Abstract
Colorectal cancer (CRC) is one of the most common types of malignancy with high morbidity and mortality rates worldwide. This biologically heterogeneous disease results in diverse therapeutic responses, thus, novel prognostic biomarkers are required to improve CRC treatment. Estrogen-related receptor α (ERRα) is a nuclear orphan receptor, which is associated with estrogen receptor α. The present study aimed to investigate the expression of ERRα in patients with CRC, and explore the association between ERRα expression and clinicopathological factors, local recurrence and prognosis. In the present study, ERRα expression was detected in 15 fresh CRC tissues using quantitative real-time polymerase chain reaction (RT-qPCR) and in 128 paraffin-embedded CRC tissues using immunohistochemistry. The associations between ERRα expression and prognosis of CRC patients were evaluated by univariate, and multivariate (Cox proportional hazards model) analysis. RT-qPCR demonstrated that the mRNA expression of ERRα in CRC tissues was significantly higher compared with that in matched normal tissues. Immunohistochemistry revealed that ERRα high expression was detected in the nuclei of cancer cells from 39.1% (50/128) of CRC tissues. ERRα expression based on immunohistochemical staining was significantly associated with tumor differentiation, tumor invasion, lymph node status and Dukes stage (all P<0.05). Furthermore, patients with high ERRα expression were significantly associated with an increased risk of recurrence and poor prognosis, compared with patients with low ERRα expression. ERRα expression was identified as an independent prognostic factor for patients with CRC. In conclusion, ERRα serves important roles in the progression of CRC and is a potential prognostic factor for patients with CRC.
Keywords: colorectal cancer, estrogen-related receptor α, prognosis, local recurrence
Introduction
Colorectal cancer (CRC) is the third most common type of malignancy and the fourth most common cause of cancer-associated mortality worldwide (1). In 2015, there were 376,300 newly-diagnosed CRC cases and 191,000 CRC-associated mortalities in China projected by the National Office for Cancer Prevention and Control, National Cancer Center (2). Despite improved precancerous screening, surgical resection, chemotherapy and radiotherapy, patients with CRC, particularly those in the advanced stages, exhibit poor prognosis with significant morbidity and mortality rates, thereby constituting a major burden on global health (3). For example, a survey from the United States of America suggested that the 5-year survival rate is 70.4% in patients with CRC with regional invasion, while in patients with distant metastasis the rate is 12.5% (4). During the development of CRC, multiple genetic mutations accumulate, and also involve genes that regulate cell proliferation and survival, thereby making CRC a biologically heterogeneous disease (5,6). Therefore, CRC exhibits diverse treatment responses in patients with similar clinicopathological parameters. Furthermore, different molecular drivers may exist in patients with CRC at the same stage, leading to varied prognosis. Thus, there is an urgent requirement to investigate the molecular markers underlying CRC and identify novel therapeutic targets for CRC treatment.
Estrogen-related receptor (ERR) is a member of the steroid nuclear hormone receptor superfamily and is involved in energy homeostasis regulation (7). ERR consists of three closely associated members ERRα, ERRβ and ERRγ. No natural estrogens have been identified to activate ERR, therefore they are classified as orphan receptors (8). Combined with transcriptional coactivator, peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α), ERRα regulates key genes coding for components of energy homeostasis, including fatty acid and glucose metabolism, mitochondrial biogenesis, and oxidative stress (7). In a previous study, ERRα was demonstrated to be expressed in 100% of the patients with CRC, and ERRα mRNA expression was elevated in tumor tissue compared with normal mucosa (9). In addition, a significantly increasing association was observed between ERRα expression in tumor tissues and TNM stages II to IV (9).
The present study aimed to investigate whether ERRα acts as an effective prognostic marker for patients with CRC. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and immunohistochemistry were performed to detect the expression of ERRα in CRC tissues, and their adjacent normal tissues. Statistical analysis was applied to evaluate the associations between ERRα expression, and clinicopathological parameters and prognosis. As a result, a high expression of ERRα was revealed to be associated with local recurrence and reduced 5-year survival rates. ERRα was identified as an independent prognostic factor for patients with CRC. The results of the present study confirm that ERRα demonstrates clinical and prognostic significance, and may also be a novel therapeutic target for CRC treatment.
Materials and methods
Patients and tissue samples
A total of 15 fresh primary CRC tissues, and their adjacent normal tissues were obtained for RT-qPCR between July 2015 and December 2015. A total of 128 paraffin-embedded primary CRC tissues and their adjacent normal tissues that were obtained between January 2005 and December 2010 were used for the immunohistochemistry assay. All specimens were collected from patients with CRC that underwent curative resection at the Department of General Surgery in Affiliated Tumor Hospital of Guangxi Medical University (Nanning, China). All the patients received no preoperative chemotherapy or radiotherapy. The 128 patients with paraffin-embedded samples included 72 males and 56 females, with a mean age of 56 years and range of 27–84 years. The histological type was determined by two experienced pathologists who reviewed the slides of CRC biopsies stained with hematoxylin and eosin according to the World Health Organization classification (10). Tumor differentiation status was divided into three types: Well, moderately and poorly differentiated. Tumor invasion was classified into T1, T2, T3 and T4 stages, and clinical status was classified into A, B, C and D stages according to the Dukes classification system (11). Postoperative follow-up was performed for each patient to monitor local recurrence and/or distal metastasis using laboratory tests (once every 3 months) and radiological examination (once every 6 months). Patients with incomplete follow-up records were excluded. The fresh specimens from 15 patients were snap-frozen and stored at −80°C for RNA isolation, and the 128 specimens were fixed with 10% formalin at 4°C for 24 h and embedded in paraffin for immunohistochemistry. All 128 patients with CRC were followed up after surgery every three months, with the follow-up deadline set at December 2015. Overall survival (OS) was defined as the interval between surgery and mortality or the last follow-up (censored data for living patients). Disease-free survival (DFS) was defined as the interval between surgery and the date of relapse. The present study was approved by the Ethics Committee of Affiliated Tumor Hospital of Guangxi Medical University and written informed consent was obtained from patients whose tissue specimens were used.
RT-qPCR
Total RNA was extracted from 15 frozen CRC tissues and their corresponding adjacent normal tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The SuperScript III Reverse Transcriptase kit (Promega Corporation, Madison, WI, USA) was used to synthesize obtained RNA into cDNA according to the manufacturer's instructions, with the temperature protocol as follows: 42°C for 30 min, 85°C for 5 sec, then holding at 4°C. The Step One Plus Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) was applied for the qPCR assay using SYBR Green mix (Takara Bio, Inc., Otsu, Japan). The thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min; followed by 40 amplification cycles of 95°C for 5 sec; annealing at 60°C for 15 sec; and elongation at 72°C for 15 sec. Ribosomal protein L13a (RPL13A) served as the internal control and the relative gene expression levels was determined by 2−ΔΔCq method (12). The primer sequences used in the present study were as follows: ERRα forward, 5′-TGCTCAAGGAGGGAGTGC-3′ and reverse, 5′-GGCGACAATTTCTGGTTCGGGTCAGGCATGGCATAG-3′; RPL13A forward, 5′-CCTGGAGGAGAAGAGGAAAGAGA-3′ and reverse, 5′-TTGAGGACCTCTGTGTATTTGTCAA-3′. Three independent experiments were performed.
Immunohistochemical staining and evaluation
The CRC tissue specimens were paraffin-embedded and processed for 4 µm-thick sections. The sections were dewaxed in xylene and rehydrated with descending series of ethanol gradient (100, 95, 90, 80 and 70%). The sections were incubated with 0.3% hydrogen peroxidase at room temperature for 25 min to block endogenous peroxidase activity, and were heated at 100°C in a 700W microwave oven for 15 min for antigen retrieval. In order to prevent nonspecific staining, the sections were pre-incubated with 10% normal goat serum (cat. no. 71-00-27; KPL; Seracare Life Sciences, Milford, MA, USA) in PBS at room temperature for 30 min. The sections were incubated with rabbit anti-ERRα primary antibody (cat. no. ab227944,1:500; Abcam, Cambridge, UK) overnight at 4°C, followed by incubation with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (cat. no. ab97051,1:200; Abcam) for 30 min at room temperature. The 3,3′-diaminobenzidine tetra-hydrochloride (liquid DAB+; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) was used to reveal antigen-antibody reactions. Finally, after the tissues were counterstained with hematoxylin at room temperature for 5 min, the sections were dehydrated and mounted. Sections incubated with PBS instead of the primary antibody served as negative controls.
Two pathologists independently evaluated the staining semi-quantitatively, who were blind to the clinical data. Any discrepancy between the two observers was assessed by a pathologist to reach the consensus. In each section, five visual fields were randomly selected for evaluation. The expression of ERRα was evaluated according to the labeling index (LI), which indicates the positive immunoreactivity of carcinoma cells. For statistical analyses, the cases that exhibited LI <10% were considered to have a low expression of ERRα, and the cases with LI >10% were considered to have a high expression of ERRα (13).
Statistical analysis
All quantitative data are presented as the mean ± standard deviation. Statistical analysis was performed using SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA). The unpaired two-tailed student's t-test was applied to compare the ERRα mRNA expression in primary CRC tumors and adjacent non-tumorous tissues. The Chi-square test was applied to assess the association between clinicopathological parameters and ERRα expression. The Kaplan-Meier estimator was performed to construct survival and local recurrence curves, and the log-rank test was applied to compare differences between groups. Significant independent prognostic factors for patients with CRC were analyzed using univariate and multivariate analysis based on the Cox proportional hazard model. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of ERRα in CRC tissues and adjacent normal tissues
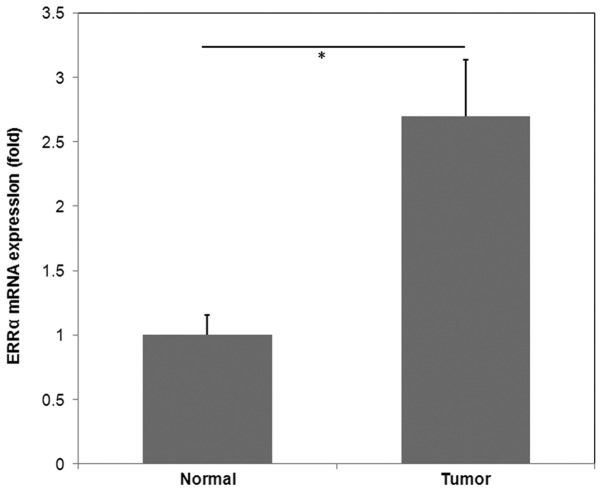
The mRNA expression of ERRα was determined by RT-qPCR analysis in 15 fresh CRC tissues and matched normal tissues. The results demonstrated that CRC tissues exhibited significantly higher ERRα expression compared with normal tissues (P<0.05; Fig. 1).
Figure 1.
ERRα mRNA expression was significantly increased in CRC tissues compared with adjacent normal tissues. Reverse transcription-quantitative polymerase chain reaction was performed to detect the mRNA expression of ERRα in CRC and adjacent normal tissues. The data are presented as the mean ± standard deviation. The relative expression of ERRα mRNA wad normalized to that of RPL13A. The relative expression level of ERRα mRNA was significantly higher in CRC tissues compared with that in adjacent normal tissues (2.70±0.44 vs. 1.00±0.15). *P<0.05. CRC, colorectal cancer; ERRα, estrogen-related receptor α; RPL13A, ribosomal protein L13a.
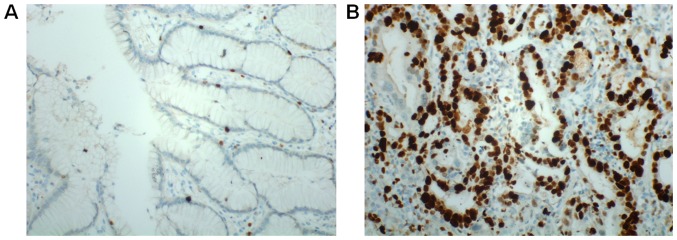
The protein expression of ERRα was determined by immunohistochemistry in 128 CRC tissues and matched normal tissues. ERRα was primarily detected in the nuclei of tumor cells. According to the results of staining evaluation, the mean values of ERRα LI were 28.7% (range, 0–83%) and 12.1% (range, 0–31%) in the 128 CRC cancer tissues and 127 adjacent normal tissues, respectively. The number of ERRα high expression colorectal cancer (ERRα LI >10%) was 50/128 cases (39.1%) (Table I), compared with 14/128 cases (10.9%) in adjacent normal tissues. ERRα expression was significantly higher in colorectal cancer tissues compared with that in adjacent normal tissues (P<0.05) (data not shown). The representative results of immunohistochemistry are presented in Fig. 2.
Table I.
Association between ERRα expression and clinicopathological parameters in patients with CRC.
| ERRα expression (n=128) | |||||
|---|---|---|---|---|---|
| Clinical features | All cases | Low (n=78) | High (n=50) | χ2 test | P-value |
| Sex | 0.469 | 0.494 | |||
| Male | 72 | 42 | 30 | ||
| Female | 56 | 36 | 20 | ||
| Age, years | 1.297 | 0.255 | |||
| <60 | 77 | 50 | 27 | ||
| ≥60 | 51 | 28 | 23 | ||
| Tumor location | 0.340 | 0.560 | |||
| Colon | 65 | 38 | 27 | ||
| Rectum | 63 | 40 | 23 | ||
| Tumor size, cm | 2.053 | 0.152 | |||
| <5 | 74 | 49 | 25 | ||
| ≥5 | 54 | 29 | 25 | ||
| Tumor differentiation | 6.386 | 0.012 | |||
| Well, moderate | 69 | 49 | 20 | ||
| Poor | 59 | 29 | 30 | ||
| Tumor invasion | 5.330 | 0.021 | |||
| T1+T2 | 70 | 49 | 21 | ||
| T3+T4 | 58 | 29 | 29 | ||
| Lymph node status | 8.412 | 0.004 | |||
| Absent | 74 | 53 | 21 | ||
| Present | 54 | 25 | 29 | ||
| Dukes stage | 7.691 | 0.006 | |||
| A+B | 78 | 55 | 23 | ||
| C+D | 50 | 23 | 27 | ||
ERRα, estrogen-related receptor α; CRC, colorectal cancer.
Figure 2.
Representative immunohistochemical staining of ERRα protein in normal tissues and CRC tissues. (A) Low expression of ERRα in normal tissues. (B) High expression of ERRα in CRC tissues. Original magnification, ×200. CRC, colorectal cancer; ERRα, estrogen-related receptor α.
Associations between ERRα expression and CRC clinicopathological parameters
To further investigate the clinical significance of ERRα in CRC, the associations between ERRα expression and CRC clinicopathological characteristics in 128 patients were statistically analyzed (Table I). Significant associations were identified between ERRα expression, and tumor differentiation (P=0.012), tumor invasion (P=0.021), lymph node status (P=0.004) and Dukes stage (P=0.006). However, no statistically significant associations between ERRα expression and other clinicopathological parameters, including gender (P=0.494), age (P=0.255), tumor location (P=0.560) and tumor size (P=0.152), were identified.
Prognostic significance of ERRα expression in CRC
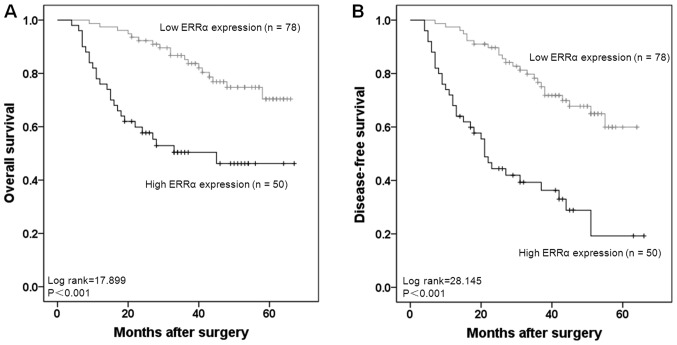
The Kaplan-Meier estimator model was applied to evaluate the prognostic significance of ERRα expression. The 5-year OS rates in patients with high ERRα expression and low expression were 50.0 and 76.9%, respectively. Patients with high ERRα expression had significantly lower OS and DFS rates compared with patients with low ERRα expression (both P<0.001; Fig. 3A and B).
Figure 3.
Kaplan-Meier estimator curves for 5-year overall survival and local recurrence in patients with colorectal cancer. (A) Patients with high ERRα expression exhibited a poorer overall survival rate compared with those with low ERRα expression (P<0.001). (B) Patients with high ERRα expression exhibited a poorer disease-free survival compared with those with low ERRα expression (P<0.001). ERRα, estrogen-related receptor α.
The univariate analysis was conducted to identify prognostic factors for CRC patients. It was revealed that in patients with CRC, ERRα expression was significantly associated with decreased rates of OS and DFS (P<0.05; Table II). Furthermore, tumor differentiation, tumor invasion, lymph node status and Dukes stage were also significantly associated with decreased rates of OS and DFS in patients with CRC (Table II). These results suggested that ERRα is a valuable prognostic factor in CRC. Therefore, multivariate analysis was performed using the Cox proportional hazards model, which demonstrated that ERRα expression was an independent prognostic factor for survival in patients with CRC [OS: Hazard ratio (HR), 2.022; 95% confidence interval (CI), 1.067–3.835; DFS: HR, 2.375; 95% CI, 1.365–4.133; Table III].
Table II.
Univariate Cox regression analyses for overall survival and disease-free survival in patients with CRC.
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Clinical features | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex | 1.289 (0.671–2.475) | 0.446 | 1.300 (0.730–2.318) | 0.373 |
| Age | 1.215 (0.644–2.292) | 0.548 | 1.201 (0.698–2.067) | 0.507 |
| Tumor location | 1.387 (0.728–2.644) | 0.320 | 1.121 (0.641–1.962) | 0.689 |
| Tumor size | 1.409 (0.718–2.768) | 0.319 | 1.267 (0.718–2.236) | 0.413 |
| Tumor differentiation | 2.217 (1.108–4.434) | 0.024 | 1.947 (1.096–3.458) | 0.023 |
| Tumor invasion | 2.156 (1.098–4.233) | 0.026 | 1.809 (1.026–3.189) | 0.041 |
| Lymph node status | 2.035 (1.037–3.995) | 0.039 | 2.012 (1.136–3.563) | 0.016 |
| Dukes stage | 2.192 (1.149–4.181) | 0.017 | 2.386 (1.350–4.217) | 0.003 |
| ERRα expression | 2.023 (1.024–3.997) | 0.043 | 2.323 (1.292–4.176) | 0.005 |
HR, hazard ratio; CI, confidence interval; ERRα, estrogen-related receptor α; CRC, colorectal cancer.
Table III.
Multivariate Cox regression analyses for overall survival and disease-free survival in patients with CRC.
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Clinical features | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Tumor differentiation | 2.347 (1.208–4.560) | 0.012 | 1.952 (1.124–3.391) | 0.018 |
| Tumor invasion | 2.300 (1.184–4.467) | 0.014 | 1.955 (1.123–3.402) | 0.018 |
| Lymph node status | 2.299 (1.197–4.417) | 0.012 | 2.122 (1.216–3.702) | 0.008 |
| Dukes stage | 2.209 (1.187–4.111) | 0.012 | 2.474 (1.436–4.263) | 0.001 |
| ERRα expression | 2.022 (1.067–3.835) | 0.031 | 2.375 (1.365–4.133) | 0.002 |
HR, hazard ratio; CI, confidence interval; ERRα, estrogen-related receptor α; CRC, colorectal cancer.
Discussion
ERRα is a key regulator in energy-driven cellular processes through the modulation of mitochondrial function and metabolism. Cancer cells demonstrate high proliferating capabilities and require constant biosynthesis of macromolecules as building blocks for the generation of new cells, thereby resulting in a high-energy demand for cancer cells (14). ERRα demonstrates key regulatory roles in a variety of malignant processes, including proliferation, invasion, metastasis and chemotherapy resistance (15–17). Since ERRα serves a crucial role in cancer initiation, development and progression, it may be speculated that ERRα holds significant clinical significance for patients with cancer. ERRα expression was reported to be elevated in a variety of cancer types. A previous study revealed increased expression of ERRα mRNA in ovarian cancer compared with healthy ovaries (18). In addition, the ERRα-positive group exhibited statistically significant reduced OS rates compared with the ERRα-negative group (18). In another study, the levels of ERRα mRNA increased with the clinical stage of ovarian cancer, thus making ERRα a prognostic factor for ovarian cancer (19). In endometrial adenocarcinoma, the expression of ERRα mRNA was positively correlated with the International Federation of Gynecology and Obstetrics stage and myometrial invasion, indicating that ERRα participates in the tumorigenesis of endometrial adenocarcinoma, and may be a promising prognostic factor (20). Through the use of immunohistochemistry on prostate cancer specimens, researchers demonstrated that nuclear ERRα expression was significantly higher in the cancerous lesions compared with that in benign epithelia (21). Elevated ERRα expression in cancerous lesions was significantly correlated with poor cancer-specific survival rates in patients with prostate cancer (21). In human breast carcinoma, ERRα immunoreactivity was significantly associated with increased recurrence and shorter survival times (22). Although ERRα expression has been reported to be associated with CRC tumor progression (9), the clinical and prognostic significance of ERRα in CRC remains unclear.
In the present study, RT-qPCR was performed to detect the mRNA expression of ERRα in 15 primary CRC tissues and adjacent normal tissues. The result demonstrated that ERRα mRNA expression was significantly higher in primary CRC tissues compared with in adjacent normal tissues. Furthermore, immunohistochemistry confirmed the results of the RT-qPCR analysis whereby an increased number of CRC tissues (81/127, 63.8%) exhibited high ERRα protein expression compared with adjacent normal tissues (34/127, 26.8%). These results were consistent with a previous study whereby elevated levels of ERRα mRNA were detected in CRC tumor tissues when compared with normal mucosa (9). Furthermore, immunohistochemistry from 127 patients with CRC indicated that ERRα expression was significantly associated with tumor differentiation, tumor invasion, lymph node status, Dukes classification and distant metastasis, suggesting that ERRα may be involved in the progression of CRC.
Prognostic biomarkers provide useful information for doctors in the clinical treatment of patients with CRC, and they are conducive for identifying patients who have a higher probability of recurrence or developing chemoresistance. In the current study, the potential for ERRα as a CRC prognostic biomarker was demonstrated. High ERRα expression was associated with lower 5-year OS and DFS compared with patients with low ERRα expression. Multivariate analysis was performed, which identified ERRα expression as an independent prognostic factor for patients with CRC. Thus, ERRα detection may be valuable for prognosis evaluation and personalized therapy in patients with CRC. Tumor recurrence is an important cause for poor survival of patients with CRC. In the present study, compared with patients with low ERRα expression, patients with high ERRα expression exhibited a higher recurrence rate. These results are in accordance with another study whereby high ERRα expression was associated with an increased risk of recurrence in patients with breast cancer (22).
A variety of molecular mechanisms may underlie the associations between ERRα expression, and recurrence and survival in CRC. Firstly, ERRα contributes to CRC recurrence through promoting CRC cell proliferation. A recent study demonstrated that ERRα significantly enhanced CRC cell proliferation, colony formation and accelerated cell cycle transition from the G1 to the S phase (23). ERRα also promotes the growth of human lung cancer cells, which is not a hormone-dependent cancer (16), indicating the effect of ERRα on cell proliferation is universal in various cancer types. MicroRNAs may negatively regulate ERRα gene expression at the post-transcriptional level and by silencing ERRα expression, miR-137 reduced the proliferation of breast cancer cells and miR-125a reduced the proliferation of oral squamous cell carcinoma cells (24,25). Secondly, ERRα may promote invasion and metastasis of colorectal cancer cells through epithelial-mesenchymal transition (EMT) process. Invasion and metastasis are important clinicopathological factors for patients with CRC with unfavorable prognosis, and they are induced by EMT at the molecular level (26). Previous studies have reported that ERRα promotes invasion and metastasis by inducing EMT in lung cancer, and ovarian cancer cells (16,27). Therefore, we hypothesize that EMT mediated by ERRα underlies the mechanism for the association between high ERRα expression and poor prognosis in patients with CRC. Thirdly, ERRα may be involved in the activation of CRC stem cells. In CRC, EMT induces the generation of CRC stem cells, which have high metastatic potential (28). As a positive regulator of EMT in several cancer types (16,27), ERRα may increase the number of CRC stem cells from residual cancer cells in patients with CRC following curative resection, thereby contributing to recurrence. A previous study revealed that ERRα, combined with PGC1α, activates the promoter of osteopontin (OPN) gene and leads to elevated OPN expression in CRC cells (29). OPN is a glycoprotein secreted by a variety of tissues and enhances cancer stem cell phenotypes in various cancer types (30,31). Higher levels of OPN are produced by macrophages when co-cultured with cluster of differentiation 44-positive CRC stem cells, subsequently increasing the tumorigenicity of the CRC cells (32). Given the fact that ERRα is able to increase the tumorigenic capacity of CRC cells (23), we hypothesize that ERRα-induced OPN expression may enhance the CRC cell phenotype and promote the tumor recurrence in patients. However, the detailed mechanisms require further investigation.
In conclusion, the results of the present study revealed that ERRα expression was higher in CRC tissues compared with in adjacent normal tissues, and ERRα expression was associated with the progression of CRC. In addition, high ERRα expression was associated with lower OS and local recurrence, and was identified as an independent prognostic factor for patients with CRC. These results suggest that ERRα is a promising therapeutic target for patients with CRC.
Acknowledgements
The present study was supported by Regional Science Fund Project of China Natural Science Foundation (grant nos. 81660498, 81360347 and 81560493), by China Postdoctoral Science Foundation, the 60th Grant Funding of General Program for the Post-Doctoral Funding Program in the Western Region (grant no. 2016M602919XB), by Guangxi Natural Science Foundation (grant nos. 2016GXNSFBA380090 and 2015GXNSFAA139128), by Self-raised Scientific Research Funds of Ministry of Health of Guangxi Province (grant nos. Z2015605 and Z2016480), by Funds of Development of Appropriate Civilization Health Technologies of Guangxi (grant no. S2017101).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381–396. doi: 10.1016/j.bpg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Kriza C, Emmert M, Wahlster P, Niederländer C, Kolominsky-Rabas P. Cost of illness in colorectal cancer: An international review. Pharmacoeconomics. 2013;31:577–588. doi: 10.1007/s40273-013-0055-4. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 5.Berg M, Søreide K. Genetic and epigenetic traits as biomarkers in colorectal cancer. Int J Mol Sci. 2011;12:9426–9439. doi: 10.3390/ijms12129426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;60:116–129. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 8.Horard B, Vanacker JM. Estrogen receptor-related receptors: Orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31:349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini A, Notarnicola M, Giannini R, Montemurro S, Lorusso D, Visconti A, Minervini F, Caruso MG. Oestrogen receptor-related receptor alpha (ERRalpha) and oestrogen receptors (ERalpha and ERbeta) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer. 2005;41:1487–1494. doi: 10.1016/j.ejca.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Bosman FT, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. 4th. Vol. 3. IARC; Lyon: 2010. [Google Scholar]
- 11.Sarma DP. The Dukes classification of colorectal cancer. JAMA. 1986;256:1447. doi: 10.1001/jama.1986.03380110053025. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Rudolph A, Toth C, Hoffmeister M, Roth W, Herpel E, Jansen L, Marx A, Brenner H, Chang-Claude J. Expression of oestrogen receptor β and prognosis of colorectal cancer. Br J Cancer. 2012;107:831–839. doi: 10.1038/bjc.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deblois G, St-Pierre J, Giguère V. The PGC-1/ERR signaling axis in cancer. Oncogene. 2013;32:3483–3490. doi: 10.1038/onc.2012.529. [DOI] [PubMed] [Google Scholar]
- 15.Bianco S, Sailland J, Vanacker JM. ERRs and cancers: Effects on metabolism and on proliferation and migration capacities. J Steroid Biochem Mol Biol. 2012;130:180–185. doi: 10.1016/j.jsbmb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Huang JW, Guan BZ, Yin LH, Liu FN, Hu B, Zheng QY, Li FL, Zhong YX, Chen Y. Effects of estrogen-related receptor alpha (ERRα) on proliferation and metastasis of human lung cancer A549 cells. J Huazhong Univ Sci Technolog Med Sci. 2014;34:875–881. doi: 10.1007/s11596-014-1367-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Wang H, Duan Z, Zou JX, Chen H, He W, Wang J. Estrogen-related receptor alpha confers methotrexate resistance via attenuation of reactive oxygen species production and P53 mediated apoptosis in osteosarcoma cells. Biomed Res Int. 2014;2014:616025. doi: 10.1155/2014/616025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun P, Sehouli J, Denkert C, Mustea A, Könsgen D, Koch I, Wei L, Lichtenegger W. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med (Berl) 2005;83:457–467. doi: 10.1007/s00109-005-0639-3. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto J, Alam SM, Jahan I, Sato E, Sakaguchi H, Tamaya T. Clinical implication of estrogen-related receptor (ERR) expression in ovarian cancers. J Steroid Biochem Mol Biol. 2007;104:301–314. doi: 10.1016/j.jsbmb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Gao M, Sun P, Wang J, Zhao D, Wei L. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16:827–833. doi: 10.1111/j.1525-1438.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 21.Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S. Increased expression of estrogen-related receptor alpha (ERRalpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer. 2007;120:2325–2330. doi: 10.1002/ijc.22363. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- 23.Bernatchez G, Giroux V, Lassalle T, Carpentier AC, Rivard N, Carrier JC. ERRα metabolic nuclear receptor controls growth of colon cancer cells. Carcinogenesis. 2013;34:2253–2261. doi: 10.1093/carcin/bgt180. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y, He F. miR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PLoS One. 2012;7:e39102. doi: 10.1371/journal.pone.0039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari A, Shivananda S, Gopinath KS, Kumar A. MicroRNA-125a reduces proliferation and invasion of oral squamous cell carcinoma cells by targeting estrogen-related receptor α: Implications for cancer therapeutics. J Biol Chem. 2014;289:32276–32290. doi: 10.1074/jbc.M114.584136. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Todosi AM, Gavrilescu MM, Aniţei GM, Filip B, Scripcariu V. Colon cancer at the molecular level-usefulness of epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med Nat Iasi. 2012;116:1106–1111. [PubMed] [Google Scholar]
- 27.Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY, Wong AS. Targeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol Ther. 2014;22:743–751. doi: 10.1038/mt.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Findlay VJ, Wang C, Watson DK, Camp ER. Epithelial-to-mesenchymal transition and the cancer stem cell phenotype: Insights from cancer biology with therapeutic implications for colorectal cancer. Cancer Gene Ther. 2014;21:181–187. doi: 10.1038/cgt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudjadi S, Bernatchez G, Beaulieu JF, Carrier JC. Control of the human osteopontin promoter by ERRα in colorectal cancer. Am J Pathol. 2013;183:266–276. doi: 10.1016/j.ajpath.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, Zhu M, Jia R, Wang H, Zhang X, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-κB-HIF-1α pathway. Oncotarget. 2015;6:6627–6640. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao G, Wang H, Li B, Huang L, Xue D, Wang X, Jin H, Wang J, Zhu Y, Lu Y, et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res. 2013;19:785–797. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]