Abstract
In a recent publication in Neuron, Choe et al. (2015) demonstrate that brain-derived neurotrophic factor (BDNF) signaling mediates salt-induced blood pressure elevation by increasing the excitability of hypothalamic vasopressin-secreting neurons. These findings suggest complex roles for BDNF in adaptive cardiovascular responses to physiological challenges and in the pathogenesis of hypertension.
Hypertension, a major risk factor for myocardial infarction and stroke, is promoted by sedentary, overindulgent lifestyles and excessive dietary sodium chloride (salt) (Egan and Stevens-Fabry, 2015). Salt consumption elevates blood pressure by increasing blood volume. Normally, when blood pressure increases, the baroreflex, a nervous system-mediated negative feedback loop, is activated and reduces heart rate and blood pressure. The baroreflex involves pressure-sensitive neurons in the aorta and carotid sinuses, which then send signals to autonomic neurons that control heart rate and blood pressure. In addition, elevated blood pressure activates inhibitory GABAergic neurons in the hypothalamus, resulting in reduced secretion of the blood pressure-elevating hormone vasopressin (Kim et al., 2011). However, regular consumption of high-salt diets can result in dysregulation of these feedback pathways. A better understanding of how high salt intake alters the signaling pathways that control vasopressin secretion may therefore lead to novel approaches for treating hypertension.
Previous studies provided evidence that a high-salt diet increases release of vasopressin from magnocellular neurosecretory cells (MNCs) in the hypothalamic supraoptic nucleus by reducing baroreceptor-mediated activation of inhibitory GABAergic input to the MNCs (Kim et al., 2011). However, the mechanism by which a high salt intake reduces inhibition of MNCs was unknown. Choe et al. (2015) “connected the dots” by noting two key extant findings: (1) other conditions that are often associated with elevated blood pressure, including chronic pain and stress, suppress the expression of the K+/Cl− co-transporter KCC2, thereby increasing neuronal excitability (Maguire, 2014); and (2) brain-derived neurotrophic factor (BDNF) increases the excitability of hippocampal neurons, in part, by downregulating KCC2 expression (Rivera et al., 2004). Choe et al. (2015) recorded ion currents in identified vasopressin-producing MNCs in hypothalamic explants from rats that had drunk either normal water or salt water for 7 days. MNCs in rats that drank salt water exhibited increased excitability and reduced inhibitory GABAergic tone. Using pharmacological agents, they further showed that high salt intake resulted in reduced expression of KCC2. Choe et al. (2015) found that levels of the phosphorylated (activated) form of the BDNF receptor TrkB were increased in the supraoptic nucleus cells of rats in the high-salt group. Importantly, infusion of a TrkB-blocking antibody into the supraoptic nucleus prevented the high-salt diet-induced increase of MNC excitability, as did suppression of BDNF production by lentivirus-mediated expression of an shRNA against the BDNF mRNA. While the authors did not establish that the latter manipulations of BDNF signaling in MNCs actually reduced blood pressure in rats consuming salt, this is their inference (Figure 1).
Figure 1. BDNF Signaling Mediates High Dietary Salt Intake-Induced Hypertension.
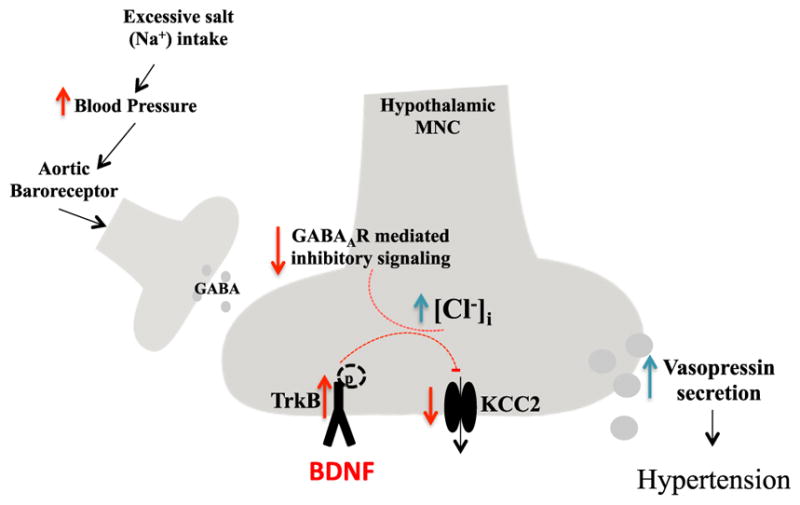
Salt consumption elevates blood pressure, which activates aortic baroreceptor-mediated GABAergic signaling in the hypothalamus, thereby reducing the secretion of vasopressin from magnocellular neurosecretory cells (MNCs). However, regular consumption of high-salt diets can impair this feedback loop. Chronic high salt intake causes downregulation of K+/Cl− co-transporter 2 (KCC2) in MNCs by a mechanism involving activation of the TrkB receptor by brain-derived neurotrophic factor (BDNF). In this way, BDNF signaling causes a collapse of the plasma membrane Cl− gradient that disables inhibitory GABAergic input to the MNCs, resulting in increased excitability of those cells and vasopressin secretion. Vasopressin increases blood pressure by acting on kidney cells to promote water retention and by promoting constriction of blood vessels.
The demonstration by Choe et al. (2015) that BDNF mediates the hyperexcitability of MNCs and excessive vasopressin secretion caused by high salt intake advances our understanding of blood pressure regulation and its dysregulation in hypertension. The new findings also add another layer of complexity to emerging roles for BDNF in the regulation of cardiovascular function and vulnerability to disease. It has been reported that mice with reduced BDNF levels (BDNF+/− mice) exhibit an elevated heart rate, and infusion of BDNF into the cerebral ventricle reduces heart rate (Wan et al., 2014). In addition, GABAergic responses are increased and glutamatergic responses are reduced in brainstem cardiovagal (cholinergic) neurons of BDNF+/− mice, suggesting that BDNF increases activity of the parasympathetic neurons to reduce heart rate (Wan et al., 2014). Because increased vagal activity also reduces blood pressure, it would be of interest to determine whether BDNF signaling in the brainstem can counteract salt-induced hypertension. BDNF may also influence cardiovascular function and vulnerability to damage by direct actions on the heart; TrkB is expressed in cardiac myocytes, BDNF improves cardiomyocyte Ca2+ handling, and mice with selective deletion of the TrkB gene in those cells exhibit impaired heart contraction and relaxation (Feng et al., 2015).
There is also evidence that interventions that increase BDNF expression, including exercise and dietary energy restriction, are cardioprotective (Marosi and Mattson, 2014). As exercise and energy restriction decrease blood pressure very effectively, it will be of interest to know how these health-promoting lifestyle factors modify the effects of high salt intake on MNC activity and vasopressin secretion (Choe et al., 2015). Exercise increases BDNF expression and TrkB activation in neurons in many regions of the brain, including several that regulate cardiovascular function (Vanevski and Xu, 2013). BDNF is best known for its critical roles in learning and memory and in the regulation of mood; in the hippocampus, BDNF enhances synaptic plasticity and neurogenesis and increases the resistance of neurons to metabolic and excitotoxic stress (Marosi and Mattson, 2014). Moreover, BDNF regulates the activities of neurons in several hypothalamic nuclei in addition to MNCs; it acts on neurons in the arcuate, ventromedial, and paraventricular nuclei to suppress hunger and increase energy expenditure (Vanevski and Xu, 2013). From an evolutionary perspective, it is crucial that the brain and cardiovascular systems function well under conditions of limited food availability. Individuals who are cognitively “sharp” and able to tolerate considerable physical exertion in such circumstances have a survival advantage. Emerging findings suggest prominent roles for BDNF in the evolution of adaptive responses to environmental challenges (Mattson, 2015). Might there have been an adaptive value to increased excitability of MNCs and vasopressin release in response to salt intake? For individuals living in hot dry climates, where dehydration during exercise could lead to hypotension and death, this is a reasonable possibility. In this view, BDNF signaling in MNCs could offset the hypotensive effects of BDNF signaling in cardiovagal neurons (Wan et al., 2014). Along these lines, chronic consumption of high-salt diets may disrupt the balance within the intricate systems that evolved to control blood pressure in harsher environments, where salt intake was lower.
BDNF is released at synapses in a tightly controlled, activity-dependent manner. In contemplating the possibility of manipulating BDNF signaling pharmacologically as an approach to hypertension and other cardiovascular disorders, it will be important to consider the overall effects of such agents on the entire nervous system. For example, a TrkB antagonist that reduces vasopressin secretion from MNCs would also impair hippocampal synaptic plasticity and neurogenesis, which would be expected to result in learning and memory deficits and anxiety/depression (Marosi and Mattson, 2014). Because of their widespread beneficial effects on health, some of which are mediated by BDNF (Marosi and Mattson, 2014), exercise and moderation in energy intake should be combined with abstention from salty foods as first-line interventions for hypertension.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging.
References
- Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. Neuron. 2015;85:549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM, Stevens-Fabry S. Nat Rev Cardiol. 2015 Published online February 17, 2015 http://dx.doi.org/10.1038/nrcardio.2015.17.
- Feng N, Huke S, Zhu G, Tocchetti CG, Shi S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, et al. Proc Natl Acad Sci USA. 2015;112:1880–1885. doi: 10.1073/pnas.1417949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, et al. J Neurosci. 2011;31:13312–13322. doi: 10.1523/JNEUROSCI.1440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Front Cell Neurosci. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Ageing Res Rev. 2015;20C:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, Payne JA, Minichiello L, Saarma M, Kaila K. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanevski F, Xu B. Front Neurosci. 2013;7:37. doi: 10.3389/fnins.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. J Neurochem. 2014;129:573–580. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]


