Abstract
The present study describes the case of a 48-year-old man who was diagnosed with lung adenocarcinoma with an epidermal growth factor receptor (EGFR) 21 L858R mutation. The patient received surgery and adjuvant chemotherapy. When multiple lung metastases appeared, icotinib was administered. Following resistance to icotinib, biopsy by endobroncheal ultrasonography for a right lung hilar lymph node revealed transformation to a neuroendocrine morphology. Neuron-specific enolase (NSE) levels were elevated, accompanied with disease progression following transformation to the neuroendocrine morphology. The post-operative and biopsy specimens were analyzed for 416 genes using next-generation sequencing, and phosphatidylinositol-3-kinase catalytic α mutation and retinoblastoma loss were evident. Five cycles of etoposide combined with cisplatin were administered and a partial response was achieved. The disease progressed again accompanied with an elevated NSE level, and bronchoscopy examination revealed small cell lung cancer (SCLC) after 3 months. The patient received chemotherapy consisting of irinotecan combined with carboplatin for two cycles and achieved stable disease. Overall, a secondary biopsy is important for the evaluation of genetic and histological changes and the selection of an appropriate treatment following tyrosine kinase inhibitor (TKI) resistance, and NSE may be useful for the early detection of SCLC transformation in cases that are resistant to EGFR-TKI therapy.
Keywords: small cell lung cancer, adenocarcinoma, transformation, tyrosine kinase inhibitor, resistance
Introduction
Lung cancer, including small-cell lung cancer (SCLC) and non-SCLC (NSCLC), which is mainly comprised of adenocarcinoma, squamous cell carcinoma and large cell carcinoma, is the most common type of malignancy and the leading cause of cancer-associated mortality worldwide (1). Adenocarcinoma is the most common type of NSCLC, and epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) therapy, including erlotinib, gefitinib or icotinib, is the gold standard of treatment for EGFR-mutant lung adenocarcinoma (2–4). The incidence of EGFR mutation in NSCLC in 2005 was higher in East Asian populations when compared with the incidence in other ethnicities (30 vs. 8%) (5). Patients with lung adenocarcinoma harboring exon 19 deletions achieved longer progression-free survival (PFS) and overall survival time (OS) compared with those with L858R mutations (6). However, patients ultimately develop acquired resistance, and the most recently identified mechanism for this is transformation to SCLC (7). Ahn et al (8) reported six cases of transformation from lung adenocarcinoma to SCLC. Erlotinib, gefitinib, afatinib and icotinib were equally as efficient as each other but exhibited different efficacy-toxicity patterns (4). EGFR-TKI-resistant SCLCs are differentiated early from the lung adenocarcinoma clones that harbor completely inactivated retinoblastoma 1 (RB1) and TP53 (9). Molecular mechanisms involved in the transformation from NSCLC to SCLC include TP53 mutations, RB1 loss, lack of EGFR expression and MYC amplification. The most studied signaling pathway is the achaete-scute homolog 1 (ASCL1) which is regulated by four different neurogenic locus notch homolog (NOTCH) receptors. NOTCH alterations promote ASCL1 and CD56 overexpression (9). These changes induce cyclin-dependent kinase 5 (CDK5) activity and inactivation of RB by phosphorylation (9). The present study reports a case of acquired resistance to icotinib therapy through transformation to SCLC. The results implicate that a secondary biopsy is important to clarify the mechanism of TKI resistance, and NSE may be useful for the early detection of SCLC transformation in cases that are resistant to EGFR-TKI therapy.
Case report
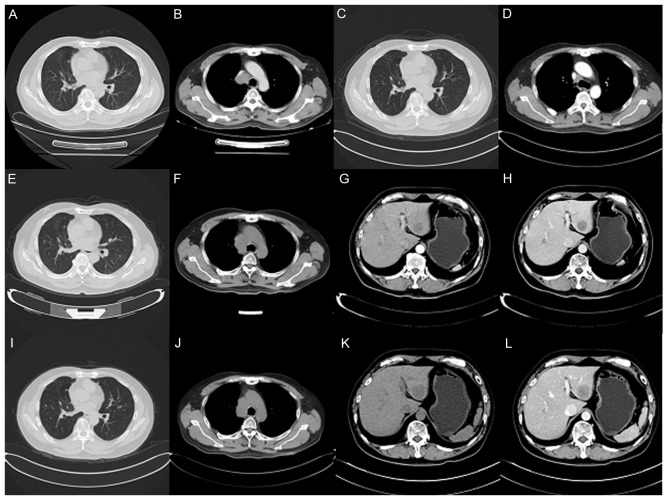
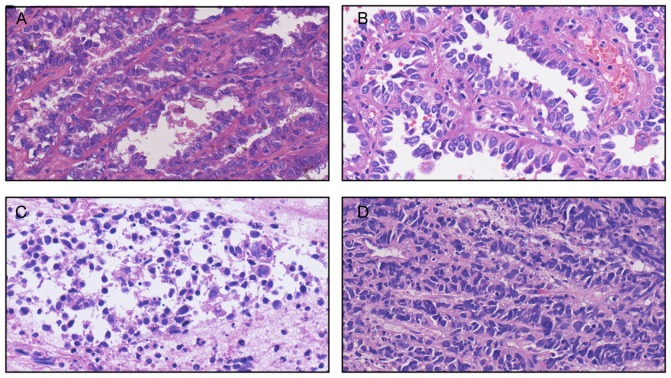
A 55-year-old man with a history of smoking was referred to Zhejiang Cancer Hospital (Hangzhou, China) September 22, 2015 due to occur lung metastasis. A right upper lobe lobectomy was performed June 26, 2009 (48-year-old). The pathological diagnosis was of lung adenocarcinoma with a mixed acinar and papillary pattern (June 26, 2009) and the stage of cancer was pT2aN1M0 (IIB) according to the eighth edition of the Tumor-Node-Metastasis classification for lung cancer (10). The results of immunohistochemistry (Primary antibody in Table I; Secondary antibody: EnVision FLEX/horseradish peroxidase; dilution, ready-to-use; cat. no., K8000, Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) (11–13) markers were as follows: Thyroid transcription factor 1 (TTF-1)(+), Napsin A(+), synaptophysin (Syn)(−) and chromogranin A(−). The patient received six cycles of adjuvant carboplatin/gemcitabine intravenously (1.6 g gemcitabine on days 1 and 8, and 100 mg carboplatin on days 1, 2 and 3, every three weeks for one cycle) chemotherapy without complication, but at 37 months post-surgery, presented with a metachronous solitary left lower lobe nodule, detected by computed tomography, and underwent a wedge resection. The tumor was ~0.9×0.8×0.7 cm, without interlobar lymph node metastasis. The pathological diagnosis using the aforementioned method was of invasive adenocarcinoma (mainly papillary accompanied with an acinar pattern; Jan 30, 2013) and was detected to harbor an EGFR 21 L858R mutation according to amplification refractory mutation system detection, as previously described (14). The patient subsequently received four cycles of pemetrexed (1,000 mg on day 1, every three weeks for one cycle). After 10 months, the patient received icotinib treatment (125 mg thrice daily) due to multiple lung metastasis, detected by computed tomography, and a complete response was achieved. After another 19 months, multiple lung metastases appeared again and the right lung hilar lymph node was determined to be enlarged on surveillance by computed tomography (Fig. 1A and B). Biopsy by endobroncheal ultrasonography for the right hilar lymph node revealed transformation to neuroendocrine carcinoma (Sep 20, 2015) and the following immunohistochemical results (Primary antibody in Table I): Cytokeratin (CK)(+), TTF-1(+), CK7 (weak positive), Ki-67(+, 40%), CD56(+), carcinoembryonic antigen (CEA)(−), Syn (weak positive), CK5/6(−), P40(−), chromogranin A(−) and Napsin A(−). The staining analysis was performed as described previously (11–13). The post-operative and biopsy specimens were analyzed for 416 genes by next-generation sequencing, and phosphatidylinositol 3-kinase catalytic α (PIK3CA) mutation and retinoblastoma (RB) loss were found (Table II). Neuron-specific enolase (NSE) level was elevated when transformation to neuroendocrine carcinoma occurred. A total of five cycles of intravenous etoposide (180 mg/d1) combined with cisplatin (45 mg day 1, day 2, day 3, every three weeks for one cycle) were administered and a partial response was achieved (Fig. 1C and D). Disease progression occurred accompanied with an elevated NSE level after 6 months (Fig. 1E-H), and bronchoscopy examination revealed SCLC in the right upper lobe (Mar 16, 2016). The patient received intravenous chemotherapy consisting of irinotecan (120 mg on days 1 and 8) combined with carboplatin (600 mg on day 1) every three weeks for two cycles, and achieved stable disease for two months (Fig. 1I-L). Subsequently, the patient was followed up once, 2–3 months later. The patient succumbed in April 2017 due to deterioration of the condition. Pathological results of the patient at different times during the study period is presented in Fig. 2. The changes in NSE and CEA concentration are presented in Fig. 3. The sequence of anticancer treatments is presented in Table III. The patient provided written informed consent for the publication of the present study.
Table I.
Antibody used in the present study.
| Antibody | Dilution | Catalog number | Supplier |
|---|---|---|---|
| TTF-1 | 1:200 | MAB-0599 | Maxim |
| Napsin A | 1:400 | NCL-L-NapsinA | Leica |
| Syn | RTU | IR660 | DAKO |
| Chromogranin A | 1:200 | MAB-0202 | Maxim |
| CK | RTU | IR 053 | DAKO |
| CK7 | RTU | IR619 | DAKO |
| Ki-67 | RTU | IR626 | DAKO |
| CD56 | RTU | IR628 | DAKO |
| CEA | RTU | IR622 | DAKO |
| CK5/6 | 1:400 | MAB-0276 | Maxim |
| P40 | 1:200 | ACI3066C | BIOCARE |
TTF-1, thyroid transcription factor 1; Syn, synaptophysin; RTU, ready to use; CK, cytokeratin; CEA, carcinoembryonic antigen.
Figure 1.
Computed tomography scans of the patient. (A and B) Aug 21, 2015: Multiple lung metastases appeared and the right lung hilar lymph node appeared enlarged, as visualized in the (A) pulmonary and (H) mediastinal windows. (C and D) Dec 21, 2015: Regression of lung metastatic neoplasm and notable shrinking of the right mediastinal lymph node, as visualized in the (C) pulmonary and (D) mediastinal windows. (E and F) Mar 16, 2016: Lung metastasis and hilar lymph node was markedly enlarged, as visualized in the (E) pulmonary and (F) mediastinal windows. (G and H) Mar 16, 2016: Emerging new liver metastasis as visualized in the (G) arterial and (H) portal phases. (I and J) May 10, 2016: Lung metastasis and enlarged mediastinal lymph node, as visualized in the (I) pulmonary and (J) mediastinal windows. (K and L) May 10, 2016: Liver metastasis as visualized in the (K) arterial and (L) portal phases.
Table II.
Genetic alteration for three specimens obtained from the patient over the study period.
| Gene | Specimen from 2009 | Specimen from 2013 | Specimen from 2015 |
|---|---|---|---|
| EGFR (L858R) | 11% | 25% | 78% |
| RB1 (Y567fs) | 6% | 20% | 57% |
| TP53 (S241C) | 20% | 34% | 73% |
| PIK3CA (E545A) | No | No | 3% |
| MYCL amplification | No | No | 21.6 times |
| RB1 loss | No | Yes | Yes |
| RAC1 amplification | 2.7 times | No | No |
Percentages refer to the percentage of cells positive for the mutation. EGFR, epidermal growth factor receptor; RB, retinoblastoma; PIK3CA, phosphatidylinositol-3-kinase catalytic α; TP53, tumor protein p53; MYCL, MYCL proto-oncogene, bHLH transcription factor; RAC1, ras-related C3 botulinum toxin substrate 1 (ρ family, small GTP binding protein Rac1).
Figure 2.
Pathological results of the patient at different times in the study period. (A) June 26, 2009: Predominant papillary/micropapillary adenocarcinoma, partly combined with acinar adenocarcinoma. (B) Jan 30, 2013: Predominant papillary adenocarcinoma and acinar adenocarcinoma. (C) Sep 20, 2015: Neuroendocrine carcinoma (inclining toward small cell lung cancer). (D) Mar 16, 2016: Small cell lung cancer.
Figure 3.
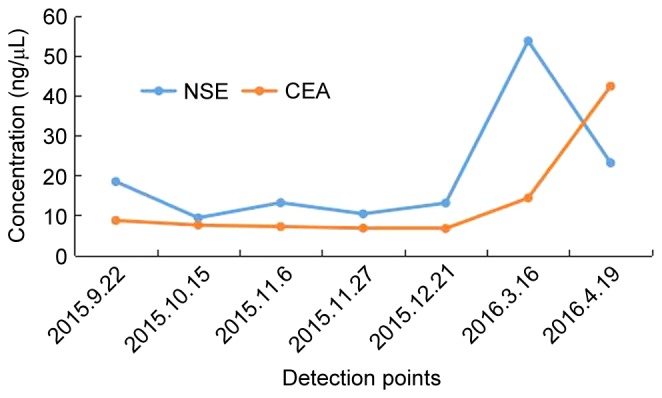
Variation curve of NSE and CEA. NSE, neuron-specific enolase; CEA, carcinoembryonic antigen.
Table III.
Sequence of anticancer treatments.
| Date | Treatment |
|---|---|
| June 2009 | Right upper lobectomy |
| December 2009 | Completed adjuvant gemcitabine-carboplatin chemotherapy |
| January 2013 | Underwent wedge resection of left lower lobe nodule |
| June 2013 | Completed pemetrexed chemotherapy |
| August 2013 | Multiple lung metastasis |
| November 2013 | Commenced icotinib treatment |
| August 2015 | Multiple lung metastasis and biopsy by endobroncheal ultrasonography for the hilar lymph node, which revealed transformation to neuroendocrine carcinoma |
| December 2015 | Completed etoposide-cisplatin chemotherapy |
| March 2016 | Multiple lung metastasis enlarged and hepatic metastasis appeared |
| May 2016 | Complete two cycles of irinotecan combined with carboplatin |
Discussion
A number of mechanisms of acquired resistance to EGFR-TKI therapy in EGFR mutant lung adenocarcinoma have been described, including the EGFR T790M mutation, EGFR amplification, MET gene amplification, PIK3CA mutation and transformation to SCLC (7). Transformation of adenocarcinoma to SCLC in patients with somatic EGFR mutations as the TKI therapy resistance mechanism has previously been reported (15), and the percentage of transformation from adenocarcinoma to SCLC was identified in 14% of cases (7).
Analysis of tumor samples and cell lines derived from resistant EGFR mutant patients revealed that RB is lost in 100% of such SCLC transformed cases, but rarely in those that remain NSCLC (16). The gene detection in the present patient following icotinib resistance also revealed RB loss and the appearance of a PIK3CA gene mutation. It is currently indicated that combined-histology tumors and transformation occur more frequently in lung cancer types with EGFR-activating mutations compared with EGFR wild-type tumors. The reason for this may be that the cell of origin of certain EGFR-mutant adenocarcinomas, type II alveolar cells, also have the potential to become SCLC (17). Mixed EGFR-mutant NSCLC/SCLC histology has been reported in a number of cases, indicating a certain degree of dynamic plasticity between the two histologies in specific cases, without the selective pressure of the EGFR TKI (18–20). The present case was verified as an adenocarcinoma, and not combined SCLC, through surgical resection and transformation from adenocarcinoma to SCLC following icotinib resistance. A secondary biopsy is important in order to evaluate the genetic and histological changes, and to select an appropriate treatment for TKI resistance. In the present patient, a secondary biopsy and gene detection were performed in order to permit the use of a more rational therapy. It is occasionally difficult to perform a second biopsy, and so NSE level may be useful for the early detection of transformation to SCLC in cases that are resistant to EGFR-TKI therapy. NSE could potentially overcome the limitations of performing biopsies on single lesions, which may miss the transformation of another metastatic lesion into SCLC (21–24). It was concluded that a secondary biopsy was important for the evaluation of genetic and histological changes and the selection of an appropriate treatment following TKI resistance, and that NSE may be useful for the early detection of SCLC transformation.
Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (grant no. LY15H290001), the Public Welfare Technology Application Studies Program of Zhejiang Province (grant no. 2016C33118), the Zhejiang Province Traditional Medical Science Fund Project of China (grant no. 2015ZA037) and the 1022 Talent Training Program of Zhejiang Cancer Hospital.
Glossary
Abbreviations
- EGFR
epidermal growth factor receptor
- NSE
neuron-specific enolase
- RB
retinoblastoma
- PIK3CA
phosphatidylinositol-3-kinase catalytic α
- SCLC
small cell lung cancer
- TKI
tyrosine kinase inhibitor
- NSCLC
non-small cell lung cancer
- CEA
carcinoembryonic antigen
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu Z, Xue C, Zhang J, Zhang J, Ma Y, et al. Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS One. 2014;9:e85245. doi: 10.1371/journal.pone.0085245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Z, Jin X, Lin B, Su H, Chen H, Fei S, Zhao L, Deng X, Xie D, Xie C. Efficacy of second-line tyrosine kinase inhibitors in the treatment of metastatic advanced non-small-cell lung cancer harboring exon 19 and 21 EGFR mutations. J Cancer. 2017;8:597–605. doi: 10.7150/jca.16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, Park K, Ahn MJ, Park WY. Transformation to small cell lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50:258–263. doi: 10.4132/jptm.2016.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, An Y, Keam B, Kim DW, Heo DS, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35:3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, Eberhardt WE, van Meerbeeck J, Rami-Porta R, Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:300–311. doi: 10.1016/j.jtho.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi G, Fabbri A, Bianchi F, Maisonneuve P, Rossi G, Barbareschi M, Graziano P, Cavazza A, Rekhtman N, Pastorino U, et al. ΔNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: A novel two-hit, sparing-material approach. J Thorac Oncol. 2012;7:281–290. doi: 10.1097/JTO.0b013e31823815d3. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–873. [PMC free article] [PubMed] [Google Scholar]
- 13.Brown AF, Sirohi D, Fukuoka J, Cagle PT, Policarpio-Nicolas M, Tacha D, Jagirdar J. Tissue-preserving antibody cocktails to differentiate primary squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of lung. Arch Pathol Lab Med. 2013;137:1274–1281. doi: 10.5858/arpa.2012-0635-OA. [DOI] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A, Kurokawa K, Tambo Y, Takato H, Ohkura N, et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370–372. doi: 10.1016/j.lungcan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto I, Araki J, Suto R, Shimada M, Nakagawa K, Fukuoka M. EGFR mutation in gefitinib responsive small-cell lung cancer. Ann Oncol. 2006;17:1028–1029. doi: 10.1093/annonc/mdj114. [DOI] [PubMed] [Google Scholar]
- 19.Tatematsu A, Shimizu J, Murakami Y, Horio Y, Nakamura S, Hida T, Mitsudomi T, Yatabe Y. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res. 2008;14:6092–6096. doi: 10.1158/1078-0432.CCR-08-0332. [DOI] [PubMed] [Google Scholar]
- 20.Lu HY, Sun WY, Chen B, Zhang YP, Cai JF, Su D, Wang Z, Zheng YQ, Ma SL. Epidermal growth factor receptor mutations in small cell lung cancer patients who received surgical resection in China. Neoplasma. 2012;59:100–104. doi: 10.4149/neo_2012_013. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Hu B, Li W, Xue J. Transformation from NSCLC to SCLC: When did it happen? Lancet Oncol. 2015;16:e309. doi: 10.1016/S1470-2045(15)00058-3. [DOI] [PubMed] [Google Scholar]
- 22.Engelman JA, Oser MG, Niederst MJ, Sequist LV. Transformation from NSCLC to SCLC: When did it happen?-Authors' reply. Lancet Oncol. 2015;16:e309–e310. doi: 10.1016/S1470-2045(15)00058-3. [DOI] [PubMed] [Google Scholar]
- 23.Norkowski E, Ghigna MR, Lacroix L, Le Chevalier T, Fadel É, Dartevelle P, Dorfmuller P, Thomas de Montpréville V. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: New insights in the era of molecular pathology. J Thorac Oncol. 2013;8:1265–1271. doi: 10.1097/JTO.0b013e3182a407fa. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Li XY, Tang Y, Xu Y, Guo WH, Li YC, Liu XK, Huang CY, Wang YS, Wei YQ. Rapid increase of serum neuron specific enolase level and tachyphylaxis of EGFR-tyrosine kinase inhibitor indicate small cell lung cancer transformation from EGFR positive lung adenocarcinoma? Lung Cancer. 2013;81:302–305. doi: 10.1016/j.lungcan.2013.04.005. [DOI] [PubMed] [Google Scholar]