Abstract
Cytotoxic T lymphocyte associated antigen 4 (CTLA-4) serves an important role in inhibiting anti-tumor immune response in the majority of solid tumors. However, a limited number of studies reported the function of CTLA-4 in luminal B HER2-negative breast cancer. Immunohistochemistry was performed to evaluate the expression of tumor and interstitial CTLA-4 in luminal B HER2-negative breast cancer tissues. The percentage of patients with tumor and interstitial CTLA-4+ was 41.2% (42/102) and 46.1% (47/102), respectively. There was a positive association between tumor CTLA-4 expression and interstitial CTLA-4 expression (P<0.05). The disease-free survival (DFS) of the tumor CTLA-4+ group was significantly shorter compared with patients with tumor CTLA-4− (mean, 89.070 vs. 39.022 months; P<0.0001). Additionally, the DFS of interstitial CTLA-4+ group was shorter compared with the interstitial CTLA-4− group (mean, 85.526 vs. 46.574 months; P<0.0001). Tumor and interstitial CTLA-4 expression may have prognostic predicting value in luminal B HER2-negative breast cancer. The present study may provide the basis for the use of a CTLA-4 blocker in patients with luminal B HER2-negative breast cancer.
Keywords: cytotoxic T lymphocyte associated antigen 4, luminal B HER2-negative breast cancer, prognosis
Introduction
Breast cancer is the second largest cause of mortality in women worldwide (1,2). Compared with western countries, the incidence in China has increased greatly since the 1990s (3,4). Breast cancer is a heterogeneous disease, with five molecular subtypes, including luminal A, luminal B HER2-negative, luminal B HER2-positive, HER2-positive and triple negative. Furthermore, the incidence, treatment and prognosis vary greatly among these five molecular subtypes (5).
A recent epidemiological study demonstrated that the luminal B HER2-negative breast cancer had the highest incidence among these five molecular subtypes, and >40% patients are Chinese women (6). Although luminal B HER2-negative breast cancer expresses hormone receptors, the curative effect of hormone therapy and conventional chemotherapy is unsatisfactory (7). Therefore, it is important to identify new targets for the treatment of luminal B HER2-negative breast cancer.
Cytotoxic T lymphocyte associated antigen 4 (CTLA-4), a CD28 homologue, consists of a short cytoplasmic tail, a signal peptide, a transmembrane domain and a cellular extracellular ligand-binding domain (8). CTLA-4 can be a competitive inhibitor for CD28, where it binds to the ligands; CD80 or CD86, resulting in inhibition of T cell activation and raising the response threshold of T cells (9). In fact, CTLA-4 has a stronger binding affinity with the two ligands compared with CD28, thus high expression of CTLA-4 may lead to inhibition of anti-tumor immune response (8). CTLA-4 may be expressed on the surface of T cells (10). However, there are some reports supporting the notion that CTLA-4 is also expressed on non-T cells, such as solid tumors (11–14). A recent study demonstrated that patients overexpressing CTLA-4 in esophageal cancer cells tend to have a poor prognosis (15).
Recent studies have also demonstrated that CTLA-4 is overexpressed in breast cancer cells (10,11). However, the association between CTLA-4 expression and prognosis in breast cancer patients is not fully elucidated, particularly for luminal B HER2-negative breast cancer. Therefore, the present study aimed to answer such questions and provide a basis for new therapeutic targets for the treatment of luminal B HER2-negative breast cancer.
Materials and methods
Patients
A total of 102 cases of patients with stage I–III luminal B HER2-negative breast cancer who underwent radical surgery between January 2008 and December 2012 at Fuzhou General Hospital of Nanjing Military Command (Fuzhou, China) were selected for the present study. The study protocol was approved by the Medical Ethics Committee of the Fuzhou General Hospital. The clinicopathological characteristics of 102 patients were collected, which included age, histological grade, lymph node, menopausal status, tumor size and stage. The selection of clinicopathological characteristics was determined by the recurrence risk factors, which are recommended by the breast cancer prognosis guideline (16). The inclusion criteria were as follows: (i) Pathologically confirmed diagnosis of luminal B HER2-negative breast cancer according to the St Gallen International Expert Consensus 2013 (17); (ii) no chemotherapy or hormone therapy prior to surgery; (iii) paraffin-embedded specimens of tumor tissues were available, (iv) informed consent was obtained; (v) follow-up was available. The median follow-up time was 43.5 months (range, 1–96 months), which included 28 patients where metastasis or local recurrence was present. The disease-free survival (DFS) rate was 72.5%. The median DFS rate was not obtained.
Immunohistochemistry (IHC)
The surgical resection specimens of 102 patients were fixed with formalin and embedded by paraffin. Continuous paraffin section (thickness, 4 µm) were deparaffinized using 100% xylene, 100% ethanol, 95% ethanol and 80% ethanol. The tissue sections were used for antigen retrieval by high-pressure with 0.01 mol/l ethylenediaminetetraacetic acid, and endogenous peroxidase activity was blocked by hydrogen peroxide at room temperature for 10 min. The tissue sections were washed in PBS three times, and then primary antibodies anti-CTLA-4 IgG (dilution, 1:200; catalog no. bs-1179R; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) and anti-Ki-67 antibody (dilution, 1:200; catalog no. bs-23105R; Beijing Biosynthesis Biotechnology Co., Ltd.) were added to the tissue sections for 60 min at room temperature. Subsequently, the tissue sections were washed with PBS three times and incubated with EliVision™ plus Polyer horseradish peroxidase (Mouse/Rabbit) IHC kit (ready-to-use dilution; catalog no. KIT 9901; Fuzhou Maixin Biotechnology Co. Ltd., Fuzhou, China) at room temperature for 30 min. Subsequently, the tissue sections were stained with 3,3-diaminobenzidine (Fuzhou Maixin Biotechnology Co. Ltd.) at room temperature for 3 min. The sections were counterstained with hematoxylin at room temperature for 15 sec, and the slides were counterstained with neutral resin for subsequent observation with an optical microscope (magnification, ×100).
Evaluation of IHC staining
A total of two independent pathologists, who were blinded to patient characteristics and clinical outcomes, evaluated results of IHC staining. The expression of CTLA-4 was evaluated with the percentage and intensity of positive tumor cells. The scores of percentage of positive tumor cells were recorded as: 0 (0–25%), 1 (26–50%), 2 (51–75%) or 3 (76–100%). The scores of intensity of positive tumor cells were recorded as: 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The scores for percentage and intensity were multiplied together to attain a final histochemical score (H-score). Then, the mean H-score was calculated from all patient samples and determined as CTLA-4 positivity (tumor CTLA-4+). The CTLA-4 positivity in interstitial lymphocytes (interstitial CTLA-4+), which was in interstitial areas adjacent to tumor nests, was evaluated as aforementioned. Ki-67 was negative if there was less than 14% of nuclei staining and positive if ≥14%, in accordance with previous studies (17,18).
Statistical analysis
The data was analyzed using SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA). The association between CTLA-4 expression and various clinicopathologic parameters were analyzed by the χ2 test. The survival probability was estimated by the Kaplan-Meier test, and the statistical significance was performed using the log-rank test. The multivariate analysis for clinicopathological characteristics features was analyzed using the Cox regression model. All tests were two sided. P<0.05 was considered to indicate a statistically significant difference.
Results
CTLA-4 expression in luminal B HER2-negative breast cancer tissues and interstitial tissues
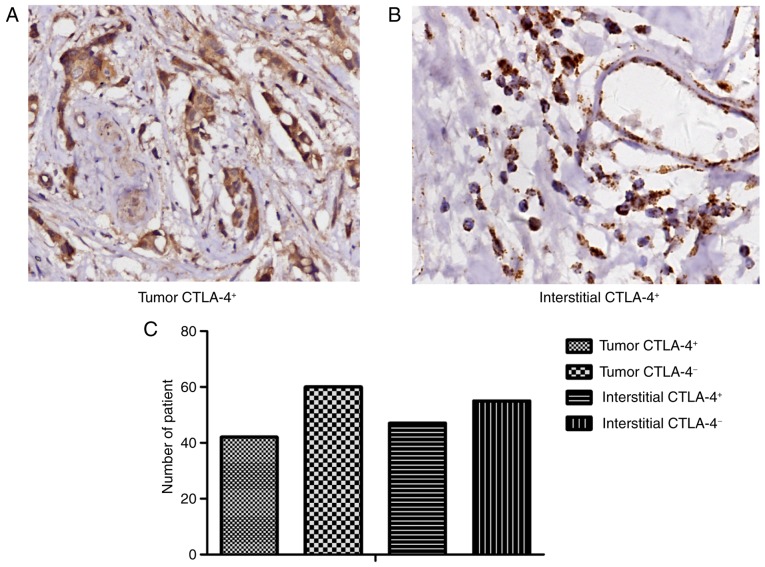
To investigate the function of CTLA-4 in progression of luminal B HER2-negative breast cancer, IHC was conducted to detect the expression of CTLA-4 in tumor and interstitial tissues. CTLA-4 was expressed in the cell membrane and cytoplasm of T lymphocytes and tumor cells (Fig. 1A and B, respectively). The percentage of patients with tumor CTLA-4+ was 41.2% (42/102), while the percentage of patients with interstitial CTLA-4+ was 46.1% (47/102; Fig. 1C). In addition, to investigate whether there is an association between the expression of tumor and interstitial CTLA-4, the χ2 test was used to demonstrate that there was a strong positive association between tumor and interstitial CTLA-4 expression (P<0.05).
Figure 1.
CTLA-4+ expression in (A) tumor and (B) interstitial tissue (magnification ×100). (C) Quantification of CTLA-4+ and CTLA-4− as analyzed by IHC. CTLA-4, cytotoxic T lymphocyte-associated antigen 4; IHC, immunohistochemistry.
Association between CTLA-4 expression and clinicopathological characteristics
The χ2 test was performed to examine the association between CTLA-4 expression and clinicopathological characteristics in luminal B HER2-negative breast cancer tissues. However, neither the expression of tumor CTLA-4 nor interstitial CTLA-4 was associated with any clinical characteristics of the patients in the present cohort, including age, histological grade, lymph node, menopausal status, tumor size and stage and Ki-67 expression (all P>0.05; Table I).
Table I.
Association between CTLA-4 expression and clinicopathological characteristics.
| Tumor CTLA-4 | Interstitial CTLA-4 | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological characteristics | n | (+) | (−) | P-value | (+) | (−) | P-value |
| Age (years) | 0.099 | 0.845 | |||||
| <35 | 8 | 6 | 2 | 5 | 3 | ||
| ≥35 | 94 | 36 | 58 | 49 | 45 | ||
| Menopausal status | 0.868 | 0.560 | |||||
| Premenopausal | 52 | 21 | 31 | 29 | 23 | ||
| Postmenopausal | 50 | 21 | 29 | 25 | 25 | ||
| Histological grade | 0.422 | 0.148 | |||||
| I | 16 | 6 | 10 | 11 | 5 | ||
| II | 58 | 27 | 31 | 32 | 26 | ||
| III | 28 | 9 | 19 | 11 | 17 | ||
| Tumor size (cm) | 0.636 | 0.243 | |||||
| ≤2 | 49 | 19 | 30 | 23 | 26 | ||
| >2 | 53 | 23 | 30 | 31 | 22 | ||
| Lymph node | 0.332 | 0.161 | |||||
| Negative | 52 | 19 | 33 | 24 | 28 | ||
| Positive | 50 | 23 | 27 | 30 | 20 | ||
| Tumor stage | 0.298 | 0.114 | |||||
| I | 29 | 10 | 19 | 11 | 18 | ||
| II | 47 | 18 | 29 | 26 | 21 | ||
| III | 26 | 14 | 12 | 17 | 9 | ||
| Ki-67 | 0.884 | 0.376 | |||||
| <14 | 9 | 3 | 6 | 3 | 6 | ||
| ≥14 | 93 | 39 | 54 | 51 | 42 | ||
CTLA-4, cytotoxic T lymphocyte-associated antigen 4.
Association between tumor/interstitial CTLA-4 expression and clinical outcomes of patients with luminal B HER2-negative breast cancer tissues
The Kaplan-Meier method was performed to examine the association between the expression of tumor/interstitial CTLA-4 and the survival of patients with luminal B HER2-negative breast cancer. In the present study, the DFS rate of patients with tumor CTLA-4+ was significantly shorter compared with patients with tumor CTLA-4− (mean, 89.070 vs. 39.022 months; P<0.0001; Fig. 2A). Additionally, the DFS rate of the patients with interstitial CTLA-4+ was considerably shorter compared with patients with interstitial CTLA-4− (mean, 85.526 vs. 46.574 months; P<0.0001; Fig. 2B). Furthermore, the multivariate cox regression analysis demonstrated that tumor CTLA-4+ was an independent predictor of shorter DFS when factors, including age, menopausal status, histological grade, tumor size, the number of lymph nodes, clinical stage, Ki-67 and interstitial CTLA-4, are controlled (P<0.001; Table II).
Figure 2.
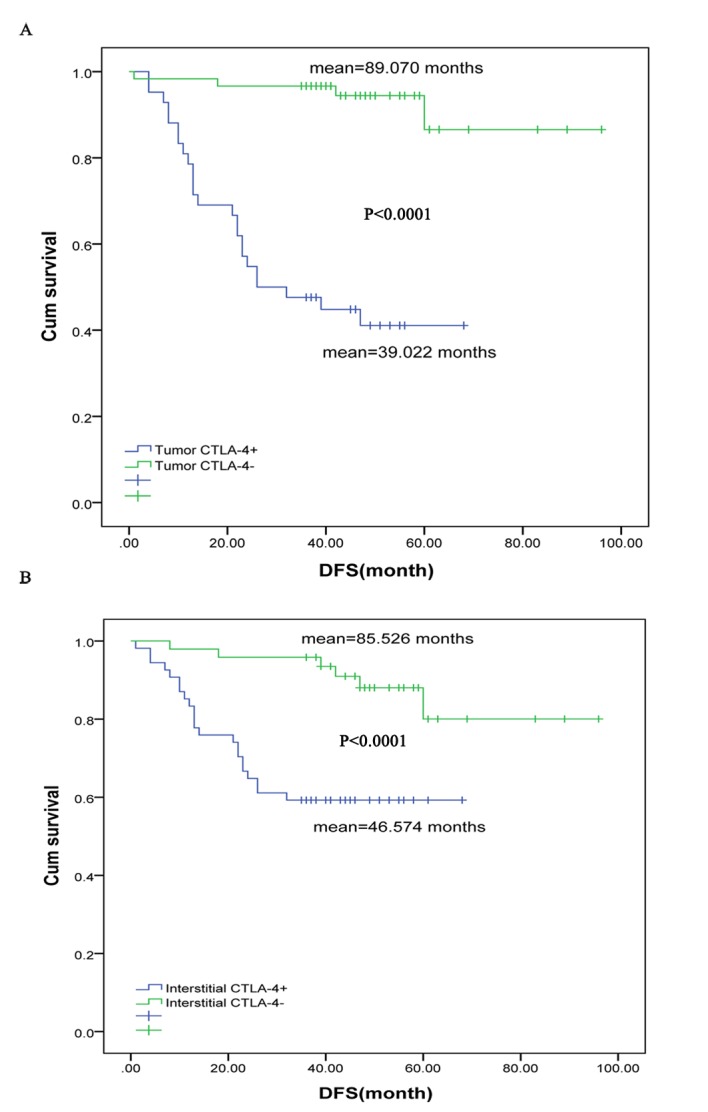
Kaplan-Meier analysis on the expression of (A) tumor and (B) interstitial CTLA and prognosis of breast cancer patients. CTLA-4, cytotoxic T lymphocyte-associated antigen 4; Cum, cumulative; DFS, disease-free survival.
Table II.
Multivariate analysis of patient survival.
| Clinicopathological characteristics | HR | 95% CI | P-value |
|---|---|---|---|
| Tumor CTLA-4 | 0.058 | 0.015–0.224 | <0.001 |
| Ki-67 | 4.406 | 0.528–36.756 | 0.171 |
| Tumor stage | 2.769 | 1.181–6.488 | 0.019 |
| Lymph node | 0.425 | 0.128–1.413 | 0.163 |
| Tumor size | 0.842 | 0.349–2.034 | 0.702 |
| Years | 2.337 | 0.471–11.591 | 0.299 |
| Menopausal status | 1.420 | 0.610–3.308 | 0.416 |
| Histological grade | 1.086 | 0.559–2.111 | 0.807 |
| Interstitial CTLA-4 | 1.413 | 0.430–4.637 | 0.569 |
CTLA-4, cytotoxic T lymphocyte-associated antigen 4; CI, confidence interval; HR, hazard ratio.
Discussion
Tumor-derived immune deregulation is a common characteristic in the majority of solid tumors, particularly for breast cancer (10). The immunosuppressive microenvironment primarily includes cytokines and immune checkpoint molecules, which leads to the blocking of anti-tumor immunity (19–21). One of these immune checkpoint molecules is the cytotoxic T lymphocyte antigen 4 (CTLA-4; CD152), a CD28 homologue which has two common ligands with CD28: B7-1 (CD80) and B7-2 (CD86). Notably, CTLA-4 has a stronger binding affinity with these two ligands compared with CD28 (8). The investigation of CTLA-4 is crucial for understanding tumor-derived immune deregulation and may give rise to novel immune therapy for breast cancer.
Previous studies have indicated that CTLA-4 was overexpressed in breast cancer cells. However, these studies did not investigate the function of CTLA-4 in different types of breast cancer, particularly luminal B HER2-negative breast cancer, which has a high incidence in Chinese women (11). In the present study, the expression of CTLA-4 was detected by IHC, and 41.2% (42/102) and 46.1% (47/102) were patients with tumor and interstitial CTLA-4+, respectively. Furthermore, the association between tumor CTLA-4 and interstitial CTLA-4 was assessed. It was demonstrated that there was a positive association of tumor CTLA-4 and interstitial CTLA-4. Subsequently, a χ2 test was performed to analyze the association between the expression of CTLA-4 and clinicopathological characteristics of the selected patients. However, the results demonstrated that the expression of neither tumor nor interstitial CTLA-4 was associated with any of the clinical characteristics assessed, including age, menopausal status, histological grade, tumor size, lymph node, tumor stage and Ki-67 expression level.
Previous studies reported that patients exhibiting positive tumor CTLA-4 expression had a better prognosis in NSCLC and gastric cancer (12,13). However, previous studies also demonstrated that CTLA-4 was a poor prognosis factor in esophageal carcinoma and breast cancer (10,19). Therefore, we hypothesized that CTLA-4 expression may be associated with the prognosis of patients with luminal B HER2-negative breast cancer. Furthermore, tumor CTLA-4+ was an independent risk factor for the prognosis of patients with breast cancer.
The results of a clinical trial for a CTLA-4 blocker, tremelimumab, for the treatment of breast cancer demonstrated that in the majority of patients, peripheral blood immune function was improved (22). Therefore, CTLA-4 may be a novel target for the treatment of luminal B HER2-negative breast cancer. However, the study by Vonderheide et al (22) demonstrated that there was no association between peripheral blood immune function and clinical outcomes (22). Tumor microenvironment may serve a greater role than immune status of the peripheral blood in anti-tumor immunotherapy. Tumor microenvironment may be taken into account when CTLA-4 becomes an immunotherapy target for breast cancer.
The present study has some limitations. More patients are required in future studies in order to confirm that CTLA-4 can be a biomarker for assessing the clinical outcomes of anti-CTLA-4 treatment in luminal B HER2-negative breast cancer. Furthermore, the association between tumor and interstitial CTLA-4, and the mechanism by which CTLA-4 enters the tumor cells were not investigated
In conclusion, the present study demonstrated a positive association between the expression of tumor and interstitial CTLA-4, which was associated with poor prognosis in luminal B HER2-negative breast cancer. The present study may provide novel therapeutic targets for patients with luminal B HER2-negative breast cancer. However, further studies are required to confirm these findings.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ML, Huang ZZ, Zheng Y. Estimates and prediction on indidence, mortality and prevalence of breast cancer in China, 2008. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:1049–1051. (In Chinese) [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network, corp-author. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si W, Li Y, Han Y, Zhang F, Wang Y, Li Y, Linghu RX, Zhang X, Yang J. Epidemiological and clinicopathological trends of breast cancer in chinese patients during 1993 to 2013: A retrospective study. Medicine (Baltimore) 2015;94:e820. doi: 10.1097/MD.0000000000000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13:221. doi: 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: An overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: Implications for prognosis. Cancer Immunol Immunother. 2015;64:853–860. doi: 10.1007/s00262-015-1696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao H, Zhang L, Yang Y, Zuo W, Bi Y, Gao W, Deng B, Sun J, Shao Q, Qu X. New Insights of CTLA-4 into its biological function in breast cancer. Curr Cancer Drug Targets. 2010;10:728–736. doi: 10.2174/156800910793605811. [DOI] [PubMed] [Google Scholar]
- 12.Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M, et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42–52. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A, Dozin B, Fontana V, Simone R, Mortara L, et al. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum Immunol. 2010;71:934–941. doi: 10.1016/j.humimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XF, Pan K, Weng DS, Chen CL, Wang QJ, Zhao JJ, Pan QZ, Liu Q, Jiang SS, Li YQ, et al. Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: Implications for prognosis. Oncotarget. 2016;7:26670–26679. doi: 10.18632/oncotarget.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehinger A, Malmström P, Bendahl PO, Elston CW, Falck AK, Forsare C, Grabau D, Rydén L, Stål O, Fernö M, South and South-East Swedish Breast Cancer Groups Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013. Acta Oncol. 2017;56:68–74. doi: 10.1080/0284186X.2016.1237778. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prihantono P, Hatta M, Binekada C, Sampepajung D, Haryasena H, Nelwan B, Asadul Islam A, Nilawati Usman A. Ki-67 expression by immunohistochemistry and quantitative real-time polymerase chain reaction as predictor of clinical response to neoadjuvant chemotherapy in locally advanced breast cancer. J Oncol. 2017;2017:6209849. doi: 10.1155/2017/6209849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emens LA. Breast cancer immunobiology driving immunotherapy: Vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch MA, Thomas KN, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu HM, Yang JL, Jiao SC, Wang JD, Li Y. TGF-β1 precursor and CD8 are potential prognostic and predictive markers in operated breast cancer. J Huazhong Univ Sci Technolog Med Sci. 2014;34:51–58. doi: 10.1007/s11596-014-1231-2. [DOI] [PubMed] [Google Scholar]
- 22.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J, Mariani GL, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]