To the Editor,
Primary sarcomas of the breast are rare tumors, accounting for less than 1% of all breast neoplasms. Leiomyosarcoma is an extremely rare subtype of breast sarcoma [1]. Prognoses for primary breast sarcoma are poor and treatment modalities are limited. Pazopanib is an oral, multitargeted tyrosine kinase inhibitor, with activity against vascular endothelial growth factors (VEGFs) 1, 2, and 3, and platelet-derived growth factors (PDGFs) [2]. Pazopanib has been approved for the treatment of advanced renal cell carcinoma and metastatic soft tissue sarcoma (STS) [2]. Hyperthermia inhibits sub-lethal cellular damage repair and improves oxygenation; thus, making it an attractive therapy for combining with radiation and/or chemotherapy to generate a potentially synergistic response. Hyperthermia has a proven benefit for treating STS [3]. Herein, we report a case of primary breast leiomyosarcoma treated with regional hyperthermia and pazopanib.
A 49-year-old woman was referred to our oncology department for chemotherapy for a primary breast leiomyosarcoma. Three months prior, she had been admitted to our surgery department because of a rapidly increasing, painless mass in the left breast that had been present for 6 months. The mass measured approximately 6 × 8 cm and was accompanied by skin necrosis. The patient was diagnosed with malignant sarcoma of the left breast on fine needle aspiration biopsy, and an F-18 fluorodeoxyglucose positron emission tomography-computed tomography (CT) scan revealed metastasis in the lung. The patient underwent a palliative total mastectomy of the left breast. Pathologic examination revealed the tumor comprised hyperchromatic spindle cells with moderate atypia arranged in fascicles, along with hemorrhage and extensive necrosis (Fig. 1A and 1B). The mitotic count was up to 18 mitoses per 10 high-power fields. On immunohistochemical analysis, the tumor cells showed strong positivity for vimentin and smooth muscle actin (Fig. 1C) and moderate positivity for desmin (Fig. 1D). The tumor cells were negative for pan-cytokeratin and S-100 protein.
Figure 1.
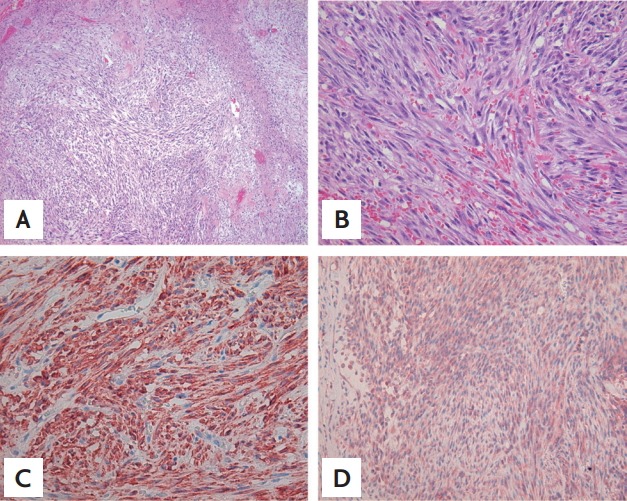
(A) A histologic examination revealed bundles of spindle-shaped cells with a fascicular growth pattern and necrosis (H&E, ×100). (B) Spindle cells showed elongated, blunt-ended nuclei with moderate atypia and mitotic figures (H&E, ×400). Immunohistochemical staining demonstrated that the tumor cells are positive for (C) smooth muscle actin (×400), and (D) desmin (×200).
The patient received palliative chemotherapy consisting of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks. After three cycles, the mass in her left chest wall recurred and the size of the lung mass had increased. The patient was transferred to our oncology department for modification of her chemotherapeutic regimen. The patient was treated with a second-line chemotherapy regimen consisting of docetaxel (75 mg/m2 on day 8) and gemcitabine (900 mg/m2 on days 1 and 8) every 3 weeks. After two cycles, a chest CT scan revealed a mass in the chest wall and the lung metastasis was more aggravated. The mass in the chest wall was measured 15 × 12 × 4 cm in size, extending in front of the left ventricle and resulting in destruction of the left 5th rib (Fig. 2A and 2C). The patient received 800 mg of pazopanib daily and hyperthermia at the left chest wall for local disease control. Hyperthermia (EHY-2000, OncoTherm, Budaörs, Hungary) was administered as combined therapy over 8 weeks, thrice a week for an average of 1 hour per session using a power output of 120 watts. After 3 weeks, the patient’s blood pressure had increased to 160/100 mmHg. She received 5 mg of amlodipine, a calcium channel blocker, and her blood pressure normalized.
Figure 2.
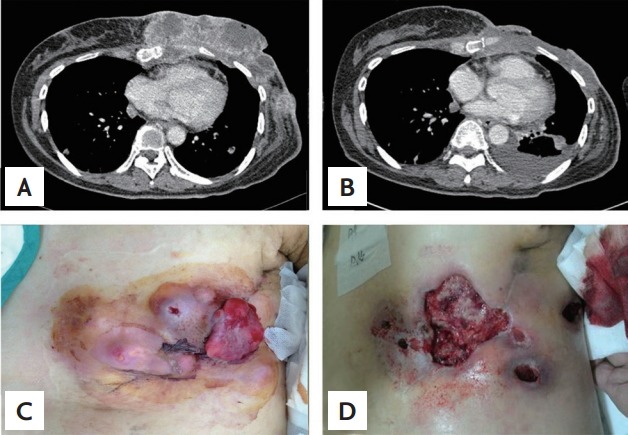
Chest computed tomography scan. (A) Before hyperthermia and pazopanib: in the left breast, there was a large, lobulated, heterogeneously enhancing mass, 15 × 12 × 4cm in size. (B) Two months later, the size of the tumor markedly decreased but that of the lung metastatic nodule mildly increased, and left pleural effusion was detected. Macroscopically, (C) before hyperthermia and pazopanib, multiple elastic hard-tumors were detected in the left breast. (D) Two months later, the size of the tumor markedly decreased but a skin defect, approximately 10 × 10 cm in size, was detected at the site where the original mass was found.
Two months later, a chest CT scan showed that the mass in the left chest wall had markedly improved, but that a skin defect measuring approximately 10 × 10 cm had appeared at the site of the previous mass. Additionally, the size of the metastatic nodules in the lung had increased slightly and pleural effusion was detected (Fig. 2B and 2D). One month later, the mass in the chest wall remained in near complete remission, but recurrent infection, bleeding, and skin pain at the defect sites developed. Overall, the patient’s general condition was very weak, and the left pleural effusion had increased. The patient discontinued pazopanib because of severe weakness and disease progression after that. She and her family requested hospice and palliative care. Three weeks later, the patient died of a sudden steep decrease in her blood pressure.
Leiomyosarcoma of the breast is an extremely rare type of neoplasm that often presents as a well-circumscribed large mass in the breast of postmenopausal women. Leiomyosarcoma is characterized by spindle-shaped cells with pleomorphic and elongated nuclei. A definitive diagnosis is established through an immunohistochemical examination that reveals positive staining for desmin, vimentin, and muscle-specific actin, and negative staining for cytokeratin, myoglobin, and S-100. This type of tumor tends to show local recurrence, and distant metastasis has been observed in 25% of patients[1]. There is no clear consensus on the best treatment modality. However, the basic treatment should include complete tumor excision with negative margins.
The conventional first-line chemotherapy for advanced STS is an anthracycline-based regimen (usually doxorubicin), administered either as monotherapy or in combination with ifosfamide. After failure of the first-line chemotherapy, chemotherapeutic options include gemcitabine alone or in combination with docetaxel, dacarbazine, eribulin, trabectedin, or pazopanib.
Pazopanib inhibits multiple receptor tyrosine kinases, including VEGF receptors, PDGF receptors, and c-kit, through which it mediates antiangiogenic and antitumor effects. The clinical efficacy of pazopanib in patients with metastatic STS was demonstrated in the pazopanib explored in soft-tissue sarcoma-a phase 3 (PALETTE) study. This study is unique because it is the first placebo controlled phase 3 trial of a tyrosine kinase inhibitor for STS. The median progression-free survival (PFS) was significantly higher in the pazopanib group (4.6 months vs. 1.6 months, p < 0.0001), and a benefit was consistently observed across all histologic subtypes; however, there was no significant difference in overall survival (OS) (p = 0.25). The best overall response was a partial response in 6% versus 0% of the pazopanib and placebo groups, respectively, and stable disease in 67% versus 38%, respectively. The most common adverse events were fatigue, diarrhea, nausea, weight loss, and hypertension. Elevation of liver enzymes was the most common laboratory abnormality [2]. In STS, biochemical analysis of patient samples from a phase II study investigating pazopanib-induced effects on serum cytokines and angiogenic factors showed that pazopanib decreases soluble VEGF receptor-2 (sVEGFR2) levels and increases placental-derived growth factor levels; changes in both of these factors are associated with pazopanib-specific toxicity, such as hypertension and thyroid stimulating hormone elevations and with poorer PFS (12 weeks) and OS (both p < 0.05). Also decreasing some cytokines were associated with better PFS and OS [4]. Further studies needs to explore potential predictive and prognostic biomarkers.
Targeted therapy and promising novel agents for the treatment of advanced STS, including sorafenib, sunitinib, ABT-510, aflibercept, cediranib, regorafenib, ridaforolimus, olaratumab, tivozanib, and immune check-point inhibitors, are being explored.
Hyperthermia is a type of treatment method in which body tissue is exposed to a slightly higher temperature (41.8°C to 42°C) to damage and kill cancer cells. In the European Society for Hyperthermic Oncology and European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group 62961 multicenter, randomized phase 3 study, this study evaluated neoadjuvant chemotherapy (etoposide, ifosfamide, and doxorubicin) alone or with regional hyperthermia for localized high risk STS. Adding regional hyperthermia to chemotherapy results in significantly better local PFS (hazard ratio [HR], 0.58; p = 0.003) and disease-free survival (HR, 0.70; p = 0.011) compared with chemotherapy alone, and the overall response is 2.3-times greater than that in patients treated with chemotherapy alone (28.8% vs. 12.7%, p = 0.002). It was the first randomized phase 3 trial to show that regional hyperthermia increased the benefit of chemotherapy. Adding regional hyperthermia to chemotherapy is a new effective treatment strategy for patients with high-risk STS [3].
A 29-year-old patient with primary refractory ovarian cancer, who was treated with a combination of pegylated liposomal doxorubicin, regional abdominal hyperthermia, and bevacizumab, remained in stable condition for 38 months. The authors described the combination of hyperthermia and bevacizumab in particular had a strong synergistic effect. They explained why inhibition of VEGF with bevacizumab led not only to the inhibition of neoangiogenesis, but also to the destruction of tumor-related blood vessels; when bevacizumab-mediated destruction of microvessels results in suboptimal perfusion, a maximum apoptosis-inducing effect might be achieved by using hyperthermia, which increases the tumor’s need for oxygen [5].
Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor that targets VEGF among other proteins. In the case described herein, we suggest that the combination of hyperthermia and pazopanib may have had a strong synergistic effect on the chest wall mass of the primary breast leiomyosarcoma. To our knowledge, this is the first report of treatment with a combination of pazopanib and regional hyperthermia for leiomyosarcoma of the breast. Further evaluation of this combination as a therapeutic option in chemo-refractory tumors or heavily pretreated patient populations is warranted.
Acknowledgments
We thank Dr. Y. N. Kim and S. J. Roh for the review of the pathology.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Karabulut Z, Akkaya H, Moray G. Primary leiomyosarcoma of the breast: a case report. J Breast Cancer. 2012;15:124–127. doi: 10.4048/jbc.2012.15.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 3.Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleijfer S, Gorlia T, Lamers C, et al. Cytokine and angiogenic factors associated with efficacy and toxicity of pazopanib in advanced soft-tissue sarcoma: an EORTC-STBSG study. Br J Cancer. 2012;107:639–645. doi: 10.1038/bjc.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietzner K, Schmuck RB, Fotopoulou C, et al. Long term combination treatment with bevacizumab, pegylated liposomal doxorubicin and regional abdominal hyperthermia in platinum refractory ovarian cancer: a case report and review of the literature. Anticancer Res. 2011;31:2675–2677. [PubMed] [Google Scholar]


