Abstract
Cognitive impairment is age-related and manageable only with early diagnosis and prevention. Moxibustion is widely accepted in East Asia as useful for preventing cognitive impairment. This systematic review of animal studies was conducted to verify the efficacy of moxibustion in preventing cognitive impairment and to elucidate the underlying mechanism. Randomized controlled animal trials that established the efficacy of moxibustion in preventing cognitive impairment were included in the analysis. Results of behavioral tests and the signaling pathways elucidated were extracted and a meta-analysis was conducted with the behavioral test results. The risk of bias was evaluated using 9 items, and reporting quality was evaluated using the ARRIVE (Animal Research: Reporting In Vivo Experiments) Guidelines Checklist. Ten trials involving 410 animals met the inclusion criteria. All studies reported the benefit of moxibustion in preventing cognitive deficits caused by Alzheimer's disease (AD). Among five studies using the Morris water maze test, a significant effect of moxibustion in decreasing the escape time was reported in three studies, increasing the crossing times in four studies, and prolonging the dwelling time in two studies. The effects of moxibustion were demonstrated to be mediated by an increase in the activity of neurotrophins and heat shock protein, modulation of the cell cycle, and suppression of apoptosis and inflammation. However, considering the small number of included studies, the lack of studies investigating entire signaling pathways, and a high risk of bias and low reporting quality, our results need to be confirmed through more detailed studies.
Keywords: Systematic review, Cognitive impairment, Prevention, Moxibustion, Animal experimentation
Graphical Abstract
INTRODUCTION
With rapid aging, the proportion of elderly individuals worldwide is estimated to almost double from 12% to 22% between 2015 and 2050 [1]. Moreover, approximately 20% of elderly individuals may develop a mental or neurological disorder, the most likely being cognitive impairment [1]. Cognitive impairment represents a diverse collection of disorders characterized by chronic and progressive neurodegeneration, resulting in high social and economic costs as well as physical and emotional burdens to patients and families [2]. Early diagnosis and adequate prevention are the best ways to manage cognitive impairment, because no current treatments can mitigate or cure the condition after it has begun [3].
Moxibustion has been widely used in East Asia for thousands of years [4,5]. Moxibustion is the burning of dried herbs, such as mugwort, at one or more relevant acupoints, thereby imparting both heat stimulation via infrared radiation [4,6] and the pharmacological action of the herbal components to the site of application [7,8]. Moxibustion has recently become popular in gynecology for managing fetal breech presentation and the pain of primary dysmenorrhea [9,10]. Moreover, moxibustion has been reported to prevent inflammation, organ dysfunction [11,12], and hormonal imbalances [13]. Moxibustion may also help prevent cognitive impairment, and prevention is currently the only suitable management technique for this disorder [14]. There have been several clinical reports about the use of moxibustion for managing cognitive impairment [15,16,17], mostly from China. After applying moxibustion as a single therapy or in combination with acupuncture and/or herbal medicine, these reports evaluated the efficacy of moxibustion by assessing outcomes such as the clinical index [16], as well as levels of inflammatory mediators [17], lipid peroxides [16], and antioxidants [15].
However, in spite of its long history of use and abundant clinical support, the efficacy of moxibustion in preventing cognitive impairment has not been fully validated. Additionally, mechanisms underlying the efficacy of moxibustion have been proposed but not verified. Although several studies have investigated the preservation of cognitive function by moxibustion, a systematic analysis of the research has not been done. Therefore, we conducted a systematic review and meta-analysis of the literature to verify the efficacy of moxibustion in preventing cognitive impairment. We focused on animal studies to analyze the reported underlying mechanisms and their levels of evidentiary support.
MATERIALS AND METHODS
Assessment of risk of bias in included studies
To evaluate the overall potential bias in the included studies, we designed a table composed of nine questions (Table 1). Using the quality assessment scoring suggested by Wever [18] and Hooijmans [19], we reorganized items necessary for checking the risk of bias while excluding those which are less valid in an animal study. The questions were grouped according to the type of bias they addressed: selection bias (items 1 and 2), detection bias (items 3), potential bias induced by baseline imbalance (items 4 and 5), performance bias (item 6 and 7), and attrition bias (items 8 and 9).
Table 1. Assessment of risk of bias in the included studies.
| Bias | Item Number | Question | Li [21] | Liu [22] | Wang [23] | Li [24] | Du et al. [25] | Li et al. [26] | Li et al. [27] | Liu et al. [28] | Liu et al. [29] | Du et al. [30] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization | 1 | Were animals randomized across groups? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | What method was used for randomization? | Y | U | U | U | U | Y | Y | U | U | U | |
| Blindness | 3 | Was the outcome assessment blinded? | U | U | U | U | U | U | U | U | U | U |
| Experimental animals | 4 | Were characteristics of experimental animals clearly described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Baseline | 5 | Were the groups similar at baseline? | Y | Y | Y | Y | U | Y | Y | Y | Y | Y |
| Homogeneity | 6 | Was each treatment homogeneous? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Was the method of outcome measurement proper and homogeneous? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Data completion | 8 | Was the number of animals excluded from analysis and the reason for exclusion clear? | U | U | U | U | U | U | U | U | U | U |
| 9 | Were the outcome data complete? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
Y, yes; N, no description; U, unclear description.
Assessment of methodological quality in included studies
The reporting quality of each study was evaluated according to the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines checklist [20]. The ARRIVE guidelines were suggested by PLOS Biology before being developed and utilized by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3R). These guidelines are believed to enhance the standard of reporting in animal studies, enabling thorough peer review and informing future research. Although we developed question items to measure the risk of bias, we also adopted the ARRIVE checklist to evaluate the overall reporting quality.
Data synthesis and statistical analysis
Data were reported as common values that could be synthesized. One reviewer (SC) then classified the data into groups and entered them into the Review Manager software (RevMan5.3, Cochrane Collaboration, Oxford, UK). Post-hoc subgroup analysis was performed. Subgroups were divided based on types of baseline test and how long conducted moxibustion pre-treatment. A second reviewer (MDC) checked the data for accuracy.
RESULTS
Fundamental study characteristics: animals
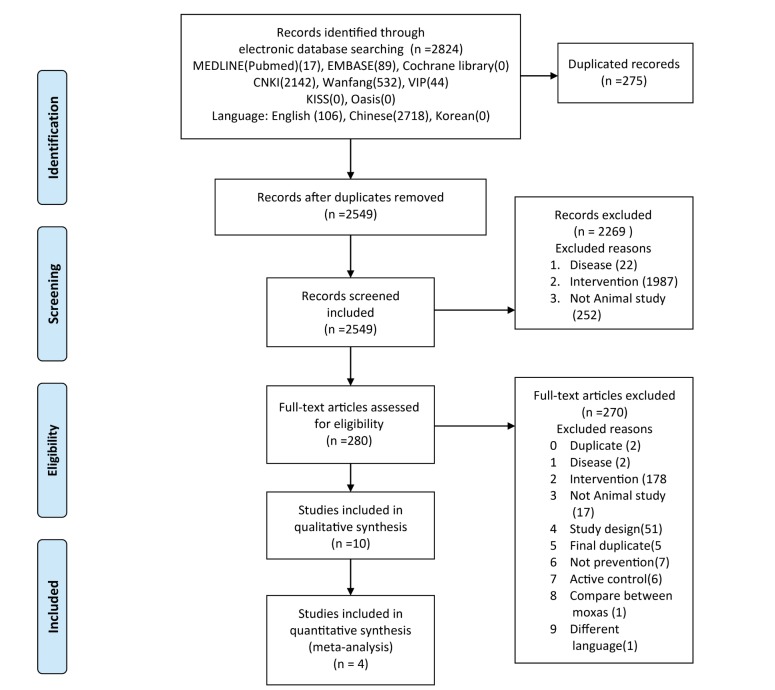
The ten studies were included in the analysis [21,22,23,24,25,26,27,28,29,30] (Fig. 1). Four studies were dissertations [21,22,23,24], and two of them [23,24] were published as original articles [31,32]. Their affiliations and authors were the same and the experimental methods and results were identical. However, two dissertations [23,24] reported more experimental results than their duplicated original articles [31,32]. Therefore, two dissertations were selected and the duplicated original articles was excluded in this review. Included ten studies used a total of 410 rats (age, 8~15 months; weight, 340~480 g) [21,22,23,24,25,26,27,28,29,30]. Sprague-Dawley (SD) rats were used in all studies except one, which used Wistar rats. Six of the studies used male rats exclusively, whereas the others used even numbers of both sexes. In all studies, cognitive impairment was induced by injecting β-amyloid (Aβ) into the hippocampus to model Alzheimer's disease (AD). In seven studies, 5 µl (1 µg/µl) of Aβ1-42 was injected and 1 µl (5 µg/µl) of Aβ25-35 was injected in three studies. Six studies described injecting Aβ into both sides of the hippocampus. In each study, ten rats were assigned to the experimental group and ten were assigned to the control group (Table 2).
Fig. 1. PRISMA flow diagram for selecting related studies. doi: 10.1371/journal.pmed1000097.
Table 2. Assessment of methodological quality in the included studies.
| Study | Animal model | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Age (month) | Weight (g) | Sample size | Induced disease | Acupoint | Moxibustion diameter (mm) | Duration (min) | Treatment method and period | |||
| Moxa (M,F) | Control (M,F) | Before inducing model | After inducing model | ||||||||
| Li [21] | SD rat | 15 | 350~480 | (100) | (100) | AD | GV20, BL23, ST36 | 8 | 5 | 18 times for 21 days | (After 1 day) 6 times for 7 days |
| Liu [22] | SD rat | 12±2 | 360±20 | (100) | (100) | AD | GV20, BL23 | 15~20 | 10 | 40 times for 56 days | (After 4 day) 11 timesfor 11 days |
| Wang [23] | SD rat | 12 | 400±50 | (100) | (100) | AD | GV20, BL23 | NR | 10 | 40 times for 56 days | |
| Li [24] | SD rat | 12±2 | 360±20 | (55) | (55) | AD | GV20, BL23 | NR | 5 | 40 times for 56 days | |
| Du et al. [25] | Wistar rat | 12 | 500±20 | (100) | (100) | AD | GV20, BL23 | 6 | 15 | 48 times for 56 days | 14 times for 14 days |
| Li et al. [26] | SD rat | 15 | 350~480 | (100) | (100) | AD | GV20, BL23, ST36 | 8 | 5 | 18 times for 21 days | (After 1 day) 6 times for 7 days |
| Li et al. [27] | SD rat | 15 | 350~480 | (100) | (100) | AD | GV20, BL23, ST36 | 8 | 5 | 18 times for 21 days | (After 1 day) 6 times for 7 days |
| Liu et al. [28] | SD rat | 12±2 | 360±20 | (55) | (55) | AD | GV20, BL23 | 6 | 10 | 18 times for 21 days | 7 times for 7 days |
| Liu et al. [29] | SD rat | 12±2 | 360±20 | (55) | (55) | AD | GV20, BL23 | NA | 5 | 40 times for 56 days | |
| Du et al. [30] | SD rat | 10±2 | 360±20 | (55) | (55) | AD | GV20, BL23 | 6 | 10 | 18 times for 21 days | 7 times for 7 days |
SD, Sprague-Dawley; M, male; F, female; Moxa, moxibustion; AD, Alzheimer's disease; NR, not reported; NGF, nerve growth factor; Trk, tropomyosin receptor kinase; BDNF, brain-derived neurotrophic factor; CDK, cyclin-dependent kinase; PGE, prostaglandin E; COX, cyclooxygenase; P-p38 MAPK, phospho-p38 mitogen-activated protein kinase.
Fundamental study characteristics: moxibustion
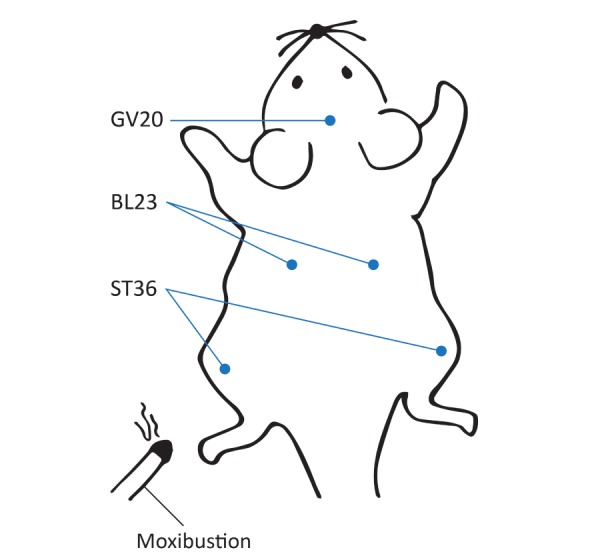
Seven of the ten studies employed moxibustion both before and after induction of the disease model. All ten studies selected GV20 and BL23 as the treatment acupoints, and three studies added ST36 (Fig. 2). When using the BL23 and ST36 acupoints, six studies applied moxibustion to both sides during the same session, and four studies treated one side per day on alternating days. All moxibustion protocols burned artemisia, which is the most popular herbal component. In nine studies, a moxibustion area 6 to 8 mm in diameter was used; only one study used an area 1.5 to 2 cm diameter. In every study, moxibustion was performed 2 to 3 cm above the surface of the acupoints as suspended moxibustion (Fig. 3). The duration of an individual treatment was 5 min (5 studies), 10 min (4 studies), or 15 min (1 study), and the treatment periods were 4 weeks (5 studies) or over 8 weeks (5 studies).
Fig. 2. Schematic showing the location of acupoints on the rat mentioned in the reviewed studies.
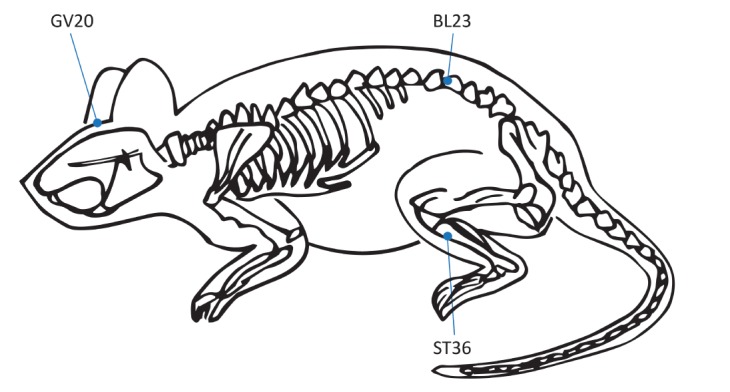
Fig. 3. Suspended moxibustion treatment. Moxibustion was performed 2~3 cm above the surface of the acupoints.
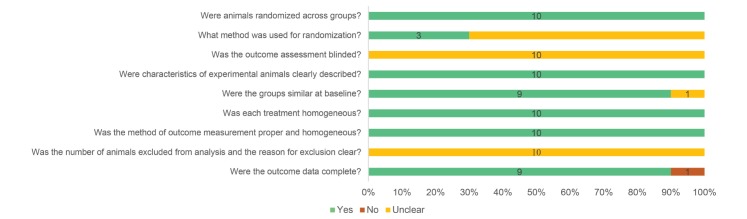
Risk of bias
From the risk of bias table that we constructed (Fig. 4), 68% of the total items satisfied the low risk of bias criteria, whereas 31% were in the “unclear” category. The risk of selection bias appeared to be low since all studies claimed that randomization was implemented; however, just three studies detailed the randomization method. None of the studies addressed whether blinding was employed during the outcome assessment, making it difficult to determine if there was detection bias. Every study described the characteristics of the experimental animals, and all except one detailed the method of adjusting the cognitive function baseline. Treatments appeared to be performed homogeneously within a given study. Because the number of animals excluded from analysis and the reasons for exclusion were not described in all of the studies, it is unclear whether or not there was a risk of bias due to selective reporting. Nine out of the ten studies recorded all outcome data. However, one study omitted one of their proposed outcomes, thus there was a high risk of selective reporting bias. Fig. 4 presents the question items for assessing the risk of bias and the results of ten studies included in this systematic review.
Fig. 4. Risks of various types of bias.
Reporting quality
The titles and abstracts of the included articles mostly met the ARRIVE guidelines (Table 3). Although objectives were expressed in all studies, six studies did not sufficiently describe the scientific evidence supporting the model. The experimental procedure was fully reported in all studies, however, just one study detailed the underlying reasons. Every study addressed housing and husbandry conditions, however, none of them assessed the welfare of the animals. Although sample size was reported in every study, none described the method of calculation. Eight studies reported the number of analyzed animals in the results section, but it was not clear whether any data had been excluded. There was no mention of adverse events and no planning to cope with them. Eight studies interpreted the result detailing the study hypotheses, current theory, and other relevant studies. With respect to limitations, two studies stated the general limitation of moxibustion research in AD, and one study added a limitation arising out of its own design. One study fully described the implications of the experimental methods, and six studies partially described them. Seven studies considered the possibility of generalization and translation from animal model to humans. All studies except one mentioned the sources of funding.
Table 3. Reporting quality assessment of the included studies based upon the ‘ARRIVE guideline’.
| Study | ARRIVE Guideline | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Introduction | Methods | ||||||||||||||||||||
| Title | Abstract | Background | Objectives | Ethical statement | Study design | Experimental procedure | Experimental animals | Housing/husbandry | Sample size | ||||||||||||
| a | b | a | b | c | a | b | c | d | a | b | a | b | c | a | b | c | |||||
| Li [21] | F | P | F | F | F | N | F | P | P | F | P | F | N | F | P | F | F | N | F | N | NA |
| Liu [22] | F | P | F | F | F | N | F | P | P | F | P | F | N | F | P | F | F | N | F | N | NA |
| Wang [23] | F | F | F | F | F | N | F | P | P | F | P | P | N | P | P | P | F | N | F | N | NA |
| Li [24] | F | P | F | F | F | N | F | F | P | F | F | F | N | F | P | F | F | N | F | N | NA |
| Du et al. [25] | F | P | F | F | F | F | F | P | P | F | P | P | N | F | P | F | F | N | F | N | NA |
| Li et al. [26] | F | P | P | P | F | N | F | F | P | F | F | F | N | F | P | P | P | N | F | N | NA |
| Li et al. [27] | F | P | P | P | F | N | F | F | P | F | F | F | N | F | P | P | P | N | F | N | NA |
| Liu et al. [28] | F | P | P | P | F | N | F | P | P | F | P | P | N | F | P | P | P | N | F | N | NA |
| Liu et al. [29] | F | P | P | N | F | F | F | P | P | F | P | P | N | F | P | P | P | N | F | N | NA |
| Du et al. [30] | F | P | P | N | F | N | F | P | P | F | P | P | P | F | P | P | P | N | F | N | NA |
ARRIVE, Animal Research: Reporting In Vivo Experiments; F, fully reported; P, partially reported; N, not reported; NA, not applicable.
Outcomes: behavioral experiments
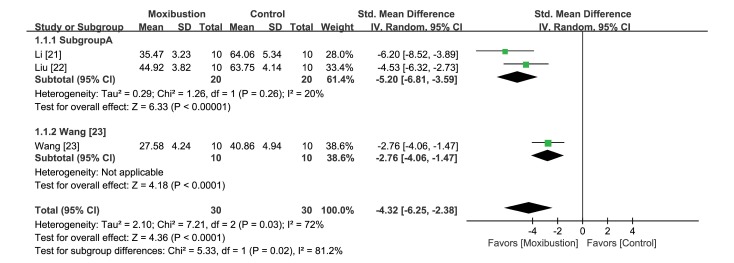
Among the included studies, five used the Morris Water Maze (MWM) test. The navigation test was conducted for 5 days, and the spatial probe test was performed on the sixth day in each study. Five studies reported escape latency, three of which [21,22,23] calculated the average of the results of the 5 days. One study [24] reported latency times for all 5 days separately, whereas the other study [25] presented this data as a graph. The three studies [21,22,23] that reported data by the same method were included in the meta-analysis (Fig. 3). From the synthesized result, moxibustion pretreatment was found to significantly decrease escape time (ΔSMD −4.32 [95% CI −6.25, −2.38], p<0.001), although the heterogeneity of this analysis was high (I2=72%) (Fig. 5).
Fig. 5. Forest plot for comparison: moxibustion versus no treatment. Outcome: escape latency in the Morris water maze test.
Due to the high heterogeneity among the analyzed studies, we conducted post-hoc subgroup analysis for escape latency and cross times. Studies were divided into subgroups according to study design, including the type of baseline test and the length of the treatment period.
Two of the three studies [21,22] had a similar design and were included in the same subgroup. The results of the subgroup analysis showed a significantly beneficial result of moxibustion (ΔSMD −5.20 [95% CI −6.81, −3.59], p<0.001). These studies also showed low heterogeneity, however, this was not statistically significant (I2=20%; p>0.05). Of note, two studies [24,25] that were not included in the meta-analysis also demonstrated significantly decreased escape latency in the moxibustion group.
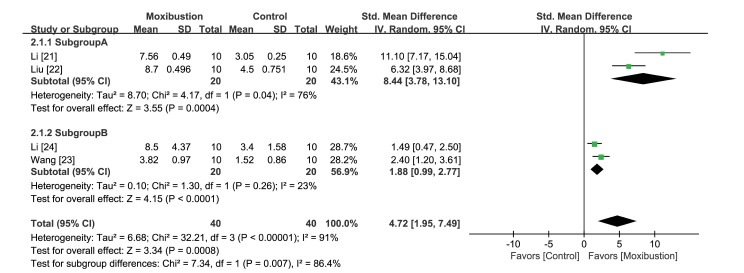
For the analysis of platform crossing times, four articles were included [21,22,23,24], whereas one study [25] that did not report numerical values was excluded. The results showed that crossing times were significantly longer in the moxibustion group (ΔSMD 4.72 [95% CI 1.95, 7.49] p<0.001), although the heterogeneity of this finding was high (I2=91%) (Fig. 6).
Fig. 6. Forest plot for comparison: moxibustion versus no treatment. Outcome: crossing times in the Morris water maze test.
Additionally, the rats of the four studies were divided into two subgroup A [21,22] and subgroup B [23,24] based on baseline tests, practice times, and treatment times. In all subgroups, longer crossing times were reported in the moxibustion group; however, subgroup A showed shorter crossing times (ΔSMD 8.44 [95% CI 3.78, 13.10] p<0.001) than subgroup B (ΔSMD 1.88 [95% CI 0.99, 2.77] p<0.001). After being divided into subgroups, the heterogeneities decreased (subgroup A: I2=76%, subgroup B: I2=23%), although the heterogeneity of subgroup A remained high.
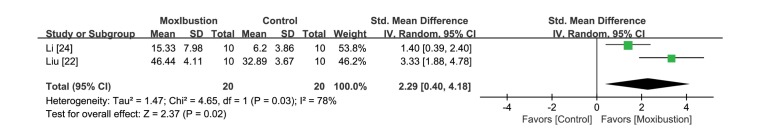
Measuring the dwelling time was pre-planned in all articles, however, only two studies [22,24] stated the dwelling time in the target quadrant. One study [21] reported their result as a modulated form with a distance proportion, and another study [23] did not report the result at all. A third study [25] only represented the results in the form of a graph. Therefore, the meta-analysis was performed with the two studies [22,24] that used the same reporting method (Fig. 7). A significantly longer dwelling time in the moxibustion-pretreated group was found (ΔSMD 2.29 [95% CI 0.40, 4.18] p<0.05, I2=78%). The three studies [21,23,25] that were not included in the meta-analysis also reported similar results.
Fig. 7. Forest plot for comparison: moxibustion versus no treatment. Outcome: dwelling times in the Morris water maze test.
Outcomes: putative signaling pathways
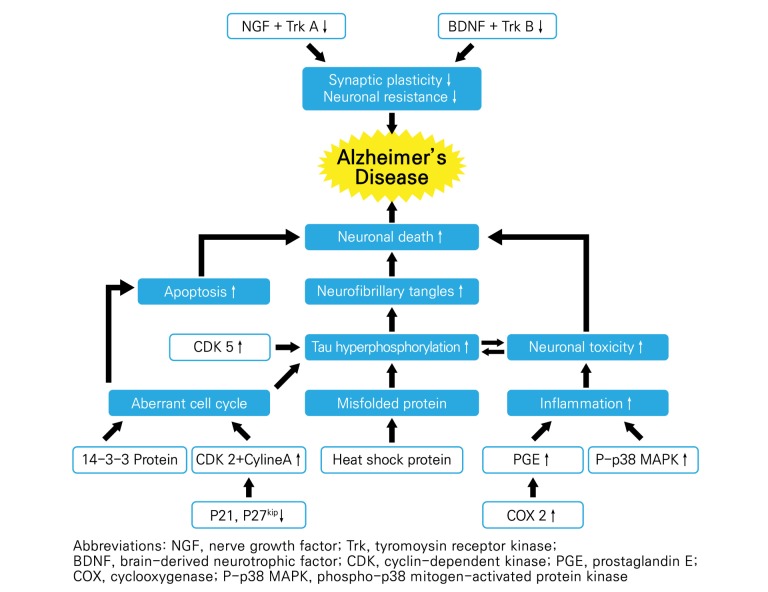
Various signaling pathways in the CA1 region of the hippocampus were examined in the ten studies (Fig. 8). Three studies [23,26,27] showed that moxibustion enhanced the activity of neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinase A (TrkA), and tropomyosin receptor kinase B (TrkB). Four studies [22,24,28,29] proposed that moxibustion might modulate the cell cycle. Three studies [24,28,29] reported decreased concentrations of cyclin A, cyclin-dependent kinase 2 (CDK2), and cyclin-dependent kinase 5 (CDK5) as well as increased concentrations of p21/cip and p27kip1 in the moxibustion-pretreated group. Additionally, Liu [22] reported increased levels of protein 14-3-3 after moxibustion treatment. Two studies [22,25] identified decreased rates of apoptosis in the moxibustion-pretreated group by examining morphological changes in the brain. Li [21] found the levels of inflammation-related factors, including prostaglandin E (PGE), cyclooxygenases (COX-2), and p38 mitogen-activated protein kinase (p38 MAPK), to be decreased in moxibustion-treated rats. Finally, Du et al. [30] observed that the levels of heat shock proteins HSP70 and HSP90 increased in the moxibustion-pretreated group, and those levels were significantly higher than the levels in normal rats (p<0.01).
Fig. 8. Putative mechanisms underlying Alzheimer's disease. The mechanisms identified in the figure represent those that could be modulated by moxibustion, according to the reviewed studies.
DISCUSSION
This systematic review and meta-analysis indicates that moxibustion might have a preventive effect against AD. In the Morris water maze test (MWM), moxibustion treatment significantly reduced the escape latency time while improving the frequency of platform crossing and dwelling time in the target quadrant. Several signaling pathways were shown to be associated with the effect of moxibustion pre-treatment on neurodegenerative disorders, such as stimulating the actions of neurotrophins and HSPs, modulating cell cycle factors, and decreasing apoptosis and inflammation.
Considerations regarding behavioral test results
The five studies that used the MWM test all used it as a behavioral test. As subtests, the navigation test and target probe test were performed, assessing two different abilities; the navigation test evaluated learning ability, whereas the target probe test assessed learning ability. For each of the tests, our meta-analysis showed significantly better results, including decreased escape latency, increased crossing times, and increased dwelling time, in the moxibustion group than in the control group. This indicates that moxibustion might prevent AD-induced cognitive impairment by improving both memory and learning ability. However, there was high heterogeneity among the results of the analyzed studies.
To investigate the reasons for heterogeneity among the studies, a post-hoc subgroup analysis was conducted by grouping studies according to study design. Three factors (baseline tests, practice times, and treatment times) were used to divide the studies into two subgroup A [21,22] and subgroup B [23,24]. First, in performing the baseline test, each subgroup chose a different test to exclude any animals showing abnormal reactions. Subgroup B adopted the MWM test, whereas subgroup A chose the Y-maze test. Second, when performing the experimental MWM test, rats in subgroup A practiced 4 times a day, whereas rats in subgroup B practiced once a day. Third, during the treatment, subgroup A used a different treatment protocol than subgroup B, applying moxibustion both before and after injection of Aβ protein. Although moxibustion is generally used for preventive purposes, the subgroup A studies performed moxibustion both before and after injecting Aβ. Their design was based on the concepts expressed by Du et al. [30], which is that it takes about 7 days to establish the dementia model after injection of Aβ; therefore, applying moxibustion before and soon after injection would encompass a preventative treatment.
Based on the subgroup analysis, both subgroups demonstrated significantly improved results for moxibustion from every subtest. Nevertheless, for subgroup A, the heterogeneity for crossing times was significantly high, whereas the heterogeneity for escape latency was low; however, this low heterogeneity was not statistically significant (p>0.05). The high heterogeneity might have been due to the small number of studies, but other factors could also have contributed to this phenomenon. In the baseline test, subgroup A used the Y-maze, but the MWM was used for the experimental test. Although adopting the Y-maze test could decrease the possibility of an effect due to pre-learning, it may not be sensitive enough to exclude outliers from the MWM test. Moreover, the different moxibustion treatment protocols could contribute to the heterogeneity. The included studies differed with respect to the diameter of moxibustion, the acupoints where it was applied, the duration of treatment per session, and the total duration of treatment. For example, Liu [22] applied moxibustion with a diameter of 15 to 20 mm, which was 2 to 3 times greater than what was used in other studies (6 to 8 mm). Considering the body size of the rat, a difference of 5 mm could result in a large variation in the results of the study. Additionally, the total length of the therapeutic period could have affected the results. However, since a treatment period of more than 8 weeks was used in all studies except one [21], the effect of treatment period length was unlikely to be significant. According to Du et al. [30], empirical evidence suggests the treatment of dementia with moxibustion can take at least 4 weeks.
Mechanisms by which moxibustion may prevent cognitive impairment: enhanced neurotrophin signaling
Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) belong to a group of growth factors known as neurotrophins [33], and both are selectively associated with one or more type of protein-tyrosine kinase (Trk). NGF specifically binds to TrkA, whereas BDNF associates with TrkB [34]. Together, BDNF and TrkB are known to play a crucial role in regulating synaptic plasticity and neuronal resistance to injury in the adult brain [35,36,37]. The enhancement of hippocampal neurogenesis and synaptic plasticity by BDNF is reported to promote the induction of long-term potentiation (LTP) in the hippocampus [38]. This cellular model of synaptic plasticity [39] underlies learning and memory formation [40,41], and a higher level of BDNF is thought to improve cognitive function [42]. Since BDNF is believed to help resist cognitive decline and because BDNF levels are reportedly lower in AD patients [42], neurotrophin supplementation may help to prevent and treat cognitive disorders [43].
Among the studies included in this meta-analysis, three investigated whether the mechanism of moxibustion in preventing neuronal loss might involve neurotrophic factors and kinases. Li et al. [26,27] reported increased expression of NGF and TrkA as well as BDNF and TrkB. Wang [23] additionally reported significantly increased numbers of NGF- and BDNF-positive cells (p<0.01) in the CA1 region of the hippocampus in moxibustion-treated animals, reinforcing the hypothesis that moxibustion prevents neuronal injury in the brain and protects brain cells.
Mechanisms by which moxibustion may prevent cognitive impairment: attenuating apoptosis
Apoptotic cell death has been accepted as a common mechanism for various neurodegenerative illnesses [44], and AD is associated with a loss of neurons [45], seemingly due to expression of proapoptotic mediators such as Aβ [46]. Apoptotic cells are characterized by morphological changes such as shrunken or fragmented cells, condensed chromatin, and cytoplasmic protuberances on the cell surface [47,48]. Among the included studies, two [22,25] identified decreased rates of apoptosis in the intervention group, suggesting that moxibustion might prevent neuronal loss by suppressing neuronal apoptosis.
Mechanisms by which moxibustion may prevent cognitive impairment: modulation of the cell cycle
A failure in regulating the cell cycle has also been proposed to drive the apoptosis of neurons [49,50]. Whereas neurons of the normal adult brain are mostly kept in the G0 phase [51], neurons in brains with AD can re-enter the cell cycle and become arrested in the G2/M phase [52]. This aberrant cell cycle response is associated with tau phosphorylation and oxidative stress, which eventually lead to apoptosis [52,53]. Among the studies included in our analysis, four [22,24,28,29] measured CDK2, cyclin A, P21/cip, P27kip, and 14-3-3 protein levels to identify the role of moxibustion in regulating the cell cycle.
Among the cyclin-dependent kinases (CDKs) that control cell cycle progression [54,55], CDK2 regulates DNA replication during the G1/S transition phase [56] by associating with cyclin A, which causes DNA assembly [57,58]. P21/cip and P27kip, which are CDK inhibitors, are involved in repairing DNA damage during the interrupted G1 phase [59]. In our systematic review, three studies [24,28,29] demonstrated down-regulated CDK2 and cyclin A. This was accompanied by up-regulated P21/cip and P27kip in the moxibustion group, indicating that moxibustion might prevent neuronal loss by regulating the G1/S transition in the cell cycle.
Additionally, protein 14-3-3 is known to modulate various cellular processes involving cell signaling, growth, apoptosis, regulation of ion channels, and neuronal function [60,61,62,63]. In the AD brain, increased amounts of protein 14-3-3 have been detected in neurofibrillary tangles. Although their interaction has not been thoroughly investigated, protein 14-3-3 is suggested to be associated with the regulation of tau phosphorylation. In fact, protein 14-3-3 might facilitate both tau phosphorylation [62] and dephosphorylation [60,61]. From the perspective of controlling the cell cycle, protein 14-3-3 is reported to regulate the G1/S and G2/M stage by modulating transcription factors [64]. Liu [22] suggested that moxibustion might prevent cognitive impairment through regulating the cell cycle and showed an increased concentration of protein 14-3-3 in the moxibustion group. Furthermore, cell cycle abnormalities may be associated with early-stage pathological changes in dementia [49], which would also explain how moxibustion acts to prevent AD.
Mechanisms by which moxibustion may prevent cognitive impairment: suppressing inflammation
In AD, neuronal inflammation is not only the result of Aβ and neurofibrillary tangles with neurodegeneration [65,66,67], but it also contributes to the pathogenesis of AD [68,69,70,71]. In cases of neuronal damage, microglia become activated for synaptic plasticity and protection [65]. However, when microglia are activated by pathological triggers, including neuronal death or aggregated proteins, they produce various neurotoxic proinflammatory factors that contribute to the development of AD [68,70]. Li [21] measured prostaglandin (PGE), cyclooxygenase-2 (COX-2), and p38 mitogen-activated protein kinase (p38 MAPK) levels to compare the degree of inflammation between groups. PGEs, which are mainly generated by COX-2 activation, are crucial for generating an inflammatory response, producing the cardinal signs of acute inflammation such as vasodilation and platelet dissolution [72]. p38 MAPK is also vital for cellular responses to external stress signals [73], by linking stress to transcription factors, which then induce target genes [74,75]. Li [21] verified that significant reductions of every component of the PGE, COX-2, and p38 MAPK system occurred in the intervention groups. Thus, moxibustion might reduce the risk of dementia by controlling inflammation.
Mechanisms by which moxibustion may prevent cognitive impairment: promoting HSPs
The accumulation of misfolded proteins is one mechanism underlying the progression of neurodegenerative diseases [76]. Among them, accumulated Aβ peptide and tau protein are pathological hallmarks of AD [77]. HSPs, particularly HSP70 and HSP90, are known to play a major role in inhibiting the aggregation of misfolded proteins [76,78,79]. Du et al. [30] observed significantly higher HSP70 and HSP90 levels in the moxibustion pretreated-group than in normal rats. Thus, moxibustion might prevent the progression of neurodegenerative diseases by assisting in the degradation or reducing the accumulation of misfolded proteins, and thus restricting tau levels.
Mechanisms by which moxibustion may prevent cognitive impairment: other mechanisms
Among the included studies, Li [24] reported decreased levels of CDK5 in the moxibustion-treated group. Although its role in cell-cycle regulation is not clear, CDK5 is thought to be implicated in neurodegeneration [80]. Under neurotoxic conditions induced by oxidative stress or Aβ peptide, p25 is generated, prolonging CDK5 activation. Thus, moxibustion may diminish Aβ-associated neurotoxicity by suppressing CDK5, which would reduce apoptosis caused by hyper-phosphorylated tau and neurofibrillary tangles.
Value and limitations of this study
There has been a strong consensus that an increase in the prevalence of dementia is inevitable in a rapidly aging society [81,82,83,84], and both long-term care and preventative strategies are needed. Moxibustion has become recognized as a preventive therapy for cognitive decline and has increasingly been used to help prevent and treat dementia. Recent studies reported that moxibustion might improve the clinical symptoms and regulate neuropeptides related in learning and memory in dementia patients [85,86]. Furthermore, its painlessness and low cost makes moxibustion particularly suitable for use in the elderly population.
Although studies have evaluated the effect of moxibustion on cognitive improvement, there have been no attempts to systematically analyze these results. Our study is the first systematic review evaluating the efficacy of moxibustion on cognitive impairment from the perspective of prevention.
There are, however, several limitations to this systematic review. First, factors that reduce the robustness of studies by increasing the risk of bias needed to be considered. A high risk of bias and low reporting quality was one of the main limitations of the original studies. The risk of bias without a blinded outcome measurement can significantly limit the validity of results. Because it was not clear whether blindness was fulfilled from selecting models to assessing outcome, there remained a high risk of assessment bias. It is also possible to overestimate the significance of findings from animal studies; therefore, it is essential to methodically investigate the effect of an intervention. Reporting bias was also suspected since descriptions of excluded animals were omitted. Furthermore, there was a potential risk of baseline differences due to omitted baseline test results. The randomization methods and sample size calculations were also generally not described, and the state of the animals and their environmental conditions needed to be described in more detail. Moreover, as studies were carried out by the same institute, they possessed a high risk of performance bias due to similarities in selecting the animal model, adopting the study protocol, performing the treatments and tests, and judging the outcomes. Most of the experiments included in this meta-analysis were conducted by one of two institutions: Hubei University of Traditional Chinese Medicine and Zhongnan Hospital of Wuhan University. Therefore, the results analyzed by this meta-analysis may not adequately represent the efficacy of moxibustion in an animal model of AD. Second, the results of the included studies are not sufficient to draw a generalized conclusion regarding every possible condition of cognitive impairment. The small number of included studies was an inevitable limitation and the results are not entirely representative of the dementia population. Furthermore, even the few studies which performed behavior tests reported the results in a different format. Therefore, the number of studies eligible for this meta-analysis was much lower than initially expected.
Furthermore, identical models established by common methods would likely have biased the results. All included studies induced AD in the animal models by injecting Aβ. Although AD is the most common cause of dementia [87], results of the studies using only AD models do not adequately reflect the various types of cognitive impairment. Additionally, these results do not sufficiently represent the range of pathological mechanisms of AD. Finally, although various pathologic mechanisms of AD have been proposed, there was no study that integrated the various mechanisms. Each study reported only two or three signaling factors, which cannot adequately represent the full mechanism of AD. Therefore, it is difficult to draw a conclusion that comprehensively addresses the mechanism of moxibustion.
It also remains controversial whether the selected signaling factors are valid indicators of AD. Among the included studies, two investigated apoptotic cell rates to measure the pathological condition of AD. Although neuronal loss is known to be associated with AD, the apoptotic mechanism of AD is quite complicated [46,88]. Several propagators and exacerbators of apoptosis are involved in the pathogenesis of AD, and sufficient evidence is not available to infer that apoptosis is the mechanism underlying the pathogenesis of AD [89]. Furthermore, vascular dementia (VD) is considered to be associated with apoptosis from secondary cell death following cerebral ischemia [44,90]. Therefore, measuring the rate of apoptosis might better reflect the pathologic condition of a VD model. To overcome this limitation, measurement of cysteine-requiring aspartate-directed proteases (caspases) might be a reasonable option [46]; this also holds true for ischemia [91].
Based on the abovementioned limitations, future studies need to be designed with higher reporting quality and a more rigorous study design. Specifically, a proper randomization method and blindness assessment should be applied. Environmental conditions, sample size calculations, the number of excluded animals, and the reasons for exclusion also need to be described. More studies performed by several different institutes using a common format to report behavioral results would reinforce the behavioral test results of this study. Furthermore, models of diverse cognitive disorders induced by diverse mechanisms might enable researchers to better conduct behavior subtest analysis. Based on previous research [91,92], an integrated study combining various factors might identify a comprehensive mechanism by which moxibustion can prevent cognitive decline. Furthermore, future systematic reviews might be discover acupoint-specific effects.
This systematic review showed that moxibustion might help prevent AD, and its mechanism of action might include increasing neurotrophin and HSP activity, regulating cell cycle factors, and/or suppressing apoptosis and inflammation. However, due to various limitations, including the small number of included studies, the high risk of bias, the lack of an integrated study design, and low reporting quality, the efficacy of moxibustion in preventing cognitive impairment remains unclear. Further rigorous and detailed studies are needed to validate the efficacy of moxibustion and to identify its underlying mechanisms.
ACKNOWLEDGEMENTS
This study was supported by the Development of a novel therapeutics for memory improvement in mild neurocognitive disorder with integrating Korean medicine and neuroimaging techniques (No. K17051) of the Korea Institute of Oriental Medicine. The authors have declared that no competing interests exist.
References
- 1.World Health Organization. Mental health and older adults [Internet] Geneva: World Health Organization; 2016. [cited 2017 Sep 26]. Available from: http://www.who.int/mediacentre/factsheets/fs381/en/ [Google Scholar]
- 2.Geldmacher DS, Kerwin DR. Practical diagnosis and management of dementia due to Alzheimer's disease in the primary care setting: an evidence-based approach. Prim Care Companion CNS Disord. 2013;15:PCC.12r01474. doi: 10.4088/PCC.12r01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivananthan SN, Lavergne MR, McGrail KM. Caring for dementia: a population-based study examining variations in guideline-consistent medical care. Alzheimers Dement. 2015;11:906–916. doi: 10.1016/j.jalz.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Shen X, Ding G, Wei J, Zhao L, Zhou Y, Deng H, Lao L. An infrared radiation study of the biophysical characteristics of traditional moxibustion. Complement Ther Med. 2006;14:213–219. doi: 10.1016/j.ctim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Pach D, Brinkhaus B, Willich SN. Moxa sticks: thermal properties and possible implications for clinical trials. Complement Ther Med. 2009;17:243–246. doi: 10.1016/j.ctim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Cao L, Mwandalima CJ, Wang Z, Liu L, Sun ZQ. Protocol for systematic review and meta-analysis: moxibustion for treating ankylosing spondylitis. Eur J Integr Med. 2017;12:142–146. [Google Scholar]
- 7.Okada K, Kawakita K. Analgesic action of acupuncture and moxibustion: a review of unique approaches in Japan. Evid Based Complement Alternat Med. 2007;6:11–17. doi: 10.1093/ecam/nem090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakita K, Shinbara H, Imai K, Fukuda F, Yano T, Kuriyama K. How do acupuncture and moxibustion act?: focusing on the progress in Japanese acupuncture research. J Pharmacol Sci. 2006;100:443–459. doi: 10.1254/jphs.crj06004x. [DOI] [PubMed] [Google Scholar]
- 9.Ewies A, Olah K. Moxibustion in breech version--a descriptive review. Acupunct Med. 2002;20:26–29. doi: 10.1136/aim.20.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Neri I, Airola G, Contu G, Allais G, Facchinetti F, Benedetto C. Acupuncture plus moxibustion to resolve breech presentation: a randomized controlled study. J Matern Fetal Neonatal Med. 2004;15:247–252. doi: 10.1080/14767050410001668644. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Chen X, Bo L, Lao L, Chen J, Yu S, Yu Z, Tang H, Yi L, Wu X, Yang J, Liang F. Moxibustion for pain relief in patients with primary dysmenorrhea: a randomized controlled trial. PLoS One. 2017;12:e0170952. doi: 10.1371/journal.pone.0170952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian XM, Lv L, Lin WB, Liang HH, Zhang Y, Wang LC. Moxibustion therapy at CV4 prevents postoperative dysuria after procedure for prolapse and hemorrhoids. Evid Based Complement Alternat Med. 2013;2013:756095. doi: 10.1155/2013/756095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao M, Wu Y, Li GQ. Regulating effect of moxibustion pretreatment on Th1/Th2 imbalance of athletes during course of heavy load training. Zhongguo Zhen Jiu. 2011;31:247–251. [PubMed] [Google Scholar]
- 14.Woo HS, Lee YH, Kim CW. The review and study trend of moxibustion. J Korean Acupunct Moxibustion Soc. 2002;19:1–15. [Google Scholar]
- 15.Li Y, Jiang G. Effects of combination of acupuncture and moxibustion with Chinese drugs on lipids peroxide and antioxidase in patients of vascular dementia. World J Acupunct Moxibustion. 1998;8:9–13. [Google Scholar]
- 16.Wang P, Yang J, Yang F, Chen H, Huang X, Li F. Clinic research of treating vascular dementia by moxibustion at head points. China J Tradit Chin Med Pharm. 2009;24:1348–1350. [Google Scholar]
- 17.Yang W, Li Y, Zhuang L, Zheng L. Effect of acupuncture-moxibustion plus Chinese medicinal herbs on plasma TXB2, 6-Keto-PGF1α in patients with vascular dementia. World J Acupunct Moxibustion. 1998;8:12–15. [Google Scholar]
- 18.Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warlé M. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012;7:e32296. doi: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooijmans CR, de Vries RB, Rovers MM, Gooszen HG, Ritskes-Hoitinga M. The effects of probiotic supplementation on experimental acute pancreatitis: a systematic review and meta-analysis. PLoS One. 2012;7:e48811. doi: 10.1371/journal.pone.0048811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X. Research on the action mechanism of p38MAPK signal pathway in treating the rats with Alzheimer disease by preventative moxibustion. Wuhan: Hubei University of Chinese Medicine; 2012. [Google Scholar]
- 22.Liu R. The effect of preventive moxibustion on expression of 14-3-3 protein and apoptosis of hippocampal neurons in rats of Alzheimer's disease. Wuhan: Hubei University of Chinese Medicine; 2012. [Google Scholar]
- 23.Wang X. The effect of moxibustion pretreatment on the expression of NGF and BDNF in hippocampal CA1 of the Alzheimer's disease model rats. Wuhan: Hubei University of Chinese Medicine; 2012. [Google Scholar]
- 24.Li J. The experimental study of the expression of prestimulation with moxibustion on CDK5, P27kip1 in rats of Alzheimer's disease rats. Wuhan: Hubei University of Chinese Medicine; 2011. [Google Scholar]
- 25.Du Y, Liu R, Sun G, Meng P, Song J. Pre-moxibustion and moxibustion prevent Alzheimer's disease. Neural Regen Res. 2013;8:2811–2819. doi: 10.3969/j.issn.1673-5374.2013.30.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Sun G, Wang S, Ma J, Wang Y, Zhang C. Effect of moxibustion pretreatment on the expression of brain-derived neurotrophic factor and its receptor TrkB in hippocampus CA1 region of rats with Alzheimer's disease. Chin J Gerontol. 2012;21:4663–4665. [Google Scholar]
- 27.Li X, Sun G, Du YJ. Effect of moxibustion pretreatment on expression of NGF and TrkA in hippocampal CA1 region of Alzheimer's disease rats Hubei. Chin J Rehabil. 2011;26:323–326. [Google Scholar]
- 28.Liu J, Du Y, Sun G. Influence of pre-stimulation with moxibustion on cells period adjustment factor-cyclinA P21/cip in AD models rats. Lishizhen Med Mater Med Res. 2011;22:2612–2613. [Google Scholar]
- 29.Liu J, Du Y, Sun G. Influence of pre-stimulation with moxibustion on cells period proteins CDK2 and p21/cip in rats with Alzheimer disease. Mod J Integr Tradit Chin West Med. 2013;29:3208–3210. 3221. [Google Scholar]
- 30.Du Y, Song J, Zhou H, Chen B, Wang S, Sun G. Influence of pre-stimulation with moxibustion on heat shock proteins in AD models rats. Liaoning J Tradit Chin Med. 2013;40:577–579. [Google Scholar]
- 31.Liu J, Du Y, Sun G. The influence of pre-stimulation with moxibustion on the ability of learning and memory in rats of Alzheimer's disease. Lishizhen Med Mater Med Res. 2014;25:1510–1511. [Google Scholar]
- 32.Wang X, Sun G, Du Y. Effect of moxibustion pretreatment on expression of NGF and BDNF in hippocampus CA1 of Alzheimer disease model rats. Chin J Rehabil. 2011;26:323–326. [Google Scholar]
- 33.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 34.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 35.Alcántara S, Frisén J, del Río JA, Soriano E, Barbacid M, Silos-Santiago I. TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J Neurosci. 1997;17:3623–3633. doi: 10.1523/JNEUROSCI.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J Neurochem. 1994;63:1021–1032. doi: 10.1046/j.1471-4159.1994.63031021.x. [DOI] [PubMed] [Google Scholar]
- 37.Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. [DOI] [PubMed] [Google Scholar]
- 38.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 39.Ye Y, Li H, Yang JW, Wang XR, Shi GX, Yan CQ, Ma SM, Zhu W, Li QQ, Li TR, Xiao LY, Liu CZ. Acupuncture attenuated vascular dementia-induced hippocampal long-term potentiation impairments via activation of D1/D5 receptors. Stroke. 2017;48:1044–1051. doi: 10.1161/STROKEAHA.116.014696. [DOI] [PubMed] [Google Scholar]
- 40.Majlessi N, Choopani S, Bozorgmehr T, Azizi Z. Involvement of hippocampal nitric oxide in spatial learning in the rat. Neurobiol Learn Mem. 2008;90:413–419. doi: 10.1016/j.nlm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 42.Coulson EJ, Bartlett PF. An exercise path to preventing Alzheimer's disease: An Editorial Highlight on ‘Exercise and BDNF reduce Ab production by enhancing alpha-secretase processing of APP’. J Neurochem. 2017;142:191–193. doi: 10.1111/jnc.14038. [DOI] [PubMed] [Google Scholar]
- 43.Nigam SM, Xu S, Kritikou JS, Marosi K, Brodin L, Mattson MP. Exercise and BDNF reduce Abeta production by enhancing alpha-secretase processing of APP. J Neurochem. 2017;142:286–296. doi: 10.1111/jnc.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldmeier PC. Prospects for antiapoptotic drug therapy of neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:303–321. doi: 10.1016/S0278-5846(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 45.Shimohama S. Apoptosis in Alzheimer's disease--an update. Apoptosis. 2000;5:9–16. doi: 10.1023/a:1009625323388. [DOI] [PubMed] [Google Scholar]
- 46.Raina AK, Hochman A, Ickes H, 2nd, Zhu X, Ogawa O, Cash AD, Shimohama S, Perry G, Smith MA. Apoptotic promoters and inhibitors in Alzheimer's disease: who wins out? Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 47.Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 48.Wyllie AH, Beattie GJ, Hargreaves AD. Chromatin changes in apoptosis. Histochem J. 1981;13:681–692. doi: 10.1007/BF01002719. [DOI] [PubMed] [Google Scholar]
- 49.Kim H, Kwon YA, Ahn IS, Kim S, Kim S, Jo SA, Kim DK. Overexpression of cell cycle proteins of peripheral lymphocytes in patients with Alzheimer's disease. Psychiatry Investig. 2016;13:127–134. doi: 10.4306/pi.2016.13.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 53.Nagy Z. The dysregulation of the cell cycle and the diagnosis of Alzheimer's disease. Biochim Biophys Acta. 2007;1772:402–408. doi: 10.1016/j.bbadis.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Goldstone S, Pavey S, Forrest A, Sinnamon J, Gabrielli B. Cdc25-dependent activation of cyclin A/cdk2 is blocked in G2 phase arrested cells independently of ATM/ATR. Oncogene. 2001;20:921–932. doi: 10.1038/sj.onc.1204177. [DOI] [PubMed] [Google Scholar]
- 55.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 56.Hengstschläger M, Braun K, Soucek T, Miloloza A, Hengstschläger-Ottnad E. Cyclin-dependent kinases at the G1-S transition of the mammalian cell cycle. Mutat Res. 1999;436:1–9. doi: 10.1016/s1383-5742(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 57.Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- 58.De Boer L, Oakes V, Beamish H, Giles N, Stevens F, Somodevilla-Torres M, Desouza C, Gabrielli B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene. 2008;27:4261–4268. doi: 10.1038/onc.2008.74. [DOI] [PubMed] [Google Scholar]
- 59.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 60.Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–762. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal-Mawal A, Qureshi HY, Cafferty PW, Yuan Z, Han D, Lin R, Paudel HK. 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. J Biol Chem. 2003;278:12722–12728. doi: 10.1074/jbc.M211491200. [DOI] [PubMed] [Google Scholar]
- 62.Umahara T, Uchihara T, Iwamoto T. Structure-oriented review of 14-3-3 protein isoforms in geriatric neuroscience. Geriatr Gerontol Int. 2012;12:586–599. doi: 10.1111/j.1447-0594.2012.00860.x. [DOI] [PubMed] [Google Scholar]
- 63.van Hemert MJ, Steensma HY, van Heusden GP. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. BioEssays. 2001;23:936–946. doi: 10.1002/bies.1134. [DOI] [PubMed] [Google Scholar]
- 64.Hermeking H, Benzinger A. 14-3-3 proteins in cell cycle regulation. Semin Cancer Biol. 2006;16:183–192. doi: 10.1016/j.semcancer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KM, Olson KE, Estes KA, Flanagan K, Gendelman HE, Mosley RL. Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders. Transl Neurodegener. 2014;3:25. doi: 10.1186/2047-9158-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tai J, Liu W, Li Y, Li L, Hölscher C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease. Brain Res. 2018;1678:64–74. doi: 10.1016/j.brainres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avila-Muñoz E, Arias C. When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer's disease. Ageing Res Rev. 2014;18:29–40. doi: 10.1016/j.arr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer's disease. Neuroscience. 2016;323:170–182. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gangwal RP, Bhadauriya A, Damre MV, Dhoke GV, Sangamwar AT. p38 Mitogen-activated protein kinase inhibitors: a review on pharmacophore mapping and QSAR studies. Curr Top Med Chem. 2013;13:1015–1035. doi: 10.2174/1568026611313090005. [DOI] [PubMed] [Google Scholar]
- 74.Cohen DM. Mitogen-activated protein kinase cascades and the signaling of hyperosmotic stress to immediate early genes. Comp Biochem Physiol A Physiol. 1997;117:291–299. doi: 10.1016/s0300-9629(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 75.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saibil HR. Chaperone machines in action. Curr Opin Struct Biol. 2008;18:35–42. doi: 10.1016/j.sbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279:17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 78.Ciechanover A, Kwon YT. Protein quality control by molecular chaperones in neurodegeneration. Front Neurosci. 2017;11:185. doi: 10.3389/fnins.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML, Prado VF, Prado MA. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front Neurosci. 2017;11:254. doi: 10.3389/fnins.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 81.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U.S. elderly population. Adv Gerontol. 2005;16:30–37. [PubMed] [Google Scholar]
- 82.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369:2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones DS, Greene JA. Is dementia in decline? Historical trends and future trajectories. N Engl J Med. 2016;374:507–509. doi: 10.1056/NEJMp1514434. [DOI] [PubMed] [Google Scholar]
- 84.Larson EB, Langa KM. What's the “Take Home” from research on dementia trends? PLoS Med. 2017;14:e1002236. doi: 10.1371/journal.pmed.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Yang J, Liu G, Chen H, Yang F. Effects of moxibustion at head-points on levels of somatostatin and arginine vasopressin from cerebrospinal fluid in patients with vascular dementia: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2010;8:636–640. doi: 10.3736/jcim20100706. [DOI] [PubMed] [Google Scholar]
- 86.Chen H, Wang P, Yang J, Liu G. Impacts of moxibustion on vascular dementia and neuropeptide substance content in cerebral spinal fluid. Zhongguo Zhen Jiu. 2011;31:19–22. [PubMed] [Google Scholar]
- 87.McGough E, Kirk-Sanchez N, Liu-Ambrose T. Integrating health promotion into physical therapy practice to improve brain health and prevent Alzheimer disease. J Neurol Phys Ther. 2017;41:S55–S62. doi: 10.1097/NPT.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 88.LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X, Raina AK, Perry G, Smith MA. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3:393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- 90.Ye Y, Zhu W, Wang XR, Yang JW, Xiao LY, Liu Y, Zhang X, Liu CZ. Mechanisms of acupuncture on vascular dementia: a review of animal studies. Neurochem Int. 2017;107:204–210. doi: 10.1016/j.neuint.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]