Abstract
AIM
To assess the effect of polyglycolic acid (PGA) plus stent placement compared with stent placement alone in the prevention of post-endoscopic submucosal dissection (ESD) esophageal stricture in early-stage esophageal cancer (EC) patients.
METHODS
Seventy EC patients undergoing ESD were enrolled in this randomized, controlled study. Patients were allocated randomly at a 1:1 ratio into two groups as follows: (1) PGA plus stent group (PGA sheet-coated stent placement was performed); and (2) Stent group (only stent placement was performed). This study was registered on http://www.chictr.org.cn (No. chictr-inr-16008709).
RESULTS
The occurrence rate of esophageal stricture in the PGA plus stent group was 20.5% (n = 7), which was lower than that in the stent group (46.9%, n = 15) (P = 0.024). The mean value of esophageal stricture time was 59.6 ± 16.1 d and 70.7 ± 28.6 d in the PGA plus stent group and stent group (P = 0.174), respectively. Times of balloon dilatation in the PGA plus stent group were less than those in the stent group [4 (2-5) vs 6 (1-14), P = 0.007]. The length (P = 0.080) and diameter (P = 0.061) of esophageal strictures were numerically decreased in the PGA plus stent group, whereas no difference in location (P = 0.232) between the two groups was found. Multivariate logistic analysis suggested that PGA plus stent placement (P = 0.026) was an independent predictive factor for a lower risk of esophageal stricture, while location in the middle third (P = 0.034) and circumferential range = 1/1 (P = 0.028) could independently predict a higher risk of esophageal stricture in EC patients after ESD.
CONCLUSION
PGA plus stent placement is more effective in preventing post-ESD esophageal stricture compared with stent placement alone in EC patients with early-stage disease.
Keywords: Esophageal cancer, Endoscopic submucosal dissection, Polyglycolic acid plus stent placement, Esophageal stricture
Core tip: This study determines the effect of polyglycolic acid (PGA) plus stent placement compared with stent placement alone in the prevention of post-endoscopic submucosal dissection (ESD) esophageal stricture in early-stage esophageal cancer (EC) patients. Our findings confirm that PGA plus stent placement is more effective in preventing post-ESD esophageal stricture compared with stent placement alone in EC patients with early-stage disease.
INTRODUCTION
Esophageal cancer (EC) is one of the most common carcinomas with high mortality and has been identified as the sixth leading cause of cancer-related death worldwide[1]. Based on 2015 global cancer statistics, an estimated 455800 new EC cases and 400200 deaths occurred in 2012. In the United States, approximately 16940 new EC cases and 15690 deaths will be seen in 2017[2,3]. Endoscopic submucosal dissection (ESD), performed as an endoscopic resection technique, has been widely utilized in the treatment of EC patients with early-stage disease, due to its minimal invasiveness and high rate of en bloc resection[4,5]. However, more than 30% of EC patients after ESD still experience postoperative esophageal stricture, which is characterized by dysphagia, dramatically decreasing the quality of life[6,7].
To prevent post-ESD esophageal stricture, various treatments have been implemented, such as polyglycolic acid (PGA) sheet, stent placement, and esophageal balloon dilatation[8]. Among these, PGA sheet, a biodegradable suture material, could be used to prevent post-ESD esophageal stricture because of its advantages of reinforcing suture and minimal scar contracture, although the limitation of instability between the PGA sheet and wound surface after long-term pasting still exists[9-11]. Another popular method is stent placement, which is frequently used with covered self-expandable metal material, and has been verified to have curative effects on refractory stricture to some extent, although its complications, such as translocation and promotion of granulation tissue proliferation, still affect its clinical outcomes in EC patients[12,13].
Although some studies on the applications of combinations of two or three treatments in the prevention of esophageal stricture after ESD have been performed, no study has explored the effect of the combination of PGA and stent placement in the prevention of post-ESD esophageal stricture[9,14]. Therefore, the aim of this study was to assess the effect of PGA plus stent placement compared with stent placement alone in the prevention of post-ESD esophageal stricture in early-stage EC patients.
MATERIALS AND METHODS
Patients
A total of 70 early-stage EC patients receiving ESD at Department of Gastroenterology, China PLA General Hospital from July 2016 to May 2017 were consecutively enrolled in this randomized controlled study. The inclusion criteria were as follows: (1) Circumferential range above 3/4; (2) Longitudinal length above 3 cm; (3) Lesion depth no more than M2; and (4) Tumor lesion could be completely removed. The exclusion criteria were as follows: (1) Patients with coagulative dysfunction, hepatic failure, renal failure, or cardiopulmonary dysfunction; (2) Patients complicated with malignant hematological disease or other solid tumors; (3) Patients who had a previous history of esophagectomy or radiation therapy; (4) Patients who were unable to complete the ESD operation; (5) Pregnant or lactating women; (6) Patients who could not be followed regularly; and (7) Patients who refused to participate in this study.
This study was approved by the Ethics Committee of China PLA General Hospital (approval No. S2016-059-01), and all participants provided written informed consent. This study was registered on http://www.chictr.org.cn (Chinese Clinical Trial Registry conducted by World Health Organization; registration No. chictr-inr-16008709).
Randomization
The randomization code was generated by a statistician using the blocked randomization method with block length set as four because of the need for allocation balance between the two groups (1:1 ratio). The documents were subsequently sent and kept in Shanghai Qeejen Bio-tech Company (a medical and statistical service company). After screening, when a patient was eligible for the study, a call was made to the Qeejen Company, and a unique subject identification number was provided from the randomized module.
Treatment
After the randomization, patients were allocated to the PGA plus stent treatment group or stent treatment group at a 1:1 ratio. In the PGA plus stent group, the patients received PGA sheet-coated stent placement to prevent esophageal stricture post ESD operation; in the stent group, the patients received only stent placement to prevent esophageal stricture post ESD operation.
ESD procedure
After intravenous anesthesia, patients with tracheal intubation and oropharyngeal tube placement underwent endoscope (Olympus, Japan) insertion to the area of the tumor lesion. Subsequently, the lesion margins were stained with iodine, and the submucosa was labeled. Then, the lesion dissections were performed, and endoscopic submucosal tunnel dissection was used for lesion stripping, followed by hemostasis.
Combination of PGA membrane and stent
According to the ESD wound length (Figure 1A), the length of stent (Derman Science, China, 1.7 cm of diameter, stainless steel, silicone rubber membrane) was selected (Figure 1B), followed by PGA sheet selection (NEOVEIL, Japan, 100 mm × 100 mm × 0.15 mm) (Figure 1C). Subsequently, the stent was coated with the PGA sheet (Figure 1D), and the covered place was designed to the ESD wound site after stent release. Then, this stent covered with a PGA sheet was mounted on the conveyer for the esophageal stent ring supporter (Figure 1E and F) and inserted under endoscopic observation (Figure 1G).
Figure 1.
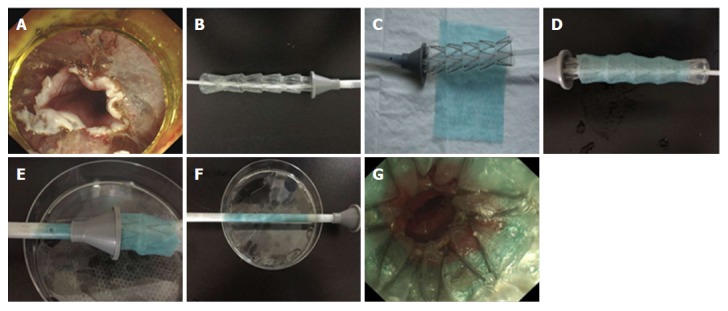
Combination of polyglycolic acid sheet and stent. A: Measurement of endoscopic submucosal dissection wound length; B: Adjustment of stent length; C: Polyglycolic acid sheet selection based on lesion length; D: Stent coating with polyglycolic acid (PGA) sheet; E-F: Stent covered with PGA sheet mounted on the stent support; G: Insertion of stent covered with PGA sheet under endoscopic observation.
Stent placement
The length of the ESD wound was measured, and then the appropriate length of metal coated stent was selected (Derman Science, China, 1.7 cm of diameter, stainless steel, silicone rubber membrane), which was beyond the top and bottom edge of the lesion by more than 2 cm. Subsequently, the guide wire was placed under the endoscope, and then the endoscope was pulled out and the conveyer was inserted for the esophageal stent ring supporter along with the guide wire for the stent placement. After stent placement, the endoscope was inserted again to observe the stent position. A chest X-ray was performed after the stent placement, and the stent position was recorded.
Stent removal
In the PGA plus stent group, the stent was removed by endoscopy at 4 wk, whereas in the stent group, the stent was removed at 8 wk after operation. Endoscopic evaluation of the ESD wound was performed (Figure 2A-C). The time to remove the stent between the two groups was different because PGA is a biodegradable suture material, which would be degraded after a period of time. According to a preliminary study with a small sample size, we found that when PGA plus stent placement was used to prevent post-ESD esophageal stricture in early-stage EC patients, the PGA was degraded at approximately 3 or 4 wk after ESD, and therefore, the previous clinical experiences suggested that removing PGA plus stent placement at 4 wk might be a good choice in EC patients.
Figure 2.
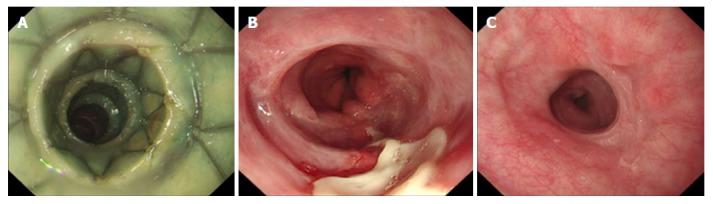
The management of one of the study patients. A: Stent covered with polyglycolic acid sheet inserted after 1 wk; B: Stent removed after 4 wk with a portion of wound not healed; C: No formation of esophageal stricture observed after 2 mo.
Assessment
The primary endpoint was esophageal stricture occurrence after ESD operation, which was defined as diameter of stricture section below 1 cm (endoscopy could not pass through) under endoscopy. The secondary endpoints were as follows: time to esophageal stricture; location, length, and diameter of esophageal stricture; and balloon dilatation times for the esophageal stricture.
Follow-up
All patients were followed through clinic visits or telephone interviews. Each patient was asked if dysphagia or other symptoms occurred at each visit or call, and if distinct dysphagia was determined, endoscopy was performed to examine the esophageal stricture. Chest X-ray examination was performed every 2 wk to monitor the position of esophageal stent, and if a stent shifted more than 2 cm, endoscopy was performed to adjust the stent to the original place.
Balloon dilatation
All patients with esophageal stricture received endoscopic balloon dilation treatment (balloon diameter: 8 mm/10 mm/12 mm/15 mm, Boston Scientific, United States). The esophageal stricture section was repeatedly dilated until the endoscope could successfully pass through at each endoscopic examination, and balloon dilation was performed every week until the endoscope could pass through the section before dilation.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM, United States) and OFFICE 2010 (Microsoft, United States). Data are mainly presented as mean value ± SD, median value (range), or count (percentage). The comparison between two groups was determined by t-test, Chi-square test, or Wilcoxon rank sum test. Factors affecting esophageal stricture occurrence were evaluated by univariate logistic regression analysis, and all factors with a P-value below 0.1 were further detected by multivariate logistic regression analysis. P < 0.05 was considered significant.
RESULTS
Study flow
In the current study, 109 EC patients were screened for eligibility, while 39 cases were excluded as follows: 21 patients for exclusions and 10 patients who refused to participate in this study (Figure 3). Subsequently, the remaining 70 patients were randomized at a 1:1 ratio into two groups as follows: PGA plus stent group and stent group. In the PGA plus stent group, one case withdrew during the study due to a lesion depth > M2 post-ESD operation, and 34 (97%) cases completed the entire study. In the stent group, there were three total withdrawals; three patients with lesion depth more than M2 after ESD operation withdrew, and 32 (91%) cases completed the entire study. Ultimately, a total of 66 EC patients completed the final analysis. After the operation, the stent was removed by endoscopy at 4 wk in the PGA plus stent group, and it was removed at 8 wk in the stent group. During the study, stent displacement was adjusted by endoscopy. Chest X-ray examination was performed every 2 wk. Endoscopy was performed to check the esophageal stricture. All patients with esophageal stricture received endoscopic balloon dilation treatment.
Figure 3.
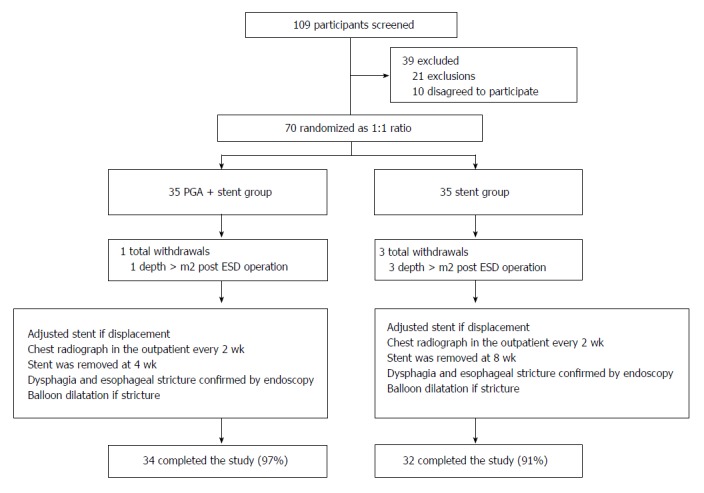
Study flow. ESD: Endoscopic submucosal dissection.
Baseline characteristics
As shown in Table 1, no difference in patients’ characteristics and tumor lesion features between the PGA plus stent group and stent group were observed (P > 0.05 for all). The numbers of patients with tumor location at the upper, middle, and lower third were 0 (0%), 12 (35%), and 22 (65%), respectively, in the PGA plus stent group, while 0 (0%), 17 (53%), and 15 (47%) in the stent group (P = 0.145). In terms of tissue depth (P = 0.331), there were 20 (59%) patients with M1 and 14 (41%) patients with M2 in the PGA plus stent group and 15 (47%) patients with M1 and 17 (53%) patients with M2 were in the stent group. The mean value of longitudinal length was 5.97 ± 2.68 cm in the PGA plus stent group and 6.06 ± 2.12 cm in the stent group (P = 0.878). Other baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics n (%)
| Parameter | PGA + stent group (n = 34) | Stent group (n = 32) | P value |
| Patients characteristic | |||
| Age (yr) | 62.74 ± 8.38 | 59.91 ± 8.80 | 0.186 |
| Gender (Male/Female) | 22/12 | 18/14 | 0.482 |
| Tumor lesion feature | |||
| Location | 0.145 | ||
| Upper third | 0 (0) | 0 (0) | |
| Middle third | 12 (35) | 17 (53) | |
| Lower third | 22 (65) | 15 (47) | |
| Tissue depth | 0.331 | ||
| M1 | 20 (59) | 15 (47) | |
| M2 | 14 (41) | 17 (53) | |
| Longitudinal length (cm) | 5.97 ± 2.68 | 6.06 ± 2.12 | 0.878 |
| Circumferential range | 0.684 | ||
| 3/4 | 12 (35) | 14 (44) | |
| 4/5 | 8 (24) | 8 (25) | |
| 1/1 | 14 (41) | 10 (31) |
Data are presented as mean ± SD or count (with or without percentage). Comparison was performed by t-test or Chi-square test. P < 0.05 was considered significant. PGA: Polyglycolic acid.
Comparison of post-ESD stricture in the two groups
The occurrence rate of patients with esophageal stricture in the PGA plus stent group was 20.5% (n = 7), which was lower than that in the stent group (46.9%, n = 15) (P = 0.024, Figure 4A). Regarding time to esophageal stricture, the mean value was 59.6 ± 16.1 d and 70.7 ± 28.6 d in the PGA plus stent group and stent groups, respectively (P = 0.174, Figure 4B).
Figure 4.
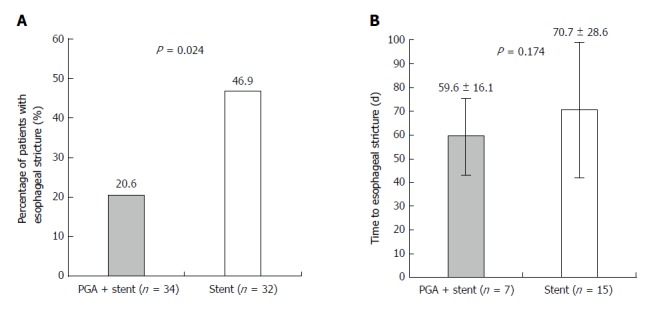
Comparison of post-endoscopic submucosal dissection stricture in polyglycolic acid plus stent and stent groups. A: Patients in the polyglycolic acid (PGA) plus stent group had a lower occurrence rate of esophageal stricture than that in the stent group; B: No difference in time to esophageal stricture was observed between the PGA plus stent and stent groups. Comparison of post-endoscopic submucosal dissection stricture in the PGA plus stent and stent groups was performed by t-test. P < 0.05 was considered significant.
Comparison of esophageal stricture features in the two groups
T-test or Wilcoxon rank sum test was used to compare esophageal stricture features between the PGA plus stent and stent groups (Table 2). The balloon dilatation times for esophageal stricture in the PGA plus stent group were less than those in the stent group [4 (2-5) vs 6 (1-14), P = 0.007]. Length (P = 0.080) and diameter (P = 0.061) of esophageal stricture were numerically decreased in the PGA plus stent group compared with the stent group, whereas there was no difference in location (P = 0.232) between the two groups (Table 2).
Table 2.
Comparison of esophageal stricture features in polyglycolic acid + stent and stent groups
| Parameter | PGA + stent group (n = 7) | Stent group (n = 15) | P value |
| Length (cm) | 0.97 + 0.59 | 1.47 + 0.59 | 0.080 |
| Diameter (cm) | 0.37 + 0.17 | 0.51 + 0.14 | 0.061 |
| Location (distance from the incisors, cm) | 31.71 + 3.20 | 29.07 + 5.20 | 0.232 |
| Balloon dilatation times | 4 (2-5) | 6 (1-14) | 0.007 |
Data are presented as mean ± SD or median (range). Comparison was performed by t-test or Wilcoxon rank sum test. P < 0.05 was considered significant. PGA: Polyglycolic acid.
Comparison of tumor lesion features in patients with esophageal stricture
As presented Table 3, comparison of tumor lesion features in patients with esophageal stricture between the PGA plus stent and stent groups was performed. No difference was observed in location (P = 0.899), tissue depth (P = 0.823), longitudinal length (P = 0.360), or circumferential range (P = 0.181) in patients with esophageal stricture between the two groups (Table 3).
Table 3.
Comparison of tumor lesion features in patients with esophageal stricture n (%)
| Parameter | PGA + stent group (n = 7) | Stent group (n = 15) | P value |
| Location | 0.899 | ||
| Upper third | 0 (0) | 0 (0) | |
| Middle third | 4 (57) | 9 (60) | |
| Lower third | 3 (43) | 6 (40) | |
| Tissue depth | 0.823 | ||
| M1 | 2 (29) | 5 (33) | |
| M2 | 5 (71) | 10 (67) | |
| Longitudinal length (cm) | 7.71 + 3.50 | 6.60 + 2.10 | 0.360 |
| Circumferential range | 0.181 | ||
| 3/4 | 0 (0) | 4 (27) | |
| 4/5 | 1 (14) | 4 (27) | |
| 1/1 | 6 (86) | 7 (47) |
Data are presented as mean ± SD or count (percentage). Comparison was determined by t-test or Chi-square test. P < 0.05 was considered significant. PGA: Polyglycolic acid.
Analysis of factors affecting esophageal stricture occurrence
Factors affecting esophageal stricture occurrence were determined by univariate logistic regression analysis (Table 4). PGA plus stent placement (P = 0.027) was correlated with a lower possibility of esophageal stricture occurrence, whereas circumferential range = 1/1 (P = 0.008) and tissue depth M2 (P = 0.017) were associated with a higher probability of esophageal stricture occurrence. Location in the middle third (P = 0.083) and longitudinal length ≥ 6 cm (P = 0.059) were two factors that appeared to be correlated with a higher risk of esophageal stricture but without statistical significance. All factors with a P-value not above 0.1 were further detected by multivariate logistic regression analysis. PGA plus stent placement (P = 0.026) was an independent predictive factor for a lower risk of esophageal stricture, whereas location in the middle third (P = 0.034) and circumferential range = 1/1 (P = 0.028) could independently predict a higher risk of esophageal stricture in EC patients after ESD.
Table 4.
Logistic analysis of factors affecting esophageal stricture occurrence
| Parameter |
Univariate logistic regression |
Multivariate logistic regression |
||||||
| P value | OR |
95%CI |
P value | OR |
95%CI |
|||
| Lower | Higher | Lower | Higher | |||||
| PGA + stent (vs stent alone) | 0.027 | 0.294 | 0.099 | 0.868 | 0.026 | 0.197 | 0.047 | 0.820 |
| Age ≥ 62 yr | 0.298 | 1.733 | 0.615 | 4.887 | - | - | - | - |
| Location - middle third (vs lower third) | 0.083 | 2.528 | 0.886 | 7.214 | 0.034 | 5.148 | 1.135 | 23.344 |
| Longitudinal length ≥ 6 cm | 0.059 | 2.779 | 0.963 | 8.019 | 0.092 | 3.826 | 0.801 | 18.270 |
| Circumferential range = 1/1 (vs others) | 0.008 | 4.333 | 1.457 | 12.888 | 0.028 | 5.113 | 1.194 | 21.892 |
| Tissue depth M2 (vs M1) | 0.017 | 3.750 | 1.264 | 11.123 | 0.069 | 3.284 | 0.912 | 11.822 |
Data are presented as P-value, OR (odds ratio), and 95%CI. Factors affecting esophageal stricture occurrence were determined by univariate logistic regression analysis, while all factors with a P-value less than 0.1 were further detected by multivariate logistic regression analysis. P-value < 0.05 was considered significant. PGA: Polyglycolic acid.
DISCUSSION
In the current study, we found that: (1) the occurrence rate of esophageal stricture in the PGA plus stent group was lower compared with the stent group, and the balloon dilatation times of esophageal stricture in the PGA plus stent group were less compared with the stent group; and (2) PGA plus stent placement could independently predict a lower occurrence rate of esophageal stricture, while location in the middle third and circumferential range = 1/1 were independent predictive factors for a higher possibility of post-ESD esophageal stricture occurrence in EC patients.
ESD, which is considered an effective method to completely resect mucosal lesions, has been popularly applied in EC patients with early-stage disease, although due to the physiological characteristics of the esophageal cavity, esophageal stricture frequently occurs after ESD[4,15,16]. Recent data indicate that the appearance of several fibroblasts and the shrinking of the natural muscle layer are present in the formation of post-ESD esophageal stricture, which suggests that fiber proliferation, scar formation, and wound contracture might contribute to the formation of post-ESD esophageal stricture[17].
Stenting has been regarded as a useful way to prevent post-ESD esophageal stricture, although a high recurrence rate still exists in most patients after removing the stent, and some patients with long-term stent placement experience several complications, such as displacement and granulation tissue hyperplasia[18,19]. In clinical practice, stent insertion has been reported to decrease the dysphagia score and the mean diameter of esophageal stricture[20]. PGA sheet, a polymer with a fiber mesh structure, could: (1) provide abundant cytoskeletons to support cell crawling during the repair process, and inhibit rejection reaction by its strong degradative function, thereby leading to a decreased risk of scar germination and stricture formation; and (2) carry cells and medicines to promote cell repair and wound healing. An interesting study revealed that PGA sheets could shield mucosal defects and prevent scarring, reducing postoperative adverse events in patients with colorectal ESD[21]. However, some limitations of PGA sheets still exist, which are easy to fall off for long-term utilization[9,10]. Therefore, both stents and PGA can decrease the risk of esophageal stricture to some extent. However, there remain several limitations. No study on the effects of the combined application of PGA and stent has been reported. Our study compared the occurrence rate of post-ESD esophageal stricture between the PGA plus stent and stent groups and indicated that the percentage of post-ESD esophageal stricture in the PGA plus stent group was lower compared with the stent group. Balloon dilatation, which is one of the most common treatments for esophageal stricture, has been identified to have good short-term efficacy, whereas the long-term curative effect is still far from satisfactory. A previous study suggested that the average usage rate of balloon dilatation is estimated to be 16 times for post-ESD esophageal stricture[22]. The results of our study also found that the balloon dilatation times for esophageal stricture in the PGA plus stent group were less than those in the stent group [4 (2-5) vs 6 (1-14) times]. Therefore, these findings indicated that PGA plus stent placement could decrease the risk of esophageal stricture and the balloon dilatation times for esophageal stricture compared with stent placement alone. Possible explanations might be as follows: (1) The stent could provide radial force to fix the PGA sheet, thereby preventing the defluvium of the PGA sheet[18,19]; and (2) The PGA sheet could increase the friction between the stent and wound surface, reducing stent displacement, and also provide cytoskeleton and carry medicine to accelerate cell repair and wound healing. Therefore, the interaction of the PGA sheet with the stent could decrease the occurrence rate of post-ESD esophageal stricture[9,11]. However, seven patients still reported the formation of esophageal stricture in the PGA plus stent group. This occurrence might be explained by the fact that during the process of wound repair, epithelial cells usually crawl from the edge of the wound to the central area. Because of the long-term repair, the PGA sheet might be degraded to result in frameless support, leading to its defluvium and increasing the occurrence rate of esophageal stricture in the central area (Figure 5A-D). As to the predictive value, the results of the present study showed that PGA plus stent placement could be an independent factor to predict a lower possibility of esophageal stricture occurrence. The possible reasons are that the stent contributes to fixing the PGA sheet and the PGA sheet increases the friction to reduce stent displacement, thereby preventing the occurrence of post-ESD esophageal stricture[9,11,18,19]. Although there is no report on the effects of PGA plus stent in the prevention of post-ESD esophageal stricture in EC patients, further studies investigating other combined treatments, such as PGA plus stent plus corticosteroids, are greatly needed. Furthermore, the results of our study also found that shorter length (P = 0.080) and diameter (P = 0.061) were observed in the PGA plus stent group compared with the stent group, which suggests that the interaction of PGA and stent might decrease the severity of the degree of esophageal stricture.
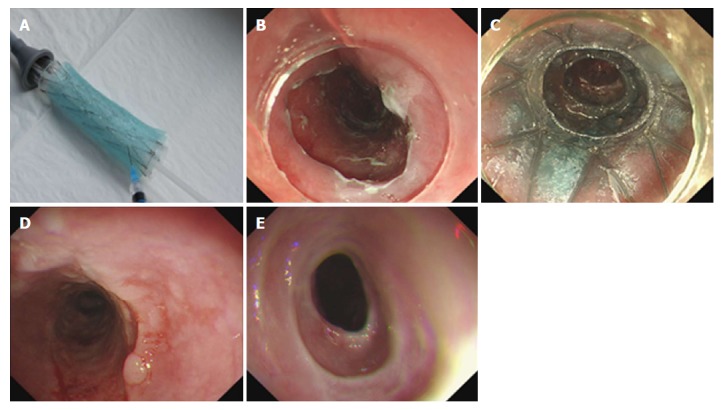
Figure 5.
The endoscopic submucosal dissection wound before and after the polyglycolic acid sheet plus stent placement. A: The esophageal lesion before endoscopic submucosal dissection (ESD); B: The mucosal defect after ESD; C: The ESD wound after the PGA sheet plus stent placement; D: The PGA sheet plus stent placement was inserted after 3 wk.
In clinical practice, glucocorticoids have established their value in inhibiting the inflammatory response, repressing collagen synthesis, and promoting collagen decomposition, thereby preventing the formation of post-ESD esophageal stricture[23,24]. Although glucocorticoid injection is a good way to decrease the occurrence of systemic adverse reactions, it still leads to several complications in EC patients after ESD, including perforation, bleeding, and mediastinal abscess[23,25]. Therefore, a PGA sheet infiltrated with a glucocorticoid (triamcinolone acetonide) might decrease the occurrence of post-ESD esophageal stricture while reducing adverse reactions. Furthermore, to optimize the methods to prevent esophageal stricture, we explored the combined application of PGA, stent, and glucocorticoid in three EC patients after ESD (Figure 6A-C). After stent removal, no formation of esophageal stricture occurred and good effectiveness was achieved during a 3 mo follow-up (Figure 6D and E). However, further study with a larger sample size and a longer follow-up period is necessary.
Figure 6.
The management of one of the study patients. A: Stent covered with polyglycolic acid (PGA) sheet was injected with glucocorticoid before insertion; B: The mucosal defect after endoscopic submucosal dissection (ESD); C: The combination of PGA, stent, and glucocorticoid was applied after 1 wk; D: The endoscopic appearance of the esophagus 4 wk after ESD; E: The endoscopic appearance of the esophagus 3 mo after ESD.
One limitation in the present study was that the total number of recruited EC patients was relatively small, which might cause lower statistical efficiency compared with a study with a large sample size. Therefore, a study with a larger sample size is needed to further confirm the efficacy of PGA plus stent placement in preventing esophageal stricture.
In conclusion, PGA plus stent placement is more effective in preventing post-ESD esophageal stricture compared with stent placement alone in EC patients with early-stage disease.
ARTICLE HIGHLIGHTS
Research background
Esophageal cancer (EC) is one of the most common carcinomas with high mortality, and more than 30% of EC patients after endoscopic submucosal dissection (ESD) still experience postoperative esophageal stricture, which dramatically decreases the quality of life.
Research motivation
The applications of combinations of two or three treatments in the prevention of esophageal stricture after ESD have been investigated in some studies. However, no study explored the effect of the combination of PGA and stent placement in the prevention of post-ESD esophageal stricture.
Research objectives
This study aimed to assess the effect of PGA plus stent placement vs stent placement alone in the prevention of post-ESD esophageal stricture in early-stage EC patients.
Research methods
About 70 EC patients undergoing ESD were enrolled in this study. Patients were allocated randomly at a 1:1 ratio into a PGA plus stent group (PGA sheet-coated stent placement was performed) and a stent group (only stent placement was performed). All patients were followed, and if dysphagia or other symptoms occurred, endoscopy was performed to examine the esophageal stricture.
Research results
The occurrence rate of esophageal stricture in the PGA plus stent group was lower than that in the stent group. Times of balloon dilatation in the PGA plus stent group were less than those in the stent group. Multivariate logistic analysis suggested that PGA plus stent placement was an independent predictive factor for a lower risk of esophageal stricture, while location in the middle third and circumferential range = 1/1 could independently predict a higher risk of esophageal stricture in EC patients after ESD.
Research conclusions
PGA plus stent placement is more effective in preventing post-ESD esophageal stricture vs stent placement alone in early-stage EC patients.
Research perspectives
The total number of recruited EC patients in the present study was relatively small. And a study with a larger sample size is needed to further confirm the efficacy of PGA plus stent placement in preventing esophageal stricture.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Chinese PLA General Hospital.
Clinical trial registration statement: This study is registered on http:// www.chictr.org.cn (No. chictr-inr-16008709).
Informed consent statement: All study participants provided written informed consent prior to study enrolment.
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Peer-review started: September 21, 2017
First decision: September 27, 2017
Article in press: January 16, 2018
P- Reviewer: Sato Y, Rodrigo L S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Ning-Li Chai, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
Jia Feng, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
Long-Song Li, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
Sheng-Zhen Liu, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
Chen Du, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
Qi Zhang, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China.
En-Qiang Linghu, Department of Gastroenterology, Chinese PLA General Hospital, Beijing 100853, China. 0572013@fudan.edu.cn.
References
- 1.Mohammed F. Esophageal cancer. N Engl J Med. 2004;350:1363–1364; author reply 1363-1364. doi: 10.1056/NEJMc033106. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg. 2017;6:88–98. doi: 10.21037/acs.2017.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu JP, Liu YJ, Tao YL, Ruan RW, Cui Z, Zhu SW, Shi W. Prevention of Esophageal Stricture After Endoscopic Submucosal Dissection: A Systematic Review. World J Surg. 2015;39:2955–2964. doi: 10.1007/s00268-015-3193-3. [DOI] [PubMed] [Google Scholar]
- 6.Shi KD, Ji F. Prophylactic stenting for esophageal stricture prevention after endoscopic submucosal dissection. World J Gastroenterol. 2017;23:931–934. doi: 10.3748/wjg.v23.i6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain D, Singhal S. Esophageal Stricture Prevention after Endoscopic Submucosal Dissection. Clin Endosc. 2016;49:241–256. doi: 10.5946/ce.2015.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ASGE Technology Committee. Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Konda V, Murad FM, Siddiqui UD, Banerjee S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311–1325. doi: 10.1016/j.gie.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi Y, Tsuji Y, Ono S, Saito I, Kataoka Y, Takahashi Y, Nakayama C, Shichijo S, Matsuda R, Minatsuki C, et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2015;47:336–340. doi: 10.1055/s-0034-1390787. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Park JC, Chung H, Shin SK, Lee SK, Lee YC. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for recurrent esophageal cancer. Endoscopy. 2016;48:E319–E320. doi: 10.1055/s-0042-117224. [DOI] [PubMed] [Google Scholar]
- 11.Iizuka T, Kikuchi D, Yamada A, Hoteya S, Kajiyama Y, Kaise M. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy. 2015;47:341–344. doi: 10.1055/s-0034-1390770. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki T, Tomita T, Takimoto M, Ohda Y, Oshima T, Fukui H, Watari J, Miwa H. Esophageal stricture after endoscopic submucosal dissection treated successfully by temporary stent placement. Clin J Gastroenterol. 2016;9:337–340. doi: 10.1007/s12328-016-0685-0. [DOI] [PubMed] [Google Scholar]
- 13.Vleggaar FP. Stent placement in esophageal cancer as a bridge to surgery. Gastrointest Endosc. 2009;70:620–622. doi: 10.1016/j.gie.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Nagami Y, Shiba M, Tominaga K, Ominami M, Fukunaga S, Sugimori S, Tanaka F, Kamata N, Tanigawa T, Yamagami H, et al. Hybrid therapy with locoregional steroid injection and polyglycolic acid sheets to prevent stricture after esophageal endoscopic submucosal dissection. Endosc Int Open. 2016;4:E1017–E1022. doi: 10.1055/s-0042-111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagami Y, Ominami M, Shiba M, Minamino H, Fukunaga S, Kameda N, Sugimori S, Machida H, Tanigawa T, Yamagami H, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis. 2017;49:427–433. doi: 10.1016/j.dld.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Kanai N, Yamato M, Ohki T, Yamamoto M, Okano T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc. 2012;76:873–881. doi: 10.1016/j.gie.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Nonaka K, Miyazawa M, Ban S, Aikawa M, Akimoto N, Koyama I, Kita H. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013;13:72. doi: 10.1186/1471-230X-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagh MS, Forsmark CE, Chauhan S, Draganov PV. Efficacy and safety of a fully covered esophageal stent: a prospective study. Gastrointest Endosc. 2012;75:678–682. doi: 10.1016/j.gie.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Shah MB, Jajoo K. Endoscopic retrieval of a migrated esophageal stent in the cecum. Endoscopy. 2010;42 Suppl 2:E245–E246. doi: 10.1055/s-0030-1255604. [DOI] [PubMed] [Google Scholar]
- 20.Cheng YS, Li MH, Chen WX, Chen NW, Zhuang QX, Shang KZ. Temporary partially-covered metal stent insertion in benign esophageal stricture. World J Gastroenterol. 2003;9:2359–2361. doi: 10.3748/wjg.v9.i10.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji Y, Ohata K, Gunji T, Shozushima M, Hamanaka J, Ohno A, Ito T, Yamamichi N, Fujishiro M, Matsuhashi N, et al. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to cover wounds after colorectal endoscopic submucosal dissection (with video) Gastrointest Endosc. 2014;79:151–155. doi: 10.1016/j.gie.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115–1121. doi: 10.1016/j.gie.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Hanaoka N, Ishihara R, Takeuchi Y, Uedo N, Higashino K, Ohta T, Kanzaki H, Hanafusa M, Nagai K, Matsui F, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007–1011. doi: 10.1055/s-0032-1310107. [DOI] [PubMed] [Google Scholar]
- 24.Nagami Y, Shiba M, Tominaga K, Minamino H, Ominami M, Fukunaga S, Sugimori S, Tanigawa T, Yamagami H, Watanabe K, et al. Locoregional steroid injection prevents stricture formation after endoscopic submucosal dissection for esophageal cancer: a propensity score matching analysis. Surg Endosc. 2016;30:1441–1449. doi: 10.1007/s00464-015-4348-x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389–1393. doi: 10.1016/j.gie.2011.07.070. [DOI] [PubMed] [Google Scholar]


