Abstract
The aim of the present study was to investigate the associations between vasculogenic mimicry (VM) and zinc finger E-box binding homeobox 1 (ZEB1) in bladder cancer. VM structure and ZEB1 expression were analyzed by cluster of differentiation 34/periodic acid Schiff (PAS) double staining and immunohistochemical staining in 135 specimens from patients with bladder cancer, and a further 12 specimens from normal bladder tissues. Three-dimensional (3-D) culture was used to detect VM formation in the bladder transitional cancer cell lines UM-UC-3 and J82, and the immortalized human bladder epithelium cell line SV-HUC-1 in vitro. ZEB1 expression in these cell lines was compared by reverse transcription-quantitative polymerase chain reaction and western blot assays. In addition, small interfering RNA was used to inhibit ZEB1 in UM-UC-3 and J82 cells, followed by 3-D culturing of treated cell lines. As a result, VM was observed in 31.1% of specimens from bladder cancer tissues, and cases with high ZEB1 expression accounted for 60.0% of patients with bladder cancer. In addition, ZEB1 expression was closely associated with VM (r=0.189; P<0.05), and also increased as the grade and stage of the tumor developed. In an in vitro assay, UM-UC-3 and J82 cells exhibited VM formation, however, SV-HUC-1 did not. Furthermore, VM-forming cancer cell lines UM-UC-3 and J82 exhibited higher ZEB1 expression. Notably, VM formation was inhibited following knockdown of ZEB1. In conclusion, ZEB1 may be associated with VM in bladder cancer and serve an important role in the process of VM formation. However, its detailed mechanism requires further study.
Keywords: vasculogenic mimicry, bladder cancer, zinc finger E-box binding homeobox 1, epithelial-mesenchymal transition, cluster of differentiation 34/periodic acid-Schiff double staining, three-dimensional culture
Introduction
With high rates of recurrence, invasion and metastasis, bladder cancer is one of the common urinary neoplasms and seriously affects human health worldwide. In China, bladder cancer is the seventh most common tumor in males and it has the highest incidence among genitourinary tumors (1). Similarly, in the US, the incidence of bladder cancer is second only to prostate cancer (2). In addition, the incidence of bladder cancer in females is lower than in males worldwide. Although bladder cancer is common, its molecular mechanisms of occurrence and progression are not yet clear. Surgery is the gold-standard treatment for bladder cancer, yet the rate of recurrence is high and the overall 5-year survival is low. In particular, therapeutic strategies for advanced bladder cancer are very limited (3). Therefore, clarifying the mechanism of proliferation, invasion and metastasis in bladder cancer, and finding new targets for therapy, has become a key focus of research.
It is well known that the biological behavior of malignant tumors is closely related to blood supply. Traditional neovascularization is common in malignant tumors, which ensures a sufficient nutrient supply over time. In recent years, some vascular targeting drugs, such as bevacizumab and Tyrosine Kinase Inhibitor, have been used in clinical therapy, but these had little effect in many malignant tumors. Some scholars have proposed that novel tumor microcirculation patterns, different from traditional angiogenesis, may exist in these malignant tumors. Vasculogenic mimicry (VM), originally discovered in melanoma (4), is a novel tumor microcirculation system that does not rely on vascular endothelial cells. It is a blood vessel-like structure, composed of a group of tumor cells, which can deliver erythrocytes and other nutrients by connecting with normal blood vessels directly. The phenomenon has been observed in many tumor types, including hepatocellular carcinoma, breast, colorectal and prostate cancer (5–8). Numerous studies (8–10) have shown that the existence of VM is closely related to the invasion, metastasis and poor prognosis of malignant tumors, which is significant in the clinic. At present, the literature about VM in bladder cancer, particularly its molecular mechanism, is very limited.
Zinc finger E-box binding homeobox 1 (ZEB1), which belongs to the ZEB family of transcription factors, consists of two zinc finger clusters, responsible for DNA binding, and a centrally-located homeodomain. ZEB1 not only promotes tumor invasion and metastasis by inducing epithelial-mesenchymal transition (EMT), but it can also regulate therapeutic resistance via different mechanisms (11). ZEB1 is overexpressed in several types of cancer, such as gastric cancer, lung cancer and prostate cancer, and it plays an important role in inducing EMT in tumor cells (12–14). EMT, which describes the process by which tumor cells escape from one site to invade the adjacent matrix and transfer to a distant site, is a key event in the progression of malignant tumors. EMT is regulated by many transcription factors, including the traditional factor ZEB1. The role of EMT in the development of VM has recently attracted attention (9,15,16). Liu et al (8) confirmed that ZEB1 can promote VM formation by inducing EMT in colorectal cancer. Similarly, ZEB2, a homologous protein of ZEB1, was found to have the same function in inducing EMT, promoting VM formation in hepatocellular carcinoma (17). Although there is no consensus about the correlation between ZEB1 expression and tumor grade, stage, invasion and metastasis in bladder cancer, numerous studies have verified that ZEB1 is significantly overexpressed in bladder cancer tissues in comparison with healthy adjacent tissues (18–20). However, it has not been reported whether ZEB1 plays a critical role in VM formation in bladder cancer. Therefore, it is necessary to study the relationship between VM, ZEB1 expression and clinical parameters in bladder cancer. More importantly, the mechanism involving ZEB1 and VM in bladder cancer must be investigated.
In the present study, we demonstrated that ZEB1 was significantly overexpressed in bladder cancer compared with normal tissue, and was positively correlated with VM. In an in vitro assay, knockdown of ZEB1 was indicated to suppress the formation of VM in bladder cancer. Furthermore, the mechanism by which ZEB1 promotes VM in bladder cancer requires further investigation.
Materials and methods
Clinical tissue samples and immunohistochemistry (IHC) staining
The current study consisted of 147 formalin-fixed and paraffin-embedded samples, of which 135 specimens (116 males and 19 females; mean age, 61.5 years; age range, 18 to 85 years) were from patients with bladder cancer and a further 12 specimens were from normal tissue adjacent to bladder cancer tissue. The samples were obtained from the First Affiliated Hospital, Sun Yat-sen University, between November 2015 and March 2017. All diagnoses were confirmed by pathology. Further clinical parameters are presented in Table I. The present study was approved by the Medical Ethics Committee of Sun Yat-sen University (Guangzhou, Guangdong, China) and written informed consent was obtained from each patient. The IHC staining assays and evaluation methods were performed as previously described (18,21). The antibody used was rabbit polyclonal ZEB1 antibody (1:200; cat. no. ab87280; Abcam, Cambridge, UK). ZEB1 expression was evaluated according to the staining intensity and extent. In brief, the staining intensity was scored as 0 (none), 1 (weak), 2 (medium) or 3 (strong), and the staining extent was scored as 0 (0–5%), 1 (6–25%), 2 (26–75%) or 3 (75–100%). Then the two scores were summed to obtain a final score. Final scores ≤3 or >3 were considered to indicate low or high expression, respectively. All samples were evaluated by two independent observers.
Table I.
Associations between vasculogenic mimicry, zinc finger E-box binding homeobox 1 expression and the clinicopathological parameters in bladder cancer.
| VM | ZEB1 expression | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Total | Positive | Negative | P-value | Low expression (scores ≤3) | High expression (scores >3) | P-value |
| Age (years) | |||||||
| <60 | 52 | 18 | 34 | 0.486 | 18 | 34 | 0.312 |
| ≥60 | 83 | 24 | 59 | 36 | 47 | ||
| Sex | |||||||
| Male | 116 | 37 | 79 | 0.626 | 48 | 68 | 0.419 |
| Female | 19 | 5 | 14 | 6 | 13 | ||
| TNM stage | |||||||
| Ta-T1 | 95 | 32 | 63 | 0.320 | 46 | 49 | 0.002 |
| T2–4 | 40 | 10 | 30 | 8 | 32 | ||
| Tumor grade | |||||||
| Low | 63 | 19 | 44 | 0.823 | 33 | 30 | 0.006 |
| High | 72 | 23 | 49 | 21 | 51 | ||
| Recurrence | |||||||
| Absent | 105 | 32 | 73 | 0.766 | 41 | 64 | 0.673 |
| Present | 30 | 10 | 20 | 13 | 17 | ||
VM, vasculogenic mimicry; ZEB1, zinc finger E-box binding homeobox 1; TNM, tumor-node-metastasis.
CD34/periodic acid Schiff (PAS) double staining
CD34/PAS double staining was performed in order to detect VM formation in paraffin-embedded sections. First, IHC staining for CD34, using a mouse monoclonal antibody (1:50; cat. no. ZM-0046; Zhongshan Goldenbridge, Beijing, China), was performed to detect endothelial cells. Then, the sections were washed in running water for 1 min and incubated with PAS for 30 min to detect the basement membrane of tubular structures. The typical characteristic of VM is a tubular structure containing red blood cells, indicated by PAS staining of the basement membrane, surrounded by tumor cells with negative CD34 staining. The number of red blood cells in the tubular structure is ≥1. All the steps were performed as previously described (22).
Cell culture and three-dimensional (3-D) culture
The immortalized human bladder epithelium cell line SV-HUC-1 and the bladder transitional cancer cell line J82 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The human bladder transitional cancer cell line UM-UC-3 was donated by Professor Chunxiao Liu (Urology Department, Zhujiang Hospital of Southern Medical University). The base media for SV-HUC-1, J82 and UM-UC-3 were F-12K, EMEN and 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), respectively. All the base media were supplemented with a final concentration of 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured at 37°C and 5% CO2.
3-D culture was used for the detection of VM formation in vitro. Firstly, 96-well culture plates were coated with 50 µl/well growth factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Then, the plates were incubated at 37°C for 2 h. Subsequently, the cells, suspended in complete medium at 3×105 cells/ml, were plated onto the surface of the Matrigel at 100 µl/well and incubated at 37°C for 4 h. The number of tube-like structures was measured in 3 random fields. The average number was calculated and statistical analysis was performed.
Small interfering RNA (siRNA) transfection
The siRNA was purchased from RiboBio Biology (Guangzhou, China). The target sequences for ZEB1 were as follows: si-ZEB1#1, GCATACACCTACTCAACTA; si-ZEB1#2, CGGACGAGAGAGAGAGTTT. A non-silencing siRNA was used as the negative control. Transfection was performed using Lipofectamine 2000 Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then, 5 µl of 20 nmol/µl siRNA was added to each well of 6-well plates, which had been seeded with cells according to the manufacturer's protocol. After incubating the cells at 37°C for 48 h, we tested the efficiency of gene knockdown by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay and western blot analysis.
RNA purification and RT-qPCR assay
Total RNA from different cells (SV-HUC-1, UM-UC-3, J82) was extracted using an E.Z.N.A® HP Total RNA Kit (Omega Bio-Tek, Norcross, GA, USA). Total RNA (1 µg) was used to synthesize cDNA with the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.). The fluorescent dye used for the qPCR assay was SYBR® Premix Ex Taq™ (Takara, Tokyo, Japan). All of the above experiments were conducted according to the protocols provided by the kit manufacturers. The primers used were as follows: ZEB1 forward, 5′-GCACCTGAAGAGGACCAGAG-3′ and ZEB1 reverse, 5′-GTGTAACTGCACAGGGAGCA-3′; GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and GAPDH reverse, 5′-TTGATTTTGGAGGGATCTCG-3′. All primers were synthesized by Thermo Fisher Scientific, Inc. The PCR conditions were 95°C for 30 sec, then a total of 40 cycles of 95°C for 5 sec, 60°C for 34 sec, then a final extension at 95°C (15 sec), 60°C (1 min) and 95°C (15 sec). The relative expression levels were calculated using the 2-Δ∆Cq method according to the following formula: ΔCq (target gene) = Cq (target gene) - Cq (control gene).
Western blot analysis
Cell lysates were collected using a total protein extract kit (KeyGen Biotech, Inc., Nanjing, China) and protein concentrations were quantified with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Proteins (30 µg/lane) were resolved by SDS-PAGE (upper gel: 5%, lower gel: 10%) (KeyGen Biotech, Inc.) and transferred to polyvinylidene difluoride (PVDF) membranes (Pierce; Thermo Fisher Scientific, Inc.). Then, the membranes were incubated overnight with primary antibodies (rabbit antibody to ZEB1, 1:250; cat. no. sc-25388; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; GAPDH, 1:5,000; cat. no. AC027; ABclonal Biotech Co., Ltd., Woburn, MA, USA) at 4°C. Subsequently, the goat anti-rabbit IgG-HRP (1:5,000, cat. no. BL003A; Biosharp, Anhui, China) was incubated at room temperature to detect protein bands in the membranes.
Statistical analysis
All data in the present study were evaluated using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Each experiment was performed at least 3 times. The relationships between VM, ZEB1 expression and clinicopathological parameters were analyzed by the Chi-square (χ2) test or Fisher's exact test. The correlation between VM and ZEB1 expression was assessed by association analysis. Student's t-test was performed to compare differences between groups in cell assays. P<0.05 was considered to indicate a statistically significant difference for all analyses.
Results
Evaluation of VM and clinicopathological characteristics in bladder cancer
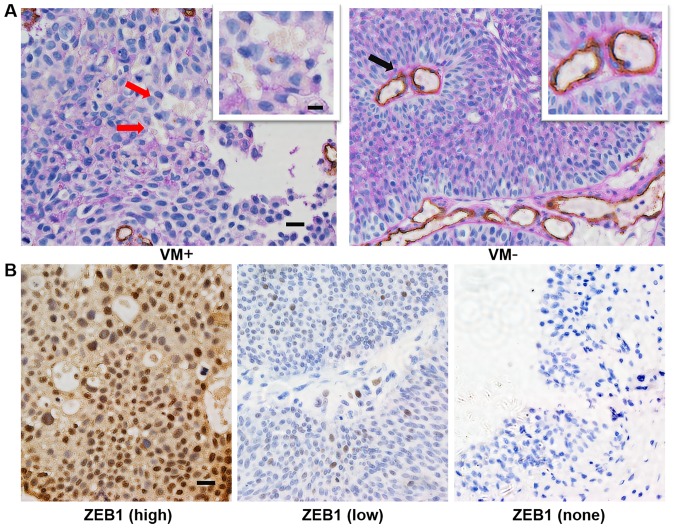
According to the aforementioned criteria, we detected VM in 135 bladder cancer cases. As shown in Table I, 42 samples from 135 cases (31.1%) in bladder cancer were VM positive. A typical VM structure, which is positive for PAS in its membrane and negative for the endothelial cell marker CD34, contains one or more red blood cells (Fig. 1A). In addition, we recorded the clinicopathological parameters of all patients, including age, sex, tumor grade, tumor stage and progression. We compared the rate of VM in different subgroups. However, the data in our study showed no significant correlations between VM and the clinicopathological parameters (Table I).
Figure 1.
CD34/PAS and ZEB1 immunohistochemistry staining of bladder cancer or normal tissues. (A) The typical appearance of VM with CD34/PAS double staining showed tubule-like structures containing red blood cells, which were positive for PAS staining and negative for CD34 (brown), as indicated by red arrows. Endothelium-dependent vessels were positive for CD34 staining (indicated by the black arrow). (B) High or low expression of ZEB1 (left or middle image) in bladder cancer tissues and no expression of ZEB1 (right image) in normal bladder tissues (magnification, ×400; scale bars, 20 µm; for insets, 10 µm). CD34, cluster of differentiation 34; PAS, periodic acid Schiff; ZEB1, zinc finger E-box binding homeobox 1; VM, vasculogenic mimicry.
VM is associated with ZEB1 overexpression in bladder cancer
In order to explore the role of ZEB1 in bladder cancer, we evaluated ZEB1 expression in all samples from patients (Fig. 1B). As shown in Table II, high expression of ZEB1 (score >3) was exhibited in 60.0% (81/135) of cases, and 31 of these cases were VM-positive (38.3%, 31/81). However, for cases with low expression of ZEB1 (score ≤3), the rate of VM was 20.4% (11/54), which was lower compared with the high-expression ZEB1 group. The difference was statistically significant (χ2=4.844, P<0.05) and VM was positively correlated with overexpression of ZEB1 (r=0.189, P<0.05). These results indicate that there is a strong correlation between VM and ZEB1 expression.
Table II.
Associations between vasculogenic mimicry and zinc finger E-box binding homeobox 1 expression in bladder cancer.
| ZEB1 expression | ||||||
|---|---|---|---|---|---|---|
| VM | High (n) | Low (n) | Total (n) | r | χ2 | P-value |
| Positive | 31 | 11 | 42 | 0.189 | 4.844 | 0.028 |
| Negative | 50 | 43 | 93 | |||
VM formation was positively associated with ZEB1 expression (r=0.189, P<0.05). VM, vasculogenic mimicry; ZEB1, zinc finger E-box binding homeobox 1.
Aberrant expression of ZEB1 is related to the stage and grade of bladder cancer
Based on its biological behavior, bladder cancer is divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). According to TNM classification, Ta-T1 tumors are classified as NMIBC and T2-4 tumors are classified as MIBC. In our study, we found that MIBC sections showed higher ZEB1 expression compared with NMIBC sections (80.0%, 32/40 vs. 51.2%, 49/95; P<0.05) (Table I). In addition, compared with the low-grade urothelial carcinoma group, ZEB1 expression was higher in those with high-grade urothelial carcinoma (70.8%, 51/72 vs. 47.6%, 30/63; P<0.05) (Table I). Notably, ZEB1 expression was absent in all 12 specimens from normal adjacent tissues (Fig. 1B). Overall, these results indicated that ZEB1 may play an important role in invasive and aggressive bladder cancer.
VM formation and ZEB1 expression in bladder transitional cancer cell lines
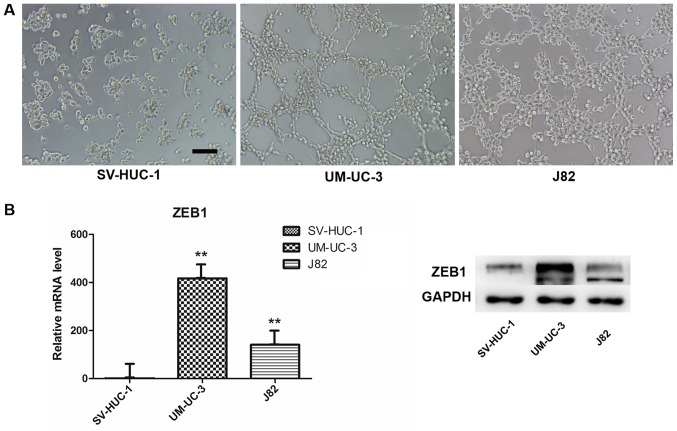
We used a well-established 3-D model to investigate VM formation in vitro. We chose the SV-HUC-1 (normal uroepithelium) and UM-UC-3 and J82 (transitional cell carcinoma) cell lines and evaluated their ability to form vessel-like tubes. The results indicated that both UM-UC-3 and J82 formed vessel-like tubes after we cultured the cells on Matrigel for 4 h (Fig. 2A). By contrast, the normal uroepithelium cells did not form these structures under the same conditions, or at higher cell density or with longer culture time. In addition, we compared ZEB1 expression in the three cell lines by RT-qPCR and western blot assays. Interestingly, both mRNA and protein expression of ZEB1 in SV-HUC-1 were lower compared with UM-UC-3 and J82 (Fig. 2B), which revealed that ZEB1 potentially promotes VM formation in bladder cancer.
Figure 2.
3D culture and ZEB1 expression. (A) Bladder transitional cancer cell lines UM-UC-3 and J82 (3×105 cells/ml) were able to form vascular channels on 3D Matrigel within 4 h following seeding; however, the immortalized human bladder epithelium cell line SV-HUC-1 (3×105 cells/ml) failed to form a similar structure even when cultured for up to 24 h (magnification, ×100; scale bar, 100 µm). (B) UM-UC-3 and J82 cells exhibited higher ZEB1 expression than SV-HUC-1 at the mRNA and protein levels. **P<0.001 vs. SV-HUC-1. ZEB1, zinc finger E-box binding homeobox 1; 3D, three dimensional.
Knockdown of ZEB1 impaired VM formation in UM-UC-3 and J82 cell lines
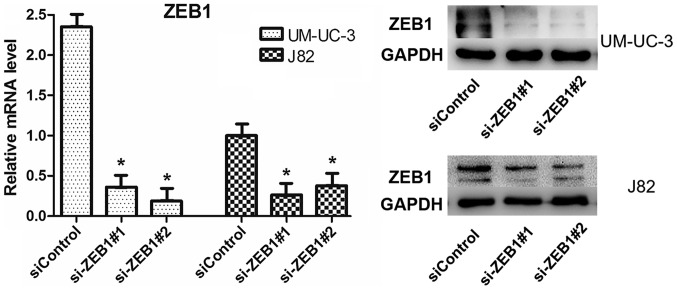
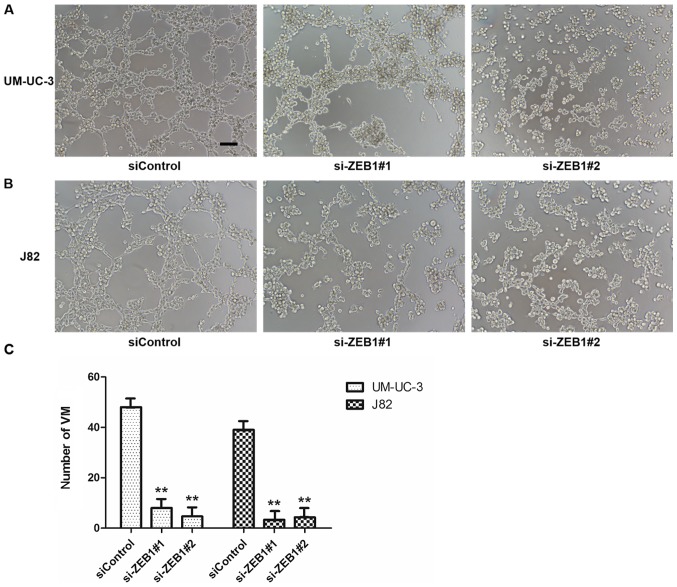
To confirm the potential role of ZEB1 in the formation of vascular networks in bladder cancer in vitro, we downregulated ZEB1 expression in UM-UC-3 and J82 cells by transfecting them with specific siRNA targeting ZEB1. We investigated the efficiency after knockdown of ZEB1 by RT-qPCR and western blot assays and ensured that the transfection method had been effective (Fig. 3). The results of 3-D culture in treated cells demonstrated that downregulation of ZEB1 in UM-UC-3 and J82 cell lines inhibited the formation of tubular structures (Fig. 4). However, the control groups exhibited lots of tubular structures. These results demonstrated that ZEB1 is an important regulatory factor for VM formation in bladder cancer.
Figure 3.
mRNA and protein expression levels of ZEB1 were significantly decreased in the UM-UC-3 and J82 cell lines. *P<0.05 vs. siControl. ZEB1, zinc finger E-box binding homeobox 1; si, small interfering RNA.
Figure 4.
Downregulation of ZEB1 by siRNA impaired VM formation on 3D Matrigel. (A) The 3D culture of UM-UC-3 following ZEB1 knockdown (magnification, ×100; scale bars, 100 µm). (B) The 3D culture of J82 following ZEB1 knockdown. (C) Statistical analysis of VM number in UM-UC-3 and J82 cells. **P<0.001 vs. siControl. ZEB1, zinc finger E-box binding homeobox 1; si, small interfering RNA; VM, vasculogenic mimicry; 3D, three dimensional.
Discussion
VM is a microcirculation pattern different from the traditional blood supply, which plays an important role in the auxiliary functions of transferring blood and other nutrients. In addition, VM is found in many solid tumors, including but not limited to the cancer mentioned above: Melanoma, hepatocellular carcinoma, colorectal and prostate cancer (4,6,8,10). Furthermore, there is a strong correlation between VM and malignant features of cancer, such as advanced stage or grade, poor differentiation and short overall survival. In our study, VM was detected in 135 specimens of bladder cancer and its positive rate was 31.1%, which is similar to a previous study (23). Despite the presence of VM in our specimens, we did not observe a significant correlation between VM and clinical parameters such as TNM stage, pathological grade and recurrence in our study. However, Zhou et al (24) reported that VM was not only closely associated with pathological grade, stage and recurrence, but also stimulated metastasis of bladder cancer, which the tumor cells may transfer to distant locations through VM. The discrepancies between these studies may be related to differences in the study populations. For instance, the patients included in the study by Zhou et al (24) were treated with radical cystectomy, but our study consisted of a large number of specimens resected from patients under transurethral resection of bladder tumor (TURBT). Factors affecting the selection of operation methods may have led to bias in the two study groups. Nevertheless, the present study confirms that VM exists in bladder cancer. Furthermore, we want to explore the molecular mechanism of VM in bladder cancer since little research has been conducted in this area.
In a previous study, VM was detected in paraffin-embedded samples of bladder cancer, but further in vitro research into its mechanism was not performed. Wang et al (25) found that human bladder transitional cancer cell lines J82 and T24 generated VM formation, and this was inhibited by downregulation of UHRF1 via miR-124. Likewise, in the current study, we confirmed that bladder transitional cancer cell lines UM-UC-3 and J82 can generate VM structures in a 3-D Matrigel culture, but the immortalized human bladder epithelium cell line SV-HUC-1 did not exhibit this ability, even when the seeding concentration was higher or the observation time was longer. Notably, both of the VM-forming cell lines, UM-UC-3 and J82, showed higher ZEB1 expression compared with SV-HUC-1 and the phenomenon was verified in IHC staining of paraffin-embedded samples. In 135 specimens resected from patients with bladder cancer, the rate of high expression of ZEB1 was 60.0%, yet a further 12 specimens from normal adjacent tissues were ZEB1-negative (P<0.05). Our results also found that MIBC (T2-4) tissue sections showed higher ZEB1 expression compared with NMIBC (Ta-T1) sections. In terms of pathological grade, ZEB1 was expressed at a higher level in the high-grade group compared with the low-grade group. These findings suggested that ZEB1 may contribute significantly to the progression of bladder cancer. Furthermore, we demonstrated that VM presentation in bladder cancer tissues was closely correlated with ZEB1 overexpression, in accordance with a previous study in colorectal carcinoma (8). However, it is unclear whether ZEB1 can regulate VM formation in bladder transitional cancer cell lines, which arouses us strong interest.
Recently, many studies have proposed that EMT is vital for VM formation and tumor progression (9). Some regulatory factors, such as Twist, Runx2 and ZEB2, play important roles in VM formation by promoting EMT (6,16,17). As a crucial EMT-inducer, ZEB1 was increased in colorectal carcinoma samples and its expression concomitantly occurred with EMT features in vivo and in vitro. Furthermore, knockdown of ZEB1 inhibited VM formation in HCT116 cells, accompanied by upregulated epithelial marker E-cadherin and downregulated mesenchymal marker vimentin expression (8). Similarly, VM was inhibited in the breast cancer cell line, Mda-MB-231, by knockdown of ZEB1 (26). To further clarify the relationship between VM and ZEB1 in bladder cancer, a 3-D culture assay was performed after transfection with a specific siRNA to decrease ZEB1 expression in bladder transitional cancer cell lines. Notably, VM formation was inhibited in both UM-UC-3 and J82 cell lines after reduction of ZEB1. However, we did not observe the phenomenon by which epithelial and mesenchymal markers in bladder transitional cancer cell lines go into reverse (data not shown), which was inconsistent with a previous study (8). We propose that ZEB1 may be an intermediate step in the VM formation process in bladder cancer, regulated by some unknown upstream molecules or affecting an unknown downstream gene, and it may not be directly associated with epithelial phenotype. Therefore, we could not observe changes in EMT markers after we inhibited ZEB1 expression in bladder cancer. In summary, ZEB1 is at least a key factor in VM formation in bladder cancer, but its detailed mechanism is unclear and worthy of further exploration.
In conclusion, the present study confirms that ZEB1 is associated with VM in bladder cancer. Moreover, ZEB1 is vital in the process of VM formation. However, our study has certain limitations. For instance, it was a retrospective study in a single center and the patients admitted were only from the last two years, meaning that there is a lack of long-term survival data. The value of VM in bladder cancer remains to be elucidated. Furthermore, we verified that ZEB1 is important for VM formation, but the mechanism of it has not been investigated thoroughly. Hence, in the future, a multicenter and prospective study must be undertaken to validate the relationship between VM and clinical features. In addition, we will explore the detailed mechanism of VM in bladder cancer and find downstream genes of ZEB1 to clarify the exact ZEB1-regulation pathway in VM formation.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 81001146) and the Science and Technology Planning Project of Guangdong Province, China (nos. 2012B031800033 and 2017A020215168).
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Mayr R, Fritsche HM, Pycha A, Pycha A. Radical cystectomy and the implications of comorbidity. Expert Rev Anticancer Ther. 2014;14:289–295. doi: 10.1586/14737140.2014.868775. [DOI] [PubMed] [Google Scholar]
- 4.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi SA, Moinfar M, Gohari Moghaddam K, Bahadori M. Practical application of angiogenesis and vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med. 2010;13:498–503. [PubMed] [Google Scholar]
- 6.Ma JL, Han SX, Zhu Q, Zhao J, Zhang D, Wang L, Lv Y. Role of twist in vasculogenic mimicry formation in hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys Res Commun. 2011;408:686–691. doi: 10.1016/j.bbrc.2011.04.089. [DOI] [PubMed] [Google Scholar]
- 7.Karroum A, Mirshahi P, Faussat AM, Therwath A, Mirshahi M, Hatmi M. Tubular network formation by adriamycin-resistant MCF-7 breast cancer cells is closely linked to MMP-9 and VEGFR-2/VEGFR-3 over-expressions. Eur J Pharmacol. 2012;685:1–7. doi: 10.1016/j.ejphar.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Sun B, Qi L, Li H, Gao J, Leng X. Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012;103:813–820. doi: 10.1111/j.1349-7006.2011.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, Luo H, Zhang J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J Cell Mol Med. 2016;20:1761–1769. doi: 10.1111/jcmm.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Lin H, Pan J, Mo C, Zhang F, Huang B, Wang Z, Chen X, Zhuang J, Wang D, Qiu S. Vasculogenic mimicry in prostate cancer: The roles of EphA2 and PI3K. J Cancer. 2016;7:1114–1124. doi: 10.7150/jca.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Sun Y, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Lu W, Huang C, Ding K, Xia D, Wu Y, Cai M. Prognostic significance of ZEB1 and ZEB2 in digestive cancers: A cohort-based analysis and secondary analysis. Oncotarget. 2017;8:31435–31448. doi: 10.18632/oncotarget.15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanrahan K, O'Neill A, Prencipe M, Bugler J, Murphy L, Fabre A, Puhr M, Culig Z, Murphy K, Watson RW. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol Oncol. 2017;11:251–265. doi: 10.1002/1878-0261.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen JE, Nathan V, Osborne JK, Farrow RK, Deb D, Sullivan JP, Dospoy PD, Augustyn A, Hight SK, Sato M, et al. ZEB1 drives epithelial-to-mesenchymal transition in lung cancer. J Clin Invest. 2016;126:3219–3235. doi: 10.1172/JCI76725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Lin P, Sun B, Zhang S, Cai W, Han C, Li L, Lu H, Zhao X. Epithelial-mesenchymal transition regulated by EphA2 contributes to vasculogenic mimicry formation of head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:803914. doi: 10.1155/2014/803914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Z, Sun B, Zhao X, Zhang Y, Gu Q, Liang X, Dong X, Zhao N. The expression and functional significance of Runx2 in hepatocellular carcinoma: Its role in vasculogenic mimicry and epithelial-mesenchymal transition. Int J Mol Sci. 2017;18:pii: E500. doi: 10.3390/ijms18030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q, An J, Dong X, Liu F, Wang Y. ZEB2 promotes vasculogenic mimicry by TGF-β1 induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Exp Mol Pathol. 2015;98:352–359. doi: 10.1016/j.yexmp.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou J, Li L, Chen Y, Zhang T, Wang X, et al. PI3K/Akt to GSK3β/β-catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell Signal. 2012;24:2273–2282. doi: 10.1016/j.cellsig.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Mahdavinezhad A, Yadegarazari R, Mousavi-Bahar SH, Poorolajal J, Jafari M, Amirzargar MA, Effatpanah H, Saidijam M. Evaluation of zinc finger E-box binding homeobox 1 and transforming growth factor-beta2 expression in bladder cancer tissue in comparison with healthy adjacent tissue. Investig Clin Urol. 2017;58:140–145. doi: 10.4111/icu.2017.58.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney PA, Wszolek MF, Rieger-Christ KM, Neto BS, Gould JJ, Harty NJ, Mosquera JM, Zeheb R, Loda M, Darling DS, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011;107:656–663. doi: 10.1111/j.1464-410X.2010.09489.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Miyake H, Nishikawa M, Fujisawa M. Expression profile of epithelial-mesenchymal transition markers in non-muscle-invasive urothelial carcinoma of the bladder: Correlation with intravesical recurrence following transurethral resection. Urol Oncol. 2015;33:110.e11–8. doi: 10.1016/j.urolonc.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31:16. doi: 10.1186/1756-9966-31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L, Wu S, Zhou L, Song W, Wang D. Expressions of CD133 and CD82/KAI1 in bladder urothelial carcinoma and their correlation with vasculogenic mimicry. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1336–1340. (In Chinese) [PubMed] [Google Scholar]
- 24.Zhou L, Chang Y, Xu L, Hoang ST, Liu Z, Fu Q, Lin Z, Xu J. Prognostic value of vascular mimicry in patients with urothelial carcinoma of the bladder after radical cystectomy. Oncotarget. 2016;7:76214–76223. doi: 10.18632/oncotarget.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y, Gu X, Meng F. MiR-124 exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015;282:4376–4388. doi: 10.1111/febs.13502. [DOI] [PubMed] [Google Scholar]
- 26.LI H, Song S, Xu Y, Zhao J, Liu H. Knockdown of ZEB1 suppresses the formation of vasculogenic mimicry in breast cancer cell line MDA-MB-231 through downregulation of Flk-1. Minerva Med. 2017;108:191–193. doi: 10.23736/S0026-4806.16.04850-3. [DOI] [PubMed] [Google Scholar]