Abstract
Astrocytic tumors, including astrocytomas and glioblastomas, are the most common type of primary brain tumors. Treatment for glioblastomas includes radiotherapy, chemotherapy with temozolomide (TMZ) and surgical ablation. Despite certain therapeutic advances, the survival time of patients is no longer than 12–14 months. Cancer cells overexpress the neuronal isoform of nitric oxide synthase (nNOS). In the present study, it was examined whether the nNOS enzyme serves a role in the damage of astrocytoma (U251MG and U138MG) and glioblastoma (U87MG) cells caused by TMZ. First, TMZ (250 µM) triggered an increase in oxidative stress at 2, 48 and 72 h in the U87MG, U251MG and U138MG cell lines, as revealed by 2′,7′-dichlorofluorescin-diacetate assay. The drug also reduced cell viability, as measured by MTT assay. U87MG cells presented a more linear decline in cell viability at time-points 2, 48 and 72 h, compared with the U251MG and U138MG cell lines. The peak of oxidative stress occurred at 48 h. To examine the role of NOS enzymes in the cell damage caused by TMZ, N(ω)-nitro-L-arginine methyl ester (L-NAME) and 7-nitroindazole (7-NI) were used. L-NAME increased the cell damage caused by TMZ while reducing the oxidative stress at 48 h. The preferential nNOS inhibitor 7-NI also improved the TMZ effects. It caused a 12.8% decrease in the viability of TMZ-injured cells. Indeed, 7-NI was more effective than L-NAME in restraining the increase in oxidative stress triggered by TMZ. Silencing nNOS with a synthetic small interfering (si)RNA (siRNAnNOShum_4400) increased by 20% the effects of 250 µM of TMZ on cell viability (P<0.05). Hoechst 33342 nuclear staining confirmed that nNOS knock-down enhanced TMZ injury. In conclusion, our data reveal that nNOS enzymes serve a role in the damage produced by TMZ on astrocytoma and glioblastoma cells. RNA interference with nNOS merits further studies in animal models to disclose its potential use in brain tumor anticancer therapy.
Keywords: nitric oxide synthase, cancer, glioblastoma, RNA interference, oxidative stress, nitric oxide
Introduction
Glioblastoma multiforme (GBM) is the most aggressive astrocytic brain tumor, with the highest degree of histological abnormality (1,2). Patients with GBM are typically associated with a poor prognosis, with a median survival of 12–14 months, according to a statistical report of nervous system tumors diagnosed in the United States between 2006 and 2010 (3,4). Current therapy consists of maximal safe resection of the tumor mass followed by radio- and chemotherapy (5). The drug of choice is temozolomide (TMZ), but the treatment yields a median survival benefit of only 2.5 months. In addition, tumor recurrence and resistance to TMZ often occur (6,7).
Cancer cells commonly present an increased metabolic activity, which results in oxidative stress (8,9). The precise role of oxidative stress in tumor biology and its implication in cancer therapy remains a complex matter. The excessive levels of reactive oxygen species (ROS) may result in cell damage and apoptosis (10). However, ROS production, which includes nitric oxide (NO), may improve the survival, growth and neoplastic phenotype of various cancer cells, including astrocytic brain tumors (11,12).
The neuronal nitric oxide synthase (nNOS) enzyme synthesizes the largest amount of NO in the body, exerting an important role in homeostasis (13). However, nNOS and NO are also involved in brain diseases and cancer pathogenesis (14,15). In general, brain tumors and peritumoral areas express NOS, which improves the blood supply required for cancer development (16,17). NO formed by nNOS contributes to angiogenesis, vasodilation and vascular permeability, thus serving a role in tumor growing and malignancy (18). Also, the non-selective NOS inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME) controlled brain tumor growth in a rat model (19).
In summary, oxidative stress is a biochemical change that affects tumor growth and cancer cells' response to antineoplastic drugs. Identifying molecules that regulate oxidative stress in TMZ-injured tumor cells will enrich our knowledge on tumor biology and responses to anticancer therapy. The present study evaluated whether NOS enzymes serve a role in the damage caused by TMZ on astrocytic tumor cells. First, the effects of TMZ (250 µM) on oxidative stress and cell viability were examined. These effects were evaluated in astrocytoma (U251MG and U138MG) and glioblastoma (ATCC U87MG) cell lines at 2, 48 and 72 h. Then, it was investigated whether NOS enzymes would affect the cell damage caused by TMZ. For that purpose, L-NAME (a nonspecific NOS inhibitor) and 7-NI (an nNOS inhibitor) were used (20,21). Finally, the strategy of RNAi was applied to explore the involvement of the nNOS enzyme in such cell responses.
Materials and methods
Cell culture
The human likely glioblastoma cell line U87MG [ATCC® HTB14™; American Type Culture Collection (ATCC), Manassas, VA, USA], named ATCC U87MG for simplicity, and the astrocytoma cell lines U251MG (European Collection of Authenticated Cell Cultures, Salisbury, UK) and U138MG (ATCC® HTB-16™), were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 1% GlutaMAX™ (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin/streptomycin solution and 250 ng/ml amphotericin B (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were seeded into 25 cm3 culture flasks and maintained at 37°C in a humidified atmosphere with 5% CO2.
Determination of cell viability
Cells were seeded in 96-well plates at a concentration of 0.5×104 cells/well. The cells were incubated with 200 µM of L-NAME (Sigma-Aldrich; Merck KGaA) or 100 µM of 7-nitroindazole (7-NI; Sigma-Aldrich; Merck KGaA) 1 h prior to the addition of 250 µM of TMZ (Orion Corporation, Espoo, Finland). After 2, 48, or 72 h, cell viability was measured by a quantitative colorimetric assay with MTT, which reveals the mitochondrial activity of living cells. Briefly, 50 µl of the MTT-labeling reagent (0.5 mg/ml) were added to each well, and the plate was incubated for an additional 3-h period. The insoluble formazan was dissolved with dimethyl sulfoxide, and MTT reduction was measured at 595 nm in an absorbance plate reader (SpectraMax® M2; Molecular Devices, LLC, Sunnyvale, CA, USA). Experiments were carried out in triplicate in 8 independent assays. Control cells without treatment were considered to exhibit 100% viability.
ROS test
The 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) (Sigma-Aldrich) assay was used to measure ROS production. Briefly, cells were seeded in 96-well plates at a density of 0.5×104 cells/well. After 24 h, cells received L-NAME or 7-NI 1 h before TMZ (250 µM) exposition for 2, 48 or 72 h. Subsequently, the medium was removed, and the cells were incubated with DCFH-DA at a final concentration of 20 µM in DMEM/F12 for 30 min at 37°C, and next washed with Dulbecco's PBS. DCFH-DA levels were measured in a microplate reader (SpectraMax® M2; Molecular Devices, LLC) with excitation and emission wavelengths set at 485 and 535 nm, respectively.
Synthetic siRNAs
The present study used a previously described siRNA named siRNAnNOShum_4400, which targets an nNOS mRNA sequence identified by the BIOPREDsi algorithm (22,23). This sequence is present in exon 28 of all nNOS splicing variants α, β and γ (5′-GCGAACGTACGAAGTGACCAA-3′; nt 4,898-4,918; NM_000620.2) (23). siRNAnNOShum_4400 was synthesized by Qiagen, Inc. as double-stranded RNA sequence with 21 nt. The present study also used the AllStars® Negative Control siRNA (Qiagen, Inc.).
Cell transfection
Transfections of three different lineages (ATCC U87MG, U251MG and U138MG) with siRNAnNOShum_4400 were carried out with Lipofectamine® 2000 Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and Opti-MEM® (Gibco; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The effect of siRNAnNOShum_4400 on nNOS mRNA content was determined by RT-qPCR as described below. The present study also evaluated the effect of siRNAnNOShum_4400 on the viability of ATCC U87MG cells injured by TMZ. To investigate this, following siRNA transfection, the medium was exchanged for DMEM/F12 (Thermo Fisher Scientific, Inc.), followed by the addition of TMZ at 250 or 500 µM. After 48 h of incubation at 37°C, cell viability was determined by MTT assay as described above. Glioblastoma cells without any treatment comprised the mock control group.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
First, total RNA was extracted with the RNeasy® Mini kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's protocol, and quantified by fluorometry (Qubit® 2.0 Fluorometer Firmware 3.11; Thermo Fisher Scientific, Inc.). The purity was considered acceptable for RNA/protein ratios above 1.8. RNA integrity was analyzed by 1% agarose gel electrophoresis using ethidium bromide (Invitrogen™; Thermo Fisher Scientific, Inc.). Complementary (c)DNA synthesis was performed from 500 ng total RNA using random primers (SuperScript® First-Strand Synthesis System for RT-PCR; Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was carried out in a 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The forward and reverse primers for nNOS were 5′-GGTGGAGATCAATATCGCGGTT-3′ and 5′-CCGGCAGCGGTACTCATTCT-3′, respectively (24). For the housekeeping gene, the GC-rich promoter binding protein 1 primers 5′-TCACTTGAGGCAGAACACAGA-3′ and 5′-AGCACATGTTTCATCATTTTCAC-3′ were used (25). Amplification products were detected via intercalation of the fluorescent dye SYBR® Green (Applied Biosystems; Thermo Fisher Scientific, Inc.). Briefly, 10 µl reaction mixture contained 5.0 µl Fast SYBR® Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), 2.0 µl cDNA (diluted 1:10), as previously described (23), and 0.4 µl each sense and antisense primer (10 pmol/µl). The PCR program included an initial denaturation at 95°C for 5 min, followed by 40 cycles of amplification (95°C for 1 min and 60°C for 1 min). Each experiment was carried out in triplicate, and the assay included non-template negative RT controls. The 2−ΔΔCq relative quantification method was used to express the RNA interference (RNAi) effects on nNOS mRNA content (26).
Nuclear staining with Hoechst 33342
Hoechst 33342 (Sigma-Aldrich; Merck KGaA) staining was used to detect injured cells. Cells were cultured in 24-well plates and transfected with siRNAnNOShum_4400 as above described. After 24 h, cells received TMZ for 48 h. Injured and non-transfected cell groups were also included as positive controls. Briefly, the cells were fixed in 4.0% paraformaldehyde for 10 min prior to staining with Hoechst 33342 (5.0 µg/ml/well) in the dark for 10 min at room temperature. Subsequently, the coverslips were washed twice with PBS, air-dried, mounted onto glass slides and observed under a fluorescence microscope (TCS SP5; Leica Microsystems, GmbH, Wetzlar, Germany). The nuclear condensation, fragmentation and bright staining of damaged cells were identified by intense local staining in the nucleus, in contrast to the diffused staining of DNA in healthy cells (27).
Statistical analysis
SPSS version 17 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. All results were expressed as means ± standard error of the mean. One-way analysis of variance with Tukey's post hoc test was applied to evaluate inter-group results. Differences between paired groups were analyzed by the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
TMZ affects astrocytoma and glioblastoma cell viability, and increases oxidative stress
All cell lines exposed to TMZ (250 µM) presented a decreased cell viability with higher levels of ROS. These effects varied in intensity according to the time points and cell lines studied. As shown in Fig. 1A, TMZ caused a progressive decline in cell viability at 2, 48 and 72 h in the ATCC U87MG cell line. The values varied from 92% (2 h) to 52% (72 h), and they were significantly different between 2, 48 and 72 h time points. The cells produced ROS at increased levels, reaching the maximum peak at 48 h (P<0.05). TMZ also affected cell viability and oxidative stress in astrocytoma cells. On U251MG cells, TMZ caused the highest cell damage at 48 h (Fig. 1B). Compared with ATCC U87MG and U138MG cell lines, the differences in cell viability among time points 2, 48 and 72 h were small (84–88%) and not statistically significant, as determined by the MTT assay. The highest oxidative stress response in U251MG cells was observed at 72 h post-TMZ. Finally, TMZ caused a progressive reduction in U138MG cell viability, with a parallel increase in ROS production (P<0.05; Fig. 1C). These two effects peaked at 72 h post-TMZ.
Figure 1.

TMZ reduces the viability of the (A) American Type Culture Collection U87MG, (B) U251MG and (C) U138MG cell lines, and increases their oxidative stress. Experiments were carried out in triplicate in eight independent assays. Circles and squares represent mean values ± standard error of the mean regarding MTT and DCFH assay results, respectively, at each time-point. Cells were injured by TMZ (250 µM) for 2, 48 or 72 h. TMZ affected cell viability and the production of ROS, as determined by MTT and DCFH assays, respectively. From 2 to 48 h post-TMZ, all cell lines exhibited a significant reduction in cell viability along with an increase in oxidative stress response. Asterisks represent statistically significant differences (*P<0.05, one-way analysis of variance followed by Tukey's post hoc test). TMZ, temozolomide; DCFH, 2′,7′-dichlorofluorescein.
nNOS-targeted siRNA reduces nNOS mRNA content in astrocytoma and glioblastoma cell lines
The present study evaluated whether the enzyme nNOS could be silenced by RNAi. For that purpose, the effect of a previously described siRNAnNOShum_4400 (37.5 nM) on nNOS mRNA content was examined at 24 h after transfection. All transfected cell lines (i.e. ATCC U87MG, U251MG and U138MG) presented their nNOS mRNA content reduced to ~50% at 24 h (Fig. 2). This strategy used to suppress nNOS expression was additionally employed in the present study to examine the role of this enzyme in TMZ-injured glioblastoma cells.
Figure 2.
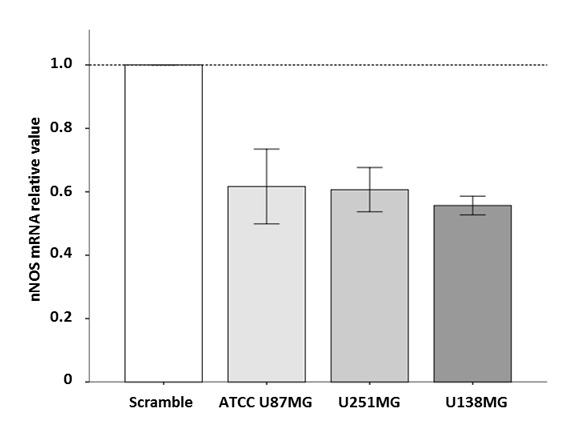
Effects of siRNAnNOShum_4400 on the nNOS mRNA content of American Type Culture Collection U87MG, U251MG and U138MG cells. Cells were transfected with siRNAnNOShum_4400 (37.5 nM) mixed with Lipofectamine® 2000 Transfection Reagent for 24 h. Reverse transcription-quantitative polymerase chain reaction results are presented as relative expression to the scramble control group (2−ΔΔCq). Bars represent the fold changes in nNOS mRNA levels relative to the scramble control group, which was arbitrarily assigned the value of 1. siRNAnNOShum_4400 reduced the content of nNOS mRNA in astrocytoma and glioblastoma cells. siRNA, small interfering RNA; nNOS, neuronal nitric oxide synthase.
Effects of NOS inhibitors on the viability and oxidative stress of ATCC U87MG cells exposed to TMZ for 48 h
Inhibiting NOS enzymes causes effects on TMZ cell damage at 48 h. As shown in Fig. 3A, L-NAME and 7-NI decreased the viability of TMZ-damaged cells by 9.6 and 12.8%, respectively (P<0.05), compared to TMZ 250 group. In parallel, these inhibitors also hampered the increase in ROS production caused by TMZ (P<0.05; Fig. 3B), compared to TMZ 250 group. As noted, 7-NI was more effective than L-NAME in improving the effects of TMZ, as well as in controlling the oxidative stress response. These data highlight a relevant role for nNOS in the actions of TMZ against glioblastoma cells.
Figure 3.
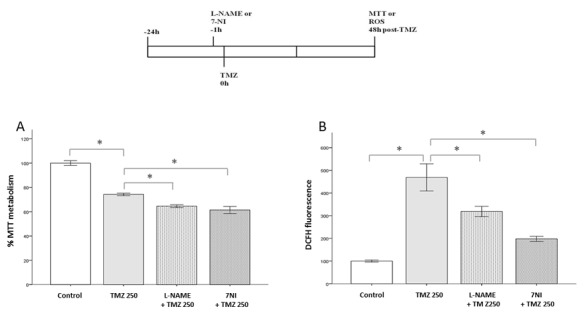
Effects of NOS inhibition on American Type Culture Collection U87MG cells exposed to TMZ for 48 h. Cell viability and ROS production were measured by MTT and DCFH-DA assays, respectively. Bars express mean ± standard error of the mean regarding the results of MTT or DCFH-DA assays. Experiments were carried out in triplicate in eight independent assays. A schematic representation of the experimental procedure is included. Briefly, either L-NAME or 7-NI was added to cell preparations 1 h before TMZ treatment. Next, the cells received TMZ (250 µM) at the time-point 0 h. The cells were then incubated for an additional period of 48 h, prior to being subjected to the MTT and DCFH-DA assays. Pretreatment with L-NAME (200 µM) or 7-NI (100 µM) in cells injured by TMZ (A) reduced their viability and (B) decreased ROS production. Asterisks represent statistically significant differences (*P<0.05, one-way analysis of variance followed by Tukey´s post hoc test). NOS, nitric oxide synthase; ROS, reactive oxygen species; TMZ, temozolomide; DCFH-DA, 2′,7′-dichlorofluorescein-diacetate; L-NAME, N(ω)-nitro-L-arginine methyl ester; 7-NI, 7-nitroindazole.
nNOS-targeted siRNA potentiates the effects of TMZ
nNOS enzyme was knocked-down by using siRNAnNOShum_4400, which was transfected 24 h before TMZ cell damage. This siRNA alone caused no significant changes in cell viability. However, siRNA-transfected cells were more susceptible to the effects of TMZ at 48 h. They presented an additional 20% decrease in cell viability compared with that of the injured mock-transfected group (70 vs. 50%, respectively; P<0.05) (Fig. 4A). Cells damaged by a higher TMZ concentration (500 µM) also presented the same response (44 vs. 26%, respectively; P<0.05) (Fig. 4B).
Figure 4.
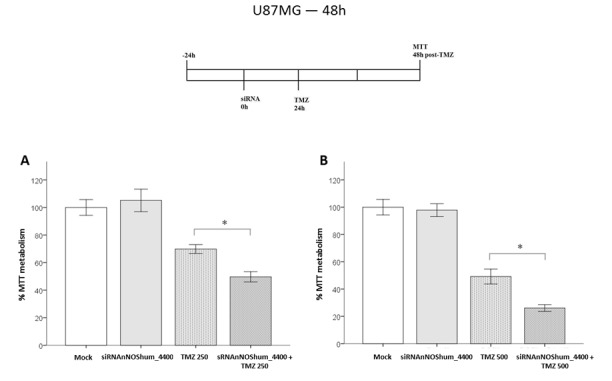
Effects of siRNAnNOShum_4400 on the viability of American Type Culture Collection U87MG cells injured by TMZ. Bars express mean ± standard error of the mean regarding the results of MTT assays. Cells were first transfected with siRNAnNOShum_4400 (37.5 nM) for 24 h. Then, cells received TMZ (250 or 500 µM). Cell viability was determined at 48 h post-TMZ by MTT assay in four independent experiments. Bars represent the absorbance values determined in treated groups normalized to the mock control group. *P<0.05 (one-way analysis of variance followed by Tukey's post hoc test) for the following groups: (A) Pretreated with siRNAnNOShum_4400 followed by TMZ 250 µM treatment vs. TMZ 250 µM treatment alone and (B) pretreated with siRNAnNOShum_4400 followed by TMZ 500 µM treatment vs. TMZ 500 µM treatment alone. siRNAnNOShum_4400 improved the effects of TMZ on cells for both drug concentrations. siRNA, small interfering RNA; nNOS, neuronal nitric oxide synthase; hum, human; TMZ, temozolomide.
nNOS-targeted siRNA increases the damage of cells caused by TMZ
The present study conducted a Hoechst 33342 analysis of TMZ-injured cells. It was observed that TMZ (250 µM) alone caused a marked damage on ATCC U87MG cells, as shown by the typical nuclear Hoechst staining (Fig. 5A). Silencing the nNOS enzyme with siRNAnNOShum_4400 caused no increase in cell apoptosis. However, cells with silenced nNOS were more susceptible to TMZ injury than control cells. As shown in Fig. 5B, the damage caused by TMZ was significantly higher in the group transfected with nNOS-targeted siRNA compared with the TMZ (250 µM) alone (P<0.05).
Figure 5.
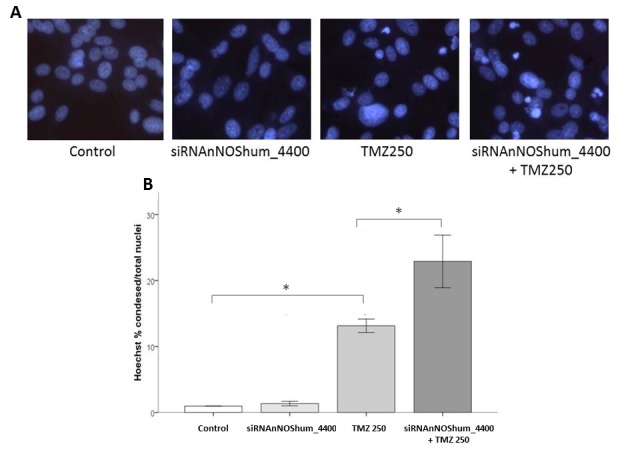
Effects of siRNAnNOShum_4400 on the injury of American Type Culture Collection U87MG cells induced by TMZ. Bars express mean ± standard error of the mean regarding Hoechst 33342 nuclear staining results. Experiments were carried out in triplicate in 8–12 independent assays. Briefly, the injury was examined in cells exposed to TMZ (250 µM) for 24 h. Transfected cell groups received siRNAnNOShum_4400 (37.5 nM) for 24 h mixed with Lipofectamine® 2000 Transfection Reagent. One of them also received 250 µM of TMZ. Cell damage was examined at 48 h post-TMZ. (A) TMZ 250 µM treatment caused a typical nuclear staining in damaged cells, which was reinforced by pretreatment with siRNAnNOShum_4400. Magnification, ×20. (B) Quantification of nuclear Hoechst 33342 staining. (*P<0.05, one-way analysis of variance followed by Tukey's post hoc test). siRNA, small interfering RNA; nNOS, neuronal nitric oxide synthase; hum, human; TMZ, temozolomide.
Discussion
Growing evidence has implicated NOS enzymes in the biology of tumor cells (28–30). The present study examined whether the nNOS enzyme affects astrocytoma and glioblastoma cell responses to TMZ cell damage. TMZ acts by forming O6-methylguanine nt, which mispairs with thymine during DNA replication. In consequence, the drug causes cell cycle arrest in G2/M phase in astrocytic tumor cells, which finally induces cell death (31). TMZ also reduces the membrane potential of the mitochondrion, releases cytochrome c from this organelle, and increases activated caspases 3 and 9 cell content (32). Oliva et al (2011) suggested that U251MG cells demonstrated an increase in ROS production at 2 and 4 h post-TMZ (33), which indicated that oxidative stress contributed to the TMZ effects on astrocytoma tumor cells, as confirmed by a protective effect obtained with the antioxidant N-acetylcysteine. Indeed, TMZ at 300 µM caused a 10-fold increase in ROS levels in TMZ-sensitive U251MG cells, but no effect occurred on resistant cells (33). The present study used TMZ at 250 µM. The drug increased oxidative stress in ATCC U87MG, U251MG and U138MG cells. The peak of ROS concentration occurred at 48 or 72 h, according to each cell line (Fig. 1). Corroborating previous studies, cell groups with increased levels of ROS exhibited a lower cell viability than those with reduced ROS levels (33,34).
In a recent study from 2016, Allen et al (32) compared the original U87MG cell line first identified at Uppsala University with the commercial U87MG cell line used in the present study (ATCC® HTB14™). Genotyping results based on short tandem repeat (STR) analysis revealed that the ATCC and Uppsala U87MG cells lines are from distinct origins (32). However, the STR profiling from the ATCC U87MG cell line is identical to that previously published by Bady et al in 2012 and the CLS Cell Line Services (35,36). In addition, the commercial ATCC U87MG cell line was identified as a cell line of central nervous system (CNS) tumor origin (Allen et al 2016), by comparing the transcriptional profiles of ATCC U87MG with those of 1,036 cell lines regarding 18 different tissue derivation lineages of the Cancer Cell Line Encyclopedia (CCLE) (37). In summary, ATCC U87MG can be classified as a bonafide human glioblastoma cell line (35).
The World Health Organization recently updated the classification of tumors of the CNS, including molecular parameters in addition to conventional histology (38). With this regard, all three lineages used in the present study are classified as astrocytic tumor cells, termed astrocytoma (U251MG and U138MG) or glioblastoma (ATCC U87MG). Glioblastoma is the most malignant astrocytic tumor (grade IV), with the following histopathology features: Cellular polymorphism, nuclear atypia, high mitotic activity, increased abnormal growth of blood vessels around the tumor, vascular thrombosis, microvascular proliferation and necrosis (1,2,39).
Although no animal model perfectly represents all aspects of human glioblastomas, the model of choice must mimic the features of the disease under investigation (40,41). The lineage used in our study, i.e. ATCC U87MG, exhibits numerous aspects of this primary brain tumor in rodent models. Intracerebral implantation of ATCC U87MG cells in nude mice resulted in the growth of tumors whose volume increased by ~50 mm3 in 39 days, presenting an infiltrative pattern with a marked neovascularization and large necrotic center areas (42,43), which indicates that the model has ‘face’ validity. The ATCC U87MG cell line has also been used for refining diagnostic techniques on histopathology and imaging of glioblastoma tumors (44–46). Regarding the ‘predictive’ validity of the model, ATCC U87MG tumors can be treated with TMZ, the drug of choice for GBM, which was employed in the present study (47,48). Besides TMZ, the ATCC U87MG cell line has also been used for pre-clinical testing of different therapeutic agents against glioblastoma (49–53). In summary, the scientific data available suggest that the ATCC U87MG cell lineage provides a glioblastoma model with invasiveness, tumor-induced necrosis and vascular alterations that mimics human glioblastomas, indicating that it is a useful experimental model for studies on tumor biology and therapeutics. Thus, the genetic differences between the ATCC U87MG cell line and the original Uppsala U87MG lineage are unlikely to affect the conclusions of the present study.
The gas NO contributes to oxidative stress in astrocytic tumors (11). NO is often referred to as a toxic and reactive molecule. However, it inhibits the apoptosis of cells associated with caspase 3-like enzymes via S-nitrosylation or cyclic guanosine monophosphate-dependent pathways (54). At mM concentrations, it also inhibits catalase and cytochrome P-450 enzymes (55). NO is released during the synthesis of L-citrulline, which is catalyzed by NOS enzymes (12). This gaseous molecule will form two metabolites: Nitrite and nitrate (13). Nitrite damages DNA strands, leading to various biochemical features observed in cancer cells such as changes in p53 activity and epidermal growth factor signaling (56–58). The increased NOS activity reported in glioma cells also contributes to oxidative stress (11). Similarly, inhibiting NOS enzymes by L-NAME resulted in a lower nitrite concentration in rat glioma tumor tissues with a decrease in tumor volume, which reinforces the benefits of reducing NOS activity in glioma cells (19,59). No previous study, however, has addressed the involvement of NOS enzymes in the damage of astrocytic tumor cells cells (astrocytoma and glioblastoma) caused by TMZ. In the present study, cells were pretreated with L-NAME to evaluate the effects of this drug on oxidative stress and viability. It was observed that NOS enzymes affect both responses. L-NAME sensitized ATCC U87MG cells to 250 µM of TMZ. The viability of glioblastoma cells and their oxidative stress levels were decreased at 48 h. Altogether, these data reveal that NOS enzymes contribute to oxidative stress in glioblastoma cells injured by TMZ. It is possible to speculate that inhibition of NOS enzymes during TMZ cell damage may converge to the same apoptotic pathway described above, thus improving the damage of glioblastoma cells.
nNOS is highly expressed in astrocytic tumors. The expression of nNOS occurs in both the tumor and peritumoral areas of brain tumors (16). Indeed, tumors with high histological grades also present increased levels of nNOS expression (18,60). Although these findings suggest a possible role for nNOS in gliomagenesis, no previous study has addressed its involvement in astrocytic tumor cells injured by TMZ. The present study evaluated the effects of the nNOS inhibitor 7-NI and a synthetic siRNA targeting nNOS. At 48 h, 7-NI decreased the viability of ATCC U87MG cells, restraining their oxidative stress response to 250 µM of TMZ (Fig. 3). The role of the nNOS enzyme in glioblastoma cell responses to TMZ was confirmed by RNAi experiments. ATCC U87MG cells subjected to nNOS silencing for 24 h were more vulnerable to TMZ compared to cells exposed to TMZ alone. They exhibited a decreased viability with an increased rate of injury (Figs. 4 and 5). The results from nNOS knock-down experiments reinforce those obtained with the nNOS inhibitor 7-NI, suggesting that this enzyme serves at least a partial role in glioblastoma cell defenses against TMZ.
Understanding the biology of astrocytic cells is a challenging effort for studies that use animal models of brain tumors (61–63). Beyond modeling tumor pathogenesis, the results from animal models have also identified potential targets for glioma chemotherapy (64–67). The present study revealed that suppressing the nNOS enzyme improves the effects of TMZ on glioblastoma cells. siRNAs designed to silence other enzymes also increase TMZ injury in astrocytic cells, as observed for DNA methyltransferases and kinases (68–70). RNAi with non-enzymatic targets was also observed to be valuable in sensitizing cells to TMZ: In a previous study, it was noted that silencing the voltage-gated potassium channel Eag1 makes glioblastoma cells more vulnerable to TMZ (71). The same effect occurred for drug resistance proteins, heat-shock proteins 90, 27 and 72, and other targets involved in cell signaling (72–77).
The studies mentioned above stand that siRNAs hold the potential to be RNAi-based drugs associated with TMZ. However, exploitation of RNAi in the clinic will depend on improvements in oligonucleotides chemical structure for stability (phosphorothioated backbones that avoid ribonuclease attack) and specificity (i.e. locked nucleic acids), as well as the development of novel carriers for siRNA delivery (78). The oligonucleotide siRNAnNOShum_4400 used in the present study has no phosphorothioate backbones and locked nucleic acids (23). Thus, we recommend adopting these chemical changes in future studies with nNOS-targeted siRNAs for improvements in the specificity and duration of silencing effects.
A significant limitation to treat brain tumors is the location where such tumors grow, i.e. the CNS, since the blood-brain barrier offers an obstacle for drug distribution (79). The introduction of siRNAs directly into brain tumor tissues by the convection-enhanced delivery (CED) technique is a viable alternative to circumvent this obstacle (80). Combining CED with the use of nanoparticles for carrying siRNAs is a promising strategy to treat glioma tumors by RNAi (81–83). Finally, a sustained delivery of siRNAs implanted in tumor tissues would be of value to control aggressive cancer types. In a previous study, biodegradable poly (lactic-co-glycolic acid) devices implanted into pancreatic tumors provided a 4-month delivery of siRNAs with significant antitumor results (84). Implantation of such delivery system following brain tumors ablation would be a promising strategy to prevent tumor recurrence.
Regarding clinical trials, at least ten RNAi-based drugs are currently in phase II and will become approved treatments in the following years (78). Of these, three clinical trials address cancer diseases, including pancreatic tumors, hepatocellular carcinomas and neuroendocrine tumors (78). It may be possible that certain RNAi-based drugs successfully tested in animal models of brain tumors could be evaluated in clinical trials.
To conclude, the present study revealed a new target for RNAi, the nNOS enzyme, which also improved the anticancer effects of TMZ on glioblastoma cells. The synthetic duplex for silencing nNOS, siRNAnNOShum_4400, merits further studies to explore its potential use for brain tumor anticancer therapy.
Acknowledgements
The present study received financial support from CAPES [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Programa Nacional de Pós-doutorado; grant no., 3731–37/2010)], CNPq [Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant no., 467467/2014-5)] and FAP-DF [Fundação de Apoio à Pesquisa do Distrito Federal (grant no., 2010/00302-9)]. The nNOS sequence used for designing siRNAnNOShum_4400 is under patent registration at the Brazilian Institute for Industrial Property-Instituto Nacional da Propriedade Industrial (patent no., INPI, BR 10 2012 032844 5).
References
- 1.Lassman AB. Molecular biology of gliomas. Curr Neurol Neurosci Rep. 2004;4:228–233. doi: 10.1007/s11910-004-0043-3. [DOI] [PubMed] [Google Scholar]
- 2.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 3.Henson JW. Treatment of glioblastoma multiforme: A new standard. Arch Neurol. 2006;63:337–341. doi: 10.1001/archneur.63.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 6.Pedretti M, Verpelli C, Mårlind J, Bertani G, Sala C, Neri D, Bello L. Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma. Br J Cancer. 2010;103:827–836. doi: 10.1038/sj.bjc.6605832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 8.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 9.Kardeh S, Ashkani-Esfahani S, Alizadeh AM. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur J Pharmacol. 2014;735:150–168. doi: 10.1016/j.ejphar.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira V, Hay N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti A, Guli C, La Torre D, Tomasello C, Angileri FF, Aguennouz M. Role of inflammation and oxidative stress mediators in gliomas. Cancers (Basel) 2010;2:693–712. doi: 10.3390/cancers2020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: Mechanism, clinical relevance, and therapeutic development. J Neurosci Res. 2014;92:1419–1424. doi: 10.1002/jnr.23431. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 14.Luo CX, Zhu DY. Research progress on neurobiology of neuronal nitric oxide synthase. Neurosci Bull. 2011;27:23–35. doi: 10.1007/s12264-011-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: Lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/A:1005912906436. [DOI] [PubMed] [Google Scholar]
- 16.Bakshi A, Nag TC, Wadhwa S, Mahapatra AK, Sarkar C. The expression of nitric oxide synthases in human brain tumours and peritumoral areas. J Neurol Sci. 1998;155:196–203. doi: 10.1016/S0022-510X(97)00315-8. [DOI] [PubMed] [Google Scholar]
- 17.Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77–89. doi: 10.1023/A:1005908805527. [DOI] [PubMed] [Google Scholar]
- 18.Tanriover N, Ulu MO, Isler C, Durak H, Oz B, Uzan M, Akar Z. Neuronal nitric oxide synthase expression in glial tumors: Correlation with malignancy and tumor proliferation. Neurol Res. 2008;30:940–944. doi: 10.1179/174313208X319099. [DOI] [PubMed] [Google Scholar]
- 19.Swaroop GR, Kelly PA, Bell HS, Shinoda J, Yamaguchi S, Whittle IR. The effects of chronic nitric oxide synthase suppression on glioma pathophysiology. Br J Neurosurg. 2000;14:543–548. doi: 10.1080/02688690020005554. [DOI] [PubMed] [Google Scholar]
- 20.Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. Pain. 1996;66:331–341. doi: 10.1016/0304-3959(96)03025-4. [DOI] [PubMed] [Google Scholar]
- 21.Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 22.Huesken D, Lange J, Mickanin C, Weiler J, Asselbergs F, Warner J, Meloon B, Engel S, Rosenberg A, Cohen D, et al. Design of a genome-wide siRNA library using an artificial neural network. Nat Biotechnol. 2005;23:995–1001. doi: 10.1038/nbt1118. [DOI] [PubMed] [Google Scholar]
- 23.Titze-de-Almeida SS, Lustosa CF, Horst CH, Bel ED, Titze-de-Almeida R. Interferon Gamma potentiates the injury caused by MPP(+) on SH-SY5Y cells, which is attenuated by the nitric oxide synthases inhibition. Neurochem Res. 2014;39:2452–2464. doi: 10.1007/s11064-014-1449-1. [DOI] [PubMed] [Google Scholar]
- 24.Dotsch J, Harmjanz A, Christiansen H, Hänze J, Lampert F, Rascher W. Gene expression of neuronal nitric oxide synthase and adrenomedullin in human neuroblastoma using real-time PCR. Int J Cancer. 2000;88:172–175. doi: 10.1002/1097-0215(20001015)88:2<172::AID-IJC4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Kwon MJ, Oh E, Lee S, Roh MR, Kim SE, Lee Y, Choi YL, In YH, Park T, Koh SS, Shin YK. Identification of novel reference genes using multiplatform expression data and their validation for quantitative gene expression analysis. PLoS One. 2009;4:e6162. doi: 10.1371/journal.pone.0006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu LC, Warters RL, Dethlefsen LA. Fluorescence studies of Hoechst 33342 with supercoiled and relaxed plasmid pBR322 DNA. Cytometry. 1985;6:191–194. doi: 10.1002/cyto.990060304. [DOI] [PubMed] [Google Scholar]
- 28.Jia W, Jackson-Cook C, Graf MR. Tumor-infiltrating, myeloid-derived suppressor cells inhibit T cell activity by nitric oxide production in an intracranial rat glioma + vaccination model. J Neuroimmunol. 2010;223:20–30. doi: 10.1016/j.jneuroim.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muntané J, La Mata MD. Nitric oxide and cancer. World J Hepatol. 2010;2:337–344. doi: 10.4254/wjh.v2.i9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm EA, Overwijk WW. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–1844. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sang DP, Li RJ, Lan Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014;35:832–838. doi: 10.1038/aps.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakubowicz-Gil J, Langner E, Badziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34:2367–2378. doi: 10.1007/s13277-013-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliva CR, Moellering DR, Gillespie GY, Griguer CE. Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production. PLoS One. 2011;6:e24665. doi: 10.1371/journal.pone.0024665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, Yang Y. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem. 2010;285:40461–40471. doi: 10.1074/jbc.M110.164046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen M, Bjerke M, Edlund H, Nelander S, Westermark B. Origin of the U87MG glioma cell line: Good news and bad news. Sci Transl Med. 2016;8:354re3. doi: 10.1126/scitranslmed.aaf6853. [DOI] [PubMed] [Google Scholar]
- 36.Bady P, Diserens AC, Castella V, Kalt S, Heinimann K, Hamou MF, Delorenzi M, Hegi ME. DNA fingerprinting of glioma cell lines and considerations on similarity measurements. Neuro Oncol. 2012;14:701–711. doi: 10.1093/neuonc/nos072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 39.Reni M, Mazza E, Zanon S, Gatta G, Vecht CJ. Central nervous system gliomas. Crit Rev Oncol Hematol. 2017;113:213–234. doi: 10.1016/j.critrevonc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Goldbrunner RH, Wagner S, Roosen K, Tonn JC. Models for assessment of angiogenesis in gliomas. J Neurooncol. 2000;50:53–62. doi: 10.1023/A:1006462504447. [DOI] [PubMed] [Google Scholar]
- 41.Stylli SS, Luwor RB, Ware TM, Tan F, Kaye AH. Mouse models of glioma. J Clin Neurosci. 2015;22:619–626. doi: 10.1016/j.jocn.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, Arap W, Huang CM, Cavenee WK. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor; Proc Natl Acad Sci USA; 1996; pp. 8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doblas S, He T, Saunders D, Pearson J, Hoyle J, Smith N, Lerner M, Towner RA. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J Magn Reson Imaging. 2010;32:267–275. doi: 10.1002/jmri.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirschner S, Murle B, Felix M, Arns A, Groden C, Wenz F, Hug A, Glatting G, Kramer M, Giordano FA, Brockmann MA. Imaging of orthotopic glioblastoma xenografts in mice using a clinical CT scanner: Comparison with Micro-CT and histology. PLoS One. 2016;11:e0165994. doi: 10.1371/journal.pone.0165994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Dong C, Shi J, Ma T, Jin Z, Jia B, Liu Z, Shen L, Wang F. Radiolabeled novel mAb 4G1 for immunoSPECT imaging of EGFRvIII expression in preclinical glioblastoma xenografts. Oncotarget. 2017;8:6364–6375. doi: 10.18632/oncotarget.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers S, Hii H, Huang J, Ancliffe M, Gottardo NG, Dallas P, Lee S, Endersby R. A novel technique of serial biopsy in mouse brain tumour models. PLoS One. 2017;12:e0175169. doi: 10.1371/journal.pone.0175169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arcella A, Oliva MA, Staffieri S, Aalberti S, Grillea G, Madonna M, Bartolo M, Pavone L, Giangaspero F, Cantore G, Frati A. In vitro and in vivo effect of human lactoferrin on glioblastoma growth. J Neurosurg. 2015;123:1026–1035. doi: 10.3171/2014.12.JNS14512. [DOI] [PubMed] [Google Scholar]
- 48.Nitta Y, Shimizu S, Shishido-Hara Y, Suzuki K, Shiokawa Y, Nagane M. Nimotuzumab enhances temozolomide-induced growth suppression of glioma cells expressing mutant EGFR in vivo. Cancer Med. 2016;5:486–499. doi: 10.1002/cam4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma; Proc Natl Acad Sci USA; 2000; pp. 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, Wong MC. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: Inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007;67:888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 51.Jin J, Choi SH, Lee JE, Joo JD, Han JH, Park SY, Kim CY. Antitumor activity of 7-O-succinyl macrolactin A tromethamine salt in the mouse glioma model. Oncol Lett. 2017;13:3767–3773. doi: 10.3892/ol.2017.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gravina GL, Mancini A, Marampon F, Colapietro A, Delle Monache S, Sferra R, Vitale F, Richardson PJ, Patient L, Burbidge S, Festuccia C. The brain-penetrating CXCR4 antagonist, PRX177561, increases the antitumor effects of bevacizumab and sunitinib in preclinical models of human glioblastoma. J Hematol Oncol. 2017;10:5. doi: 10.1186/s13045-016-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong X, Zhao H, Liang S, Zhou D, Zhang W, Yuan L. Gene delivery of apoptin-derived peptide using an adeno-associated virus vector inhibits glioma and prolongs animal survival. Biochem Biophys Res Commun. 2017;482:506–513. doi: 10.1016/j.bbrc.2016.10.059. [DOI] [PubMed] [Google Scholar]
- 54.Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. doi: 10.1016/j.tox.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 55.Brunelli L, Yermilov V, Beckman JS. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic Biol Med. 2001;30:709–714. doi: 10.1016/S0891-5849(00)00512-8. [DOI] [PubMed] [Google Scholar]
- 56.Cobbs CS, Whisenhunt TR, Wesemann DR, Harkins LE, Van Meir EG, Samanta M. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63:8670–8673. [PubMed] [Google Scholar]
- 57.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 58.Zhang P, Wang YZ, Kagan E, Bonner JC. Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J Biol Chem. 2000;275:22479–22486. doi: 10.1074/jbc.M910425199. [DOI] [PubMed] [Google Scholar]
- 59.Oyoshi T, Nomoto M, Hirano H, Kuratsu J. Pathodynamics of nitric oxide production within implanted glioma studied with an in vivo microdialysis technique and immunohistochemistry. J Pharmacol Sci. 2003;91:15–22. doi: 10.1254/jphs.91.15. [DOI] [PubMed] [Google Scholar]
- 60.Broholm H, Rubin I, Kruse A, Braendstrup O, Schmidt K, Skriver EB, Lauritzen M. Nitric oxide synthase expression and enzymatic activity in human brain tumors. Clin Neuropathol. 2003;22:273–281. [PubMed] [Google Scholar]
- 61.Agnihotri S, Burrell KE, Wolf A, Jalali S, Hawkins C, Rutka JT, Zadeh G. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp (Warsz) 2013;61:25–41. doi: 10.1007/s00005-012-0203-0. [DOI] [PubMed] [Google Scholar]
- 62.Lenting K, Verhaak R, Ter Laan M, Wesseling P, Leenders W. Glioma: Experimental models and reality. Acta Neuropathol. 2017;133:263–282. doi: 10.1007/s00401-017-1671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Resende FF, Bai X, Del Bel EA, Kirchhoff F, Scheller A, Titze-de-Almeida R. Evaluation of TgH(CX3CR1-EGFP) mice implanted with mCherry-GL261 cells as an in vivo model for morphometrical analysis of glioma-microglia interaction. BMC Cancer. 2016;16:72. doi: 10.1186/s12885-016-2118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, McKay RM, Parada LF. Malignant glioma: Lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: From molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 66.Kegelman TP, Hu B, Emdad L, Das SK, Sarkar D, Fisher PB. In vivo modeling of malignant glioma: The road to effective therapy. Adv Cancer Res. 2014;121:261–330. doi: 10.1016/B978-0-12-800249-0.00007-X. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Jiang T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Kato T, Natsume A, Toda H, Iwamizu H, Sugita T, Hachisu R, Watanabe R, Yuki K, Motomura K, Bankiewicz K, Wakabayashi T. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther. 2010;17:1363–1371. doi: 10.1038/gt.2010.88. [DOI] [PubMed] [Google Scholar]
- 69.Shervington A, Patel R. Silencing DNA methyltransferase (DNMT) enhances glioma chemosensitivity. Oligonucleotides. 2008;18:365–374. doi: 10.1089/oli.2008.0128. [DOI] [PubMed] [Google Scholar]
- 70.Wen X, Huang A, Liu Z, Liu Y, Hu J, Liu J, Shuai X. Downregulation of ROCK2 through nanocomplex sensitizes the cytotoxic effect of temozolomide in U251 glioma cells. PLoS One. 2014;9:e92050. doi: 10.1371/journal.pone.0092050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sales TT, Resende FF, Chaves NL, Titze-De-Almeida SS, Báo SN, Brettas ML, Titze-De-Almeida R. Suppression of the Eag1 potassium channel sensitizes glioblastoma cells to injury caused by temozolomide. Oncol Lett. 2016;12:2581–2589. doi: 10.3892/ol.2016.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruickshanks N, Shervington L, Patel R, Munje C, Thakkar D, Shervington A. Can hsp90alpha-targeted siRNA combined with TMZ be a future therapy for glioma? Cancer Invest. 2010;28:608–614. doi: 10.3109/07357901003630967. [DOI] [PubMed] [Google Scholar]
- 73.Jakubowicz-Gil J, Langner E, Badziul D, Wertel I, Rzeski W. Silencing of Hsp27 and Hsp72 in glioma cells as a tool for programmed cell death induction upon temozolomide and quercetin treatment. Toxicol Appl Pharmacol. 2013;273:580–589. doi: 10.1016/j.taap.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Paul-Samojedny M, Pudelko A, Kowalczyk M, Fila-Daniłow A, Suchanek-Raif R, Borkowska P, Kowalski J. Combination therapy with AKT3 and PI3KCA siRNA enhances the antitumor effect of temozolomide and carmustine in T98G glioblastoma multiforme cells. BioDrugs. 2016;30:129–144. doi: 10.1007/s40259-016-0160-y. [DOI] [PubMed] [Google Scholar]
- 75.Qian C, Li P, Yan W, Shi L, Zhang J, Wang Y, Liu H, You Y. Downregulation of osteopontin enhances the sensitivity of glioma U251 cells to temozolomide and cisplatin by targeting the NF-κB/Bcl-2 pathway. Mol Med Rep. 2015;11:1951–1955. doi: 10.3892/mmr.2014.2951. [DOI] [PubMed] [Google Scholar]
- 76.Tivnan A, Zakaria Z, O'Leary C, Kögel D, Pokorny JL, Sarkaria JN, Prehn JH. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front Neurosci. 2015;9:218. doi: 10.3389/fnins.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q, Du J, Xu B, Xu L, Wang X, Liu J, Wang J. Silence of bFGF enhances chemosensitivity of glioma cells to temozolomide through the MAPK signal pathway. Acta Biochim Biophys Sin (Shanghai) 2016;48:501–508. doi: 10.1093/abbs/gmw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Titze-de-Almeida R, David C, Titze-de-Almeida SS. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res. 2017;34:1339–1363. doi: 10.1007/s11095-017-2134-2. [DOI] [PubMed] [Google Scholar]
- 79.de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 80.Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH. Convection-enhanced delivery to the central nervous system. J Neurosurg. 2015;122:697–706. doi: 10.3171/2014.10.JNS14229. [DOI] [PubMed] [Google Scholar]
- 81.Cohen ZR, Ramishetti S, Peshes-Yaloz N, Goldsmith M, Wohl A, Zibly Z, Peer D. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano. 2015;9:1581–1591. doi: 10.1021/nn506248s. [DOI] [PubMed] [Google Scholar]
- 82.Danhier F, Messaoudi K, Lemaire L, Benoit JP, Lagarce F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int J Pharm. 2015;481:154–161. doi: 10.1016/j.ijpharm.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 83.Tsujiuchi T, Natsume A, Motomura K, Kondo G, Ranjit M, Hachisu R, Sugimura I, Tomita S, Takehara I, Woolley M, et al. Preclinical evaluation of an O(6)-methylguanine-DNA methyltransferase-siRNA/liposome complex administered by convection-enhanced delivery to rat and porcine brains. Am J Transl Res. 2014;6:169–178. [PMC free article] [PubMed] [Google Scholar]
- 84.Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A, Domb A, Harari G, David EB, Raskin S, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]


