Abstract
This study aimed to investigate the expression levels of microRNA-195 (miRNA-195) in different types of patients with cholangiocarcinoma (CCA) and its correlation with the prognosis. Serum samples were collected from different types of patients with CCA (I, II, III, IV) and normal cases, followed by detection of expression of miRNA-195 using quantitative polymerase chain reaction (qPCR). The serum samples of 204 patients with CCA, including 75 cases of type I, 68 cases of type II, 35 cases of type III and 26 cases of type IV and 200 healthy subjects were selected. The baseline clinicopathological data of patients with CCA were assessed and recorded, and patients were followed up constantly. The receiver operating characteristic (ROC) curve was established, and the area under the ROC curve (AUC) was calculated to evaluate the difference of miRNA-195 expression levels between patients with CCA and normal controls. Survival curves were set up for groups with high and low expression levels via the Kaplan-Meier method, and the log-rank test was used to evaluate the difference of survival curves between the two groups. The expression of miR-195 in patients with CCA was significantly lower than that in the normal control group, with a sensitivity of 0.78 and a specificity of 0.76, and it was positively correlated with the pathological grade of CCA. Additionally, the expression level of serum miRNA-195 was associated with lymph node metastasis (P=0.009) and tumor-node-metastasis (TNM) classification (P=0.010). The survival analysis revealed that the prognosis in patients with CCA in types III and IV was poorer than that in those with types I and III who had a low expression of miRNA-195 (P=0.0026). The results show that miR-195 is an important marker that reflects the malignant degree of CCA, and it is expected to be a reference marker to determine the prognosis of CCA.
Keywords: miRNA, cholangiocarcinoma, prognosis, qPCR, ROC
Introduction
Cholangiocarcinoma (CCA) is a highly aggressive tumor derived from bile duct epithelial cells. In recent decades, the incidence and mortality rate of CCA have been on the increase worldwide (1,2). The survival rate and prognosis of CCA patients are not ideal due to the early invasion and metastasis (3). Although radical surgery is the only therapy for CCA, the disease is difficult to be controlled and treated given that patients are diagnosed in the middle or late stage when the disease is identified (4). Thus, it is imperative to further understand the pathogenesis of CCA, in order to search for a new diagnostic and therapeutic method (5,6).
MicroRNA (miRNA) is a type of endogenous, small, non-coding single-stranded RNA molecule that regulates the expression of target genes after its transcription, and it is involved in the regulation of multiple physiological and pathological functions (7). Previous findings have shown that the abnormal expression of miRNA-195 is associated with breast cancer (8), lung cancer (9) and many other tumors, but the role of miRNA-195 in CCA has not been reported yet. Therefore, this study aimed to investigate the expression level and prognostic value of miRNA-195 in different pathological grades of CCA, and to provide a direction for further exploration of the potential targets of CCA gene therapy.
Materials and methods
Clinical data
In total, 204 CCA patients admitted to the Third People's Provincial Hospital of Henan Province (Zhengzhou, China) from March, 2009 to May, 2015 were selected. CCA patients with different pathological types were divided into two groups using magnetic resonance imaging (MRI), according to the Bismuth-Corlette typing method. In groups of type I and II, there were 59 males and 74 females, aged 56–68 years, with an average age of 62.2±65.7 years. In groups of type III and IV, there were 30 males and 41 females, aged 65–73 years, with an average age of 69.3±73.8 years. In addition, 200 healthy subjects examined at the Third People's Provincial Hospital of Henan Province were enrolled as the control group. There were no statistically significant differences in age and sex in each group (P<0.05).
The study was approved by the Ethics Committee of the Third People's Provincial Hospital of Henan Province, and informed consent was obtained from the selected subjects or family members. The study was in line with the Medical Ethics Standards.
Collection of samples
Peripheral venous blood (2.5 ml) was collected from the elbow of patients, followed by anticoagulation using ethylene diaminetetraacetic acid dipotassium (EDTA-K2) and preserved in a refrigerator at −80°C. EDTA-K2 anticoagulant blood (2 ml) was extracted by the transfer liquid gun and added into a centrifuge tube, followed by centrifugation at 1,260 × g at room temperature for 10 min. Then, the serum was taken and placed into the high-speed centrifuge for centrifugation at 8,000 × g for 10 min. Finally, the serum was collected and reserved in a refrigerator at −80°C.
Main reagents
The experimental material TRIzol reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA); Deoxyribonuclease I (DNase I) (RNase-free) reagent was purchased from Thermo Fisher Scientific; and ReverTra Ace qPCR RT kit and SYBR-Green Real-Time PCR Master Mix were purchased from Thermo Fisher Scientific. The internal primers were produced by Shanghai Invitrogen Biotechnology Co., Ltd., Shanghai, China (Table I).
Table I.
Reverse transcription of miRNA-195 and RT-PCR primers.
| Item | Primer sequence |
|---|---|
| miRNA-195 | |
| Reverse transcription primer | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGCCAATAT-3′ |
| Upstream primers | 5′-ACACTCCAGCTGGGTAGCAGCACAGAAAT-3′ |
| Downstream primers | 5′-TGGTGTCGTGGAGTCG-3′ |
| GAPDH | |
| Upstream primers | 5′-CAGGGCTGCTTTTAACTCTGGTAA-3′ |
| Downstream primers | 5′-GGGTGGAATCATATTGGAACATGT-3′ |
Reverse transcription of mRNA into complementary DNA (cDNA)
miRNA of total RNA was reversely transcribed into cDNA according to the protocol of the miRNA reverse transcription kit. The reaction conditions of reverse transcription of miRNA-195 were: 16°C for 30 min, 42°C for 30 min and 85°C for 5 min; the reaction conditions and system of internal reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were conducted according to the instructions of ReverTra Ace qPCR RT kit. Subsequently, three duplicate wells were made in all the samples.
Data analysis
The fluorescence signals of each reaction well were collected quantitatively, and the Cq values were recorded. The expression of miRNA-195 in CCA tissue relative to the normal tissue adjacent to the tumor was expressed as 2−∆∆Cq. ΔCq is the difference of Cq values of the target gene to the internal reference GAPDH in each sample, namely ∆Cq = Cq (miR-195) - Cq (GAPDH), ∆∆Cq = (Cq miR-195 - Cq GAPDH) tumor - (Cq miR-195 - Cq GAPDH) normal. ∆∆Cq <1 indicated that the expression level of miR-195 in the bile duct tissue was lower than that in the paired pericancerous tissues, while ∆∆Cq >1 indicated that the expression level of miR-195 in bile duct tissue was higher than that in paired pericancerous tissues.
Statistical analysis
Statistical Product and Service Solutions 21.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used to process data in this experiment. Enumeration data were analyzed using the Chi-square test. Measurement data with normal distribution were expressed as mean ± SD and analyzed by Student's t-test. Pearson's correlation analysis was used for the determination of the correlation of the expression of miR-195 with clinical features of CCA. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of miR-195 in the serum of CCA patients and healthy controls
In this experiment, the results of the detection of miR-195 expression levels in the serum of CCA patients and healthy controls, qPCR showed that the expression level of miR-195 in the serum of CCA patients (1.671±0.955) was lower than that of in the serum of healthy controls (3.574±1.751), and the difference was statistically significant (t=3.237, P=0.014) (data not shown).
Expression levels of miR-195 among the different groups of patients with CCA
In this experiment, CCA patients with different pathological degrees were divided into two groups (I, II; III, IV). The expression level was 2.874±0.612 in Group I and II and 1.985±0.434 in Group III and IV (data not shown). The results indicated that the expression level was distinctly reduced in the groups with high pathological degree (III, IV) compared with that in the groups with low pathological degree (I, II). In addition, the receiver operating characteristic (ROC) curve analysis revealed that the value of miR-195 in predicting the risk of CCA was high, the area under the ROC curve (AUC) was 0.78, and 95% confidence interval (CI) was 0.729–0.834 (Fig. 1).
Figure 1.
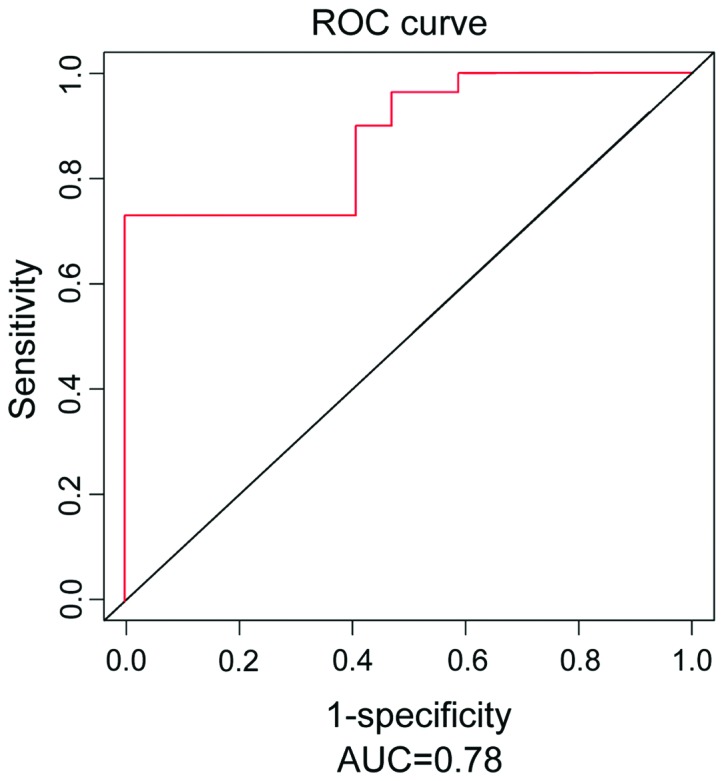
ROC curves of miR-195 in the serum of CCA and the normal control group. The value of miR-195 in predicting the risk of CCA is high, AUC is 0.78, and 95% confidence interval (CI) is 0.729–0.834.
Relationship of the expression level of miRNA-195 in serum with the clinicopathological features of patients with CCA
The relationship of the expression of miRNA-195 with the clinicopathological features [sex, age, degree of differentiation and tumor-node-metastasis (TNM) staging] of patients with CCA were analyzed. According to the design in this study, the CCA patients were divided into two groups (I, II; III, IV), and the results indicated that the expression level of the serum miRNA-195 was closely associated with TNM staging and lymph node metastasis. The expression of miRNA-195 in the serum was gradually decreased with the increase of pathological staging, and the differences had statistical significance (χ2=6.547, P=0.010; χ2=7.147, P=0.009). There were no correlations between the expression of miRNA-195 and the serum and clinical features of CCA including age, sex and degree of differentiation, and the differences were not statistically significant (Table II).
Table II.
Clinicopathological features of patients with CCA.
| Clinicopathological features | Case | Relative expression level of miR-195 (I, II; III, IV) | P-value |
|---|---|---|---|
| Age (years) | 0.651 | ||
| <60 | 120 | 2.443±0.874; 2.034±0.385 | |
| ≥60 | 84 | 2.351±0.752; 1.985±0.543 | |
| Sex | 0.746 | ||
| Male | 9 | 2.364±0.494; 1.821±0.610 | |
| Female | 115 | 2.211±0.529; 1.901±0.577 | |
| Degree of differentiation | 0.068 | ||
| Well | 155 | 2.089±0.564; 1.964±0.341 | |
| Moderate + Poor | 49 | 1.936±0.436; 1.794±0.386 | |
| TNM staging | 0.010 | ||
| I/II | 140 | 1.842±0.428; 1.754±0.367 | |
| III/IV | 64 | 1.695±0.614; 1.681±0.315 | |
| Lymph node metastasis | 0.009 | ||
| Positive | 34 | 1.684±0.447; 1.594±0.541 | |
| Negative | 170 | 1.867±0.761; 1.710±0.611 |
Relationship of miRNA-195 with the 5-year survival rate of the patients with CCA
The results of the Kaplan-Meier survival curve analysis indicated that 5-year survival rate in the serum miRNA-195 Group I and II was higher than that in Group III and IV, and the difference was statistically significant (P=0.0026) (Fig. 2).
Figure 2.
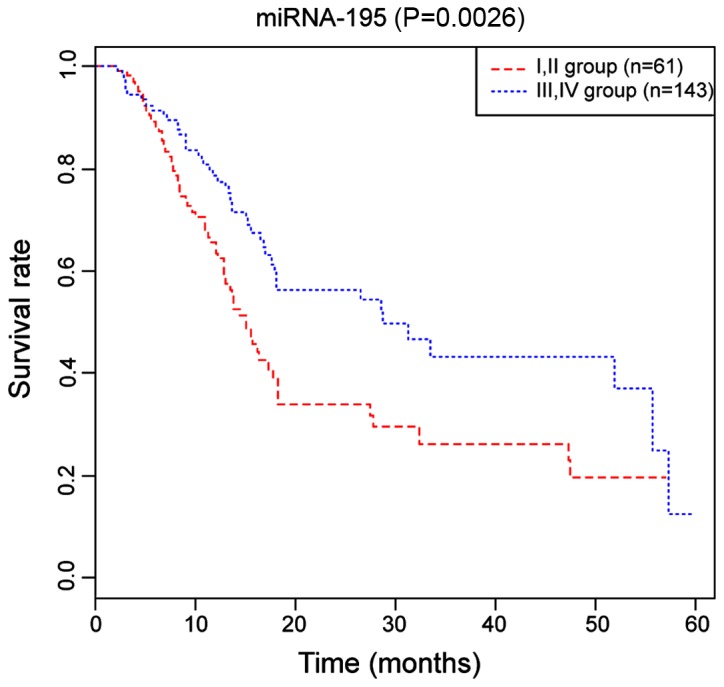
The Kaplan-Meier survival curves of the miRNA-195 Group I and II (61 patients) as well as Group III and IV (143 patients) among CCA patients. The 5-year survival rate in Group I and II is higher than that in Group III and IV among CCA patients (P=0.0026) (P<0.05).
Discussion
At present, MRI and computed tomography (CT) are common imaging methods used in the diagnosis of CCA, but the disease cannot be diagnosed in the early stage, thus leading to missed diagnosis and misdiagnosis easily. Therefore, this study aimed to diagnose and prevent disease through miRNAwhich is regarded as an independent prognostic factor. Numerous studies have shown that miRNA is abnormally expressed in some tumors, such as breast cancer (10), rectal cancer (11), lung cancer (12) and thyroid carcinoma (13) and can be used as an effective diagnostic index and assures stable detection. In addition, miRNA is a tumor marker that has broad prospects and can indicate the tumor status to some extent (14,15).
By detecting and comparing expression levels of miRNA-195 between the CCA patients and healthy controls in this study, it was found that miRNA-195 was downregulated in the CCA patients compared with that in the healthy controls. The study demonstrated that miRNA-195 is a diagnostic index that has certain sensitivity and specificity in CCA patients. In this study, the expression of miRNA-195 in the serum and clinical features of CCA patients was analyzed, resulting in the serum miRNA-195 being closely associated with TNM staging and lymph node metastasis, which may be due to its inhibitory effect on the migration and metastasis of tumor cells. In addition, there was no correlation with age, sex and degree of differentiation. The relationship of the expression of miRNA-195 in the serum with the 5-year survival rate in patients with CCA was analyzed in this study. The results showed that the serum of miRNA-195 Group I and II was higher than that in Group III and IV.
miRNA-195 is a non-coding RNA molecule with a length of 22 nucleotides, which is closely related to the occurrence and progression of various tumors. miRNA-195 has the tissue and space specificity, which can promote or inhibit cancer genes in different types of cancers, thus playing different roles. Previous findings have shown that miR-195 inhibited cancer metastasis in vitro and in vivo by partially targeting cell cycle gene CyclinD1 (CCND1) in osteosarcoma thus inhibiting the occurrence of cancer (16). Additionally, miR-195 was downregulated in hepatocellular carcinoma and adrenocortical carcinoma as a tumor suppressor (17). However, it was upregulated in chronic lymphocytic leukemia and breast cancer as an oncogene (10,18,19).
The present study investigated the expression of miRNA-195 in the serum of CCA patients and its clinical value in the diagnosis and prognosis of CCA, suggesting that serum miRNA-195 was highly expressed in CCA, which had a certain specificity. Notably, the expression of miRNA-195 in the serum was positively associated with the clinical analysis in patients with CCA, which had a relatively high clinical value in diagnosis and prognosis. This study further demonstrated that the expression of miRNA was differentially expressed, and it is required to explore its specific molecular mechanism in exerting the role as an oncogene in CCA.
In summary, the results of the present study have shown that miRNA-195 is lowly expressed in the serum of CCA patients compared with that in the control group. miRNA-195 in serum can be used as an effective diagnostic index for CCA. The expression of miRNA-195 in serum is closely related to the clinical staging of the CCA patients and the 5-year survival rate in serum miRNA-195 Group I and II is higher than that in Group III and IV. The present study lays a certain foundation for further research on the mechanism of occurrence and progression of CCA, and provides a potential target for the early diagnosis and prognosis of CCA.
Acknowledgements
Not applicable.
Funding
This study was supported by Henan Medical Science and Technology Project. Project name: Measurement and clinical application of 3.0T high field 3D MRCP biliary anatomy. Project nο. 201503218. Project manager Qingliang Chen.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QC and CW analyzed and interpreted the patient data, and were the major contributors in writing the manuscript. HZ and ZL performed the experiment and participated in the design of the study. YL participated in the analysis and discussion of the data. YC and XX were responsible for the collection of the data and the follow-up management of the patients. YZ was responsible for the statistical analysis of the data. SL and XH were the major contributors in designing the methods. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Third People's Provincial Hospital of Henan Province. Signed written informed consents were obtained from the patients and/or guardians. The study was in line with the Medical Ethics Standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: Epidemiology, molecular pathogenesis and genetic risk associations. Linchuang Zhongliuxue Zazhi. 2016;5:61. doi: 10.21037/cco.2016.10.09. [DOI] [PubMed] [Google Scholar]
- 3.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairweather M, Balachandran VP, D'Angelica MI. Surgical management of biliary tract cancers. Linchuang Zhongliuxue Zazhi. 2016;5:63. doi: 10.21037/cco.2016.10.03. [DOI] [PubMed] [Google Scholar]
- 5.Høgdall D, O'Rourke CJ, Taranta A, Oliveira DV, Andersen JB. Molecular pathogenesis and current therapy in intrahepatic cholangiocarcinoma. Dig Dis. 2016;34:440–451. doi: 10.1159/000444562. [DOI] [PubMed] [Google Scholar]
- 6.Jiang XM, Li ZL, Li JL, Zheng WY, Li XH, Cui YF, Sun DJ. LncRNA CCAT1 as the unfavorable prognostic biomarker for cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2017;21:1242–1247. [PubMed] [Google Scholar]
- 7.Zen K, Zhang CY. Circulating microRNAs: A novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 8.Luo Q, Wei C, Li X, Li J, Chen L, Huang Y, Song H, Li D, Fang L. MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncol Rep. 2014;31:1096–1102. doi: 10.3892/or.2014.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yongchun Z, Linwei T, Xicai W, Lianhua Y, Guangqiang Z, Ming Y, Guanjian L, Yujie L, Yunchao H. MicroRNA-195 inhibits non-small cell lung cancer cell proliferation, migration and invasion by targeting MYB. Cancer Lett. 2014;347:65–74. doi: 10.1016/j.canlet.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Heneghan HM, Miller N, Kelly R, Newell J, Kerin MJ. Systemic miRNA-195 differentiates breast cancer from other malignancies and is a potential biomarker for detecting noninvasive and early stage disease. Oncologist. 2010;15:673–682. doi: 10.1634/theoncologist.2010-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksen AHM, Sørensen FB, Andersen RF, Jakobsen A, Hansen TF. Association between the expression of microRNAs and the response of patients with locally advanced rectal cancer to preoperative chemoradiotherapy. Oncol Lett. 2017;14:201–209. doi: 10.3892/ol.2017.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 13.Forte S, La Rosa C, Pecce V, Rosignolo F, Memeo L. The role of microRNAs in thyroid carcinomas. Anticancer Res. 2015;35:2037–2047. [PubMed] [Google Scholar]
- 14.Letelier P, Riquelme I, Hernández AH, Guzmán N, Farías JG, Roa JC. Circulating MicroRNAs as biomarkers in biliary tract cancers. Int J Mol Sci. 2016;17:17. doi: 10.3390/ijms17050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz-Quintero B. Cell-free microRNAs in blood and other body fluids, as cancer biomarkers. Cell Prolif. 2016;49:281–303. doi: 10.1111/cpr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han K, Chen X, Bian N, Ma B, Yang T, Cai C, Fan Q, Zhou Y, Zhao TB. MicroRNA profiling identifies MiR-195 suppresses osteosarcoma cell metastasis by targeting CCND1. Oncotarget. 2015;6:8875–8889. doi: 10.18632/oncotarget.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, et al. MiR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15:7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 18.Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, Falcão RP, Zago MA. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40:1435–1440. doi: 10.1590/S0100-879X2007001100003. [DOI] [PubMed] [Google Scholar]
- 19.Hannafon BN, Sebastiani P, de las Morenas A, Lu J, Rosenberg CL. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011;13:R24. doi: 10.1186/bcr2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


