Abstract
Ras-related protein (Rap)2a and Rap2b are members of the GTP-binding protein family, and serve an important function in tumor progression. However, the associations between Rap2c and cancer cell functions have not yet been reported. Osteosarcoma is a type of bone cancer; its high degree of invasion is considered to be a major treatment challenge. The present study first investigated the biological role of Rap2c in human osteosarcoma cells and investigated the underlying mechanism of Rap2c on osteosarcoma cell migration and invasion. The results of the present study demonstrated that Rap2c overexpression promoted the migratory and invasive ability of cancer cells, and increased the activity of matrix metalloproteinase-2 (MMP2). Correspondingly, the knockdown of Rap2c inhibited tumor cell migration and invasion, whereas alterations to Rap2c had no effect on osteosarcoma cell proliferation or rate of apoptosis. Furthermore, Rap2c overexpression may decrease the protein level of tissue inhibitor of metalloproteinases 2 and increase the phosphorylation level of protein kinase B (Akt). Collectively, these results indicated that Rap2c has a key function in tumor migration and invasion, and the Akt signaling pathway may be involved in Rap2c-induced MMP2 expression.
Keywords: Ras-related protein Rap2c, osteosarcoma cell, invasion, migration, matrix metalloproteinase-2
Introduction
Osteosarcoma is one of the most common forms of childhood cancer, and is characterized by its poor overall prognosis and high mortality rate (1). Osteosarcoma is a highly aggressive neoplasm typically composed of spindle cells and it metastasizes predominantly to the lungs (2). Thus, the development of novel curative strategies to prevent lung metastasis is highly desirable. Accordingly, one of the key aims in current osteosarcoma research is to further understand the underlying molecular mechanisms of invasion and to provide an experimental basis for the development of therapeutics for osteosarcoma.
Ras-related proteins regulate various cellular processes, including cell adhesion, differentiation, cell cycle control, cytoskeletal organization and metabolic turnover (3,4). Ras-related proteins transform between active GDP-bound and inactive GTP-bound forms (4). Ras-related proteins comprise a large family of small molecular weight guanine nucleotide binding proteins that includes five different members: Ras-related protein (Rap)-1a, Rap1b and Rap2a, Rap2b, Rap2c (5,6). Previous studies have demonstrated that Rap is able to enhance metastasis in breast and prostate cancer cells (7,8), and it has been previously revealed that the Rap2 family members' full-length sequence open reading frame contains 561 bp, encoding 186 amino acids (9). Subsequent studies have demonstrated that Rap2 is a regulator of LFA-1-mediated migration and is highly expressed in human thyroid cancer cells (8,10). Thus, decreasing Rap2 activity may indicate a novel therapeutic approach. U2OS is one of the most commonly used types of osteosarcoma cell and is representative of human osteosarcoma cells (11). Accordingly, U2OS cell lines in which Rap2c expression was silenced or overexpressed were constructed to investigate the effects of Rap2c on the invasion of osteosarcoma cells. In the present study, the function of Rap2c in regulating the proliferation and apoptosis of U2OS cells was analyzed. Furthermore, the effect of Rap2c on the migration of U2OS and the activity of matrix metalloproteinase-2 (MMP2) were detected. Finally, in order to explore the underlying mechanisms by which Rap2c is involved in osteosarcoma, the expression levels of B cell lympphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), tissue inhibitor of metalloproteinases 2 (Timp2), protein kinase B (Akt) and phosphorylated (p)-Akt473 were examined in U2OS cells. The data demonstrated that increasing Rap2c significantly promoted the invasion and migration of U2OS cell in vitro, and increased the expression level of p-Akt473. The data indicated that Rap2c may serve as a novel therapeutic target for osteosarcoma.
Materials and methods
Cell line and culture conditions
U2OS (human osteosarcoma cell line) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in an incubator containing 5% CO2.
Plasmid construction
Total RNA was extracted from U2OS cells using the Qiagen RNeasy kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA), and the first-strand cDNA was synthesized using the PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). Then, Rap2c cDNA was amplified using Taq polymerase (Tiangen Biotech Co., Ltd., Beijing, China). The primers sequences were as follows: forward, 5′-AAGCTTATGAGGGAATACAAG-3′ and reverse, 5′-GAATTCTTACTGGACGACAC-3′. Consensus sequences for the restriction enzymes EcoRI and HindIII (Takara Biotechnology Co., Ltd., Dalian, China) are underlined. cDNA was then subcloned into pcDNA3.1 at the EcoRI and HindIII sites. The identity of the clones were verified using sequencing.
Overexpression of Rap2c
Cells were transfected with pcDNA3.1-control or pcDNA3.1-Rap2c expression plasmids using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and grown to 90% confluence according to the manufacturer's protocol. The quantity of plasmids used for transfection was 4 µg for each 35 mm culture dish. The medium containing the transfection reagents was removed 4 h after transfection and replaced with fresh medium. Cells were then collected 24 h after transfection and used in the following experiments.
Knockdown of Rap2c
Rap2c-targeted small interfering RNA (siRNA) was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and transfected using siLentFect lipid reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol. Briefly, non-specific control siRNA, Rap2c#1, Rap2c#2 or Rap2c#3 siRNAa were transfected by siLentFect when cells reached ~50% confluence. A total of 6 h after transfection, the medium containing transfection reagents was removed and fresh medium containing FBS was added. Cells were harvested for subsequent experiments 48 h after transfection. The quantity of the siRNAs used for transfection was 200 pmol for each 35 mm culture dish. The three types of siRNA duplexes targeting human Rap2c that were used are listed in Table I. A non-specific siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′) was transfected as a control.
Table I.
siRNA duplexes used for the knockdown of Rap2c.
| siRNA | siRNA duplexes, 5′-3′ |
|---|---|
| Rap2c#1 | CAGGAUAUCAAGCCAAUGATT |
| UCAUUGGCUUGAUAUCCUGTT | |
| Rap2c#2 | GAAGCAAGAUCAGUGUUGUTT |
| ACAACACUGAUCUUGCUUCTT | |
| Rap2c#3 | GAGAUCGUCAGGCAAAUGATT |
| UCAUUUGCCUGACGAUCUCTT |
siRNA, small interfering RNA; Rap2c, Ras-related protein 2c.
Western blotting
Following transfection with expression plasmids for 24 h or siRNA for 48 h, total proteins were extracted from U2OS cells using lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and the concentration of protein was determined using enhanced BCA protein assay kit (Beyotime Institute of Biotechnology). Total proteins were boiled with loading buffer (Beyotime Institute of Biotechnology) and 100 µg protein per lane were loaded and separated by 12.5% SDS-PAGE for electrophoresis and then transferred onto nitrocellulose membrane. Following blocking with 5% non-fat milk (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature in TBS containing 0.1% Tween-20 (TBST) for 2 h, the membranes were incubated at 4°C overnight with primary antibodies against the following proteins: Rap2c (anti-rabbit; 1:1,000; cat. no. ab138300; Abcam, Cambridge, UK), B cell lymphoma-2 (Bcl-2; anti-rabbit; 1:500; cat. no. sc-492; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Bcl-2-associated X protein (Bax; anti-rabbit; 1:500; cat. no. sc-493; Santa Cruz Biotechnology, Inc.), tissue inhibitor of metalloproteinases 2 (Timp2; anti-rabbit; 1:1,000; cat. no. 5738; Cell Signaling Technology, Inc., Danvers, MA, USA), Akt (anti-rabbit; 1:2,000; cat. no. 9272s; Cell Signaling Technology, Inc.), p-Akt473 (anti-rabbit; 1:1,000; cat. no. 4060s; Cell Signaling Technology, Inc.) and β-actin (anti-mouse; 1:2,000; sc-58673; Santa Cruz Biotechnology, Inc.). Following incubation with horseradish peroxidase-conjugated anti-mouse IgG (1:10,000; cat. no. P/N926-80010; LI-COR Biosciences, Lincoln, NE, USA) or anti-rabbit IgG (1:10,000; cat. no. P/N926-80011; LI-COR Biosciences) at 37°C for 2 h at room temperature, membranes were then washed and scanned on the Odyssey Two-Color Infrared Imaging System (LI-COR Biosciences). The densitometric analysis for the quantification of the bands was performed using ImageJ software (version 1.46; National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Following transfection, U2OS cells were seeded (5×103/well) into 96-well culture plates. Cell proliferation was detected using Cell Counting kit-8 (CCK-8; Vicmed Biotech Co., Ltd., Xuzhou, China; http://vicmed2013.bioon.com.cn/) every 24 h for up to 96 h. In total, 100 µl serum-free culture medium and 10 µl CCK-8 solutions were added into each well and cells were incubated at 37°C for 2 h. The absorbance was measured at 490 nm using an ELX-800 spectrometer reader (BioTek Instruments, Inc., Winooski, VT, USA). Each experiment was performed in triplicate.
Detection of apoptosis using flow cytometry
U2OS cells were plated in six-well plates and transfected with expression plasmids for 24 h, or siRNA for 48 h. Cells were collected and labeled using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocol, and the apoptosis fraction was analyzed using a FACScan cytometer (BD Biosciences). Data was analyzed using FlowJo software (FlowJo LLC, Ashland, OR, USA).
Wound-healing assay
For the wound-healing assay, U2OS cells were seeded into 6-well plates in culture medium and were grown to 90% confluence. Following transfection with Rap2c plasmid or Rap2c siRNAs, a wound was made by pulling a 200 µl pipette tip along the center of the plate; the confluent monolayer was washed three times with PBS to remove cell debris. The wound monolayer was captured at 0 and 24 h using a light microscope (Nikon Corporation, Tokyo, Japan) at ×100 magnification. The rate of wound healing was determined by comparing the wound width at 24 h to the wound width at 0 h.
Migration and invasion assay
Cell migration and invasion assays were performed using Transwell units with 8.0 µm-pore polycarbonate filters (Corning Incorporated, Corning, NY, USA). For the migration assay, the underside of a Transwell filter was coated with 10 µg/ml fibronectin from human plasma (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C overnight. In brief, a total of 1×105 U2OS cells were seeded in the upper chamber, and 600 µl DMEM supplemented with 10% FBS was placed in the lower compartment. After being cultured at 37°C in a CO2 incubator for 24 h, the cells in the upper chamber were fixed in methanol for 30 min and stained with leucocrystal violet (Beyotime Institute of Biotechnology) for 15 min at room temperature. Cells in the upper chamber were removed with a cotton swab and the number of cells that migrated across the membrane was counted in five randomly selected microscopic fields in at a minimum of three independent experiments. For the invasion assay, a similar protocol was performed using Matrigel-coated (BD Biosciences) instead of Transwell chambers. The cells that suspended into the well bottom were quantified after 48 h incubation at 37°C. Cells in the upper chamber were subjected to microscopic inspection and then photographed randomly at ×100 magnification.
Gelatin zymography
It has been reported that matrix metalloproteinases (MMPs) serve a pivotal role in cellular invasion and metastasis (12). Therefore, the activity of matrix metalloproteinase MMP2 secreted by U2OS cells was evaluated using gelatin zymography. Briefly, 40 µl of serum-free DMEM was denatured in SDS buffer under non-reducing conditions (63 mM TrisHCl, 10% glycerol, 2% SDS and 0.0025% bromophenol blue; Beyotime Institute of Biotechnology) without heating and electrophoresed for 3 h in 10% SDS-PAGE with 0.1% gelatin (Sigma-Aldrich; Merck KGaA). The SDS-PAGE gels were renatured by incubating the gel in renaturing buffer (2.5% Triton X-100) for 30 min twice at room temperature and equilibration in developing buffer (50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij) for 48 h at 37°C. The gels were rinsed in staining buffer (0.1% Coomassie Brilliant Blue R-250, 40% ethanol, and 10% acetic acid; Sigma-Aldrich; Merck KGaA) and then in de-staining buffer (10% ethanol and 7.5% acetic acid). Regions of protease activity appeared as bands against a dark blue background.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA). All quantitative data are presented as mean ± standard deviation (SD). The differences between groups were analyzed using unpaired Student's t-test when only 2 groups were compared, or one-way ANOVA analysis of variance when >2 groups were compared. The Student-Newman-Keuls test was used as a post hoc test following ANOVA. All experiments were performed at least three times, unless otherwise indicated. P<0.05 was considered to indicate a statistically significant difference.
Results
Rap2c has no effect on cell proliferation
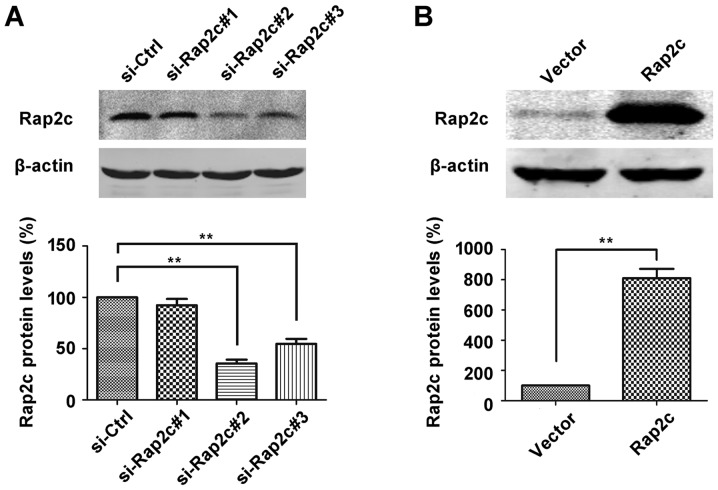
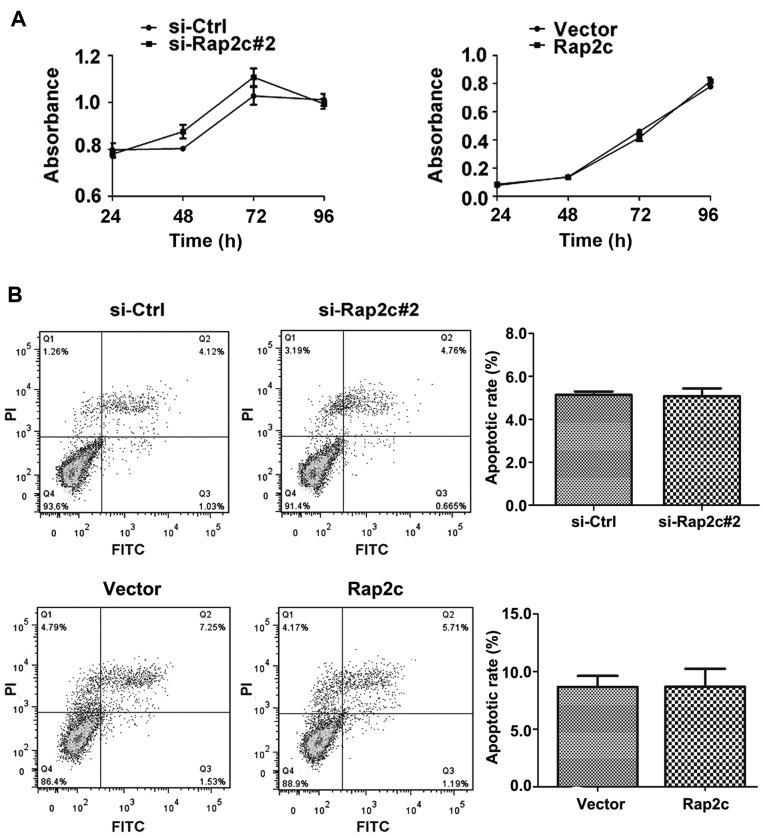
To investigate the oncogenic role of Rap2c, siRNA targeting Rap2c or pcDNA3.1-Rap2c expression plasmid was transiently transfected into U2OS cells and Rap2c protein expression was evaluated using western blot analysis. As illustrated in Fig. 1A, the expression level of Rap2c protein was significantly downregulated in si-Rap2c#2 and si-Rap2c#3 groups compared with the si-control group. However, si-Rap2c#2 exhibited the most marked effect in decreasing the expression levels of Rap2c and was therefore used in subsequent experiments. Furthermore, the expression of Rap2c was significantly upregulated following transfection of U2OS cells with the Rap2c expression plasmid (Fig. 1B). A CCK-8 assay was performed following the transfection of U2OS cells with Rap2c siRNA or a Rap2c expression plasmid. The results demonstrated that there was no significant difference in the rate of proliferation between the Rap2c-knockdown cells and those treated with the negative control siRNA (Fig. 2A). Similarly, the overexpression of Rap2c had no effect on cell proliferation, when compared with the rate of proliferation in cells transfected with the empty control vector (Fig. 2A).
Figure 1.
Knockdown and overexpression of Rap2c in U2OS cells. (A) U2OS cell lines were transfected with three sets of Rap2c-targeted siRNA, and Rap2c protein expression was measured using western blotting. **P<0.01 vs. si-Ctrl. (B) U2OS cell lines were transfected with pcDNA3.1-control or pcDNA3.1-Rap2c expression plasmids, then the Rap2c protein expression level was measured using western blot analysis. **P<0.01 vs. vector. Rap2c, Ras-related protein Rap2c; siRNA, small interfering RNA; Ctrl, control; vector, empty vector.
Figure 2.
Effects of Rap2c overexpression and knockdown on U2OS cell proliferation. (A) CCK-8 assay was performed with U2OS cells following transfection with the indicated siRNAs or a pcDNA3.1-Rap2c expression plasmid at 24, 48, 72 and 96 h. (B) Apoptotic cells were detected using Annexin V-FITC/PI staining using flow cytometry. siRNA, small interfering RNA; siCtrl, negative control siRNA; Rap2c, Ras-related protein Rap2c; Vector, empty vector; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Rap2c has no effect on the apoptosis of cancer cells
To investigate whether Rap2c influences carcinoma cell survival, cell apoptosis was analyzed using flow cytometry with Annexin V-FITC/PI. Following the transfection of U2OS cells with Rap2c siRNA or a Rap2c expression plasmid, no significant difference in the proportion of cells undergoing apoptosis was observed between either of the Rap2c-treatment groups and the control groups (Fig. 2B).
Rap2c promotes invasion and migration of osteosarcoma cell
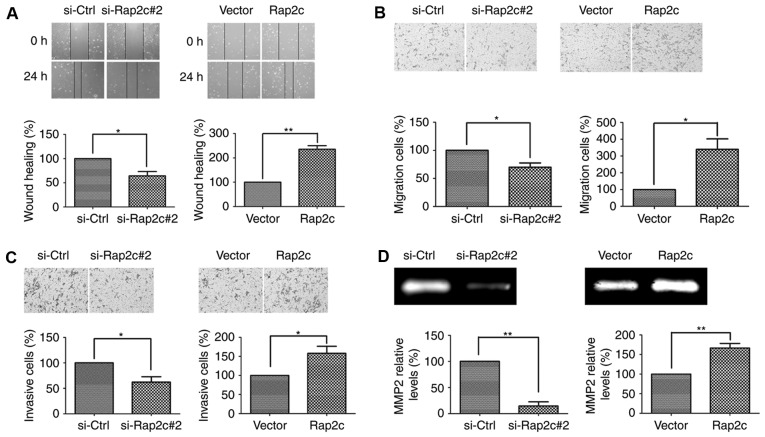
The effects of Rap2c on the migration of U2OS cells was measured using wound-healing assays. The results of these assays demonstrated that decreased wound closure was observed in Rap2c-knockdown U2OS cells. By contrast, overexpression of Rap2c increased the degree of wound closure in U2OS cells (Fig. 3A). Moreover, Transwell cell migration assays demonstrated that knockdown of Rap2c markedly decreased the migratory ability of U2OS cells. Conversely, overexpression of Rap2c promoted the migratory ability of U2OS cells (Fig. 3B). Furthermore, the invasive ability of Rap2c cells was measured using transwell cell invasion assays, the results of which demonstrating that Rap2c promoted U2OS cells invasion (Fig. 3C). These results indicated that Rap2c may have a significant effect on cell migration and invasion in vitro.
Figure 3.
Effect of Rap2c overexpression and knockdown on the invasion and migration of U2OS cells. (A) Wound-healing assays were performed in U2OS cells transfected with a Rap2c expression plasmid or siRNA (magnification, ×100). *P<0.05 vs. si-Ctrl; **P<0.01 vs. Vector. (B) Cell migration was performed following transfection with siRNA or a pcDNA3.1-Rap2c expression plasmid (magnification, ×100). *P<0.05 vs. si-Ctrl or Vector. (C) Cell invasion was measured following transfection with Rap2c expression plasmids or siRNA (magnification, ×100). *P<0.05 vs. si-Ctrl or Vector. (D) The relative enzyme activity of cleaved-MMP2 was measured by a gelatin zymography assay following transfection with siRNA or a Rap2c expression plasmid. **P<0.01 vs. si-Ctrl or Vector. siRNA, small interfering RNA; MMP2, matrix metalloproteinase-2; Ctrl, control; Vector, empty vector.
Rap2c upregulates the MMP2 activity and downregulates the protein level of Timp2
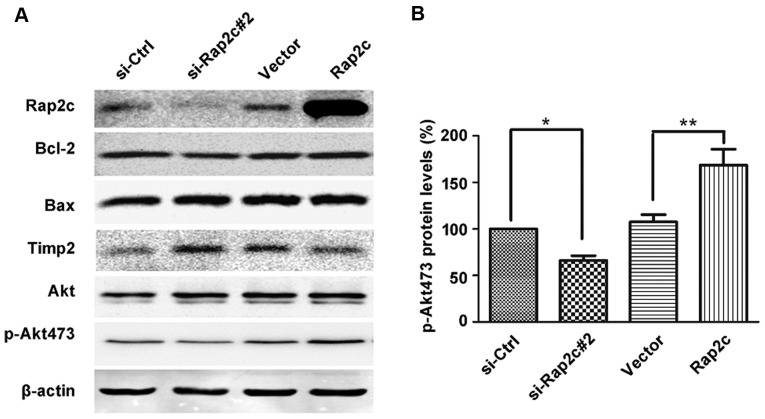
The present study revealed that MMP2 secretion was increased in Rap2c-overexpressing U2OS cells and decreased in Rap2c-knockdown U2OS cells (Fig. 3D). Thus, Rap2c may promote migration and invasion of osteosarcoma cells via the upregulation of MMPs activities. To further validate this conclusion, the protein level of Timp2 was analyzed. As expected, the results of the present study demonstrated that the level of Timp2 protein was markedly increased in U2OS cells following the knockdown of Rap2c (Fig. 4A). These results demonstrated that Rap2c upregulated MMP2 activity and downregulated levels of Timp2 protein, which may subsequently have led to an increase in migration and invasion in U2OS cells. In addition, the expression levels of Bcl-2 and Bax were not altered in Rap2c-transfected cells (Fig. 4A); this result was consistent with the previous proliferation and apoptosis data.
Figure 4.
Akt phosphorylation is involved in Rap2c-mediated cancer cell migration and invasion. (A) Western blot analysis of the indicated protein levels in U2OS cells overexpressing Rap2c or with Rap2c knockdown. (B) Densitometry analysis of p-Akt473. *P<0.05 vs. si-Ctrl, **P<0.01 vs. Vector. Rap2c, Ras-related protein Rap2c; p-Akt, phosphorylated protein kinase B; Ctrl, control; Timp2, tissue inhibitor of metalloproteinase 2; Bcl-2, B-cell lymphoma-2; Bax, Bcl-associated X.
Akt phosphorylation is involved in Rap2c-mediated migration and invasion of osteosarcoma cells
Tumor growth and metastasis are caused by highly integrated multistep cellular events regulated by various signaling molecules, including PI3K/Akt (13). Emerging evidence reveals that MMP2 secretion and the invasion of tumor cells are associated with PI3K/Akt signaling pathway (13–15). Tumor angiogenesis and invasion are triggered via the abundant secretion of MMPs by tumor cells; Akt is a key regulator of the intracellular processes promoting cell growth, migration and survival. It has been identified that the activation of Akt pathway stimulates MMP production by cancer cells, and that pharmacological inhibition of Akt can suppress the expression of MMP2 (16,17). To understand whether Rap2c regulates U2OS cell migration and invasion via Akt, the expression levels of Akt proteins were detected in the present study. Results revealed that Rap2c overexpression increased the phosphorylation of Akt, which has a single residue at serine 473 (p-Akt473). Correspondingly, the downregulation of Rap2c decreased p-Akt473 expression (Fig. 4A and B).
Discussion
Rap2c is a member of the Ras superfamily of small GTPases (7). Ras gene mutations are involved in a variety of human tumors and are poor prognostic indicators for patient survival (18,19). Ras gene proteins can present in two states: The inactive state, in which the GTP has been hydrolyzed to GDP, and an active state, in which GTP is bound to Ras. In physiological conditions, the active isoform of Ras gene induces cell proliferation through the Ras-dependent kinase cascade (20). Aberrant Rap activation leads to cancer cell proliferation and tumorigenesis, thereby contributing to several types of malignancy, including prostate and thyroid cancer (21,22). The Rap small GTPases are characterized important regulators of cell adhesion and serve a key function in cell adhesion (23,24). It has been previously reported that Rap2a and Rap2b are involved in tumor progression and act as an oncogene in human cancer (25–27). However, until recently, little was known about the function of Rap2c protein in mammalian cells.
In the present study the following experiments were used: Cell proliferation assay, wound-healing assay, migration assay, invasion assay and western blotting, which were used to investigate the function of Rap2c on U2OS cells. Results from the present study demonstrated that upregulation of Rap2c promotes the invasive and migratory capacities of cancer cells. However, CCK-8 and Annexin V-FITC/PI flow cytometry assays revealed that Rap2c has no effect on the proliferation or rate of apoptosis in U2OS cells. Consistent with this result, Rap2c did not alter the expression levels of Bax or Bcl-2. These results indicated that Rap2c might promote cancer cell motility. Furthermore, results demonstrated that Rap2c regulated the expression of Timp2, MMP2 and p-Akt.
MMPs, secreted by tumor cells, can degrade the extracellular matrix and serve an important function in tumor progression (12,28). MMP-2 and MMP-9, commonly associated with tumor metastasis and invasion, have been regarded as biomarkers for various types of cancer (29). The present results demonstrated that the siRNA-mediated downregulation of Rap2c significantly decreased the expression of MMP-2, whereas Rap2c overexpression increased it. Increased MMP2 secretion may be involved in mediating the effect of Rap2c-enhanced migration and invasion of cancer cells. Timps have the ability to inhibit the catalytic activity of MMPs and Timp2 is a specific inhibitor of MMP2 (30). MMP2 and Timp2 serve important functions in mediating tumor cell metastasis (31,32). The present study evaluated the association between Rap2c and Timp2 expression. The data demonstrated that knockdown of Rap2c significantly increased Timp2 protein expression. By contrast, the expression of Timp2 was markedly downregulated in U2OS cells following Rap2c overexpression, which was consistent with the upregulation of MMP2 induced by Rap2c.
To fully elucidate the mechanisms involved in the upregulation of MMP2 by Rap2c, the signaling pathways of MMPs associated with cell invasion were investigated. Previous studies revealed that the Akt signaling pathway may be involved in metastatic potential and mediated the activity of MMPs (33,34). Thus, the present study investigated whether Rap2c expression could modulate the protein level of Akt in order to contribute to evidence regarding the molecular pathways leading to Rap2c-mediated upregulation of MMP2. Results demonstrated that overexpression of Rap2c enhanced the phosphorylation of Akt, whereas and the knockdown of Rap2c expression contributed to a reduction of p-Akt protein levels. These results indicate that the Akt signaling pathway may be involved in Rap2c-induced MMP2 expression. Therefore, it is likely that this signaling molecule is not the only one responsible for Rap2c-induced MMP2 expression and the invasion of tumor cells. Further studies and the use of other osteosarcoma cells are required to uncover other critical signaling molecules involved in these events.
Collectively, this study provided evidence that Rap2c may influence cancer cell invasion and migration. Overexpression of Rap2c promoted the migration and invasion of U2OS cells, and decreased Rap2c expression may contribute to the inhibition of cancer cell invasion and migration. In addition, MMP2 activity and the Akt signaling pathway may be involved in Rap2c-mediated tumor metastasis. Future work will be required to elucidate whether this pathway occurs in vivo, and whether there are other signaling pathways involved. To conclude, the results of the present study provide information on a potential therapeutic target that mediates the invasiveness of osteosarcoma.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81572349), Jiangsu Provincial Medical Talent, the Science and Technology Department of Jiangsu Province (grant nos. BK20141149 and BK20150217) and Education Departmental Nature Science Foundation of Jiangsu Province (grant no. 15KJB320018).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence; Mayo Clin Proc; 2012; pp. 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang S, Ren Z, Han X, Yang J, Shan L, Li L, Wang B, Zhang Q, Mu T, Chen K, et al. PLA2G16 expression in human osteosarcoma is associated with pulmonary metastasis and poor prognosis. PLoS One. 2015;10:e0127236. doi: 10.1371/journal.pone.0127236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal V, Linder ME. Biochemical characterization of RGS14: RGS14 activity towards G-protein alpha subunits is independent of its binding to Rap2A. Biochem J. 2006;394:309–315. doi: 10.1042/BJ20051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albright CF, Giddings BW, Liu J, Vito M, Weinberg RA. Characterization of a guanine nucleotide dissociation stimulator for a ras-related GTPase. EMBO J. 1993;12:339–347. doi: 10.1002/j.1460-2075.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokoch GM. Biology of the Rap proteins, members of the ras superfamily of GTP-binding proteins. Biochemical J. 1993;289:17–24. doi: 10.1042/bj2890017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasheva E, Janoueix-Lerosey I, Tavitian A, de Gunzburg J. Characterization of the Ras-related RAP2A protein expressed in the baculovirus-insect cell system: Processing of the protein in insect cells and comparison with the bacterially produced unprocessed form. Biochem Biophys Res Commun. 1994;198:973–982. doi: 10.1006/bbrc.1994.1139. [DOI] [PubMed] [Google Scholar]
- 7.Itoh M, Nelson CM, Myers CA, Bissell MJ. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007;67:4759–4766. doi: 10.1158/0008-5472.CAN-06-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong X, Korch C, Meinkoth JL. Histone deacetylase inhibitors upregulate Rap1GAP and inhibit Rap activity in thyroid tumor cells. Endocr Relat Cancer. 2011;18:301–310. doi: 10.1530/ERC-10-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Wang X, Deng C, Lv X, Fan Y, Men J, Liang C, Yu X. Molecular cloning and characterization of a novel ras-related protein (rap2) from Clonorchis sinensis. Parasitol Res. 2011;108:1021–1026. doi: 10.1007/s00436-010-2147-9. [DOI] [PubMed] [Google Scholar]
- 10.Stanley P, Tooze S, Hogg N. A role for Rap2 in recycling the extended conformation of LFA-1 during T cell migration. Biol Open. 2012;1:1161–1168. doi: 10.1242/bio.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Cui X, Yuan H, Zhang B, Meng C, Zhao D. Effects of distinct drugs on gene transcription in an osteosarcoma cell line. Oncol Lett. 2017;14:4694–4700. doi: 10.3892/ol.2017.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS, Lee SH, Park IC, Rhee CH, Hong SI. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66:8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- 14.Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue KH, Yang SF, Lu KH. Selaginella tamariscina (Beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food Chem Toxicol. 2013;59:801–807. doi: 10.1016/j.fct.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Chen T, Zhang J, Mao Q, Li S, Xiong W, Qiu Y, Xie Q, Ge J. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2011;103:181–190. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- 16.de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor-1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szpon L, Stal A, Zawadzki M, Lis-Nawara A, Kielan W, Grzebieniak Z. K-ras gene mutation as an early prognostic marker of colon cancer. Pol Przegl Chir. 2016;88:15–19. doi: 10.1515/pjs-2016-0021. [DOI] [PubMed] [Google Scholar]
- 19.Tao LY, Zhang LF, Xiu DR, Yuan CH, Ma ZL, Jiang B. Prognostic significance of K-ras mutations in pancreatic cancer: A meta-analysis. World J Surg Oncol. 2016;14:146. doi: 10.1186/s12957-016-0888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S, et al. The role of RAS oncogene in survival of patients with lung cancer: A systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res. 2009;69:4962–4968. doi: 10.1158/0008-5472.CAN-08-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschuler DL, Ribeiro-Neto F. Mitogenic and oncogenic properties of the small G protein Rap1b; Proc Natl Acad Sci USA; 1998; pp. 7475–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod SJ, Shum AJ, Lee RL, Takei F, Gold MR. The Rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization, and Pyk2 tyrosine phosphorylation in B lymphocytes. J Biol Chem. 2004;279:12009–12019. doi: 10.1074/jbc.M313098200. [DOI] [PubMed] [Google Scholar]
- 24.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 25.Bigler D, Gioeli D, Conaway MR, Weber MJ, Theodorescu D. Rap2 regulates androgen sensitivity in human prostate cancer cells. Prostate. 2007;67:1590–1599. doi: 10.1002/pros.20644. [DOI] [PubMed] [Google Scholar]
- 26.Prabakaran I, Grau JR, Lewis R, Fraker DL, Guvakova MA. Rap2A is upregulated in invasive cells dissected from follicular thyroid cancer. J Thyroid Res. 2011;2011:979840. doi: 10.4061/2011/979840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie X, Liu H, Wang M, Ding F, Xiao H, Hu F, Hu R, Mei J. miR-342-3p targets RAP2B to suppress proliferation and invasion of non-small cell lung cancer cells. Tumour Biol. 2015;36:5031–5038. doi: 10.1007/s13277-015-3154-3. [DOI] [PubMed] [Google Scholar]
- 28.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Wang X, Zhong W, Ren X, Sha X, Fang X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: Preparation and in vitro and in vivo evaluation. Int J Nanomedicine. 2016;11:1643–1661. doi: 10.2147/IJN.S101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulukuri SM, Patibandla S, Patel J, Estes N, Rao JS. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene. 2007;26:5229–5237. doi: 10.1038/sj.onc.1210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannelli G, Erriquez R, Fransvea E, Daniele A, Trerotoli P, Schittulli F, Grano M, Quaranta M, Antonaci S. Proteolytic imbalance is reversed after therapeutic surgery in breast cancer patients. Int J Cancer. 2004;109:782–785. doi: 10.1002/ijc.20009. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Mo N, Li L, Cao Y, Wang W, Liang Y, Deng H, Xing R, Yang L, Ni C, et al. Surgery-induced hippocampal angiotensin II elevation causes Blood-brain barrier disruption via MMP/TIMP in aged rats. Front Cell Neurosci. 2016;10:105. doi: 10.3389/fncel.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao J, Du H, Li W, Liu F, Lu J, Yang X, Cui W. Anthocyanins protected hearts against ischemic injury by reducing MMP-2 activity via Akt/P38 pathways. Am J Transl Res. 2016;8:1100–1107. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Li X, Li C, He C, Ren B, Deng Q, Gao W, Wang B. Aurora-A modulates MMP-2 expression via AKT/NF-κB pathway in esophageal squamous cell carcinoma cells. Acta Biochim Biophys Sin (Shanghai) 2016;48:520–527. doi: 10.1093/abbs/gmw030. [DOI] [PMC free article] [PubMed] [Google Scholar]