Abstract
Adult neurogenesis occurs in the dentate gyrus in the mammalian hippocampus. These new neurons arise from neural precursor cells named radial glia-like cells, which are situated in the subgranular zone of the dentate gyrus. Here, we review the emerging topic of precursor heterogeneity in the adult subgranular zone. We also discuss how this heterogeneity may be established during development and focus on the embryonic origin of the dentate gyrus and radial glia-like stem cells. Finally, we discuss recently developed single-cell techniques, which we believe will be critical to comprehensively investigate adult neural stem cell origin and heterogeneity.
Keywords: radial glial cells, dentate gyrus, stem cell heterogeneity
Introduction
The dentate gyrus (DG) is a V-shaped structure in the hippocampus, which is located in the medial temporal cortex of mammals. The addition of newborn neurons to the DG, unlike other areas of the brain, such as the neocortex where neurons are generated only during embryonic development, continues throughout life through a process named adult neurogenesis 1, 2. Interestingly, adult neurogenesis in the DG has been observed in all studied mammals, including humans, suggesting that there may be some evolutionarily conserved function of adult hippocampal neurogenesis 3– 6. Indeed, animal models have shown that adult neurogenesis in the DG plays important roles in both cognitive and affective behaviors, such as spatial memory learning and retention, pattern separation, and memory clearance 7– 11.
Adult-born neurons in the DG are derived from a population of neural stem cells (NSCs) named radial glia-like cells (RGLs) 1. RGLs express some astrocyte and stem cell markers and can generate both granule neurons and astrocytes but typically not oligodendrocytes 12– 15. These RGLs retain the capacity to divide and generate new neurons throughout life, even in the aging animal 16, 17. Recently, more and more studies have shown that rather than being a homogeneous population of identical cells, the RGL population is made up of multiple subpopulations of RGLs that differ in their morphology and how they react to external stimuli 18. In this review, we discuss recent discoveries concerning adult neurogenesis in the DG and focus on RGL heterogeneity. Furthermore, we review current knowledge about the embryonic and early postnatal development of the DG and RGL origins given that NSC heterogeneity may be established during development. Finally, we discuss current single-cell analysis techniques that could be used to answer a multitude of remaining questions that concern RGL heterogeneity and its origin.
Classic homogeneous radial glia-like cell population model
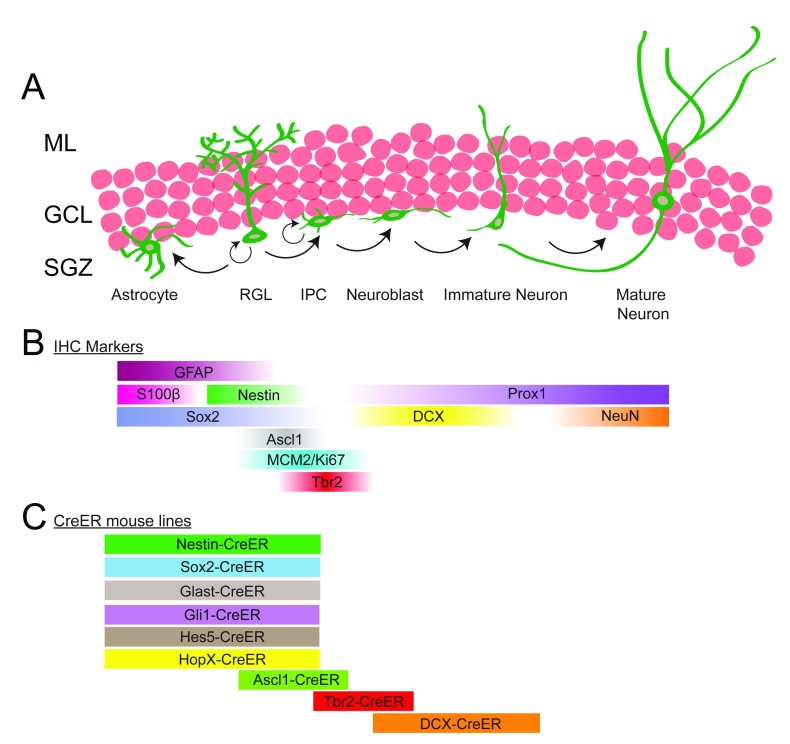
Multiple studies have indicated that RGLs (also known as type 1 cells) are putative NSCs, which generate dentate granule neurons in the adult DG 14, 15, 19, 20. RGLs are similar in appearance to radial glial cells of the embryonic brain and share many markers expressed by NSCs in the embryo, including Nestin, glial fibrillary acidic protein (GFAP), and sex-determining region Y-box 2 (Sox2) 13. The somas of the RGLs are situated in the subgranular zone (SGZ) of the DG, a region between the granule cell layer (GCL) and the hilus ( Figure 1A). RGLs have a bushy radial process, which extends through the GCL to the molecular layer and terminates with end-feet on both synapses and vasculature 21, 22.
Figure 1. Lineage progression of adult neurogenesis.
( A) Radial glia-like cells (RGLs), situated in the subgranular zone (SGZ) of the dentate gyrus (DG), have the potential to both self-renew and give rise to astrocytes and neurons. Each RGL has a bushy process that extends through the granule cell layer (GCL) and ends in the molecular layer (ML). In the process of generating neurons, RGLs divide to generate intermediate progenitor cells (IPCs), which are highly proliferative and lineage-restricted to the neuronal fate. IPCs progress through a series of steps and eventually differentiate into mature neurons which integrate into the existing neuronal networks. ( B) Immunohistochemical (IHC) markers that can be used to distinguish different stages of the lineage progression in adult neurogenesis in the DG. ( C) Many Cre-ER transgenic mouse lines have been used to label different cell types throughout the process of neurogenesis. Most of the Cre-ER lines that induce recombination in RGLs also label astrocytes but to varying degrees. Tbr2-CreER and doublecortin (DCX)-CreER lines label neural precursors with no contamination of astrocytes or neural stem cells, while the Ascl1-CreER line labels both RGLs and IPCs.
RGLs are generally a quiescent population of precursor cells that only occasionally divide 14, 23, 24. However, when RGLs undergo cell division, they can divide either symmetrically or asymmetrically multiple times, suggesting that they retain the capacity to self-renew 13, 20. During the process of neurogenesis, RGLs divide and give rise to intermediate progenitor cells (IPCs), which express the T-box brain protein 2 (Tbr2/Eomes) ( Figure 1A, B). IPCs have short multipolar processes, are lineage-restricted, and undergo limited rounds of division 25, 26. IPCs then give rise to bipolar neuroblasts, which express doublecortin (DCX). Neuroblasts migrate tangentially along the SGZ before migrating short distances radially into the GCL, where they mature into functional Prox1-positive dentate granule neurons 15.
Quiescent RGLs are difficult to label using developmental lineage-tracing methods, such as thymidine analogues—5-bromo-2'-deoxyuridine (BrdU) and 5-ethynyl-2'-deoxyuridine (EdU)—or retrovirus, because these techniques label dividing cells only 27. However, RGLs can be labeled using multiple tamoxifen-inducible CreER T2 mouse lines ( Figure 1C). Interestingly, the use of different mouse lines has started to reveal the complexity and heterogeneity of the progenitor cells in the adult DG. For example, a study using the Nestin-CreER T2 and glutamate aspartate transporter (Glast)-CreER T2 mouse lines has shown that while the cells labeled using both lines contribute to neurogenesis under homeostasis, only Glast-CreER T2-labeled RGLs contributed to increased proliferation after running and repopulation after injury 28. Additionally, studies using the Hes5-CreER T2 and Sox2-CreER T2 lines have suggested the presence of a horizontal and non-radial NSC in the DG 19, 20. These and other studies have provided evidence that subpopulations of RGLs with different properties coexist in the adult DG, and current work has been focusing on identifying and distinguishing these populations.
Modern heterogeneous radial glia-like cell population model
Given the accumulating evidence that RGLs are not identical in the adult mouse DG 28, 29, a central question in the field of adult neurogenesis arises: how do discrete subtypes of RGLs in the niche differ in their capacity to self-renew and differentiate? For example, it is possible that a distinct population of RGLs is responsible for generating neurons while another is responsible for generating astrocytes. These possibilities have not been exhaustively explored yet, but recent data clearly suggest that the SGZ consists of RGLs with different morphologies and behaviors.
The adult mouse DG can be divided along its longitudinal axis into the septal pole (the dorsal region) and the temporal pole (the ventral region) 30. Interestingly, the septal and temporal DGs function differently, from the systems level all the way down to the molecular level 31. For example, the septal region of the DG has been shown to be involved with spatial learning while the temporal region is involved in emotional behavior and motivation 32, 33. Similarly, many properties of neurogenesis are dependent on their location along the septo-temporal axis of the DG. For example, the density of RGLs and neuroblasts is lowest in the temporal region of the DG 34. Additionally, the tempo of neurogenesis is faster in the septal region of the DG 35. At the molecular level, there is a gradient in the expression of the Wingless/INT (Wnt) inhibitor Frizzled-related protein 3 (sFRP3), and the highest expression is observed in the temporal pole 36. Deletion of sFRP3 leads to activation of RGLs, suggesting a potential niche mechanism for generating regional heterogeneity in the adult DG 37. In vitro studies also suggest different neurosphere-forming capacities of the neural progenitors along the septo-temporal axis. Treatment of neurospheres with norepinephrine and KCl was shown to increase the number of neurospheres generated from the temporal region, while neurospheres from the septal region were unaffected 38. These data indicate that there are distinct populations of RGLs in the adult DG, which respond differently to niche signaling.
Currently, there are no defined NSC markers to distinguish these different RGL populations in vivo. Instead, morphological differences have been used as one approach to distinguish between different subtypes of RGLs. A recent study used careful analysis of confocal images to show that RGLs can be divided into two classes on the basis of their morphology 29. Cells of the most common type, termed type α cells in the study, possess longer and less branched processes compared with the less prevalent type β cells. Lineage tracing showed that the type α cells could give rise to neurons, astrocytes, and type β cells but that type β cells did not proliferate. This suggests that type α cells are hierarchically above type β cells, but it is not known whether all type α cells have the potential to give rise to the type β cell over time. Future studies should investigate whether the population of type α cells is homogeneous or consists of multiple cell types.
Return to quiescence after cell division is considered a hallmark of slowly cycling, self-renewing adult stem cells and can be assessed using thymidine analogues, such as BrdU and EdU. These labels get incorporated into the DNA of cells during the S phase of the cell cycle. If the cell continues to undergo multiple divisions, the label gets diluted to an undetectable level. In contrast, the presence of label-retaining RGLs after long chase periods indicates that these cells have returned to quiescence after dividing when the EdU or BrdU was administered. For example, one study administered a single injection of BrdU to Nestin-GFP mice and showed that the RGLs that incorporated BrdU quickly diluted the label, and no label-retaining RGLs were found after a 15-day chase 16. This can be put in contrast to other studies, which used longer BrdU pulses and found label-retaining RGLs after longer chases 12, 19, 39. Thus, the ability to label RGLs that return to quiescence depends on the experimental paradigm used. Another reason for the conflicting conclusions concerning the existence of label-retaining RGLs is because most studies have been performed at the population level, where rare populations may get overlooked. For example, it is possible that not all RGLs are able to return to quiescence and that those that can return to quiescence represent a small subpopulation of RGLs in the adult DG. Therefore, a high-resolution understanding of the heterogeneity in the SGZ will require a combination of techniques, including single-cell lineage tracing of specific subpopulations.
Clonal analysis using the Nestin-CreER T2 mouse line has revealed that at least some Nestin + RGLs can self-renew multiple times and are multipotent (generating both neurons and astrocytes) under physiological conditions in the adult DG. Importantly, the Nestin-CreER T2-labeled RGLs are able to return to quiescence after activation 13. Nestin protein is present in most, if not all, RGLs in the adult DG, but it is not known whether the cells labeled under clonal analysis conditions represent the majority of the RGL pool or compose a small subpopulation. It should also be noted that different Nestin-CreER T2 lines have varying specificity and could potentially label different subtypes 40.
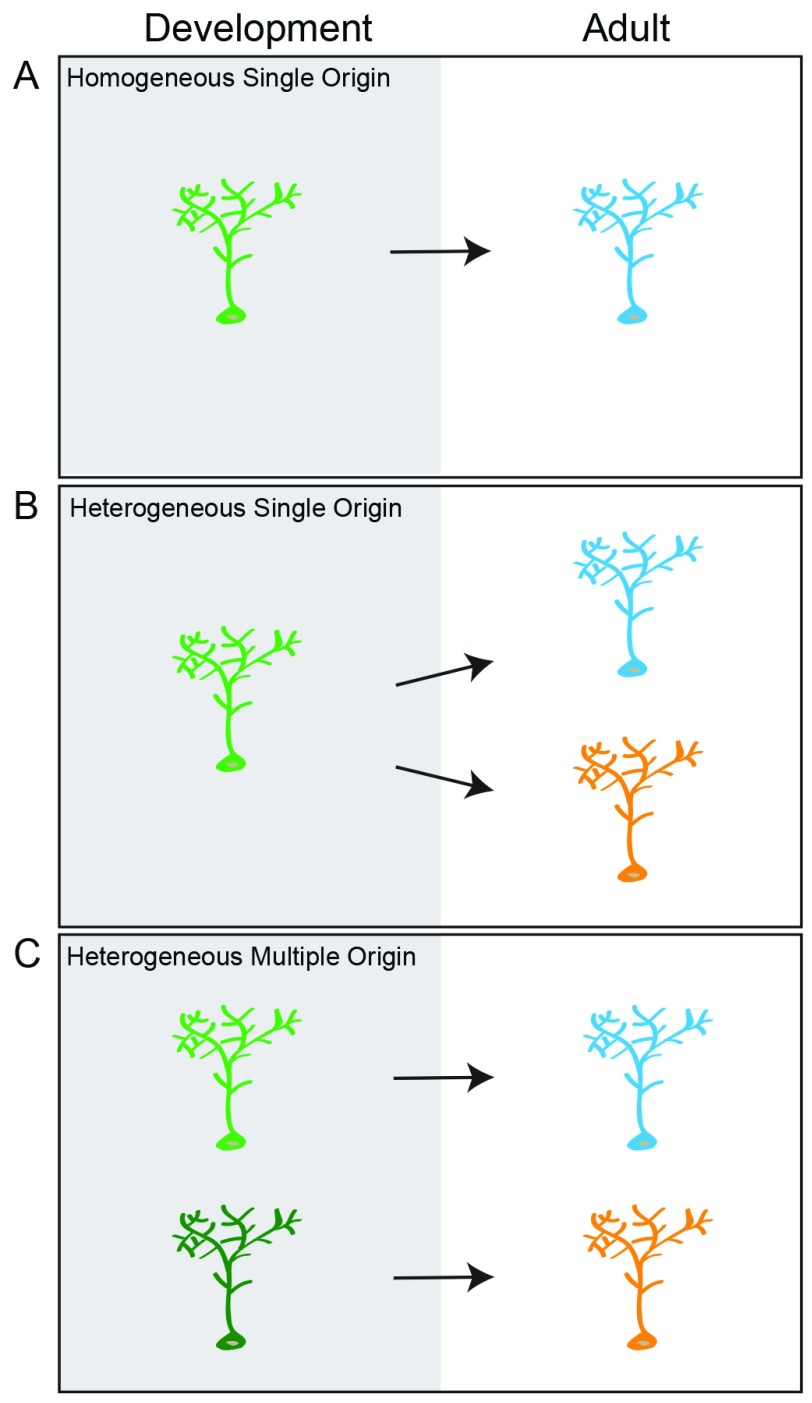
Stem cell heterogeneity has been more rigorously studied in the adult SVZ. The putative NSCs in the adult SVZ are the type B1 cells. These cells express GFAP and have a radial morphology. The type B1 cells can be divided into two groups—the quiescent neural stem cells (qNSCs) and activated neural stem cells (aNSCs)—which can be distinguished by their transcriptional profiles 41, 42. Quiescence was associated with a lack of Nestin expression and high glycolytic and lipid metabolism, whereas activation was associated with upregulation of Nestin expression and high protein synthesis and differentiation priming. The qNSCs give rise to the aNSCs, which in turn generate progeny that migrate to the olfactory bulb and become different types of granular cells and periglomerular interneurons 43, 44. Different types of interneurons are derived from specific subpopulations of type B1 cells located in distinct areas of the ventricular wall. Type B1 cells from different regions of the ventricular wall remain restricted to their lineages even when transplanted into other areas of the ventricular wall, suggesting that they are intrinsically different from each other 44, 45. Furthermore, SVZ progenitors that generate astrocytes are found in distinct domains of the SVZ 46. Clonal lineage tracing from development into adulthood has revealed that regionally specified embryonic NSCs give rise to distinct subpopulations of type B1 cells, suggesting that heterogeneity in the adult SVZ is established embryonically ( Figure 2C) 47. Embryonic DG development has not been studied as extensively as cortical development, and it will be necessary to examine the ontogenesis of the DG to get a complete understanding of when and how adult RGL heterogeneity is established.
Figure 2. Developmental origin of adult neural stem cell heterogeneity.
There are three potential models for the developmental origin of adult neural stem cells. ( A) Homogeneous single origin: all radial glia-like cells (RGLs) in the adult dentate gyrus (DG) belong to a single population of stem cells that have similar potentials and make similar fate choices. These cells have a common developmental progenitor. ( B) Heterogeneous single origin: there are multiple populations of RGLs in the adult DG that have distinct potentials and may make different fate choices, but these cells have a common developmental precursor. ( C) Heterogeneous multiple origin: there are distinct populations of RGLs in the adult DG, and they are generated by different lineage-restricted precursors.
Origin of adult neural stem cells in the dentate gyrus
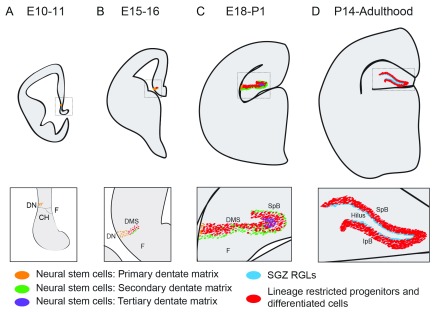
The origin of the adult RGLs in the DG remains largely unknown. Population studies using thymidine analogues, reporter mice, and immunohistological methods suggest that DG precursors originate from a region called the primary dentate neuroepithelia or primary dentate matrix. Identified by Altman and Bayer 48, this putative origin of the DG is situated around the dentate notch, a small indentation in the ventricular wall of the medial pallium, which is visible at embryonic day 11 (E11) in mice ( Figure 3A) 49, 50. At around E15, a stream of cells, seemingly originating from this area, start to migrate toward the pial surface into the dentate primordium. This stream, called the dentate migratory stream (DMS), contains both GFAP +Sox2 + NSCs and Tbr2 + IPCs ( Figure 3B) 50, 51. The proliferating cells in the DMS are termed the secondary dentate matrix 48 ( Figure 3A, B). At around E18, Prox1 + granule neurons appear in what will become the suprapyramidal blade (SpB) of the DG ( Figure 3C) 49, 52. At this stage, the secondary dentate matrix is found on the outside of the granule cell layer, while the proliferating NSCs in the hilar region are termed the tertiary dentate matrix. It has been hypothesized that the primary and secondary dentate matrices contribute to embryonic neurogenesis while the tertiary matrix generates the adult RGLs 52– 54. It is possible that the secondary matrix generates granule cells in the outer layer of the DG, since work using mouse chimeras has shown that granule cells in this part of the DG are derived from a different pool of progenitors compared with the neurons in the inner layers 55.
Figure 3. Development of the dentate gyrus.
( A) Around embryonic day 10–11 (E10–11), the putative dentate neuroepithelium is situated adjacent to the dentate notch (DN), a small indentation of the ventromedial ventricular wall, which in turn is placed caudal to the cortical hem (CH), neighboring the fimbria (F). These neural stem cells (NSCs) are sometimes referred to as the primary dentate matrix. ( B) During embryonic neurogenesis (E15–16), a stream of neural progenitors appears medial to the DN forming the dentate migratory stream (DMS). The DMS consists of both lineage-restricted neural progenitor cells (Tbr2 +) and NSCs (expressing Sox2, Nestin, and GFAP). The DMS leads to the dentate primordium. ( C) At about E18, the upper blade of the dentate gyrus (also called the suprapyramidal blade, or SpB) starts to form and contains post-mitotic Prox1 + neurons, as well as a layer of NSCs that are located subpially, on the upper part of the SpB blade. These NSCs are defined as the secondary dentate matrix, and the NSCs ventral to the SpB are termed the tertiary dentate matrix. ( D) At about postnatal day 14 (P14), both the SpB and the infrapyramidal blade (IpB) have formed. At this stage, the secondary and tertiary matrices are gone, and the remaining NSCs—now referred to as radial glia-like cells (RGLs)—are located in the subgranular zone (SGZ), on the border between the granule cell layer and the hilus. This structure and morphology are maintained throughout the rest of the animal’s life.
Although no lineage-tracing studies have examined the early embryonic origin of adult DG RGLs, fate mapping studies using the Gli1-CreER mouse line have shown that a subset of developmental precursors to adult RGLs become sonic hedgehog-responsive around E17.5 56. In this study, Li et al. observed that the sonic hedgehog-responsive cells were located in the ventral hippocampus and that these cells migrated into the dorsal hippocampus and generated neurogenic RGLs in the adult animal, suggesting a ventral-to-dorsal NSC migration pattern 56.
At postnatal day 14 (P14), the infrapyramidal blade (IpB) has formed, giving the DG its characteristic V-shape ( Figure 3D). By this time, the secondary matrix has disappeared with most of the proliferating NSCs found in the SGZ, where they remain into adulthood 50, 52. Immunohistochemical analysis of known markers for adult neurogenesis has suggested that the SGZ is morphologically adult-like by P14, but comprehensive analysis of the potential of individual NSCs during different stages of development is still lacking 52.
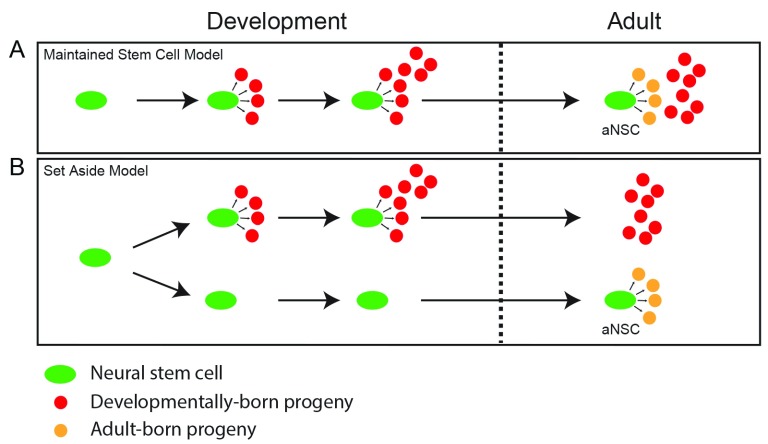
In the adult SVZ, type B1 cells are derived from developmental radial glia cells 57. In the original model, NSCs that generated neurons throughout development became type B1 cells in the adult and retained their NSC properties ( Figure 4A) 58, 59. However, two recent studies have shown that NSCs that ultimately give rise to adult type B1 cells may contribute to developmental neurogenesis to a limited degree but are largely set aside to become quiescent at E13.5–E15.5 until they become active again postnatally ( Figure 4B) 47, 60. To examine the early contribution (before E15.5) of the developmental precursors of the adult type B1 cells, Fuentealba et al. made use of a retroviral-mediated lineage-tracing method, in which the NSCs were labeled with a genetic barcode 47. Sequencing of cells from different areas of the brain revealed that some type B1 cells shared a common progenitor with neurons in other brain areas, including the cortex and striatum. However, as of yet, the identity and location of the developmental type B1 cell precursor are not known, and there is no way to distinguish between these cells and the other more transient developmental NSCs. RNA sequencing of the type B1 cell precursors could reveal novel prospective markers, which then could be used to target these cells for lineage tracing in vivo.
Figure 4. Developmental origin of adult neural stem cells (NSCs).
There are two models that explain how adult NSCs are generated during development. It should be noted that these models are not mutually exclusive but can coexist. ( A) Maintained stem cell model: NSCs that produce mature cell types to populate the dentate gyrus (DG) during development remain in the adult DG and transition into more quiescent activated NSCs (aNSCs) which generate neurons in the adult DG. ( B) Set aside stem cell model: a subset of NSCs are set aside in a quiescent state during development and do not participate in populating the DG with mature cell types. Once the DG is formed, these NSCs are the aNSCs and can reactivate to generate neurons in the adult DG.
Some key aspects of DG development are strikingly different from embryonic cortical development 61. First, most neurogenesis in the developing cortex takes place during the embryonic stage, whereas neurogenesis in the DG occurs mostly postnatally, and peak neurogenesis takes place during the first two weeks after birth 52. Secondly, the NSCs in the developing cortex and the adult SVZ are continuously in contact with the cerebrospinal fluid (CSF) in the ventricular system, whereas NSCs in the developing and adult DG migrate away from the ventricular niche and must maintain their stem cell properties without contact with the CSF. These traits make the development of the DG exceptional because a neurogenic niche that can support NSCs must be maintained in the postnatal brain, which is less conducive to precursor maintenance, and away from the ventricular system. Understanding how a neurogenic environment is established away from the ventricles in the adult brain is of great interest to the field of regenerative medicine, which aims to develop therapies that use NSCs to replace lost neurons in the diseased or injured brain 62.
So far, the embryonic origin of adult RGLs in the DG has not been identified. It remains unknown whether adult RGL precursors contribute to neurogenesis during the development of the DG and then continue to be active into adulthood or whether a subset of NSCs are set aside during development, as they are in the SVZ, to then become reactivated during adulthood ( Figure 4A, B). To determine which model is correct, the fate choices of individual NSCs should be determined at different stages of development. It will also be important to examine whether the NSCs that give rise to the DG also generate neurons in other areas of the hippocampus or cortex or whether they become lineage-restricted at an early stage of development.
Using single-cell analyses to investigate adult radial glia-like cells
Owing to the sparsity, heterogeneity, and dynamic nature of adult NSCs, it is difficult to study these cells using conventional population-level analyses. In order to identify different subtypes of RGLs in the adult DG, one has to examine the cells in the SGZ on a single-cell level. The last decade has seen a vast increase in new single-cell technologies, such as single-cell RNA sequencing, clonal lineage tracing, and in vivo imaging. Here, we discuss these techniques and how they might be used for the study of NSCs in the developing and adult DG at the single-cell level.
Single-cell sequencing of transcriptomes and epigenomes
Recent technical advancements in single-cell transcriptome and epigenome profiling technologies have made it possible for researchers to commence deciphering heterogeneous populations of stem cells in different tissues, including NSCs 63. In both the embryonic and the adult brain, molecular signatures identified through single-cell RNA sequencing have been used to detect previously unknown cell types and to identify novel markers for subpopulations of NSCs.
In the developing human brain, the outer radial glia represent a population of cells which are thought to give rise to most cortical neurons. Though clearly important for the development of the human brain, the molecular features of these cells were not known. To address this question, researchers performed RNA sequencing, which has revealed a multitude of new markers for the outer radial glia 64, 65. The new markers have been used to identify outer radial glial cells in in vitro culture experiments, demonstrating the predictive accuracy of the data generated 66. In the adult DG, single-cell RNA sequencing of Nestin-CFP-expressing cells in the DG 67 revealed that, on the basis of their transcriptome, quiescent RGLs can be divided into different groups, which represent progressive stages in a developmental trajectory. Additionally, this study revealed the molecular signatures of the active RGLs and early IPCs. Markers which are strongly expressed in distinct groups of cells at specific time points, and no other cell types in the DG, will be good candidates for lineage-tracing experiments to determine the long-term behavior of these cells (see below).
The field of single-cell RNA sequencing is rapidly progressing. In these first studies, the number of sequenced cells numbered in the hundreds. But the development of new techniques, such as Drop-seq, means that many more cells can be sequenced at a reasonable cost 68, 69. Some populations of stem cells might be quite rare such that increasing the number of sequenced cells will increase the resolution and potentially lead to the discovery of new subpopulations. This, together with future improvements in sequencing depth and coverage, will further illuminate the complex heterogeneity of different stem cell populations.
In addition to RNA sequencing, which examines differences in transcriptomes, analysis of the epigenetic landscape of cells can further reveal differences between cell populations. Technologies such as bisulfite sequencing to determine DNA methylation 70; assay for transposase-accessible chromatin sequencing (ATAC-seq), which reveals chromatin accessibility 71; and analysis of chromosome structure on a single-cell level 72 are available to examine epigenetic regulation on a single-cell level.
Single-cell sequencing techniques are still in their infancy but are rapidly becoming more efficient and reliable. In the coming years, we might even be able to perform both RNA sequencing and multiple epigenome profilings on the same cell. In addition, there are recent developments of technologies for profiling epitranscriptomes and appreciation of their critical role in neurogenesis 73. These methodologies ultimately will reveal further layers of heterogeneity within NSC populations.
Single-cell lineage tracing
While single-cell RNA sequencing may reveal novel markers for subpopulations of RGLs in the DG, it can reveal only the molecular signature of a transient state. Long-term lineage tracing is needed to determine the lineage potential of these subpopulations over time. Lineage tracing on a clonal level has been performed in the adult DG using the Nestin-CreER T2 mouse line and has revealed that these RGLs can self-renew and generate both neurons and astrocytes 13. This technique has also been combined with genetic manipulations to examine the role of genes, such as PTEN, sFRP3, γ 2-subunit-containing GABA A receptors, and NF1, in regulating quiescent NSC behavior 13, 15, 37, 74.
Single-cell lineage tracing could also be used to characterize the behavior of different populations of stem cells in a tissue by using different CreER mouse lines. For example, although both Nestin and GLAST are expressed by most, if not all, RGLs at the protein level, the Nestin-CreER T2 and GLAST-CreER T2 mouse lines label RGLs with different behaviors at the population level 28. It would be interesting to compare these drivers in a clonal analysis experiment to investigate potential differences in fate choice or maintenance with higher resolution.
Single-cell lineage tracing is also a powerful tool for the study of brain development. Clonal analysis using the Mosaic Analysis with Double Markers (MADM) system has been used to examine the development of the cortex and thalamus 75, 76. The MADM system is a two-color system, in which cells in G 2-X phase that express Cre recombinase undergo Cre-mediated inter-chromosomal recombination, which can lead to RFP expression in one daughter cell and GFP expression in the other daughter cell 77. These two sister cells and their progeny then can be traced separately over time to assess their fate choices. The MADM system would be very useful to determine the origin and behavior of NSCs during DG development. Cre-driver mouse lines need to be screened for labeling the developmental precursors of the RGLs.
In vivo imaging
To get a complete understanding of stem cell behavior, researchers are now aiming to image stem cells in vivo. This would enable the examination of individual stem and progenitor cells over time in a living animal 78. Many technical hurdles remain before this can be done, especially when it comes to the DG, which is situated deep in the hippocampus.
Recent technical advancements for in vivo imaging have been performed in zebrafish, a teleost fish in which neurons are generated in many areas of the adult central nervous system 79. The brain of the teleost fish develops through outward bending or eversion with the result that the adult NSCs, which have radial glia-like morphology, have their soma on the outside of the brain, close to the surface, making the NSCs easier to visualize. Additionally, some zebrafish lines lack pigment, making them more transparent and thus enabling deep tissue imaging with high resolution, making it possible to image single NSCs over time in a live animal by using confocal microscopy. This technique has made it possible for researchers to study the fate choices made by individual NSCs in different brain areas for up to one month 80, 81.
Since the DG and SVZ are situated deep inside the mammalian brain, this makes them difficult to access for imaging without injuring the brain. Nevertheless, in vivo imaging has been used to study the behavior of adult-born neurons in the DG 82. In this study, investigators used the Nestin-CreER T2 line crossed with a tdTomato reporter line and waited 6 weeks after tamoxifen injection, meaning that the tdTomato + cells were 6 weeks or younger. Calcium imaging was performed on these cells, and investigators found that newborn neurons actively participate in encoding information and are more active and less spatially tuned compared with the more mature granule cells. Another study used retrovirus to label and birth-date newborn neurons to examine dendritogenesis over time 83. Efforts should be made to image the behavior of NSCs in the adult DG, but this will have many technical challenges which need to be overcome. Possibly, the biggest hurdle will be that the DG is situated deep in the brain, under the cortex, and using current imaging strategies will undoubtedly lead to significant injury that might alter the behavior of the NSCs. Once these issues have been overcome, in vivo imaging will be a powerful tool to determine the behavior and characteristics of individual RGLs.
Concluding remarks
Adult stem cell heterogeneity has garnered increasing attention in the last decade 18, 84– 86. Recent studies have shown that the NSCs in the SGZ of the DG can be distinguished by differences in their morphology, lineage potential, and function during tissue maintenance and repair. Future studies will be needed to determine whether these variations are due to the presence of multiple, restricted populations or to different states within the same population in vivo. Another important question is whether stem cell heterogeneity is established during development or adulthood, which will require a better understanding of the embryonic origin of the adult RGLs. Investigators should continue to use single-cell techniques as discussed in this review, such as clonal analysis and single-cell sequencing, to address these questions and to determine when and how stem cell heterogeneity is established in the adult DG.
Acknowledgments
We thank K.M. Christian for comments and members of the Ming and Song laboratories for discussion.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Andreas Faissner, Department of Cell Morphology and Molecular Neurobiology, Faculty of Biology and Biotechnology, Ruhr-University, Bochum, D-44780, Germany
Stavros Taraviras, Department of Physiology, School of Medicine, University of Patras, Patras, 26504, Greece
Funding Statement
The research in the authors’ laboratory has been supported by the National Institutes of Health (R37NS047344, P01NS097206, U19MH106434, and R01AG057497 to HS and R01MH105128, U19AI131130, and R35NS097370 to G-lM) and an EMBO postdoctoral fellowship and a grant from the Swedish Research Council (to DAB).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Bond AM, Ming G, Song H: Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17(4):385–95. 10.1016/j.stem.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Gonçalves JT, Schafer ST, Gage FH: Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167(4):897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 3. Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. : Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- 4. Hevner RF: Evolution of the mammalian dentate gyrus. J Comp Neurol. 2016;524(3):578–94. 10.1002/cne.23851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ming GL, Song H: Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spalding KL, Bergmann O, Alkass K, et al. : Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–27. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Akers KG, Martinez-Canabal A, Restivo L, et al. : Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. 10.1126/science.1248903 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Anacker C, Hen R: Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18(6):335–46. 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christian KM, Song H, Ming GL: Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–62. 10.1146/annurev-neuro-071013-014134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitamura T, Inokuchi K: Role of adult neurogenesis in hippocampal-cortical memory consolidation. Mol Brain. 2014;7:13. 10.1186/1756-6606-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahay A, Wilson DA, Hen R: Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–8. 10.1016/j.neuron.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Beckervordersandforth R, Deshpande A, Schäffner I, et al. : In vivo targeting of adult neural stem cells in the dentate gyrus by a split-cre approach. Stem Cell Reports. 2014;2(2):153–62. 10.1016/j.stemcr.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonaguidi MA, Wheeler MA, Shapiro JS, et al. : In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–55. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Seri B, García-Verdugo JM, McEwen BS, et al. : Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun GJ, Zhou Y, Ito S, et al. : Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat Neurosci. 2015;18(12):1722–4. 10.1038/nn.4159 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Encinas JM, Michurina TV, Peunova N, et al. : Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8(5):566–79. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Kuhn HG, Dickinson-Anson H, Gage FH: Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonaguidi MA, Stadel RP, Berg DA, et al. : Diversity of Neural Precursors in the Adult Mammalian Brain. Cold Spring Harb Perspect Biol. 2016;8(4):a018838. 10.1101/cshperspect.a018838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lugert S, Basak O, Knuckles P, et al. : Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–56. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 20. Suh H, Consiglio A, Ray J, et al. : In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2 + neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–28. 10.1016/j.stem.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moss J, Gebara E, Bushong EA, et al. : Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci U S A. 2016;113(18):E2536–45. 10.1073/pnas.1514652113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Palmer TD, Willhoite AR, Gage FH: Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94. [DOI] [PubMed] [Google Scholar]
- 23. Kronenberg G, Reuter K, Steiner B, et al. : Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467(4):455–63. 10.1002/cne.10945 [DOI] [PubMed] [Google Scholar]
- 24. Mignone JL, Kukekov V, Chiang AS, et al. : Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469(3):311–24. 10.1002/cne.10964 [DOI] [PubMed] [Google Scholar]
- 25. Berg DA, Yoon K, Will B, et al. : Tbr2-expressing intermediate progenitor cells in the adult mouse hippocampus are unipotent neuronal precursors with limited amplification capacity under homeostasis. Front Biol. 2015;10(3):262–71. 10.1007/s11515-015-1364-0 [DOI] [Google Scholar]
- 26. Hodge RD, Kowalczyk TD, Wolf SA, et al. : Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28(14):3707–17. 10.1523/JNEUROSCI.4280-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ming GL, Song H: Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. 10.1146/annurev.neuro.28.051804.101459 [DOI] [PubMed] [Google Scholar]
- 28. DeCarolis NA, Mechanic M, Petrik D, et al. : In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23(8):708–19. 10.1002/hipo.22130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebara E, Bonaguidi MA, Beckervordersandforth R, et al. : Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells. 2016;34(4):997–1010. 10.1002/stem.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amaral DG, Witter MP: The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–91. 10.1016/0306-4522(89)90424-7 [DOI] [PubMed] [Google Scholar]
- 31. Wu MV, Sahay A, Duman RS, et al. : Functional differentiation of adult-born neurons along the septotemporal axis of the dentate gyrus. Cold Spring Harb Perspect Biol. 2015;7(8):a018978. 10.1101/cshperspect.a018978 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Bannerman DM, Grubb M, Deacon RM, et al. : Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139(1–2):197–213. 10.1016/S0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- 33. Bannerman DM, Rawlins JN, McHugh SB, et al. : Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–83. 10.1016/j.neubiorev.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 34. Jinno S: Topographic differences in adult neurogenesis in the mouse hippocampus: a stereology-based study using endogenous markers. Hippocampus. 2011;21(5):467–80. 10.1002/hipo.20762 [DOI] [PubMed] [Google Scholar]
- 35. Piatti VC, Davies-Sala MG, Espósito MS, et al. : The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci. 2011;31(21):7715–28. 10.1523/JNEUROSCI.1380-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Sun J, Bonaguidi MA, Jun H, et al. : A septo-temporal molecular gradient of sfrp3 in the dentate gyrus differentially regulates quiescent adult hippocampal neural stem cell activation. Mol Brain. 2015;8:52. 10.1186/s13041-015-0143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jang MH, Bonaguidi MA, Kitabatake Y, et al. : Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell. 2013;12(2):215–23. 10.1016/j.stem.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Jhaveri DJ, O'Keeffe I, Robinson GJ, et al. : Purification of neural precursor cells reveals the presence of distinct, stimulus-specific subpopulations of quiescent precursors in the adult mouse hippocampus. J Neurosci. 2015;35(21):8132–44. 10.1523/JNEUROSCI.0504-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Urbán N, van den Berg DL, Forget A, et al. : Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science. 2016;353(6296):292–5. 10.1126/science.aaf4802 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Sun MY, Yetman MJ, Lee TC, et al. : Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER T2 lines. J Comp Neurol. 2014;522(5):1191–208. 10.1002/cne.23497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Codega P, Silva-Vargas V, Paul A, et al. : Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82(3):545–59. 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Llorens-Bobadilla E, Zhao S, Baser A, et al. : Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell. 2015;17(3):329–40. 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Lledo P, Valley M: Adult Olfactory Bulb Neurogenesis. Cold Spring Harb Perspect Biol. 2016;8(8): pii: a018945. 10.1101/cshperspect.a018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merkle FT, Mirzadeh Z, Alvarez-Buylla A: Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–4. 10.1126/science.1144914 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Merkle FT, Fuentealba LC, Sanders TA, et al. : Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17(2):207–14. 10.1038/nn.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Tsai HH, Li H, Fuentealba LC, et al. : Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–62. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Fuentealba LC, Rompani SB, Parraguez JI, et al. : Embryonic Origin of Postnatal Neural Stem Cells. Cell. 2015;161(7):1644–55. 10.1016/j.cell.2015.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Altman J, Bayer SA: Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990;301(3):325–42. 10.1002/cne.903010302 [DOI] [PubMed] [Google Scholar]
- 49. Li G, Kataoka H, Coughlin SR, et al. : Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136(2):327–35. 10.1242/dev.025742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seki T, Sato T, Toda K, et al. : Distinctive population of Gfap-expressing neural progenitors arising around the dentate notch migrate and form the granule cell layer in the developing hippocampus. J Comp Neurol. 2014;522(2):261–83. 10.1002/cne.23460 [DOI] [PubMed] [Google Scholar]
- 51. Hodge RD, Nelson BR, Kahoud RJ, et al. : Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci. 2012;32(18):6275–87. 10.1523/JNEUROSCI.0532-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicola Z, Fabel K, Kempermann G: Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat. 2015;9:53. 10.3389/fnana.2015.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Altman J, Bayer SA: Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301(3):365–81. 10.1002/cne.903010304 [DOI] [PubMed] [Google Scholar]
- 54. Li G, Pleasure SJ: The development of hippocampal cellular assemblies. Wiley Interdiscip Rev Dev Biol. 2014;3(2):165–77. 10.1002/wdev.127 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Martin LA, Tan SS, Goldowitz D: Clonal architecture of the mouse hippocampus. J Neurosci. 2002;22(9):3520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li G, Fang L, Fernández G, et al. : The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78(4):658–72. 10.1016/j.neuron.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Merkle FT, Tramontin AD, García-Verdugo JM, et al. : Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101(50):17528–32. 10.1073/pnas.0407893101 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Goldman SA, Zukhar A, Barami K, et al. : Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30(4):505–20. [DOI] [PubMed] [Google Scholar]
- 59. Kriegstein A, Alvarez-Buylla A: The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Furutachi S, Miya H, Watanabe T, et al. : Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci. 2015;18(5):657–65. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Yao B, Christian KM, He C, et al. : Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016;17(9):537–49. 10.1038/nrn.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lie DC, Song H, Colamarino SA, et al. : Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. 10.1146/annurev.pharmtox.44.101802.121631 [DOI] [PubMed] [Google Scholar]
- 63. Shin J, Ming GL, Song H: Decoding neural transcriptomes and epigenomes via high-throughput sequencing. Nat Neurosci. 2014;17(11):1463–75. 10.1038/nn.3814 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Pollen AA, Nowakowski TJ, Chen J, et al. : Molecular identity of human outer radial glia during cortical development. Cell. 2015;163(1):55–67. 10.1016/j.cell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Thomsen ER, Mich JK, Yao Z, et al. : Fixed single-cell transcriptomic characterization of human radial glial diversity. Nat Methods. 2016;13(1):87–93. 10.1038/nmeth.3629 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Qian X, Nguyen HN, Song MM, et al. : Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165(5):1238–54. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Shin J, Berg DA, Zhu Y, et al. : Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015;17(3):360–72. 10.1016/j.stem.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Klein AM, Mazutis L, Akartuna I, et al. : Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161(5):1187–201. 10.1016/j.cell.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Macosko EZ, Basu A, Satija R, et al. : Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161(5):1202–14. 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Smallwood SA, Lee HJ, Angermueller C, et al. : Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11(8):817–20. 10.1038/nmeth.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Cusanovich DA, Daza R, Adey A, et al. : Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348(6237):910–4. 10.1126/science.aab1601 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Nagano T, Lubling Y, Stevens TJ, et al. : Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature. 2013;502(7469):59–64. 10.1038/nature12593 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Yoon KJ, Ringeling FR, Vissers C, et al. : Temporal Control of Mammalian Cortical Neurogenesis by m 6A Methylation. Cell. 2017;171(4):877–889.e17. 10.1016/j.cell.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Song J, Zhong C, Bonaguidi MA, et al. : Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489(7414):150–4. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Gao P, Postiglione MP, Krieger TG, et al. : Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell. 2014;159(4):775–88. 10.1016/j.cell.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Shi W, Xianyu A, Han Z, et al. : Ontogenetic establishment of order-specific nuclear organization in the mammalian thalamus. Nat Neurosci. 2017;20(4):516–28. 10.1038/nn.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Zong H, Espinosa JS, Su HH, et al. : Mosaic analysis with double markers in mice. Cell. 2005;121(3):479–92. 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Park S, Greco V, Cockburn K: Live imaging of stem cells: answering old questions and raising new ones. Curr Opin Cell Biol. 2016;43:30–7. 10.1016/j.ceb.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Than-Trong E, Bally-Cuif L: Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia. 2015;63(8):1406–28. 10.1002/glia.22856 [DOI] [PubMed] [Google Scholar]
- 80. Barbosa JS, Sanchez-Gonzalez R, Di Giaimo R, et al. : Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science. 2015;348(6236):789–93. 10.1126/science.aaa2729 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Dray N, Bedu S, Vuillemin N, et al. : Large-scale live imaging of adult neural stem cells in their endogenous niche. Development. 2015;142(20):3592–600. 10.1242/dev.123018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Danielson NB, Kaifosh P, Zaremba JD, et al. : Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron. 2016;90(1):101–12. 10.1016/j.neuron.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gonçalves JT, Bloyd CW, Shtrahman M, et al. : In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci. 2016;19(6):788–91. 10.1038/nn.4301 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Donati G, Watt FM: Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16(5):465–76. 10.1016/j.stem.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 85. Goodell MA, Nguyen H, Shroyer N: Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol. 2015;16(5):299–309. 10.1038/nrm3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li L, Clevers H: Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–5. 10.1126/science.1180794 [DOI] [PMC free article] [PubMed] [Google Scholar]