insulin | 8 | NR | NR | 36.4 | 8.0 | NR | 73.1 |
| placebo + insulin | 10 | NR | NR | 36.3 | 9.8 | NR | 72 | |||
| Shah et al.49 | 12–16 | Titration | pioglitazone 45 mg per day + insulin | 12 | 58.0 | 84.0 | 36.7 | 7.6 | NR | 105.0 |
| placebo + insulin | 13 | 58.0 | 84.0 | 36.7 | 7.8 | NR | 114.0 | |||
| Yasunari et al.53 | 48 | Titration | pioglitazone 15 mg bid + insulin + OADs | 22 | 56.0 | 81.8 | 25.5 | 8.6 | 13.4 | 37.2 |
| insulin ± OADs | 26 | 57.2 | 69.2 | 26.9 | 8.6 | 14.8 | 39.6 | |||
| Yilmaz et al.54 | 24 | Titration | rosiglitazone 8 mg qd + insulin | 15 | 57.6 | 53.3 | 30.7 | 9.6 | 12.1 | 41.9 |
| insulin | 19 | 61.5 | 36.8 | 28.2 | 8.7 | 17.9 | 42.7 | |||
Abbreviations: NR, not reported; OAD, oral antidiabetic drugs; SU, sulfonylurea; TZD, thiazolidinedione; DPP4i, dipeptidyl peptidase-4 inhibitor; bid, twice a day; qd, once a day; qw, once weekly; a, median; ‘insulin’ denotes multiple types of insulin preparations.
Quality assessment of the included studies
Twenty-seven15,18,20,22–24,26,28,30,31,33,34,36,38,40,41,43,44,47–49,51,54,57–59,61 (54%) and twenty-three13,14,20–24,26,28,32,34,36,40,49–51,53,54,57–61 (46%) of the 50 studies did not describe their methods of generating a random number and of allocation concealment, respectively. In the assessment for the blinding of patients and personnel, 8 trials20,21,33,36,46,53,54,56 (16%) were evaluated as high-risk, as they were open-label trials. For the risk of incomplete outcome data, most studies12–16,18,20,22,24–35,37,38,40–45,47,48,50–52,55–61 (80%) were rated as low-risk. Three studies57–59 were considered open to a high risk of selective reporting bias since we used their data from the Clinicaltrials.gov website only, and those data were not regarded as complete reports. Most of the trials12–22,24–48,50–56,60,61 (90%) had balanced baseline characteristics between the treatment groups. The detailed results of the assessment are found in Supplementary Figure 1.
Efficacy outcomes
We initially compared the primary outcome values from the common control group among the included studies. The pooled weighted mean changes in HbA1c from baseline in the control group were −0.16% (95% CI, −0.33% to 0.01%), −0.55% (−0.82% to −0.27%), −0.05% (−0.21% to 0.10%), and −0.11% (−0.32% to 0.09%) in the DPP4i, GLP-1RA, SGLT2i, and TZD studies, respectively, with a large extent of within-group heterogeneity (I2 = 94.5%, 97.9%, 94.7%, and 80.5%, respectively) (Supplementary Figure 2A). Considerable between-group heterogeneity was also observed (P < 0.0001). Greater reduction of HbA1c in the control group was noted in some studies with GLP-1RA or TZD treatments and those also adopted some type of insulin titration method. An inherited relationship was observed between the change in the HbA1c in the control group and the treatment difference in the changes of the HbA1c (P = 0.02) (Supplementary Figure 2B). Therefore, we took this modification effect into account by adjusting for the value in the control group. Age, sex, and BMI at baseline also had significant relationships with the treatment difference in the change of HbA1c (P < 0.05 for all) (Supplementary Figure 3).
In the adjusted analysis, all 4 add-on anti-diabetic agents showed a greater reduction of HbA1c compared to the control group: DPP4i, −0.54% (95% CrI, −0.68% to −0.39%); GLP-1RA, −0.84% (−1.00% to −0.69%); SGLT2i, −0.66% (−0.84% to −0.48%); and TZD, −0.73% (−0.93% to −0.52%) (Table 2). The difference in change of HbA1c between the GLP-1RA and DPP4i groups was significant (−0.30%; −0.52% to −0.09%). GLP-1RA showed the highest probability of being the best for glycemic control (Table 3). The unadjusted analysis also suggested a similar trend but failed to show a significant difference between GLP-1RA and DPP4i (Supplementary Table 1). The results were confirmed by sensitivity analyses (Supplementary Tables 2 and 3).
Table 2.
Pairwise results of comparisons between antidiabetic agents as an add-on to pre-existing insulin therapy from network meta-analyses adjusted by study-level covariates.
| Difference in mean change of HbA1c from baseline (95% credible interval), % | ||||
|---|---|---|---|---|
| Control | −0.54(−0.68, −0.39) | −0.84(−1.00,−0.69) | −0.66(−0.84,−0.48) | −0.73(−0.93,−0.52) |
| — | DPP4i | −0.30(−0.52,−0.09) | −0.11(−0.36, 0.12) | −0.18(−0.44, 0.07) |
| — | — | GLP-1RA | 0.19(−0.06, 0.43) | 0.11(−0.16, 0.40) |
| — | — | — | SGLT2i | −0.07(−0.33, 0.20) |
| — | — | — | — | TZD |
| Difference in mean change of FPG from baseline (95% credible interval), mg/dL [mmol/L] | ||||
| Control | −11.42(−15.47, −7.36) [−0.63(−0.86, −0.41)] |
−9.96(−14.55, −5.38) [−0.55(−0.81, −0.30)] |
−24.14(−29.64, −18.54) [−1.34(−1.65, −1.03)] |
−19.57(−24.78, −14.25) [−1.09(−1.38, −0.79)] |
| — | DPP4i | 1.48(−4.58, 7.42) [0.08(−0.25, 0.41)] |
−12.72(−19.53, −5.82) [−0.71(−1.08, −0.32)] |
−8.12(−14.79, −1.37) [−0.45(−0.82, −0.08)] |
| — | — | GLP-1RA | −14.17(−21.69, −6.73) [−0.79(−1.20, −0.37)] |
−9.61(−16.65, 2.38) [−0.53(−0.92, 0.13)] |
| — | — | — | SGLT2i | 4.61(−2.93, 12.11) [0.26(−0.16, 0.67)] |
| — | — | — | — | TZD |
| Difference in mean change of body weight from baseline (95% credible interval), kg | ||||
| Control | −0.10(−0.83, 0.64) | −2.20(−2.87, −1.57) | −1.75(−2.65, −0.85) | 2.58(1.63, 3.48) |
| — | DPP4i | −2.11(−3.11, −1.15) | −1.65(−2.81, −0.47) | 2.67(1.46, 3.82) |
| — | — | GLP-1RA | 0.46(−0.64, 1.58) | 4.78(3.63, 5.90) |
| — | — | — | SGLT2i | 4.32(2.98, 5.60) |
| — | — | — | — | TZD |
| Relative proportion of participants attaining HbA1c levels of <7% (95% credible interval) | ||||
| Control | 2.68(1.80, 3.81) | 3.70(2.89, 4.67) | 1.83(0.64, 3.90) | 2.18(1.12, 3.58) |
| — | DPP4i | 1.43(0.93, 2.15) | 0.72(0.21, 1.69) | 0.84(0.39, 1.51) |
| — | — | GLP-1RA | 0.50(0.16, 1.11) | 0.60(0.29, 1.02) |
| — | — | — | SGLT2i | 1.48(0.42, 3.81) |
| — | — | — | — | TZD |
| Difference in mean change of daily insulin dose from baseline (95% credible interval), IU/day | ||||
| Control | −3.87(−7.70, −0.10) | −8.61(−12.34, −5.00) | −4.64(−9.62, 0.32) | −11.97(−15.48, −8.41) |
| — | DPP4i | −4.76(−9.68, 0.16) | −0.79(−6.99, 5.51) | −8.09(−13.26, −2.88) |
| — | — | GLP-1RA | 3.94(−2.23, 10.14) | −3.36(−8.33, 1.74) |
| — | — | — | SGLT2i | −7.29(−13.45, −1.12) |
| — | — | — | — | TZD |
| Relative risk of hypoglycemia (95% credible interval) | ||||
| Control | 1.06(0.85, 1.32) | 1.37(1.08, 1.71) | 1.22(0.88, 1.67) | 1.94(1.39, 2.62) |
| — | DPP4i | 1.30(0.94, 1.77) | 1.15(0.78, 1.70) | 1.83(1.23, 2.65) |
| — | — | GLP-1RA | 0.89(0.60,1.32) | 1.41(0.95, 2.06) |
| — | — | — | SGLT2i | 1.59(1.01, 2.49) |
| — | — | — | — | TZD |
Abbreviations: DPP4i, dipeptidyl peptidase-4 inhibitor; GLP-1RA, glucagon like peptide-1 receptor agonists; SGLT2i, sodium-glucose co-transporter 2 inhibitor; TZD, thiazolidinedione; FPG, fasting plasma glucose.
Table 3.
Probabilities (%) of being the highest-ranked group for each study outcome
| DPP4i | GLP-1RA | SGLT2i | TZD | |
|---|---|---|---|---|
| Reduction of HbA1c from baseline | 0.13 | 77.14 | 4.21 | 18.52 |
| Reduction of FPG from baseline | 0.00 | 0.00 | 88.94 | 11.06 |
| Reduction of body weight from baseline | 0.00 | 79.64 | 20.36 | 0.00 |
| Proportion of HbA1c <7% | 4.64 | 88.76 | 4.14 | 2.46 |
| Reduction of insulin dose from baseline | 0.00 | 9.55 | 0.79 | 89.61 |
| Risk of hypoglycemia | 0.00 | 3.82 | 1.88 | 94.24 |
Abbreviations: DPP4i, dipeptidyl peptidase-4 inhibitor; GLP-1RA, glucagon like peptide-1 receptor agonists; SGLT2i, sodium-glucose co-transporter 2 inhibitor; TZD, thiazolidinedione; FPG, fasting plasma glucose.
Forty-one studies12,13,15–18,22,24–27,29–36,38,39,41–48,50–61 assessed changes in the FPG level from baseline. All groups showed a significantly greater reduction in FPG than the control group in the adjusted analysis: DPP4i, −11.42 mg/dL (−15.47 to −7.36 mg/dL) [−0.63 mmol/L (−0.86 to −0.41 mmol/L)]; GLP1-RA, −9.96 mg/dL (−14.55 to −5.38 mg/dL) [−0.55 mmol/L (−0.81 to −0.30 mmol/L)]; SGLT2i, −24.14 mg/dL (−29.64 to −18.54 mg/dL) [−1.34 mmol/L (−1.65 to −1.03 mmol/L)]; TZD, −19.57 mg/dL (−24.78 to −14.25 mg/dL) [−1.09 mmol/L (−1.38 to −0.79 mmol/L)] (Table 2). SGLT2i showed a significantly greater reduction in FPG compared to DPP4i and GLP1-RA. SGLT2i was ranked as the best treatment for FPG reduction (Table 3). Sensitivity analysis with missing covariate imputation was not required for this outcome.
Thirty-eight trials12,13,15–18,20–22,25,27,30,32–36,40–56,58–61 assessed changes in body weight from baseline. GLP-1RA and SGLT2i decreased body weight significantly more than DPP4i (−2.11 kg, −3.11 to −1.15 kg; and −1.65 kg, −2.81 to −0.47 kg, respectively), whereas TZD showed an increase in body weight compared to DPP4i (2.67 kg, 1.46 to 3.82 kg) (Table 2). The difference between the DPP4i and the control group was not significant. GLP-1RA had the highest probability of being ranked the first for body weight reduction, followed by SGLT2i (Table 3). The results were consistent in the sensitivity analysis after the imputation of missing covariates (Supplementary Table 2).
Thirty-two trials12,15,16,18,20,21,23,24,26,28,30–35,37,38,41–48,50,51,55,56,59,61 described the proportion of participants attaining the target HbA1c level (7.0%). GLP-1RA, DPP4i, and TZD groups showed a significantly higher proportion of achieving the target HbA1c level than the control (RR = 3.70, 2.89–4.67; RR = 2.68, 1.80–3.81; and RR = 2.18, 1.12–3.58, respectively), among which GLP-1RA showed the highest probability of being the best for reaching the target HbA1c level (Tables 2 and 3). Only 2 SGLT2i studies37,51 were included in the covariate-adjusted analysis, because the other SGLT2i studies had some missing covariates. When we reanalyzed the results with an imputation of the missing covariates, all 4 groups showed a significant increase in the rate of achieving the target HbA1c level compared to the control group (Supplementary Table 2).
For the change in insulin doses from baseline, 34 studies13,15,16,18,20–22,24,25,27,28,32–34,39–42,44–56,58,59,61 were included in the analysis. DPP4i, GLP-1RA, and TZD showed a significant reduction in the insulin dose compared to the control group (−3.87 IU/day, −7.70 to −0.10 IU/day; −8.61 IU/day, −12.34 to −5.00 IU/day; and −11.97 IU/day, −15.48 to −8.41 IU/day, respectively) (Table 2). The insulin-sparing effects of TZD were significantly greater than those of DPP4i and SGLT2i. TZD was ranked the highest in terms of its insulin-sparing effect, followed by GLP-1RA (Table 3). A sensitivity analysis after imputing missing covariates confirmed the results (Supplementary Table 2).
Hypoglycemia
Thirty-four studies12,13,15,16,18,20–22,24–26,29–31,33–35,38,39,41–45,48,50–52,55–57,59–61 were included in the analysis. The risk of hypoglycemia was not greater in the DPP4i or SGLT2i groups than in the control group. Hypoglycemia risk with TZD was found to be significantly greater than with DPP4i and SGLT2i (RR = 1.83, 1.23–2.65 and RR = 1.59, 1.01–2.49, respectively) (Table 2). The TZD group was ranked the highest for risk of hypoglycemia (Table 3). The risk of hypoglycemia was not associated with any potential covariates, and therefore no adjustment was required for this analysis.
Discussion
Through a current systematic review and network meta-analysis based on 50 RCTs including 15,494 participants, we report the best available evidence comparing the efficacy and safety among different types of non-insulin anti-diabetic agents as an add-on to pre-existing insulin therapy in patients with inadequately controlled T2DM. The principal findings of our study are as follows: (1) GLP-1RA showed the greatest effect on HbA1c reduction, followed by TZD, SGLT2i, and DPP4i; (2) the reduction in FPG was higher with SGLT2i than with DPP4i and GLP1-RA; (3) GLP-1RA and SGLT2i were associated with body weight reduction, whereas TZD increased body weight; (4) TZD and GLP-1RA reduced total daily insulin requirements; (5) the risk of hypoglycemia was increased with TZD and GLP-1RA.
The studies included in our analysis had different baseline characteristics and showed significant within-group and between-group heterogeneity. We found that age, sex, and BMI at baseline were associated with placebo-subtracted HbA1c reduction in a linear relationship; therefore, we adjusted for those potential confounding factors as covariates. In addition, since it was not possible to adjust for various methods of insulin titration among the included studies, we instead adjusted for the treatment effects of the control group. After adjustment for the covariates, GLP-1RA was at the top rank of the hierarchy. In addition, the results were similar in the sensitivity analyses, with GLP-1RA showing the highest probability of being the best for glycemic control. Consistent with the primary outcome, GLP-1RA also showed the highest rank for achieving HbA1c levels <7.0%. This might be explained by the complementary effects of GLP-1RA with insulin therapy62.
The FPG-lowering efficacy of SGLT2i was significantly greater than that of DPP4i and GLP-1RA, and tended to be greater than that of TZD. SGLT2i drugs act on the kidneys and increase glucose excretion through the urine, which is mediated by an insulin-independent mechanism; therefore, they reduce both FPG and postprandial glucose (PPG)63. In contrast, GLP-1RA agents have different FPG- and PPG-lowering efficacy, depending on their duration of action. Short-acting GLP-1RA drugs drastically decelerate gastric emptying, leading to a marked reduction in the PPG excursion. In contrast, long-acting GLP-1RA drugs have little effect on gastric emptying, but their long-lasting effect on pancreatic beta-cell and alpha-cell function contributes to the predominant reduction in FPG levels64. Because about half of the GLP-1RA studies used long-acting GLP-1RA agents, and the rest of the studies used short-acting GLP-1RA agents, the FPG-lowering effect of add-on GLP-1RA might be mixed.
Weight gain is commonly associated with insulin therapy, particularly with intensive insulin therapy65. In this network meta-analysis, GLP-1RA appeared to be the most effective for weight reduction, which is attributed to decreasing appetite and promoting satiety64. SGLT2i was ranked lower than GLP-1RA, possibly because the weight-loss effect of SGLT2i is compensated by increased appetite and calorie intake66,67. Overall, the reported effects of weight loss with GLP-1RA and SGLT2i, weight gain with TZD, and no effect for DPP4i were preserved when combined with preexisting insulin therapy in patients with T2DM62,63,68.
The insulin-sparing effect, which was pre-defined as the reduction in the total required daily dose of insulin from baseline, was greater in TZD and GLP-1RA. The greater potency of GLP-1RA and TZD in glycemic control and their insulin-sensitizing effects may contribute to reducing insulin dosage62,69. Differences in the insulin titration algorithm may also have contributed to the different insulin-sparing effect across the studies. More than half of the studies of TZD and GLP-1RA adopted insulin dose titration regimens, whereas most studies of SGLT2i and DPP4i adopted stable insulin dose regimens. The placebo-subtracted insulin-sparing effects in studies with active insulin dose titration tend to be greater than in studies with stable insulin dose regimens.
In the current study, SGLT2i and DPP4i were suggested to have a potentially lower risk of hypoglycemia than the other 2 agents. In general, SGLT2i drugs do not induce hypoglycemia because they increase plasma glucagon concentrations and decrease plasma insulin concentrations70,71. DPP4i drugs also have a minimal risk of hypoglycemia due to their glucose-dependent mechanism of action regarding the regulation of insulin and glucagon secretion62. Theoretically, TZD and GLP-1RA drugs seldom provoke hypoglycemia by themselves unless combined with insulinotrophic agents64,72. In this study, however, TZD and GLP-1RA agents showed an increased risk of hypoglycemia in comparison to the control group. The greater glucose-lowering potency of TZD and GLP-1RA and adopting an active insulin dose titration regimen might contribute to the increased risk. Because the definitions of hypoglycemia varied widely across the included studies, the comparative risk of hypoglycemia among these agents must be carefully interpreted.
The study has several limitations. First, the current study is based on indirect comparisons using a common control. Network meta-analyses are typically required to address inconsistencies between the results of direct and indirect comparisons. However, since no study has conducted head-to-head comparisons among GLP-1RA, DPP4i, SGLT2i, and TZD, it was not possible to evaluate consistency between the results of direct and indirect comparisons. We therefore primarily explored the heterogeneity among the values of HbA1c reduction in the control group, which possibly indicates a lack of comparability among the included studies. We then identified as many potential confounders as possible and adjusted for their effect in the final analysis. Second, the patients in the studies included in this study used various baseline insulin regimens, but we could not isolate the effects of the studied medications on top of the basal insulin therapy regimen, which is the most common practice of insulin therapy at present. Finally, due to lack of information, we could not assess drug-specific safety outcomes, such as gastrointestinal adverse events and urinary tract infections.
Based on the evidence from our network meta-analysis, we may suggest patient-centered guidance for non-insulin anti-diabetic agents in patients with inadequately controlled T2DM on pre-existing insulin therapy. For those who need to reduce both HbA1c and body weight, GLP-1RA might be an optimal choice. Fixed-ratio combinations of insulin and GLP-1RA are currently available to improve glycemic control and to minimize the risk of weight gain33,56. For patients who need to lower their FPG level, SGLT2i might be a good treatment option due to its superior FPG-lowering efficacy without increasing weight gain or hypoglycemia. It also has the advantage of body weight reduction. Even though DPP4i was relatively less efficacious for HbA1c reduction, it rarely increased the risk of hypoglycemia. Therefore, DPP4i may be considered for patients with relatively less severe hyperglycemia but with a high risk of hypoglycemia. Lastly, TZD might be taken into consideration in patients with severe insulin resistance requiring high doses of insulin.
Since a previous study which compared insulin monotherapy with the addition of various oral glucose-lowering agents to insulin did not present the comparative efficacy among non-insulin anti-diabetic agents in patients with inadequately controlled T2DM with insulin therapy73, our systematic review and network meta-analysis provides evidence-based suggestions for selecting an appropriate non-insulin antidiabetic agent based on patients’ clinical characteristics. Nonetheless, RCTs directly comparing the efficacy and safety of these agents should be undertaken.
Electronic supplementary material
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HC15C3357).
Author Contributions
Y.M.C., S.H., J.-H.Y. and S.H.M. contributed to the study concept and design. J.-H.Y. and S.H.M. performed the systematic literature search and extracted data. J.-H.Y., S.H.M. and S.H. did analysis and interpretation of data. J.-H.Y., S.H.M., Y.M.C. and S.H. wrote the first draft and C.H.A., Y.M.C. and S.H. critically revised the manuscript for important intellectual content. Y.M.C. and S.H. were the study supervisors.
Competing Interests
Y.M.C. received research grants from AstraZeneca and LG Chemical. S.H. received a consultation fee from Novartis. The other authors disclose no potential conflicts of interest relevant to this study.
Footnotes
Jeong-Hwa Yoon and Se Hee Min contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22443-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young Min Cho, Email: ymchomd@snu.ac.kr.
Seokyung Hahn, Email: hahns@snu.ac.kr.
References
- 1.Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc. 2003;78:459–467. doi: 10.4065/78.4.459. [DOI] [PubMed] [Google Scholar]
- 2.Edelman S, Pettus J. Challenges associated with insulin therapy in type 2 diabetes mellitus. Am J Med. 2014;127:S11–16. doi: 10.1016/j.amjmed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, et al. Consensus Statement By The American Association of Clinical Endocrinologists And American College of Endocrinology On The Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr Pract. 2017;23:207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 5.Higgins, J. P. T. & Green, S. Cochrane handbook for Systematic Reviews of Interventions, www.cochrane-handbook.org.
- 6.Glenny, A. M. et al. Indirect comparisons of competing interventions. Health technology assessment9, 1–134, iii–iv (2005). [DOI] [PubMed]
- 7.Jansen JP, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2014;17:157–173. doi: 10.1016/j.jval.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC medicine. 2013;11:159. doi: 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoaglin DC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value in health. 2011;14:429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in medicine. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 11.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Ahmann A, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes, obesity & metabolism. 2015;17:1056–1064. doi: 10.1111/dom.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki, E. et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: Results of the interim analysis of 16-week double-blind treatment period. Journal of Diabetes Investigation (2016). [DOI] [PMC free article] [PubMed]
- 14.Asnani S, et al. Pioglitazone restores endothelial function patients with type 2 diabetes treated with insulin. Metabolic Syndrome and Related Disorders. 2006;4:179–184. doi: 10.1089/met.2006.4.179. [DOI] [PubMed] [Google Scholar]
- 15.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Current Medical Research and Opinion. 2012;28:513–523. doi: 10.1185/03007995.2012.665046. [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes. Annals of Internal Medicine. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, et al. Troglitazone use in insulin-treated type 2 diabetic patients. Diabetes Care. 1998;21:1455–1461. doi: 10.2337/diacare.21.9.1455. [DOI] [PubMed] [Google Scholar]
- 18.Buse JB, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) Diabetes Care. 2014;37:2926–2933. doi: 10.2337/dc14-0785. [DOI] [PubMed] [Google Scholar]
- 19.Cefalu WT, et al. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. 2015;38:1218–1227. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wit HM, et al. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT) Diabetologia. 2014;57:1812–1819. doi: 10.1007/s00125-014-3302-0. [DOI] [PubMed] [Google Scholar]
- 21.Distiller LA, Nortje H, Wellmann H, Amod A, Lombard L. A 24-week, prospective, randomized, open-label, treat-to-target pilot study of obese type 2 diabetes patients with severe insulin resistance to assess the addition of exenatide on the efficacy of U-500 regular insulin plus metformin. Endocrine Practice. 2014;20:1143–1150. doi: 10.4158/EP14067.OR. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca V, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 23.Franc S, et al. Type 2 diabetes patients on metformin and well-controlled basal: Can supplementary vildagliptin control residual prandial hyperglycaemia? VIBE study results. Diabetologia. 2015;58:S388. [Google Scholar]
- 24.Hanefeld M, Pfutzner A, Forst T, Kleine I, Fuchs W. Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: the PIOCOMB study. Cardiovascular diabetology. 2011;10:65. doi: 10.1186/1475-2840-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksen K, et al. Efficacy and safety of the PPARγ partial agonist balaglitazone compared with pioglitazone and placebo: A phase III, randomized, parallel-group study in patients with type 2 diabetes on stable insulin therapy. Diabetes/Metabolism Research and Reviews. 2011;27:392–401. doi: 10.1002/dmrr.1187. [DOI] [PubMed] [Google Scholar]
- 26.Hirose T, Suzuki M, Tsumiyama I. Efficacy and Safety of Vildagliptin as an Add-on to Insulin with or without Metformin in Japanese Patients with Type 2 Diabetes Mellitus: A 12-week, Double-Blind, Randomized Study. Diabetes Therapy. 2015;6:559–571. doi: 10.1007/s13300-015-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodis HN, et al. Effect of peroxisome proliferator-activated receptor γ agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. 2006;29:1545–1553. doi: 10.2337/dc05-2462. [DOI] [PubMed] [Google Scholar]
- 28.Hollander P, Yu D, Chou HS. Low-dose rosiglitazone in patients with insulin-requiring type 2 diabetes. Archives of Internal Medicine. 2007;167:1284–1290. doi: 10.1001/archinte.167.12.1284. [DOI] [PubMed] [Google Scholar]
- 29.Kadowaki T, et al. Efficacy and safety of sitagliptin add-on therapy in Japanese patients with type 2 diabetes on insulin monotherapy. Diabetology International. 2013;4:160–172. doi: 10.1007/s13340-013-0109-z. [DOI] [Google Scholar]
- 30.Kaku K, Mori M, Kanoo T, Katou M, Seino Y. Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: A randomized, double-blind, 12-week, placebo-controlled trial followed by an open-label, long-term extension phase. Expert Opinion on Pharmacotherapy. 2014;15:2121–2130. doi: 10.1517/14656566.2014.956722. [DOI] [PubMed] [Google Scholar]
- 31.Kothny W, et al. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2013;15:252–257. doi: 10.1111/dom.12020. [DOI] [PubMed] [Google Scholar]
- 32.Lind M, et al. A randomised double-blind trial of liraglutide to control HbA1c in subjects with type 2 diabetes treated with multiple daily insulin injections (MDILiraglutide trial) Diabetologia. 2015;58:S37. doi: 10.1007/s00125-014-3397-3. [DOI] [PubMed] [Google Scholar]
- 33.Lingvay I, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2diabetes: The DUAL v randomized clinical trial . JAMA - Journal of the American Medical Association. 2016;315:898–907. doi: 10.1001/jama.2016.1252. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu C, et al. A Randomized Clinical Trial to Evaluate the Efficacy and Safety of Co-Administration of Sitagliptin with Intensively Titrated Insulin Glargine. Diabetes Therapy. 2015;6:127–142. doi: 10.1007/s13300-015-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattoo V, et al. Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: Results of a six-month, randomized, double-blind, prospective, multicenter, parallel-group study. Clinical Therapeutics. 2005;27:554–567. doi: 10.1016/j.clinthera.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Naka KK, et al. Rosiglitazone improves endothelial function in patients with type 2 diabetes treated with insulin. Diabetes & vascular disease research. 2011;8:195–201. doi: 10.1177/1479164111408628. [DOI] [PubMed] [Google Scholar]
- 37.Neal B, et al. Efficacy and safety of canaglif lozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 38.Ning G, et al. Vildagliptin as add-on therapy to insulin improves glycemic control without increasing risk of hypoglycemia in Asian, predominantly Chinese, patients with type 2 diabetes mellitus. Journal of Diabetes. 2016;8:345–353. doi: 10.1111/1753-0407.12303. [DOI] [PubMed] [Google Scholar]
- 39.Raskin P, et al. Rosiglitazone Clinical Trials Study Group: A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds L, Konz E, Frederich R, Anderson J. Rosiglitazone amplifies the benefits of lifestyle intervention measures in long‐standing type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2002;4:270–275. doi: 10.1046/j.1463-1326.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 41.Riddle MC, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: A 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddle MC, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenstock J, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2015;17:936–948. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstock J, et al. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1c without causing weight gain or increased hypoglycaemia. Diabetes, Obesity and Metabolism. 2009;11:1145–1152. doi: 10.1111/j.1463-1326.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 46.Sato, S. et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 Diabetes: The EDIT randomized trial. PLoS ONE10 (2015). [DOI] [PMC free article] [PubMed]
- 47.Seino, Y. et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: A 36-week, randomized, double-blind, parallel-group trial. Journal of Diabetes Investigation (2016). [DOI] [PMC free article] [PubMed]
- 48.Seino Y, et al. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes, Obesity and Metabolism. 2012;14:910–917. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah PK, et al. Effects of intensive insulin therapy alone and in combination with pioglitazone on body weight, composition, distribution and liver fat content in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2011;13:505–510. doi: 10.1111/j.1463-1326.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vilsboll T, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2010;12:167–177. doi: 10.1111/j.1463-1326.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 51.Wilding JP, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes care. 2009;32:1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilding JP, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Annals of internal medicine. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 53.Yasunari E, et al. Efficacy of pioglitazone on glycemic control and carotid intima-media thickness in type 2 diabetes patients with inadequate insulin therapy. Journal of Diabetes Investigation. 2011;2:56–62. doi: 10.1111/j.2040-1124.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yilmaz H, Gursoy A, Sahin M, Guvener Demirag N. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and rosiglitazone or insulin and acarbose in type 2 diabetes. Acta Diabetologica. 2007;44:187–192. doi: 10.1007/s00592-007-0004-9. [DOI] [PubMed] [Google Scholar]
- 55.Yki-Järvinen H, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: A ≥ 52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–3881. doi: 10.2337/dc12-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–1980. doi: 10.2337/dc16-1495. [DOI] [PubMed] [Google Scholar]
- 57.Clinicaltrials.gov. Efficacy and Safety Study of Teneligliptin (MP-513) in Combination With Insulin in Patients With Type 2 Diabetes. https://clinicaltrials.gov/ct2/show/NCT02081599. Accessed March 29, 2017.
- 58.Clinicaltrials.gov. Phase III Insulin Add-On Asia Regional Program - ST. https://clinicaltrials.gov/ct2/show/NCT02096705. Accessed March 29, 2017.
- 59.Clinicaltrials.gov. A Study of Dulaglutide (LY2189265) in Participants With Type II Diabetes (AWARD-9). https://clinicaltrials.gov/ct2/show/NCT02152371. Accessed March29, 2017.
- 60.Inagaki N, et al. Efficacy and safety of canagliflozin in combination with insulin: a double-blind, randomized, placebo-controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovascular Diabetology. 2016;15:89. doi: 10.1186/s12933-016-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shankar RR, et al. Sitagliptin added to stable insulin therapy with or without metformin in Chinese patients with type 2 diabetes. J Diabetes Investig. 2016 doi: 10.1111/jdi.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 63.Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–151. doi: 10.1016/S2213-8587(13)70050-0. [DOI] [PubMed] [Google Scholar]
- 64.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 65.Holman RR, et al. Three-Year Efficacy of Complex Insulin Regimens in Type 2 Diabetes. New England Journal of Medicine. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 66.Polidori D, Sanghvi A, Seeley RJ, Hall KD. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity (Silver Spring) 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrannini G, et al. Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–531. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 69.Schoonjans K, Auwerx J. Thiazolidinediones: an update. Lancet. 2000;355:1008–1010. doi: 10.1016/S0140-6736(00)90002-3. [DOI] [PubMed] [Google Scholar]
- 70.Merovci A, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrannini E, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizos CV, Elisaf MS, Mikhailidis DP, Liberopoulos EN. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf. 2009;8:15–32. doi: 10.1517/14740330802597821. [DOI] [PubMed] [Google Scholar]
- 73.Vos, R. C. et al. Insulin monotherapy compared with the addition of oral glucose‐lowering agents to insulin for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control. The Cochrane Library (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.