Abstract
p60 is a subunit of katanin involved in microtubule-severing. Previous studies of p60 were primarily focused on microtubule regulation and cell cycle regulation. More recent research has demonstrated that katanin p60 possesses a function in prostate cancer bone metastasis; however, its role in breast cancer bone metastasis remains unclear. In the present study, immunohistochemistry was used to analyze the expression of katanin p60 in primary and bone metastatic breast cancer. The role of up- and downregulated katanin p60 was investigated using cell proliferation, and migration experiments. Overall, katanin p60 was highly expressed in breast cancer bone metastatic tissue compared with primary tumor tissue. In breast cancer cells, overexpression of katanin p60 inhibited cell proliferation, but promoted cell migration, whereas silencing katanin p60 expression promoted cell proliferation but inhibited cell migration. Overall, the present study indicated that katanin p60 serves a role in cell proliferation and migration, and thus may be a novel therapeutic target for prevention of breast cancer metastasis.
Keywords: katanin p60, breast cancer, cell proliferation, bone metastasis
Introduction
Breast cancer is among the most commonly diagnosed types of malignant tumor in women worldwide (1). Bone is the most common site of distant metastasis in patients with breast cancer and >70% of all patients with breast cancer eventually develop bone metastases, which presents clinical challenges (2). There is an increased risk of mortality for patients with breast cancer once bone metastasis has occurred (3). Therefore, elucidation of the mechanism of breast cancer bone metastasis is required for the identification of novel therapeutic targets for the prevention or control of bone metastasis.
Metastasis is associated with the migratory ability of tumor cells. Pseudopodia form through the rearrangement of the cell cytoskeleton and serve key roles in cell migration (4). Microtubules are essential for pseudopodia extension and regulation of cell movement, katanin is an ATPase that causes microtubule degradation (5–7). Katanin consists of p60 and p80 subunits (8); p80 targets the p60 subunit to the centrosome, and promotes the microtubule-severing activity of p60 (9). Research has demonstrated that leucine zipper tumor suppressor 2 (LAPSER1) and katanin p80 co-localize in the centrosome, and are involved in mitosis and cell movement (10). However, the activity of the p60 subunit remains poorly characterized and requires further research.
p60 is a 60-kDa enzymatic subunit containing an ATPases associated with diverse cellular activities (AAA) domain, which is responsible for microtubule-severing activity (11) and directly regulates microtubule-severing by phosphorylating katanin p60 at the Ser131 site (12). E3 ubiquitin ligases participate in the degradation of katanin p60 (13,14). In the context of disease, research into p60 function has primarily focused on the role of p60 in neurogenesis (15–17). To the best of our knowledge, the expression of katanin p60 in tumor metastasis has only been reported in prostate cancer (18), and its role in breast cancer is unknown. In the present study, the distribution and expression of katanin p60 in clinical breast cancer specimens was investigated, and it was determined whether katanin p60 was involved in breast cancer cell proliferation or promotion of breast cancer bone metastasis.
Materials and methods
Cell culture
The triple negative breast cancer cell line, MDA-MB-231 and metastatic invasive ductal carcinoma cell line, MCF-7 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin (Beyotime Institute of Biotechnology, Shanghai, China) at 37°C in 5% CO2.
Tissue samples and immunohistochemistry
The primary breast cancer specimens and breast cancer bone metastases specimens were divided into primary, and metastasis groups, each containing 10 specimens. All tissues were obtained from the Xiangyang Central Hospital between April 2013 and November 2016; the mean age of the patients was 61.2±13.1 years (37–83 years), written informed consent was obtained from all patients. The present study was approved by the Xiangyang Central Hospital Ethics Committee (Xiangyang, China). Paraffin tissue sections of 5 µm thickness were dewaxed with xylene and rehydrated with graded alcohol, antigen retrieval was performed using 10 mM citrate buffer pH 6.0 (3 mg/ml trisodium citrate, 0.4 mg/ml citric acid; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Then sections were treated with 3% hydrogen peroxide at room temperature for 15 min and blocked with 5% sheep serum (Beyotime Institute of Biotechnology) at 37°C for 30 min. Subsequent to an overnight incubation at 4°C with anti-katanin p60 primary antibody (dilution 1:200; cat no. ab111881; Abcam, Cambridge, UK), an ABC kit (cat no. SA1022; Boster Biological Technology, Pleasanton, CA, USA) was used for protein visualization. ImageJ software (version 1.46; National Institutes of Health, Bethesda, MD, USA) was used to evaluate the mean optical density of the immunohistochemical staining for each group.
Katanin p60 plasmids and transfection
Katanin p60 is encoded by the gene katanin catalytic subunit A1 (KATNA1; GenBank Accession no. 007044). The pcDNA3.1 and pcDNA3.1/KATNA1 plasmids were designed and constructed by Chongqing Weisiteng Biomedical Science and Technology Co., Ltd. (Chongqing, China). MDA-MB-231 and MCF-7 cells were seeded in 6-well plates at a density of 1.5×105 cells/well, and transfected with pcDNA3.1 or pcDNA3.1/KATNA1 when the cell confluence reached 50–70%. Two cell lines were transfected under the same condition. A total of 1800 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) was added to each well, followed by a mixture of 2 µg plasmid DNA, 6 µl X-tremeGENE transfection reagent (Roche, Basel, Switzerland) and 200 µl Opti-MEM, the control group was cultured only with Opti-MEM. Following an incubation of 5 h at 37°C, cells were collected at different time points for subsequent studies. The transfection efficiency was detected by western blotting and reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
shRNA
The shRNAs were constructed and identified by Chongqing Weisiteng Biomedical Science and Technology Co., Ltd., and the sequences were as follows: shRNA-KATNA1, forward, 5′-GGUUCAGAUGGAUGGUGUUTT-3′ and reverse, 5′-AACACCAUCCAUCUGAACCTT-3′); shRNA-negative control (NC), forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′). A total of 9 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) diluted in Opti-MEM and 3 µg shRNA-NC or shRNA-KATNA1 (0.5 µg/µl) diluted in Opti-MEM were mixed for 20 min, then the mixture was added into MDA-MB-231 or MCF-7 cells (6-well plates) for 6 h at 37°C, the transfection efficiency be detected by western blotting analysis and RT-qPCR.
RNA isolation and RT-qPCR
Cells were collected after transfected with plasmids or shRNAs for 48 h. At room temperature, total RNA was extracted with TRIzol (Thermo Fisher Scientific, Inc.) for 5 min, reacted with chloroform for 2–3 min, isopropanol for 10 min and 75% ethanol for 1 min sequentially, then RNA was dissolved in 0.1% diethyl pyrocarbonate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The concentration and purity of RNA were measured using a spectrophotometer (GE Healthcare, Chicago, IL, USA). Reverse transcription was performed using PrimeScript™ 1st strand cDNA Synthesis Kit (cat no. 6610A; Takara Biomedical Technology, Beijing, China), for 10 min at 25°C, 50 min at 42°C and 5 min at 85°C. qPCR analysis was performed using SYBR® Premix DimerEraser™ (Perfect Real Time; cat no. RR091A; Takara Biomedical Technology). The primer sequences were as follows: Katanin p60, forward, 5′-TAAACTGGACAGCACTCCCTTG-3′ and reverse, 5′-CCTGGTGAGGGTCTTCGTTC-3′; actin, forward, 5′TGACGTGGACATCCGCAAAG-3′ and reverse, 5′-CTGGAAGGTGGACAGCGAGG-3′. The thermocycling parameters were as follows: 94°C for 4 min; 35 cycles of 94°C for 20 sec, 60°C for 30 sec and 72°C for 30 sec. The relative expression of katanin p60 was obtained according to the 2−ΔΔCq method (19).
Western blot analysis
After transfected with shRNAs or plasmids for 48 h, cells were lysed using radioimmunoprecipitation assay buffer containing 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS (0.1 ml/1×106 cells; Beyotime Institute of Biotechnology). Total protein was extracted and its concentration was measured using a BCA protein assay kit (Beyotime Institute of Biotechnology). Extracted proteins (50 µg) were separated by SDS-PAGE (5% stacking gels, 12% resolving gels) and transferred into a nitrocellulose membrane. Subsequent to blocking with 5% skim milk (GE Healthcare, Chicago, IL, USA) for 2 h at room temperature, the membranes were washed and incubated with anti-katanin p60 antibody (dilution, 1:500; Abcam, Cambridge, UK) and anti-GADPH antibody (dilution, 1:1,000; Abcam) overnight at 4°C. Then the membranes were washed with 1X TBST buffer (pH 7.6; 2.42 g/l Tris, 8 g/l NaCl, 0.5 ml Tween-20) and incubated with the peroxidase-conjugated anti-rabbit secondary antibody (dilution, 1:1,000; cat no. A0545; Sigma-Aldrich; Merck KGaA) for 90 min at room temperature, an ECL chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.) was used to visualize the proteins. Densitometry was performed using ImageJ software (version 1.46; National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Un-transfected group, negative control and shRNA-KATNA1 or pcDNA3.1/KATNA1-transfected cells were seeded into 96-well plates at 1×104 cells/well for 24, 48, 72 and 96 h. Cells were cultured with 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) for 4–6 h, then 150 µl dimethyl sulfoxide was added (Amresco, LLC, Solon, OH, USA) and incubated for 10 min. Subsequently, the absorbance was measured using a multimode reader at 490 nm. Each experiment was repeated three times for construction of the growth curve.
Cell migration assay
Un-transfected group, negative control and shRNA-KATNA1 or pcDNA3.1/KATNA1-transfected cells were cultured for 24 h. Then, 2.5×104 cells were added to the upper chamber of Transwell filters, a total of 200 µl DMEM supplemented with 5% BSA (Gibco; Thermo Fisher Scientific, Inc.) was added to each lower chamber, and incubated for 24 h at 37°C. The cells were fixed with 70% formaldehyde for 30–60 min and stained with 0.1% crystal violet for 30 min (Sigma-Aldrich; Merck KGaA) at room temperature, and the number of migrated cells was counted using ImageJ software (version 1.46; National Institutes of Health).
Statistical analysis
All data were analyzed by SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± standard error. Student's t-test was used to determine the statistical significance. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of katanin p60 in breast cancer and bone metastasis
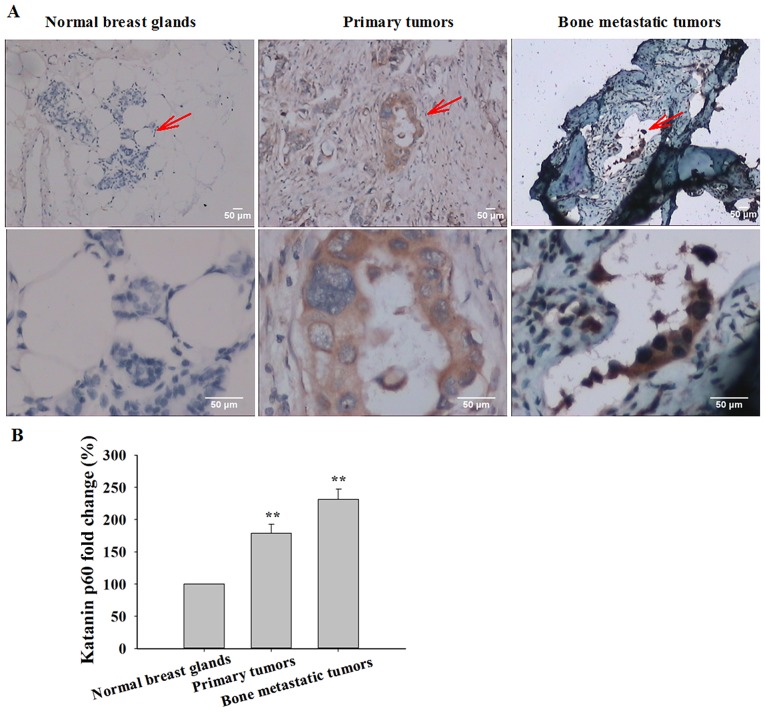
It has been demonstrated that the expression of katanin p60 contributes to the progression of prostate cancer, indicated that katanin p60 may also serve an important role in breast cancer (18). To test this hypothesis, immunohistochemical staining of tissues with an anti-katanin p60 antibody (Table I) was performed. Low expression of katanin p60 was exhibited in healthy breast tissue, and katanin p60 expression was primarily identified in the cytoplasm (Fig. 1A). The expression of katanin p60 was significantly increased in primary breast cancer tissue compared with healthy control tissue (178.96±13.81%; P=0.001; Fig. 1B). In bone metastatic breast cancer, the expression of katanin p60 was further increased when compared with healthy breast tissue (231.48±16.00%; P=0.001), and with primary breast cancer (P=0.023; Fig. 1B). Together, these data indicate that katanin p60 may function in the regulation of breast cancer cell proliferation and migration.
Table I.
Immunohistochemical staining of katanin p60.
| Tissue type | No. of patients | Mean optical density | Standard error | P-value (compared with healthy breast) |
|---|---|---|---|---|
| Healthy breast | 10 | 0.1687 | 0.0074 | |
| Primary breast tumor | 10 | 0.3019 | 0.0233 | 0.001 |
| Bone metastatic tumor | 10 | 0.3905 | 0.0270 | 0.001 |
Figure 1.
Immunostaining of katanin p60 in normal breast glands, primary breast and bones metastatic tumors (n=10/group). (A) The expression of katanin p60 in healthy breast, primary breast cancer and bone metastatic tissues. The red arrows indicate the enlarged area. (B) Quantitative analysis demonstrated that the expression of katanin p60 protein increased significantly in primary and bone metastatic tissues compared with healthy breast tissue, **P<0.01 vs. normal breast..
Up- or downregulation of katanin p60
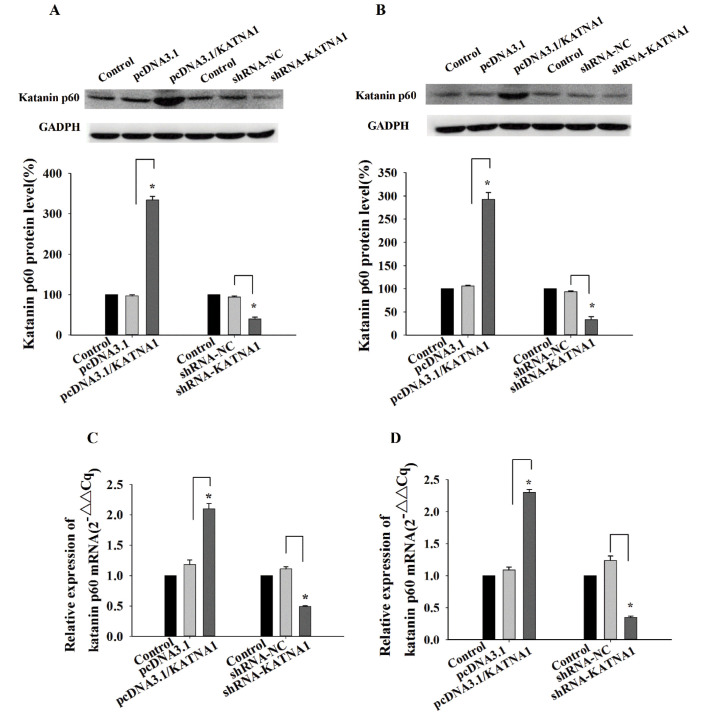
To determine the function of katanin p60 in breast cancer cells, plasmids and shRNAs were utilized to up- or downregulate its expression. RT-qPCR and western blotting were used to detect the expression of katanin p60 at the mRNA, and protein levels. In MDA-MB-231 cells and MCF-7 cells, the expression of katanin p60 was significantly increased following transfection with pcDNA3.1/KATNA1 (Fig. 2). In addition, the protein and mRNA levels of katanin p60 were significantly decreased following transfection with shRNA-KATNA1 (Fig. 2).
Figure 2.
Effect of up- or downregulation on MDA-MB-231 and MCF-7 cells. Western blot demonstrating the effects of pcDNA3.1/KATNA1 or shRNA-KATNA1 transfection on katanin p60 protein expression in (A) MDA-MB-231 and (B) MCF-7 cells. *P<0.05 vs. pcDNA3.1 or shRNA-NC. Reverse transcription-quantitative polymerase chain reaction was used to determine the expression of katanin p60 following transfection with pcDNA3.1/KATNA1 or shRNA-KATNA1 in (C) MDA-MB-231 and (D) MCF-7 cells. *P<0.05 vs. pcDNA3.1 or shRNA-NC. shRNA, short hair pin RNA; NC, negative control; KATNA1, katanin catalytic subunit A1.
Function of katanin p60 on cell proliferation
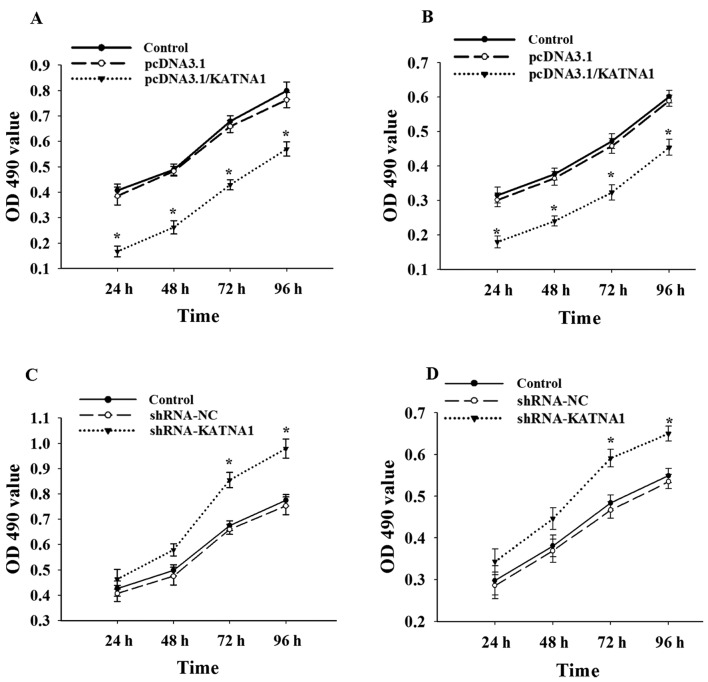
To further investigate whether katanin p60 was involved in the regulation of cell proliferation, cells were collected at 24, 48, 72 and 96 h after transfection with plasmids or shRNAs (Fig. 3). The present study demonstrated no significant difference in cell proliferation between MDA-MB-231 and MCF-7 cells. Upregulation of katanin p60 expression with pcDNA3.1/KATNA1 reduced cell proliferation, the percentage of proliferating cells at 24 h was 40.72±2.54% for MDA-MB-231 cells and 56.82±1.34% for MCF-7 cells (Fig. 3A and B). No significant differences in cell proliferation were identified compared with the shRNA-NC group at 24 or 48 h following transfection with shRNA-KATNA1; however, the percentage of proliferating cells at 72 h was significantly increased, at 126.64±0.88% in MDA-MB-231 cells and 122.03±0.56% in MCF-7 cells (Fig. 3C and D). This indicates that katanin p60 may serve a role in breast cancer cell proliferation.
Figure 3.
Katanin p60 in cell proliferation. Upregulated expression of katanin p60 inhibited the proliferation of (A) MDA-MB-231 and (B) MCF-7 cells. *P<0.05 vs. pcDNA3.1. Cell proliferation was increased in (C) MDA-MB-231 and (D) MCF-7 following transfected with shRNA-KATNA1. *P<0.05 vs. shRNA-NC. shRNA, short hair pin RNA; NC, negative control; OD, optical density; KATNA1, katanin catalytic subunit A1.
Katanin p60 is necessary for cell migration
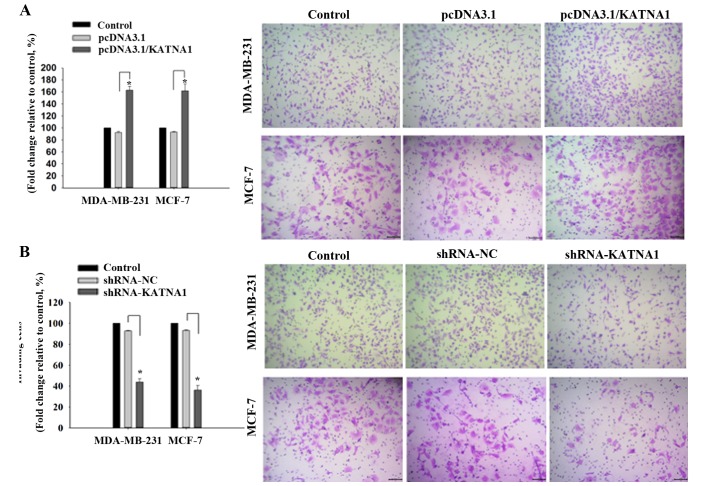
To investigate whether katanin p60 was involved in cell migration, plasmids or shRNAs were transfected into cells to achieve up- and downregulation of p60, respectively, and the resultant effects on cell migration were analyzed. It was demonstrated that upregulation of katanin p60 expression accelerated cell migration in MDA-MB-231 and MCF-7 cells compared with pcDNA3.1 cells, with fold changes of 162.95±6.20% and 161.84±11.23% (Fig. 4A). Downregulated expression of katanin p60 reduced cell mobility in MDA-MB-231 cells (43.78±3.28%) and MCF-7 cells (36.18±4.23%) compared with shRNA-NC cells (Fig. 4B). No significant difference was identified in the metastatic rate between the two cell lines. These results indicated that katanin p60 may promote tumor cell spread to other sites, and are supportive of the aforementioned results achieved in breast cancer tissue specimens and bone metastasis tissue specimens.
Figure 4.
Regulation of katanin p60 levels adjusted cell migration. (A) The number of cells stained with crystal violet demonstrated the capacity of migration in each group. The number of cells transfected with pcDNA3.1/KATNA1 increased notably, and overexpression of katanin p60 significantly increased cell migration in MDA-MB-231 and MCF-7 cells. *P<0.05 vs. pcDNA3.1. (B) After transfected with shRNA-KATNA1, the number of cells decreased and silencing of katanin p60 significantly inhibited cell migration. *P<0.05 vs. shRNA-NC. shRNA, short hair pin RNA; NC, negative control; KATNA1, katanin catalytic subunit A1. The length of the scale bar was 100 µm.
Discussion
Katanin p60 contains an N-terminal domain that is connected with microtubules, which are required for cell motility and spindle formation (11). Previous studies have reported that katanin p60 oligomerization increases the affinity of katanin for microtubules, thus increasing microtubule degradation (20,21). In the present study, it was demonstrated that katanin p60, a member of the AAA ATPase family, was expressed differently between healthy breast tissue specimens, primary and bone metastatic breast cancer specimens. The expression of katanin p60 in bone metastatic breast cancer was significantly higher, which was positively associated with tumor metastasis, indicating that katanin p60 may serve a role in breast cancer cell proliferation and metastasis.
The present study demonstrates that the expression levels of katanin p60 mRNA and protein increased significantly subsequent to transfection with pcDNA3.1/KATNA1. Simultaneously, the number of breast cancer cells decreased. Downregulation of katanin p60 expression using shRNA resulted in an increased cell number. These results indicate that katanin p60 expression and cell proliferation are positively associated. Previous research has reported an association between katanin p60 expression and cell proliferation (18). Spindle length is controlled by katanin in mitosis and meiosis, essential for chromosome segregation and cytokinesis (22). Katanin p60 has also been demonstrated to facilitate microtubule instability (23). Microtubule binding by the katanin p60 subunit is important for katanin in targeting the spindle poles (24). These studies suggest that katanin p60 may affect cell division by regulating spindle length.
Cell migration is an essential process in tumor metastasis. Previous studies have indicated that pseudopodial protrusion is associated with tumor cell migration and invasion (25,26). Drosophila katanin p60 is reported to regulate the interactions of the cortical-microtubule plus-end, which is involved in cell migration (27). The appropriate distribution and content of katanin p60 has been demonstrated as critical for neuronal migration (28). The present study confirmed that overexpression of katanin p60 significantly promoted cell migration, and reduced expression inhibited cell migration in MDA-MB-231 and MCF-7 cell lines. Therefore, it is hypothesized that katanin p60 may affect breast cancer cell migration by regulating the formation of cellular pseudopodia.
Overall, the present study supports the concept that katanin p60 functions in cell proliferation and migration. A previous study reported that purine-type compounds interact with katanin p60, which induce cell death of NSCLC cells (29). Together, these results suggest that katanin p60 is required for breast cancer cell proliferation and bone metastases, and, therefore, has exciting potential as a therapeutic target. Future research by this group will focus on the association between katanin p60, spindle and cellular pseudopodia in breast cancer cells, in order to further elucidate the regulatory mechanisms of katanin p60.
Acknowledgements
The current study was supported by the Hubei Health and Family Planning Commission Programs (grant no. WJ2015MB185) and the Hubei Nature Science Foundation (grant no. 2014CFC1077).
References
- 1.Kozlow W, Guise TA. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Cetin K, Christiansen CF, Sværke C, Jacobsen JB, Sørensen HT. Survival in patients with breast cancer with bone metastasis: A Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open. 2015;5:e007702. doi: 10.1136/bmjopen-2015-007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabi IR. The polarization of the motile cell. J Cell Sci. 1999;112:1803–1811. doi: 10.1242/jcs.112.12.1803. [DOI] [PubMed] [Google Scholar]
- 5.Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. A microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/S0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- 7.Bershadsky AD, Vasiliev JM. Mechanisms of regulation of pseudopodial activity by the microtubule system. Symp Soc Exp Biol. 1993;47:353–373. [PubMed] [Google Scholar]
- 8.McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, Solowska JM, Qiang L, Karabay A, Baird D, Baas PW. Regulation of microtubule severing by katanin subunits during neuronal development. J Neurosci. 2005;25:5573–5583. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudo H, Maru Y. LAPSER1 is a putative cytokinetic tumor suppressor that shows the same centrosome and midbody subcellular localization pattern as p80 katanin. FASEB J. 2007;21:2086–2100. doi: 10.1096/fj.06-7254com. [DOI] [PubMed] [Google Scholar]
- 11.Johjima A, Noi K, Nishikori S, Ogi H, Esaki M, Ogura T. Microtubule severing by katanin p60 AAA+ ATPase requires the C-terminal acidic tails of both alpha- and beta-tubulins and basic amino acid residues in the AAA+ ring pore. J Biol Chem. 2015;290:11762–11770. doi: 10.1074/jbc.M114.614768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitehead E, Heald R, Wilbur JD. N-terminal phosphorylation of p60 katanin directly regulates microtubule severing. J Mol Biol. 2013;425:214–221. doi: 10.1016/j.jmb.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings CM, Bentley CA, Perdue SA, Baas PW, Singer JD. The Cul3/Klhdc5 E3 ligase regulates p60/katanin and is required for normal mitosis in mammalian cells. J Biol Chem. 2009;284:11663–11675. doi: 10.1074/jbc.M809374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SW, Oh KH, Park E, Chang HM, Park JM, Seong MW, Ka SH, Song WK, Park DE, Baas PW, et al. USP47 and C terminus of Hsp70-interacting protein (CHIP) antagonistically regulate katanin-p60-mediated axonal growth. J Neurosci. 2013;33:12728–12738. doi: 10.1523/JNEUROSCI.0698-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Ye Y, Ji Z, Tan M, Li S, Zhang J, Guo G, Lin H. Katanin p60 promotes neurite growth and collateral formation in the hippocampus. Int J Clin Exp Med. 2014;7:2463–2470. [PMC free article] [PubMed] [Google Scholar]
- 17.Korulu S, Yildiz-Unal A, Yuksel M, Karabay A. Protein kinase C activation causes neurite retraction via cyclinD1 and p60-katanin increase in rat hippocampal neurons. Eur J Neurosci. 2013;37:1610–1619. doi: 10.1111/ejn.12185. [DOI] [PubMed] [Google Scholar]
- 18.Ye X, Lee YC, Choueiri M, Chu K, Huang CF, Tsai WW, Kobayashi R, Logothetis CJ, Yu-Lee LY, Lin SH. Aberrant expression of katanin p60 in prostate cancer bone metastasis. Prostate. 2012;72:291–300. doi: 10.1002/pros.21431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hartman JJ, Vale RD. Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science. 1999;286:782–785. doi: 10.1126/science.286.5440.782. [DOI] [PubMed] [Google Scholar]
- 21.Rasi MQ, Parker JD, Feldman JL, Marshall WF, Quarmby LM. Katanin knockdown supports a role for microtubule severing in release of basal bodies before mitosis in Chlamydomonas. Mol Biol Cell. 2009;20:379–388. doi: 10.1091/mbc.E07-10-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo M, Shimodaira T, Kasama T, Hata Y, Echigo A, Okabe M, Arai K, Makino Y, Niwa S, Saya H, Kishimoto T. Katanin p60 contributes to microtubule instability around the midbody and facilitates cytokinesis in rat cells. PLoS One. 2013;8:e80392. doi: 10.1371/journal.pone.0080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNally KP, Bazirgan OA, McNally FJ. Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci. 2000;113:1623–1633. doi: 10.1242/jcs.113.9.1623. [DOI] [PubMed] [Google Scholar]
- 25.Shankar J, Messenberg A, Chan J, Underhill TM, Foster LJ, Nabi IR. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010;70:3780–3790. doi: 10.1158/0008-5472.CAN-09-4439. [DOI] [PubMed] [Google Scholar]
- 26.Guirguis R, Margulies I, Taraboletti G, Schiffmann E, Liotta L. Cytokine-induced pseudopodial protrusion is coupled to tumour cell migration. Nature (Lond) 1987;329:261–263. doi: 10.1038/329261a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Grode KD, Stewman SF, Diaz-Valencia JD, Liebling E, Rath U, Riera T, Currie JD, Buster DW, Asenjo AB, et al. Drosophila Katanin is a microtubule depolymerase that regulates cortical-microtubule plus-end interactions and cell migration. Nat Cell Biol. 2011;13:361–370. doi: 10.1038/ncb2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyo-Oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, Toyoshima YY, Tokuoka SM, Ishii S, Shimizu T, Muramatsu M, et al. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14:3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- 29.Kuo TC, Li LW, Pan SH, Fang JM, Liu JH, Cheng TJ, Wang CJ, Hung PF, Chen HY, Hong TM, et al. Purine-type compounds induce microtubule fragmentation and lung cancer cell death through interaction with Katanin. J Med Chem. 2016;59:8521–8534. doi: 10.1021/acs.jmedchem.6b00797. [DOI] [PubMed] [Google Scholar]