Abstract
Oomycetes are eukaryotic pathogens infecting animals and plants. Amongst them Saprolegnia parasitica is a fish pathogenic oomycete causing devastating losses in the aquaculture industry. To secure fish supply, new drugs are in high demand and since fish experiments are time consuming, expensive and involve animal welfare issues the search for adequate model systems is essential. Galleria mellonella serves as a heterologous host model for bacterial and fungal infections. This study extends the use of G. mellonella for studying infections with oomycetes. Saprolegniales are highly pathogenic to the insects while in contrast, the plant pathogen Phytophthora infestans showed no pathogenicity. Melanisation of hyphae below the cuticle allowed direct macroscopic monitoring of disease progression. However, the melanin response is not systemic as for other pathogens but instead is very local. The mortality of the larvae is dose-dependent and can be induced by cysts or regenerating protoplasts as an alternative source of inoculation.
Keywords: Galleria mellonella, Host model, Oomycetes, Saprolegnia parasitica
Graphical abstract
Highlights
-
•
Galleria mellonella serves as a heterologous host model system for Saprolegniales.
-
•
The melanisation of the larvae is local around the growing hyphae.
-
•
Regenerating protoplasts can be used as an alternative inoculum to cysts.
1. Introduction
Oomycetes are eukaryotic organisms that are morphologically similar to fungi but genetically related to brown algae (Baldauf et al., 2000). The vast majority of oomycete species are pathogenic to animals and plants, significantly affecting the agri- and aquaculture industries, but also responsible for wiping out wild populations (van West, 2006). The most devastating oomycete affecting salmon farming is Saprolegnia parasitica because it infects fish eggs as well as salmonid fish (Stueland et al., 2005, van West, 2006, van den Berg et al., 2013). However, also other fish in fresh water and arthropods such as crayfish and river insects are susceptible to S. parasitica and other Saprolegnia species (Krugner-Higby et al., 2010, Sarowar et al., 2014). The disease caused by Saprolegniaceae is called saprolgeniosis (or saprolegniasis) and once the infection is established, cotton-like white to grey patches on the skin, especially around the fins, as well as in the gills can be observed with destruction of the epidermis (Khoo, 2000, Bruno et al., 2011). While the infection progresses, fish become lethargic and lose their equilibrium (Bruno et al., 2011). Final death of the fish occurs due to an osmotic shock caused by haemodilution (Roberts, 1993, Torto-Alalibo et al., 2005).
While research on plant pathogenic oomycetes allows in planta infection assays without ethical objections (Kanneganti et al., 2007, Bhaskar et al., 2009), studies on fish pathogens such as S. parasitica on its native salmonid hosts are expensive, time consuming and raise animal welfare issues. Moreover, it is difficult to replicate the complex conditions under which infections naturally occur (Kales et al., 2007, Minor et al., 2014). Due to the economic impact of S. parasitica, Saprolegnia diclina and other Saprolegnia species in hatcheries, eggs are also frequently used for challenge experiments (Songe et al., 2016, Eissa et al., 2013). However, these studies aim more at replicating conditions of outbreaks on eggs specifically than investigating the host–pathogen interaction and insights of the defence system gained from egg experiments cannot simply be transferred to fish. Another way to study the immune response to saprolegniosis is the work with cell lines (Kales et al., 2007, de Bruijn et al., 2012, Belmonte et al., 2014); although the humoral immunity component is absent in cell line studies (Magnadóttir, 2006). Hence, a reliable and simple model system to screen potential compounds against Saprolegnia species and to study virulence factors under controlled conditions is needed.
Due to their low cost, easy handling, short reproductive cycles and simple housing, insects and nematodes have become increasingly popular as alternative model hosts in infection assays (Cook and McArthur, 2013). For many fungal pathogens, such as the human opportunistic pathogen Candida albicans, the greater wax moth (G. mellonella) is used as an alternative approach to classical mouse model system (Chamilos et al., 2007). The insects are economical, simple to maintain and viable at a wide range of temperatures allowing experimentation at temperatures more similar to the natural conditions in freshwater ecosystems (10–20 °C). G. mellonella can be used without ethical concerns and results can be obtained within days. Similar to the skin of vertebrates, the cuticle of G. mellonella provides the first defence barrier against pathogens (Magnadóttir, 2006). In the insect itself, the pathogen has to overcome the interconnected cellular and humoral immune response contained in the haemolymph, the blood analogue of insects (Matha & Áček, 1984; Brennan et al., 2002). Hence, the insect immune system comprises important similarities to the vertebrate immune system at a structural and functional level (Zhao and Kanost, 1996, Rock et al., 1998, Wittwer et al., 1999, Kavanagh and Reeves, 2004, Lemaitre and Hoffmann, 2007) and therefore provides a potential model system to study the pathogenicity of S. parasitica.
Here we report on the use of G. mellonella as a novel in vivo model to study animal pathogenic oomycetes. The virulence of two Saprolegnia species was determined through the mortality rates of infected insects. Progression of infection was monitored enzymatically and by visual inspection as well as histological examinations. Since the production of zoospores or cysts can be challenging, especially for newly discovered species, we show that protoplasts can also be used as an inoculum.
2. Material and methods
2.1. Galleria mellonella
Sixth instar larvae of G. mellonella (Lepidoptera: pyralidae) (Livefood UK Ltd. Somerset, UK) were kept in wood shavings in the dark at 12 °C. Prior to a challenge experiment, healthy insects without black or grey marks visible on the skin and a swift response to stimulation (being flipped on their backs) were separated and used. Twenty larvae for each group were inoculated with 10 μL of cysts or protoplasts or as a negative control with 10 μL PBS via the last left pro-leg into the haemolymph with a 10 μL microsyringe (VWR, 549-0199) as described elsewhere (Mylonakis et al., 2007; Bergin et al., 2006). Following inoculation, larvae were incubated at 24 °C (optimal in vitro growth temperature for S. parasitica) in the dark. Insects were investigated as indicated. An insect was scored as dead when it did not show a response to physical stimulation with forceps.
2.2. Oomycete strain maintenance, zoospore/cyst and protoplast production
S. parasitica C65 (CBS223.65) was originally isolated from young pike (Esox lucius), S. parasitica N12 (VI-02736) originated from parr of Atlantic salmon in Scotland (Lochailort) (Jiang et al., 2013). Saprolegnia delica (DON160516) was isolated from caddisfly larvae (Rhyacophila dorsalis) sampled from the river of Don near Monymusk, Scotland. Phytophthora infestans (P88069) was originally isolated from tomato in the Netherlands (Kamoun et al., 1998). Stock cultures of each strain were kept at 12 °C on potato-dextrose agar. For growth tests, agar plugs (⌀ 0.5 cm) were cut from the stock cultures and mycelial growth was tracked over time.
For the production of zoospores, an agar plug of mycelia was grown in pea broth (125 g pea/L) at 24 °C. Sporulation of 3 d old mycelia was induced by washing 3 times with deionised water followed by 24 h incubation in Tap:tank water (sterile water from a fish tank diluted 1:2 in tap water) at 24 °C. Zoospores were separated from sporulating mycelia through 40 μm cell strainers and encysted during the washing procedure.
For the production of protoplasts, 24 h old mycelium was digested in 0.5 M sorbitol supplemented with cellulose (5 mg/mL, Sigma–Aldrich, C8546) and lysing enzymes (5 mg/mL, from Trichoderma harzianum, Sigma–Aldrich, L1412) at 200 rpm at 25 °C for 1 h. Protoplasts were separated from non-degraded mycelia through 40 μm cell strainers and washed 3 times in 0.5 M sorbitol to remove digestive enzymes and small debris from the disrupted mycelia. Before injections, protoplasts were allowed to regenerate in LB medium at 24 °C for 2 h.
For injections into G. mellonella, cysts and protoplast were concentrated by centrifugation, counted and diluted in PBS to the final concentration as indicated.
2.3. Histological examination
Insect preparation for paraffin embedding was performed essentially as described elsewhere (Perdoni et al., 2014). Briefly, 50 μL of 4 % PFA/PBS was injected into the last right pro-leg before immersing insects in 4 % PFA overnight at 4 °C. Fixed insects were embedded in either paraffin or OCT embedding matrix (Cellpath, KMA-0100-00A). Samples were cut in 10 μm sections on a cryostat (Leica 1850) at −20 °C.
Staining with GMS (Grocott methamine-silver) was performed by the NHS Grampian Biorepository (Aberdeen, Scotland). Briefly, slides were deparaffinised, hydrated and oxidised in 2 % chromic acid for 5 min. Treatments in 1 % sodium metabisulfit (1 min), quickly in methenamine silver solution and 0.5 % gold chloride followed before a final incubation in 5 % sodium thiosulfate (3 min). Counterstaining was performed with working light green solution. Slides were thoroughly rinsed with water between individual steps.
For PAS stain slides were deparaffinised, hydrated and oxidised in 0.5 % periodic acid schiff (PAS) for 5 min. Followed by an incubation in Schiff reagent for 15 min and a counter stain with Mayer's Haematoxylin Solution for 1 min. Slides were thoroughly rinsed with water between individual steps. Slides were scanned on a Zeiss Axio Scan Z1.
For whole mount microscopy insects were opened and the cuticle spread out on a glass slide after removing internal organs. Samples were inspected on an inverted microscope and images were taken using a Lumenera Infinity2 camera.
2.4. DNA extraction
For PCR amplification, DNA was isolated from mycelia and infected larvae of G. mellonella. A piece of pea-broth-cultured mycelium or a cut larvae were transferred into 2 mL tubes with glass beads (425–600 μm) and 800 μL DNA extraction buffer (Sarowar et al., 2014) containing RNAseA and homogenized in a FastPrep-24™ 5G tissue homogenizer (MP Biomedicals, 4 × 40 s, 6 m/s, 2 min breaks). After disruption samples were incubated at 55 °C for 30 min followed by a centrifugation to remove debris at 10,000 × g for 10 min. The supernatant was used for DNA extraction by a phenol-chloroform method as described elsewhere (Zelaya-Molina et al., 2011). Briefly, for DNA extraction from insects an additional phenol extraction step was performed. DNA was precipitated in 1 mL isopropanol overnight at −20 °C and pelleted by centrifugation at 4 °C. Pellets were washed twice with 70 % ethanol before air dried at room temperature for 5 min, followed by resuspension in nuclease-free water.
2.5. PCR
Standard PCR for ITS amplification was performed with the GoTaq polymerase according to manufacturer's instructions (Promega) with 0.2 μM primer each (ITS4 (5′- TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAGTCGTAACAAGG-3′)) with Tm = 58 °C and an elongation time of 1 min.
2.6. Phenoloxidase activity
For measuring the phenoloxidase (PO) activity challenged larvae were incubated on ice for 10 min. 10 μL haemolymph were collected from the tail between the prolegs and diluted with 25 μL PBS. Cells were pelleted (5 min, 500 × g, 4 °C) and 10 μL of the supernatant was added to 200 μL l-DOPA (10 mM). To allow substrate conversion samples were incubated for 1 h at 30 °C. Absorption of melanin was measured at 490 nm.
3. Results
3.1. Injection of cysts of S. parasitica strains results in killing and colonization of G. mellonella larvae
Cysts of S. parasitica were collected and injected into the haemocoel of healthy larvae. For comparison, the reference strain C65 and N12, of which the latter one is significantly more virulent in Atlantic salmon, were used (Jiang et al., 2013). Although both strains show the same in vitro growth rate on agar plates (Fig. 1A), their virulence to moth larvae differs. Consistent with a higher pathogenicity of N12 in Atlantic Salmon, N12 resulted in a higher mortality rate of Galleria melonella compared to C65 (Fig. 1B). During the first 24 hpi no mortalities were observed, while on day 2 both strains caused rapid mortality. Although both strains were able to cause almost 100 % cumulative mortality within 72 h (day 3), infections with N12 were more progressive than with C65 (75 % and 50 % mortality after 48 h, respectively). As expected, the progression of the infection process is faster when more cysts are injected (Fig. 1C). When injecting germinated cysts from the insect pathogenic oomycete S. delica into G. mellonella, mortality rates were increased and killing occurred even within 24 hpi (Fig. 1D). In contrast, cysts of the plant pathogenic oomycete P. infestans were unable to kill larvae, even when the concentration of cysts was increased to 2450 per insect (Fig. S1). In none of the challenges a transmission from dead insects to healthy larvae could be observed. Mycelia could only be re-isolated from oomycete- but not from PBS-treated insects.
Fig. 1.
Mortality of G. mellonella after injection with cysts of different oomycete isolates. (A) S. parasitica strains N12 (dashed line) and C65 (solid line) show the same in vitro growth rate on agar plates. (B) G. mellonella was injected with 100 cysts of either N12 (▲) or C65 (■). Infections with N12 progress faster compared to C65 while the cumulative mortality rate after 3 dpi is the same. (C) The survival rate of G. mellonella at 48 hpi decreases with an increasing concentration of injected cysts of S. parasitica N12. (D) Comparison of S. parasitica C65 (●) with the insect pathogenic S.delica (■) and the plant pathogenic P.infestans (◆). While S. delica causes a higher mortality rate, G. mellonella is not susceptible to cysts of P. infestans even after increasing the amount of injected cysts (Fig. S1).
3.2. Phenotype of Saprolegnia infection in G. mellonella
A key part of the humoral immune response of G. mellonella comprises the formation of brown-coloured melanin by phenoloxidases to encapsulate invading pathogens and thereby preventing their growth by inducing starvation (Gillespie et al., 1997, Cerenius and Söderhäll, 2004, Eleftherianos and Revenis, 2011, González-Santoyo and Córdoba-Aguilar, 2012). Due to the obvious difference between yellowish healthy larvae and the melanin induced brown colour of infected insects, the progression of an infection can be followed macroscopically (Fig. 2). When comparing larvae infected with S. parasitica strains C65 or N12, the stages of infection are very similar (Fig. 2A). Up to 24 hpi the insects are generally asymptomatic with a high vitality as no signs of infection can be seen and the response to physical stimuli is unchanged. The first visible signs of infection appear when black lines in the abdomen and thorax are formed. These are attempts from the larvae to encapsulate the mycelium by local melanisation (Fig. 2B). If the network of mycelium becomes visible underneath the cuticle, the corresponding insect will die within the next 24 h. As the infection progresses, black patches are expanding and melanisation is detected at additional sites. On day 2, the whole insect begins to turn dark while the mycelium expands throughout the whole larvae and the first mortalities occur. Until death of G. mellonella clinical signs for both S. parasitica strains are the same. However, for a short time after death, mycelium of C65 penetrates through the cuticle from the inside of the insect and is exposed to the environment (Fig. 2C). Shortly after, the dead insects begin to shrink and shrivel. In contrast, the cuticle of insects infected with N12 is not perforated by mycelium (Fig. 2A). Within 72 hpi the cumulative mortalities peak for N12 as well as C65 and the insects that do not yet show any signs of infection tend to survive and keep their yellowish appearance (Fig. 2D). In contrast, dead insects are entirely black after 3 d. The same phenotype can be observed also with other Saprolegniales (Fig. 2D). However, after injection with cysts of P. infestans, a faint melanisation of the whole larvae is observed that does not change over time while the mortality rate is very low (Fig. 2D).
Fig. 2.
Progress of infection with Saprolegnia in G. mellonella. (A) Disease progression is associated with increasing melanisation over time. First signs of infections caused by S. parasitica are localised pigmentations around the mycelium. Later the entire insect turns dark until death. While S. parasitica N12 infected insects keep their shape, S. parasitica C65 penetrates the cuticle and insects shrink. Infection with P. infestans only causes a weak systemic melanisation of larvae that does not change over the time. (B) Local melanisation of the thorax of an S. parasitica C65 infected G. mellonella at early stages. (C) Penetration of the cuticle by S. parasitica C65 after the insect died. The penetration leads to water evaporation and concomitant shrinking of the body. (D) Progress of Saprolegnia infection over time after infection of G. mellonella with cysts of S. parasitica C65, N12 and S. delica. After infection with P. infestants early systemic melanisation appears only in some insects and shows little progression.
3.3. Histopathology of G. mellonella suffering from infection with Saprolegnia
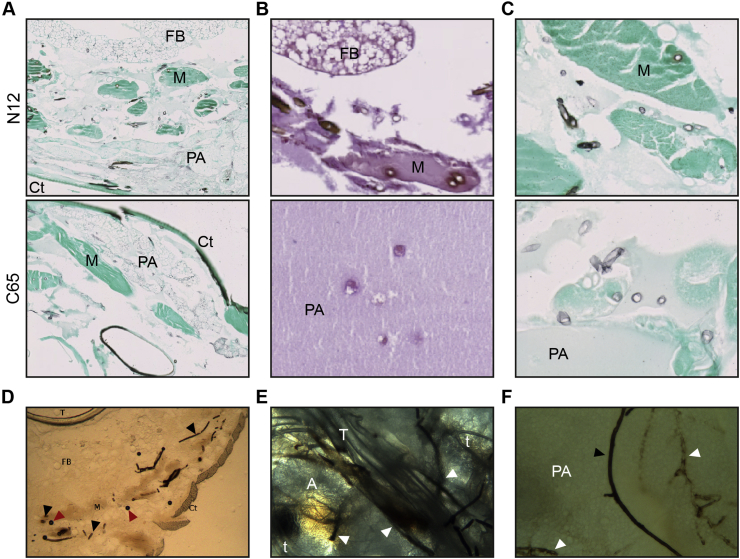
In order to study changes in the insect tissue, histological sections from S. parasitica infected insects were microscopically examined. C65 as well as N12 were both found in subcuticular tissues (including the peripheral fat body), trachea and particularly in muscle but was absent in the gut-associated/perivisceral fat-body (Fig. 3A). Hyphae of S. parasitica C65 as well as N12 were GMS positive while in the PBS-injected control group no hyphal structures could be detected (Fig. S2A). This was also confirmed by the Periodic acid Schiff (PAS) stain in which mycelium appears as dark pink (Fig. 3B). On average hyphal structures of C65 and N12 have a diameter of 6 μm and 7.5 μm, respectively, which is very similar to the tracheoles of G. mellonella. However, while hyphal structures are very consistent in diameter, tracheoles differ because of their narrowing tips and in addition, they are GMS negative. As a reaction of the insect immune system to the infection and as already seen macroscopically, melanisation also appears microscopically as brown halos around GMS stained mycelium (Fig. 3C). Although the phenotypes in G. mellonella caused by an S. parasitica C65 and N12 infection are very similar (Fig. 2A), N12 mycelium is more frequently and more intensely melanised than C65 is (Fig. 3C). Due to the high melanisation of hyphae of S. parasitica it is possible to observe mycelia without any stain of sections (Fig. 3D) as well as whole-mount unprocessed tissue (Fig. 3E). The whole-mount processing is very time efficient and allows for easy distinguishing between hyphae and trachea since they differ in colour, size and shape. Furthermore, the examination of unstained samples confirms a gradual melanisation process since partially melanised hyphae can be detected at various sites (Fig. 3F).
Fig. 3.
Histopathology of Saprolegnia infection in G. mellonella. (A) GMS stain of tissues of infected insects with S. parasitica N12 and C65. Mycelium appears as black lined circles (black arrows) which cannot be detected in the control (Fig. S2A). Both strains cluster at the musculature (M) and the trachea (T), spreading into the peripheral adipose tissue (PA) below the cuticle (Ct). The gut associated fat-body (FB) was mostly free of mycelia. (B) In PAS stained samples, S. parasitica N12 and C65 appear dark pink with brown halos when the mycelium is melanised. (C) Beside the same localisation in the musculature, N12 mycelium is stronger and more frequently melanised compared to C65. (D) The high melanisation of S. parasitica N12 allows the detection of mycelium in unstained cryo-sections of infected insects (black arrow heads). Tracheoles (red arrow heads) have a larger diameter than mycelium. M: muscle, Ct: cuticle, FB: fat-body, T: trachea. (E) Whole mount preparation of a life insect showing clinical signs after infection with S. parasitica. Mycelium (arrow heads) is easier to distinguish from trachea (T) or tracheoles (t) compared to cryo-sections. (F) Peripheral adipose (PA) tissue from a whole mount preparation including one fully (black arrow head) and partially melanised hyphae (white arrow heads).
3.4. Detection of S. parasitica in challenged G. mellonella
The activity of melanin-producing phenoloxidases (PO) in the haemolymph can also be measured biochemically in vitro and is used for the determination of the systemic immune response in G. mellonella (Dubovskiy et al., 2013). Although the level of melanisation is different for S. parasitica strains N12 and C65, the PO activity in the haemolymph is identical (Fig. S2B). However, 24 hpi the activity is below the PO activity measured in PBS-injected samples. Therefore, this assay is to be considered as not suitable for the detection of an infection with an oomycete.
In order to confirm results of macroscopic and microscopic examinations of the Saprolegnia infections, PCR was used to detect also minimal amounts of S. parasitica DNA. Primers covering the high copy number ITS (internal transcribed spacer) region were used for DNA amplification. Amplification from a pure oomycete strain resulted in the usual size of 800 bp for the ITS region while in non-challenged insects the ITS region (1.5 kb) of G. mellonella was amplified (Fig. 4A). Hence, as infections progress the intensity of the G. mellonella band decreases and the S. parasitica band appears (Fig. 4B).
Fig. 4.
Detection of S. parasitica inside infected G. mellonella. (A) PCR reaction (ITS-4/5) of a pure culture of S. parasitica N12 resulted in a band of 800 bp while for non-infected insects a band of 1500 bp appears. (B) Time course of the infection progress of G. mellonella infected with S. parasitica. PCR was run on whole sample DNA extractions. As the infection proceeds, the ITS-4/5 primer favour the S. parasitica product in challenged insects after 24 h while the band of G. mellonella gradually disappears. The 800 bp fragment from infected insects was sequenced and matched to the ITS region of S. parasitica.
3.5. G. mellonella infection with protoplasts of S. parasitica
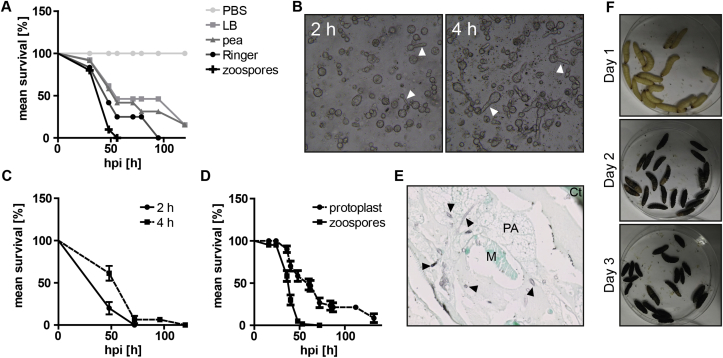
Cysts are the natural vehicle of most oomycete infections. However, spore release can be unreliable in terms of numbers of cells and the time of release, which complicates the performance of infection challenges with G. mellonella. Therefore, protoplast injections were explored as an alternative source of infection. The protoplast regeneration buffer used was found to be a critical parameter for successful protoplast recovery rates and thereby for infectiousness (Fig. 5A). In addition, regeneration of protoplasts before infection is beneficial for the infection process of G. mellonella but only up to a particular length of the germ tubes (Fig. 5B and C). Finally, after optimisation of the regeneration medium and time, mortality rates of G. mellonella challenged with either protoplast or cysts of S. parasitica C65 were comparable (Fig. 5D). Additionally, infections caused by protoplasts were indistinguishable from cysts infections in disease progression and during histological examinations (Fig. 5E and F). Although protoplasts are not a natural source of infection, they can be a viable alternative to cysts in order to study oomycete infections in G. mellonella.
Fig. 5.
Protoplast as an alternative inoculum for S. parasitica. (A) Effect of regeneration medium on protoplast virulence. Prior injections, protoplast were regenerated in different buffers as indicated. Mortality of G. mellonella after injection with 200 protoplast of S. parasitica C65 was measured over time. (B) Protoplast of S. parasitica C65 recovering and germ tube formation (arrow heads) is progressed after 4 h compared to 2 h. (C) Effect of regeneration time on the virulence of protoplasts. The virulence of regenerated protoplast is stronger after 2 h (●) compared to 4 h (■). (D) Cumulative mortality of G. mellonella 48 hpi is indistinguishable between infections with protoplast (●) or cysts (■) of S. parasitica C65. (E) Histological examination of G. mellonella infected with S. parasitica protoplasts revealed the same localisation of mycelium (arrow heads) compared to infections with cysts. M: muscle, Ct: cuticle, PA: peripheral adipose tissue, T: trachea. (F) Progress of Saprolegnia infection in G. mellonella over time after inoculation of protoplasts is the same compared to cysts-injected insects (Fig. 2D).
4. Discussion
After injection, cysts or protoplasts from S. parasitica are able to establish an infection in the alternative heterologous host G. mellonella. The resulting clinical symptoms can be used to distinguish between different strains of S. parasitica. The susceptibility of G. mellonella seems to be restricted to animal pathogenic oomycetes because after infection with P. infestans we did not observe significant mortalities.
Compared to small aquatic organisms like river insects, G. mellonella does not have to be captured for inspection and inoculation is more convenient because of the size of the insects. In addition, disease progression is more isolated and free of external influences. Furthermore, compared to Drosophila melanogaster, G. mellonella is susceptible to a broad range of pathogens and no immune-deficient mutants have to be used (Brennan et al., 2002, Perdoni et al., 2014). The zoospore/cysts production in oomycetes for infection experiments can be quite challenging and a final working protocol requires a lot of optimisation for newly discovered strains. After regeneration, protoplast share many morphological similarities with germinated cysts in our study. Indeed, injections with protoplasts replicated infections with cysts indistinguishably and therefore provide a reliable alternative as infection agents (Butt et al., 1996).
In previous reports, S. parasitica N12 was found to be one of the most virulent cultured strains (Stueland et al., 2005) which is in agreement with the slightly higher mortality of G. mellonella compared to infection with S. parasictica C65 in our study. Challenges of G. mellonella with a higher inoculum of the human pathogenic C. albicans, Staphylococcus aureus, Shigella spp. or Aspergillus fumigatus, resulted in earlier clinical signs but a lower overall mortality rate compared to infections with the Saprolegniales tested in this study (Desbois and Coote, 2011, Li et al., 2013, Barnoy et al., 2017, Mulvihill et al., 2017). Furthermore, the infection of G. mellonella with animal pathogenic oomycetes (S. parasitica and S. delica) is more efficient than with the plant pathogenic oomycete, P. infestans. While shrunken carcasses of insects challenged with S. parasitica C65 become hard and dry, presumably due to water evaporation through the perforated cuticle, insects injected with cysts from P. infestans show reduced response rates and no significant mortality. It was possible to detect melanised mycelia in surviving insects (Fig. S3) which might indicate the capability of G. mellonella to cope with a mild infection with P. infestans.
Although melanisation is a key part of the immune system of the insect, no PO activity could be detected in the haemolymph from G. mellonella infected with S. parasitica (Fig. S2B) which is in contrast to studies with other fungi and bacteria where PO activity was detected (Harding et al., 2012, Grizanova et al., 2014, Kryukov et al., 2016). However, in contrast to infections with human fungi, the immune response to S. parasitica is very local around melanised hyphae and is not systemic. Thus, it is likely that initially only a small amount of POs is activated locally when haemocytes are locally committed and found less in the circulation (Lavine and Strand, 2002). Hence, the PO activity assay is an unsuitable tool to measure the immune response to oomycete infections.
Although the insect's immune system is mainly limited to an innate defence response (Hillyer, 2016), G. mellonella is still a reasonable model system for infections with Saprolegnia since it has been shown that S. parasitica is mainly facing the innate immunity of fish because the adaptive immune system is suppressed and the progression of infection is often too fast for an immune response (Belmonte et al., 2014). The first macroscopic sign of the humoral immune response is the melanisation and thereby encapsulation of subcuticular hyphae (Cerenius and Söderhäll, 2004, Eleftherianos and Revenis, 2011, González-Santoyo and Córdoba-Aguilar, 2012), which is considered as a point of no return because in our studies none of the larvae were able to recover from the infection. At later stages of infection also the haemolymph becomes pigmented, indicating a systemic response. Interestingly, although S. parasitica N12 is slightly more pathogenic, the melanisation response is stronger and thereby likely causing a stronger immune response when compared to injections with C65. Melanin is produced by phenoloxidases (PO) which are expressed as inactive zymogens (Cerenius and Söderhäll, 2004). POs are not secreted but released by the rupture of haemocytes into the extracellular space where they are activated by for example components of the pathogen's cell wall (Cerenius and Söderhäll, 2004, Kanost and Gorman, 2008). However, the activity of POs does not always correlate with resistance to an infection since in some cases an excessive response can lead to the death of the insect (González-Santoyo and Córdoba-Aguilar, 2012). Nonetheless, reports indicate a positive correlation of virulence in G. mellonella and mammals (Jander et al., 2000, Brennan et al., 2002). Understanding of the melanin production in G. mellonella in response to Saprolegniales might also increase the understanding of infections in crayfish, which immune system also comprises melanisation to combat infections (Noonin et al., 2010).
Histological examinations revealed the spread of mycelium occurs segmentally and primarily along sub-cuticular regions close to muscles and from there into adjacent tissues with the least mycelium in tissues most distal from the site of infection. Interestingly, even in highly infected insects hardly any mycelium was detected in the gut-associated/perivisceral fat body which is the main source for the production of antimicrobial peptides in G. mellonella (Arrese and Soulages, 2010).
In this study, G. mellonella was established as an alternative model host to study the virulence of animal pathogenic oomycetes in vivo. G. mellonella offers a low cost, fast replicative model systems without ethical concerns that allow screening experiments before performing animal (fish) experiments.
Acknowledgment
Our work is supported by the University of Aberdeen (AW, PvW); BBSRC (BB/M026566/1 & BB/P020224/1: PvW); BBSRC (BB/N005058/1 & BB/J018333/1: FT & PvW); NERC (NE/P010873/1: PvW). We would like to acknowledge Joan Wilson and Bill Mathieson at NHS Grampian Biorepository (Aberdeen, Scotland) for help with histological experiments and Kevin McKenzie and his team at the Microscopy Core facility at the University of Aberdeen for assistance with microscopy and histology.
Corresponding editor: Matthew Charles Fisher
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.funbio.2017.12.011.
Contributor Information
Andreas Wuensch, Email: a.wuensch.12@aberdeen.ac.uk.
Franziska Trusch, Email: franziska.trusch@abdn.ac.uk.
Nurul A. Iberahim, Email: r01nabi@abdn.ac.uk.
Pieter van West, Email: p.vanwest@abdn.ac.uk.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Arrese E.L., Soulages J.L. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf S.L., Roger A.J., Wenk-Siefert I., Doolittle W.F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- Barnoy S. The Galleria Mellonella larvae as an in vivo model for evaluation of Shigella virulence. Gut Microb. 2017;13:1–16. doi: 10.1080/19490976.2017.1293225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte R. Role of pathogen-derived cell wall carbohydrates and prostaglandin E2 in immune response and suppression of fish immunity by the oomycete Saprolegnia parasitica. Infect. Immun. 2014;82:4518–4529. doi: 10.1128/IAI.02196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microb. Infect. 2006;8:2105–2112. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bhaskar P.B. Agrobacterium-mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002;34:153–157. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Bruno D.W., Van West P., Beakes G.W. vol. 3. CABI International; Wallingford, UK: 2011. Saprolegnia and other oomycetes; pp. 669–720. (Fish Diseases and Disorders). [Google Scholar]
- Butt T.M., Hajek A.E., Humber R.A. Gypsy moth immune defenses in response to hyphal bodies and natural protoplasts of entomophthoralean fungi. J. Invertebr. Pathol. 1996;68:278–285. doi: 10.1006/jipa.1996.0097. [DOI] [PubMed] [Google Scholar]
- Cerenius L., Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Chamilos G. Role of mini-host models in the study of medically important fungi. Lancet Infect. Dis. 2007;7:42–55. doi: 10.1016/S1473-3099(06)70686-7. [DOI] [PubMed] [Google Scholar]
- Cook S.M., McArthur J.D. Developing Galleria mellonella as a model host for human pathogens. Virulence. 2013;4:350–353. doi: 10.4161/viru.25240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn I. Immune gene expression in trout cell lines infected with the fish pathogenic oomycete Saprolegnia parasitica. Dev. Comp. Immunol. 2012;38:44–54. doi: 10.1016/j.dci.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Desbois A.P., Coote P.J. Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011;66:1785–1790. doi: 10.1093/jac/dkr198. [DOI] [PubMed] [Google Scholar]
- Dubovskiy I.M. More than a colour change: insect melanism, disease resistance and fecundity. Proc. Biol. Sci. 2013;280:20130584. doi: 10.1098/rspb.2013.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa A.E. Detection of Saprolegnia parasitica in eggs of angelfish Pterophyllum scalare (Cuvier–Valenciennes) with a history of decreased hatchability. Int. J. Vet. Sci. Med. 2013;1:7–14. [Google Scholar]
- Eleftherianos I., Revenis C. Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 2011;3:28–33. doi: 10.1159/000321931. [DOI] [PubMed] [Google Scholar]
- Gillespie J.P. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- González-Santoyo I., Córdoba-Aguilar A. Phenoloxidase: a key component of the insect immune system. Entomol. Exp. Appl. 2012;142:1–16. [Google Scholar]
- Grizanova E.V. Contributions of cellular and humoral immunity of Galleria mellonella larvae in defence against oral infection by Bacillus thuringiensis. J. Invertebr. Pathol. 2014;119:40–46. doi: 10.1016/j.jip.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Harding C.R. Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. 2012;80:2780–2790. doi: 10.1128/IAI.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016;58:102–118. doi: 10.1016/j.dci.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 2000;182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R.H.J. Distinctive repertoire of potential virulence genes in the genome of the oomycete fish pathogen Saprolegnia parasitica. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales S.C. Response of the rainbow trout monocyte/macrophage cell line, RTS11 to the water molds Achlya and Saprolegnia. Mol. Immunol. 2007;44:2303–2314. doi: 10.1016/j.molimm.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Kamoun S. Resistance of nicotiana benthamiana to phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell. 1998;10:1413–1426. doi: 10.1105/tpc.10.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T.D., Huitema E., Kamoun S. In planta expression of oomycete and fungal genes. Meth. Mol. Biol. 2007;354:35–43. doi: 10.1385/1-59259-966-4:35. [DOI] [PubMed] [Google Scholar]
- Kanost M.R., Gorman M.J. Phenoloxidases in insect immunity. Insect immunol. 2008;1:69–96. [Google Scholar]
- Kavanagh K., Reeves E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004;28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Khoo L. Fungal diseases in fish. Seminars Avian Exot. Pet Med. 2000;9:102–111. [Google Scholar]
- Krugner-Higby L. Ulcerative disease outbreak in crayfish Orconectes propinquus linked to Saprolegnia australis in Big Muskellunge Lake, Wisconsin. Dis. Aquat. Org. 2010;91:57–66. doi: 10.3354/dao02237. [DOI] [PubMed] [Google Scholar]
- Kryukov V.Y. Fungal infection dynamics in response to temperature in the lepidopteran insect Galleria mellonella. Insect Sci. 2016 doi: 10.1111/1744-7917.12426. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lavine M.D., Strand M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002;32(10):1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Li D.D. Using Galleria mellonella-Candida Albicans infection model to evaluate antifungal Agents. Biol. Pharm. Bull. 2013;36:1482–1487. doi: 10.1248/bpb.b13-00270. [DOI] [PubMed] [Google Scholar]
- Magnadóttir B. Innate immunity of fish (overview) Fish Shellfish Immunol. 2006;20:137–151. doi: 10.1016/j.fsi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Matha V., Áček Z. Changes in haemocyte counts in Galleria mellonella (L.) (Lepidoptera: Galleriidae) larvae infected with Steinernema sp. (Nematoda: Steinernematidae) Nematologica. 1984;30:86–89. [Google Scholar]
- Minor K.L. A putative serine protease, SpSsp1, from Saprolegniaparasitica is recognised by sera of rainbow trout, Oncorhynchus mykiss. Fungal Biol. 2014;118:630–639. doi: 10.1016/j.funbio.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E.D. Functional investigation of iron-responsive microsomal proteins, including MirC, in Aspergillus fumigatus. Front. Microbiol. 2017;8:418. doi: 10.3389/fmicb.2017.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E., Casadevall A., Ausubel F.M. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonin C. Melanization and pathogenicity in the insect, Tenebrio molitor, and the crustacean, Pacifastacus leniusculus, by Aeromonas hydrophila AH-3. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdoni F. A histological procedure to study fungal infection in the wax moth Galleria mellonella. Eur. J. Histochem. EJH. 2014;58:2428. doi: 10.4081/ejh.2014.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.J. Ulcerative dermal necrosis (UDN) in wild salmonids. Fish. Res. 1993;17:3–14. [Google Scholar]
- Rock F.L. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarowar M.N. Reprint of: Saprolegnia strains isolated from river insects and amphipods are broad spectrum pathogens. Fungal biol. 2014;118:579–590. doi: 10.1016/j.funbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Songe M.M. A thicker chorion gives ova of Atlantic salmon (Salmo salar L.) the upper hand against Saprolegnia infections. J. Fish. Dis. 2016;39:879–888. doi: 10.1111/jfd.12421. [DOI] [PubMed] [Google Scholar]
- Stueland S. Morphological and physiological characteristics of Saprolegnia spp. strains pathogenic to Atlantic salmon, Salmo salar L. J. Fish. Dis. 2005;28:445–453. doi: 10.1111/j.1365-2761.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- Torto-Alalibo T. Expressed sequence tags from the oomycete fish pathogen Saprolegnia parasitica reveal putative virulence factors. BMC Microbiol. 2005;5:46. doi: 10.1186/1471-2180-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg A.H. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 2013;27:33–42. [Google Scholar]
- van West P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist. 2006;20:99–104. [Google Scholar]
- Wittwer D. Presence of IL-1-and TNF-like molecules in Galleria mellonella (Lepidoptera) haemocytes and in an insect cell line Fromestigmene acraea (Lepidoptera) Cytokine. 1999;11:637–642. doi: 10.1006/cyto.1998.0481. [DOI] [PubMed] [Google Scholar]
- Zelaya-Molina L.X. Easy and efficient protocol for oomycete DNA extraction suitable for population genetic analysis. Biotechnol. Lett. 2011;33:715–720. doi: 10.1007/s10529-010-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Kanost M.R. In search of a function for hemolin, a hemolymph protein from the immunoglobulin superfamily. J. Insect Physiol. 1996;42:73–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.