Abstract
The three cell lines, designated as gastric cancer (GC)1401, GC1415 and GC1436 were derived from peritoneal effusions from patients with gastric adenocarcinoma. Cell lines were established in tissue culture and in immunodeficient, non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice. All cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. These cell lines were grown as an adherent monolayer with doubling time ranging between 25 h (GC1436 cell line) and 30–34 h (GC1401 and GC1415, respectively). All cells showed morphological features of epithelial-like cells, forming sheets of polygonal cells. Chromosomal analysis showed that the modal numbers ranged from 52 (GC1401), 51–56 (GC1415) and 106 (GC1436). High heterogeneity, resulting from several structural and numerical chromosomal abnormalities were evident in all cell lines. The surface marker expression suggested a tumor origin of the cells, and indicated the intestinal phenotype of a GC (CD10+, MUC1). All three cell lines were tumorigenic but not metastatic, in vivo, in NOD/SCID mice. The lack of metastatic potential was suggested by the lack of aldehyde dehydrogenase 1A1 activity. In conclusion, these newly established GC cell lines widen the feasibility of the functional studies on biology of GC as well as drug testing for potential therapeutic purposes.
Keywords: gastric adenocarcinoma, ALDH, Her-2/neu
Introduction
Gastric cancer (GC) is the fifth most common malignancy in the world and the third in cancer-related death (1). One of the reasons behind high mortality is that patients at the time of diagnosis are usually at an advanced tumor stage, hence the need for new, more effective diagnostic and therapeutic approaches is urgent. Also, despite different therapies (surgery, chemotherapy, targeted therapy) the prognosis for patients is still poor (2). Among others, the reason for this poor prognosis may lie in the biological heterogeneity of cancer cells, comprising their morphology and, more importantly, their functionality (3). Established tumor cancer cell lines are a very useful tool for studying cancer cell biology, heterogeneity or sensitivity to different drugs and therapies. A comprehensive collection of well-described cell lines should reflect the diversity of GC and provide adequate models for its study. Up to date, just a few GC cell lines are available, most of them being established from Asian patients (4–6), where this type of cancer is most prevalent.
In the present study we characterize three, new cell lines established from ascitic fluids of Caucasian patients with GC. The karyotype (by conventional G-banding) of these cells, their phenotype [including tumor-associated antigens (TAA) such as c-Met, Her-2/neu, Tag72, EMA, Epithelial Antigen and EMMPRIN], mRNA expression profile (Her-2/neu, MAGE-1) and growth in immunodeficient mice are presented. In addition, the expression and activity of aldehyde dehydrogenase (ALDH) isoforms relevant to metastatic potential of cancer cells are documented (7–11).
In conclusion, the presented data widens the current knowledge on GC cells and provides a liable laboratory model for anticancer drug testing and tumor proliferation studies.
Materials and methods
Origin of cell lines
Tumor cell lines were established from carcinomatous ascites of three patients with advanced GC diagnosed at the First Department of General Gastrointestinal and Oncology Surgery of the Jagiellonian University Medical College (Krakow, Poland). Patients provided their informed, written consent in the present study. The study was approved by the Jagiellonian University Ethical Committee (KBET/491/B/2003). Ascites were harvested into sterile bottles with heparin, centrifuged at 110 × g for 5 min. Thereafter both the cells and the ascitic fluids were collected. The ascitic fluids were filtered and kept at −80°C until use. The cell pellet was resuspended (1×106/ml) in DMEM medium with high glucose (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 5% fetal bovine serum (FBS; Biowest, Nuaille, France), 40% of autologous ascitic fluid and 50 µg/ml gentamycin (Sigma-Aldrich; Merck KGaA). When the cells started to grow rapidly, the ascitic fluid, after a period of gradual decrease, was completely withdrawn from the culture. The cells were incubated at 37°C in 5% CO2 atmosphere and regularly tested for Mycoplasma sp. contamination by PCR-ELISA kit (Roche, Mannheim, Germany) and for endotoxin contamination by the Limulus test (Charles River Laboratories, Wilmington, MA, USA) according to manufacturer's instruction.
For analysis of cellular morphology, an inverted phase-contrast microscope (Olympus, Tokyo, Japan) was used.
Doubling time (growth curves)
Cells (1×105/ml) in medium supplemented with 5% FBS and 50 µg/ml gentamycin (further referred as complete medium) were seeded in duplicates into 24-well plates (BD Falcon, Franklin Lakes, NY, USA). Every 24 h, over a period of 5 days, cells were harvested and counted. The doubling time was estimated from the growth curves during the exponential phase of cells' growth.
Karyotyping analysis
The dividing cells, at the exponential growth phase (after 18 or 24 h), were exposed to the colcemid solution (0,25 µg/ml culture medium; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min. Then, the cells were transferred into 10 ml conical centrifuge tubes and centrifuged at 400 × g for 10 min, after which the supernatant was removed and cells were suspended in 8–9 ml of prewarmed (37°C) hypotonic solution (20 mM potassium chloride (KCl) and 10 mM sodium citrate (Na3C6H5O7; POCH S.A., Gliwice, Poland) with simultaneous vortexing. Next, the cells were incubated at 37°C for 30 min and centrifuged at 400 × g for 10 min. The supernatant was removed without disrupting the pellets and cells were suspended in 8–9 ml cold (4°C) fixative solution 3:1 (methanol:glacial acetic acid ratio) again with simultaneous vortexing. The fixation was repeated 3 times. Finally, cells were resuspended in 1–2 ml of fixative solution and about 0,3 ml was dropped on a microscopic slide. The spread slides were dried for at least overnight at 37°C until staining. The G-banding was used as the routine cytogenetic technique. The metaphase chromosome staining was performed with gentle digestion in the trypsin solution [0,25% in 1X phosphate-buffered saline (PBS), POCH S.A.] and the Giemsa stain (Sigma-Aldrich; Merck KGaA). The karyotypes for gastric adenocarcinoma cell lines were analyzed with the OLYMPUS BX51 microscope and the CytoVision Master 3.0 software (Olympus) according to the 2013 ISCN international guidelines (12).
Immunophenotyping
The following fluorescein (FITC)-, allophycocyanin (APC)-or phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibodies (mAbs) were used: Anti-CD10, -CD11a, CD11c, -CD18, -CD33, -CD40, -CDD44std, -CD44v5, -CD44v6, -CD54, -CD61, -CD62P, -CD86, -CD133, -CD206 (MR), -EGFR, -Her-2/neu, -HLA-DR, HLA class I, -CCR5, -CCR6, Her-2/neu all from BD Pharmingen (San Diego, CA, USA); anti-CD29, -CD36, -CD51, -CD58 from Immunotech (Marseille, France); anti-c-MET, -CCR1, -CCR2, -CCR3, -CCR7, -CXCR1, -CXCR2, -CXCR4 from R&D (Abington, UK); anti-Tag72, -Mucin1 (EMA, CD227), -EMMPRIN from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and anti-Epithelial Antigen, -Epithelial Membrane Antigen (EMA) from DAKO (Heverlee, Belgium). Isotype controls included appropriate FITC-, APC- or PE-labeled mouse IgG1, IgG2a or IgG2b. Cells were incubated with mAbs or isotype controls for 20 min at 4°C, washed, resuspended in PBS and analyzed by flow cytometry (FACS Canto; BD Biosciences Immunocytometry Systems, San Jose, CA, USA) using FACS DiVa software.
Western blotting
Cells were lysed in M-PER lysing buffer (Pierce; Thermo Fisher Scientific, Inc.) containing protease inhibitor cocktail (Roche). 20 µg of isolated protein was mixed with NuPAGE LDS Sample Buffer (4X; Thermo Fisher Scientific, Inc.) and NuPAGE Sample Reducing Agent (10X; Thermo Fisher Scientific, Inc.). Samples were heated (70°C, 10 min) and electrophoresed in 14% polyacrylamide gel containing SDS (Bio-Rad, Hercules, CA, USA). Next, electrophoresed samples were transferred onto the polyvinylidene fluoride membrane (Bio-Rad). Then, after blocking for 1 h at room temperature in Tris buffered saline (TBS) with 0,1% Tween-20 (Sigma-Aldrich; Merck KGaA) and 1% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA), the membranes were incubated overnight at 4°C with the following antibodies: Goat anti-human ALDH1A1 (clone L-15), ALDH1A2 (clone N-20), ALDH1A3 (clone C-13), ALDH2 (clone N-14) and mouse anti-human ALDH3A1 (clone B-8), rabbit anti-EMMPRIN (clone N-19), -panCEA (clone: H-300), -MAGE-A1 (clone: FL-309) and -GAPDH (clone: 14C10) (all Santa Cruz Biotechnology, Dallas, TX, USA). As a loading control, rabbit anti-human GAPDH (Cell Signaling Technology, Inc., Danvers, MA, USA) was used. After incubation, membranes were washed in TBS supplemented with BSA and Tween-20 and incubated for 1 h at room temperature with either goat anti-rabbit or goat anti-mouse (dilution 1:4,000) secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.). The protein bands were visualized with the SuperSignal West Pico Chemiluminescence Substrate kit according to the manufacturer's protocol (Pierce; Thermo Fisher Scientific, Inc.) and analyzed with KODAK GEL LOGIC 1500 Digital Imaging System (KODAK, Rochester, NY, USA).
Detection of ALDH activity
The ALDEFLUOR kit (StemCells Technologies, Grenoble, France) was used for identification of cells with ALDH activity. Cells were incubated (45 min, 37°C, final concentration 1×106/ml) with the Assay Buffer containing BODIPY-amino-acetaldehyde (BAAA; final concentration 1 µM)-a fluorescent substrate for ALDH. Cells able to process the BAAA substrate to its fluorescent form, BODIPY-aminoacetate (BAA), were considered as ALDH positive (ALDH+). To confirm specificity of ALDH depended reaction cells were additionally incubated with specific ALDH inhibitor, diethylaminobenzaldehyde (DEAB). Cells incubated with DEAB only served as a negative control. After treatment, cells were washed and suspended in ice-cold PBS supplemented with 0.5% of BSA and verapamil (50 µM; Sigma-Aldrich; Merck KGaA) to block Abcg2 transporters activity and prevent active efflux of the ALDEFLUOR product from viable cells.
Determination of Her-2/neu and MAGE-1, −2 mRNA expression in the cell lines using nested quantitative PCR (qPCR)
The isolation of total RNA and qPCR for MAGE-1, −2 and β-actin was performed as previously described (13). For detection of HER-2/neu mRNA qPCR was performed using the following primers: Sense-5′-CCTCTGACGTCCATCATCTC-3′ and antisense-5′-ATCTTCTCGTGCCGTCGCTT-3′. The cycle profile for HER-2/neu PCR run was: Initial denaturation at 95°C for 10 min, then denaturation at 95°C for 0 sec, annealing at 60°C for 35 sec, and elongation at 72°C for 35 sec for 35 cycles, followed by final extension at 72°C for 2 min. The results were normalized with β-actin data and expressed as CT. To verify amplified product, melting curve analysis using the LightCycler software was performed for each sample.
Xenografts in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice
Cells (1×106 of each cell line, viability over 95%) suspended in 200 µl of saline were injected subcutaneously (s.c.) into dorsal region of 8-week old NOD/SCID mice (5 mice per group; Charles River Laboratories, Sulzfeld, Germany). Every three days, tumor's diameter was measured with a caliper and its volume (v) was calculated according to the formula: v=ab2/2, where a is the longest dimension, b is the perpendicular width. When moribund, the tissues were examined macroscopically for metastasis in various organs and then processed for histological examination (14). The study was approved by the Ist Local Ethical Committee on Animal Testing (no. 128/2012).
Histological analysis
Subcutaneous GC1401, GC1415 and GC1436 tumors in NOD/SCID mice and other organs (spleen, liver, lung, lymph nodes) of tumor-bearing mice were cut out, divided into several portions and fixed in 10% buffered formalin. Some of them, after routine processing, were embedded in paraffin. 3 µm thick sections were stained with H&E according to manufacturer's protocol.
Statistical analysis
The non parametric Kruskal-Wallis test was performed using GraphPad InStat version 4.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Growth characteristics
The cells were obtained from carcinomatous ascites of patients diagnosed with gastric adenocarcinoma (Table I). All patients were evaluated as non-resectable, with peritoneal spread and no liver or lung metastases.
Table I.
Patients' characteristics.
| Cells | Age | Gender | Stage (TNM) | Histology |
|---|---|---|---|---|
| GC1401 | 72 | F | IV (T4NXM1) | Adenocarcinoma |
| GC1415 | 73 | M | IV (T4NXM1) | Tubular adenocarcinoma combined with signet ring cell carcinoma |
| GC1436 | 19 | F | IV (T4NXM1) | Mucinous adenocarcinoma |
F, female; M, Male.
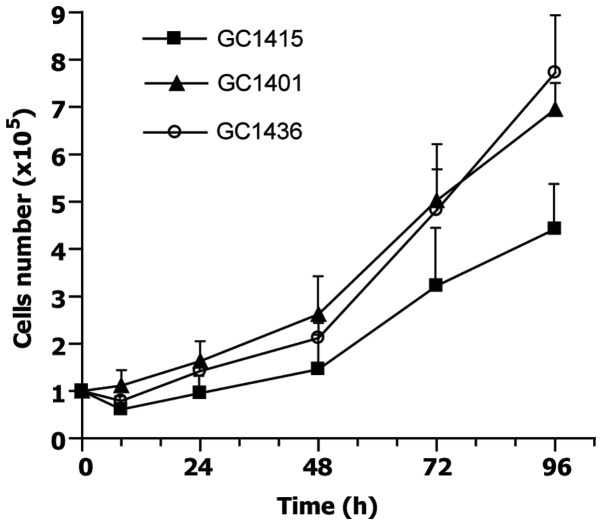
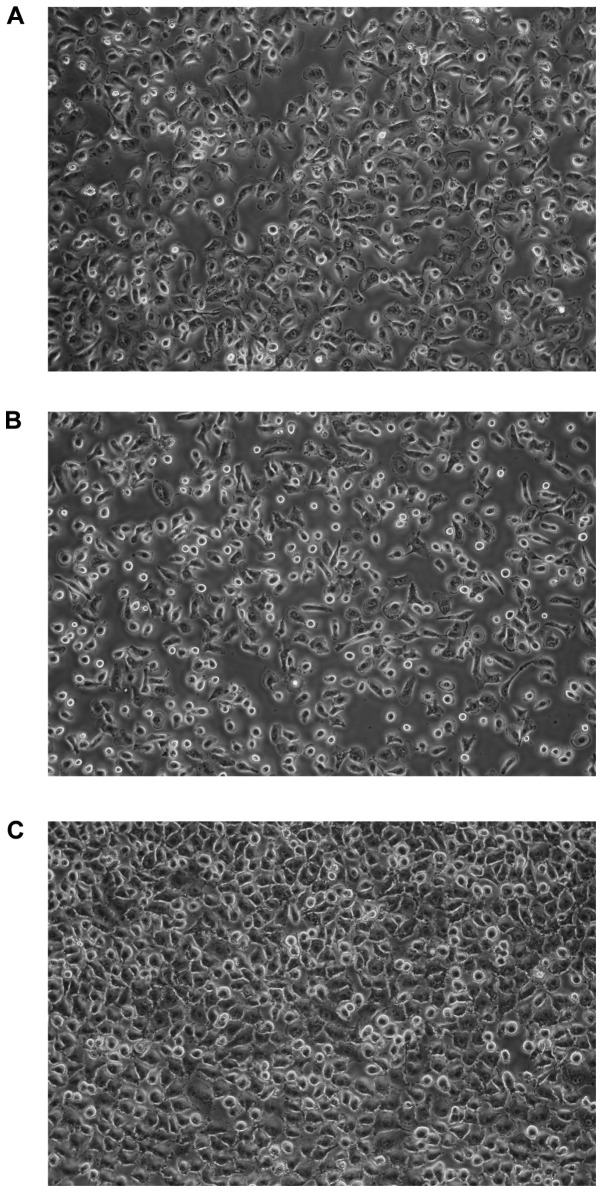
At the initiation of the culture, some of the cells adhered to the plastic surface, while the others did not. In the case of GC1401 and GC1415 cells, negligible proliferation of tumor cells was observed for several (6–8) weeks, instead, the proliferation of fibroblasts was seen. After this period of adaptation to the in vitro conditions, both GC1401 and GC1415 cells started to grow rapidly. In contrast, the GC1436 cells were rapidly growing from the beginning. With successive passages, the number of fibroblasts gradually decreased, to be finally replaced by tumor cells. Doubling time, estimated at exponential phase of growth, for GC1401 and GC1415 cell lines was about 30 and 34 h, respectively, and about 25 h for GC1436 cells (Fig. 1). The differences in doubling time were not significant. All cells exhibited morphologic features of epithelial-like cells, creating the sheets of polygonal cells which attached to the culture flask and formed monolayer at confluence (Fig. 2).
Figure 1.
Growth curves of GC1401, GC1415 and GC1436 cell lines. Cells were cultured in duplicates and counted every 24 h. The differences in doubling time were not statistically significant.
Figure 2.
Phase-contrast photomicrographs of monolayers of (A) GC1401, (B) GC1415 and (C) GC1436 cell lines. Original magnification ×400.
Karyotyping
Karyotyping analysis showed a great complexity of all three gastric adenocarcinoma cell lines. The identified variety involves especially structural chromosomal aberrations. Additionally, high hyperdiploidy of tumor cell lines were detected; from 51–56 and 52 chromosomes for GC1415 and GC1401 to 91–106 chromosomes for GC1436. The best cytogenetic characterization was done for the GC1401 cell line, because of the presence of only two subclones (Table II). The karyotype description according to the international guidelines was also possible for this cell line. The exact result for the GC1436 cell line was not allowed due to high heterogeneity of the cells and a low resolution of the karyotype. Additionally, several structural and numerical chromosomal abnormalities were evident in all cell lines (Table III).
Table II.
Karyotypes of gastric adenocarcinoma cell lines.
| Cell lines | Karyotype (ISCN 2013) |
|---|---|
| GC1401 | 52,X,+der(1)t(1;17?)(p13;q11.2?),dup(1)(p13p32),+2,der(6)t(6;17?)(p12;q11.2?),+der(7)t(7;?)(p11.2;?)?add(7) (q32?),+der(10)t(10;18?)(p11.2;p11.2?),der(12)t(12;14?)(q22;q22?),?dup(14)(q13q24),der(15)t(15;21?) (p13;q11.2?),der(16)?add(16) (q22 or q24),der(19)?add(19)(p13.3),rob(21;21)(q10;q10),+2mar[11]/52,X,+der(1) t(1;17?)(p13;q11.2?),dup(1)(p13p32),+2,der(6) t(6;17?)(p12;q11.2?),+der(7)t(7;?)(p11.2;?)?add(7)(q32?),+der(10) t(10;18?)(p11.2;p11.2?),der(12)t(12;14?)(q22;q22?),-13,?dup(14) (q13q24),der(15)t(15;21?)(p13;q11.2?),der(16)? add(16)(q22 or q24),der(19)?add(19)(p13.3),rob(21;21)(q10;q10),+3mar[5] |
| GC1415 | 51–56,X,+der(1)t(1;17?)(p13;q11.2?),+2,+der(4)?(q),+5,der(6)t(6;17?)(p12;q11.2?),+7,-8,der(12)?add(12) (p13),? dup(14)(q13q24),der(15)t(15;21?)(p13;q11.2?),rob(21;21)(q10;q10),+mar[cp7] |
Table III.
Common chromosomal aberrations in gastric adenocarcinoma cell lines.
| Cell lines | GC1401 | GC1415 | GC1436 |
|---|---|---|---|
| Ploidy | 52 chromosomes | 51–56 chromosomes | 91–106 chromosomes |
| Chromosomal aberrations | +der(1)t(1;17?)(p13;q11.2?) | +der(1)t(1;17?)(p13;q11.2?) | +der(1)t(1;17?)(p13;q11.2?) |
| dup(1)(p13p32) | dup(1)(p13p32) | ||
| +2 | +2 | ||
| der(6)t(6;17?)(p12;q11.2?) | der(6)t(6;17?)(p12;q11.2?) | der(6)t(6;17?)(p12;q11.2?) | |
| +der(10)t(10;18?)(p11.2;p11.2?) | der(10)t(10;18?)(p11.2;p11.2?) | ||
| der(12)?add(12)(p13) | der(12)?add(12)(p13) | ||
| der(12)t(12;14?)(q22;q22?) | der(12)t(12;14?)(q22;q22?) | ||
| ?dup(14)(q13q24) | ?dup(14)(q13q24) | ?dup(14)(q13q24) | |
| der(15)t(15;21?)(p13;q11.2?) | der(15)t(15;21?)(p13;q11.2?) | ||
| der(19)?add(19)(p13.3) | der(19)?add(19)(p13.3) | ||
| rob(21;21)(q10;q10) | rob(21;21)(q10;q10) | rob(21;21)(q10;q10) |
Expression of surface determinants
The expression of surface determinants on cells from in vitro cultures was evaluated using a wide range of mAbs (Table IV). All three cell lines showed a similar pattern of surface determinants with similar levels of expression. All cell lines were HLA-class I positive (100% of cells) and HLA-DR negative. CD29 and CD51 integrins and CD58 of the Ig superfamily were expressed on all cells. The majority of GC1401, GC1415 and GC1436 cells possessed the expression of CD10, CD40, CD44 and CD61 determinants. There were differences between the cell lines in the expression of CD44 variants. The lowest level of v5 and v6 was noticed on GC1401 cells (about 3 and 34%, respectively). GC1415 cells were positive in ~10% for the v5 and in ~50% positive for the v6. The highest level of the v5 (app. 40%) and the v6 (app. 60%) positive cells was among GC1436 cells. Less than 10% of cells were positive for CD33 and 10–20% were positive for CD86. The other determinants tested (i.e., CD11a, c, CD18, CD36, CD54, CD62P, CD133, CD206) were not detected on the cells of all three cell lines.
Table IV.
Expression of selected surface markers on gastric adenocarcinoma cell lines.
| Surface marker | GC1401 (% of cells) | GC1415 (% of cells) | GC1436 (% of cells) |
|---|---|---|---|
| CD10 | 50 | 70–80 | 80–90 |
| CD29 | 100 | 97–100 | 100 |
| CD33 | 1 | 8 | 9 |
| CD40 | 91–99 | 85–100 | 65–85 |
| CD44 | 92–97 | 88–93 | 79–83 |
| CD44v5 | 3 | 10 | 41 |
| CD44v6 | 24–44 | 38–70 | 48–80 |
| CD51 | 100 | 95–99 | 96–99 |
| CD58 | 100 | 89–99 | 92–100 |
| CD61 | 90–95 | 87–97 | 97 |
| CD86 | 11–20 | 12–17 | 13–18 |
| HLA-ABC | 100 | 100 | 100 |
| CCR3 | 3 | 10 | 3 |
| CCR6 | 6–15 | 10 | 10 |
| CCR7 | 2 | 1–2 | 1 |
| CXCR1 | 5 | 3 | 3 |
| CXCR4 | 2–3 | 3–4 | 3 |
| c-MET | 98 | 97 | 98 |
| Her-2/neu | 63–98 | 92–98 | 98 |
| Tag72 | 0 | 0 | 2–3 |
| Epithelial Antigen | 0 | 4–7 | 2–9 |
| Mucin1(CD227) | 2–4 | 4–7 | 5–14 |
| EMMPRIN | 7–17 | 26–66 | 16–24 |
CD11a, CD11c, CD18, CXCR2, CCR1, CCR2, CCR5, CD206, HLA-DR, CD133, CD62P, CD54, CD36 were not detected.
The cells were comparably positive for CCR3 expression which fluctuated between 5 to 25% of positive cells and CCR6 was present on 6–15% of cells. Very low percent of CCR7 (1–2%), CXCR1 and CXCR4 (up to 5%) and the lack of CCR1, 2, 5, and CXCR2 expression was observed on cells of all cell lines.
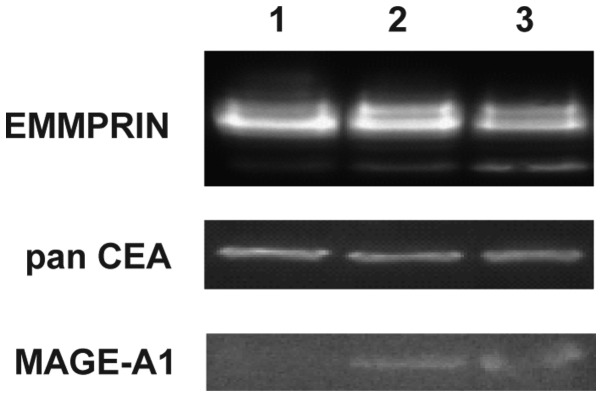
The highest level of EMMPRIN positive cells was observed in GC1415 cell line (up to 66%), whereas GC1401 and GC1436 cell lines were up to 24% positive. These results corroborate with western blotting data where EMMPRIN was shown to be present in all three cell lines (Fig. 3). Mucin1 (EMA) was detected on a small population of GC1401, GC1415 and GC1436 cells (below 14%). Almost all cells of the three cell lines were Her-2/neu and c-MET positive. Tag72 was detected on a very low percent (app. 3%) of GC1436 cells and was not present on GC1401 and GC1415 cells. Epithelial Antigen was detected on GC1415 and GC1436, however, the percentage of positive cells was very low (less than 9% of cells). Presence of panCEA was confirmed by western blotting in all tested cell lines. Expression of MAGE-1 was detected by western blotting in GC1415 and 1436 only (Fig. 3).
Figure 3.
Expression of EMMPRIN, panCEA and MAGE-A1 in gastric cell line detected by Western blotting. 1-GC1401, 2-GC1415 and 3-GC1436. Lane 1, GC1401; lane 2, GC1415; and lane 3, GC1436.
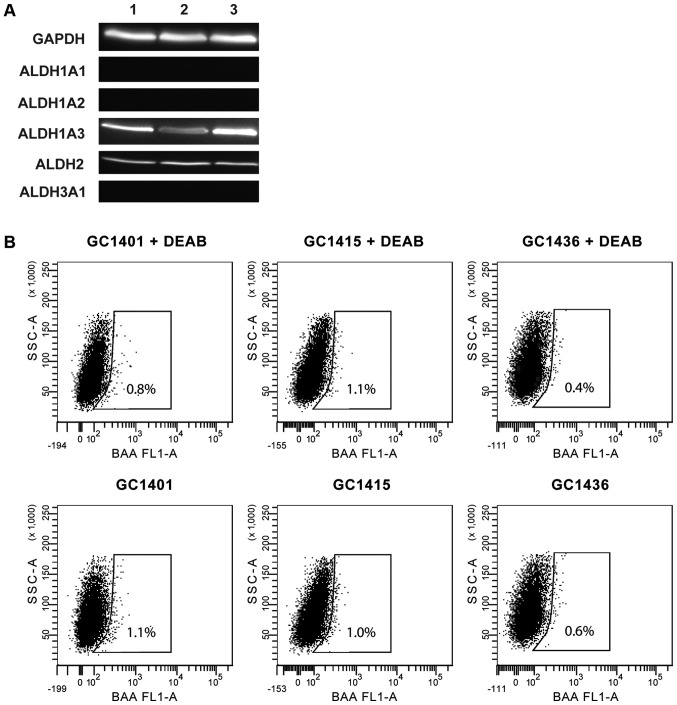
Expression and activity of ALDH
ALDH expression was assessed by western blotting. All three tested cell lines expressed ALDH1A3 and ALDH2 isoforms, however, none of them expressed ALDH1A1, ALDH1A2 and ALDH3A1 isoforms (Fig. 4A). The ALDH activity measured by flow cytometry was very low (Fig. 4B); less than 1% of cells showed such activity. This observation is consistent with the data that ALDH1A1 is the main enzyme of the ALDH family responsible for the enzymatic activity as determined by the ALDEFLUOR assay.
Figure 4.
Aldehyde dehydrogenase (ALDH) protein activity and expression in gastric cancer (GC) cell lines. (A) Western blot analysis was used to compare the expression of different ALDH isozymes GC1401 (lane 1), GC1415 (lane 2) and GC1436 (lane 3) GC cell lines. GAPDH served as loading control. (B) ALDH activity in GC cell lines. Flow cytometric graphs show the fluorescence intensity of reacted ALDH substrate in the absence and presence of diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor. Gated regions indicated ALDH+ cells.
Determination of MAGE-1, −2 and Her-2/neu mRNA expression by nested qPCR
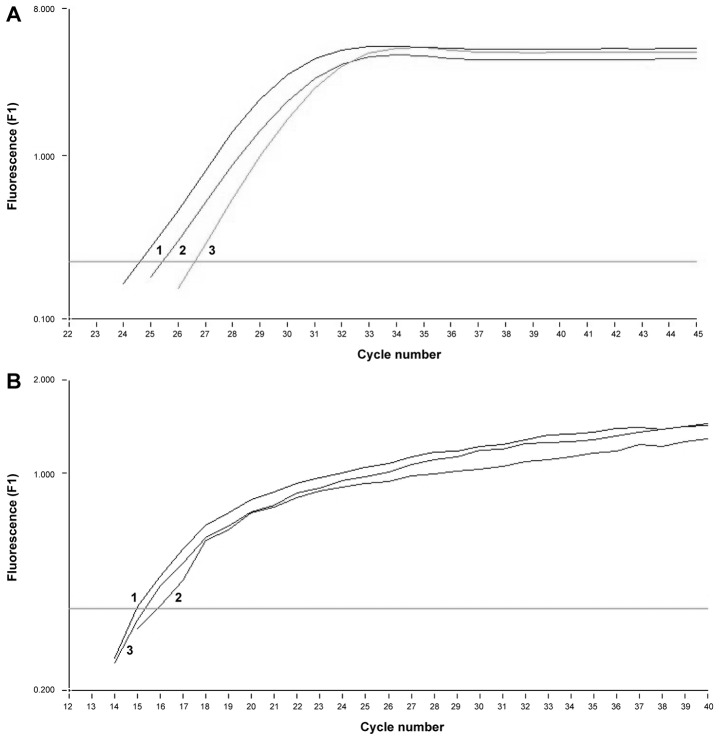
In all three tumor cell lines similar amounts of mRNA for MAGE-1, −2 and Her-2/neu were detected. To verify the amplified product, melting curve analysis was performed for each sample confirming the presence of MAGE-1 mRNA and Her-2/neu (respectively Fig. 5).
Figure 5.
Expression of mRNA for tumor markers: (A) Her-2/neu and (B) MAGE-1 in GC1401 (1), GC1415 (2), GC1436 (3) tumor cells. Negative control is out of scale. One representative experiment of 3 performed is presented.
Tumorigenicity and metastasis evaluation
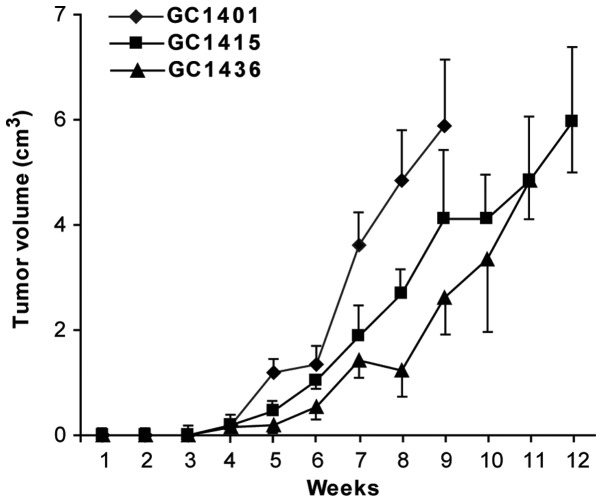
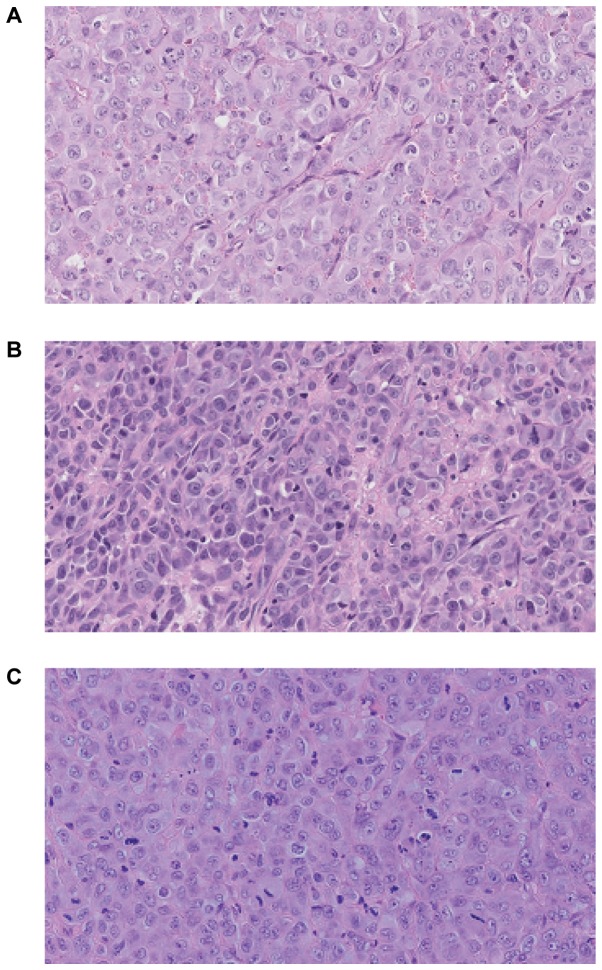
All three cell lines were tumorigenic in vivo in NOD/SCID mice. The transplantation of cells from in vitro culture led usually to the formation of tumors in 13 of 15 mice (~82–89%). Following s.c. injection of 1×106 tumor cells, palpable encapsulated tumors were observed within 3–4 weeks (Fig. 6). The differences in tumor growth were not statistically significant. 100% mortality was noticed at week 13. Fig. 7 presents hematoxylin-eosin staining of the tumor sections after 8 weeks of the tumor growth in vivo. Histologically in all cases malignant neoplasm of epithelial origin was observed.
Figure 6.
Tumor growth of GC1401, GC1415 and GC1436 cells in NOD/SCID mice. Tumor volume was calculated every 3 days and presented in time relation. One experiment of two performed is shown. The observed differences were not statistically significant. NOD/SCID, non-obese diabetic/severe combined immunodeficiency.
Figure 7.
Subcutaneous injection of GC cells into NOD/SCID mice led to primary tumor development at the site of injection. Histological analysis of section stained with hematoxylin and eosin of tumor established in NOD/SCID is presented (A) GC1401, (B) GC1415 and (C) GC1436. Magnification ×200. NOD/SCID, non-obese diabetic/severe combined immunodeficiency.
No macro- and microscopic metastasis to lungs, liver, peritoneum, spleen, kidneys or lymph nodes were observed.
Discussion
There are GC cell lines already established and characterized, however, due to the heterogeneity of cancer cells each newly characterized cell line may provide new data useful for anticancer therapy. The presented manuscript describes the characterization of three new cell lines established from the malignant ascites of Caucasian patients diagnosed with gastric adenocarcinoma. The use of primary tumors as a source of cancer cells encounters several problems, e.g., the need for mechanical or enzymatic disruption which often leads to cell damage, which is not the case, when ascites is being used. Also, the addition of autologous ascitic fluid improves the culture conditions and cell survival (especially at the beginning), which is probably due to the presence of growth-promoting factors (15). Doubling time of established cell lines fall in the range described for commercially available GC cell lines e.g., KATO, ATCC®HTB-103, ATCC®CRL-5973 and ATCC®CRL-5973 (21–36 h) (16).
Based on karyotyping analysis, a great cytogenetic complexity in the investigated cell lines was observed. Numerous structural and ploidy aberrations were evident in all three cell lines. This observation is in accordance with high ploidies of well-defined human stomach-derived cell lines in the American Type Culture Collection which are mostly hypotetraploid (ATCC®HTB-103, ATCC®CRL-5973, ATCC®CRL-5974). To the authors' best knowledge, all of the identified chromosomal changes are reported for the first time, and will be subjected to further research. It has been indicated that 53–94% of advanced gastric adenocarcinomas have an abnormal chromosomal number (17,18). The chromosomal alterations have been recorded in gastric adenocarcinomas as gains: 1q, 3q, 7p, 7q, 8q, 9q, 10p, 11q, 13q, 17q, 19q, 20q and losses: 1p, 4p, 4q, 5q, 6q, 9p, 10, 13, 17p, 18q (19–25). The double minute chromosomes were not found in GC cell lines, but were described in ATCC®CRL-5971, ATCC®CRL-5973 and ATCC®CRL-5974 (but not KATO-III). Additionally, aneuploidy has been detected as a potentially unfavorable prognostic marker often associated with high proliferative activity and metastatic potential (17,18).
A complex karyotype is often reported in aggressive cancers with a tendency to metastasize (26–28). The structural rearrangements are one of the activation modes of protooncogenes and may also be a reason for suppressor genes inactivation, which in turn may induce and drive the neoplastic process. The classical karyotyping has limitations, primarily with the resolution of the study and the quality of metaphase chromosomes. Nevertheless, conventional banding remains the best technique for the evaluation balanced aberrations.
The phenotype analysis revealed the presence of HLA class I, CD40 (member of TNFR superfamily) and some adhesion molecules of the Ig superfamily (CD58), integrins (CD29, CD51, CD61) and CD44 on almost all cells of all three cell lines. The level of these markers did not change throughout the culture. CD44 variants 5 and 6 were also present, but their expression has varied in the course of culture. These markers were already detected in solid tumors including GC as well as GC cell lines (e.g., KATO-III, SNU-5, SNU-16) (29,30). Their high expression was observed in more expanding tumors (31) and was correlated with decreased patients' survival (32–35).
Among chemokine receptors, CCR3, CCR6, CCR7, CXCR1 and CXCR4 were present at very low levels on the cells of all cell lines. Chemokine receptors are involved in different activities of tumor cells such as migration, invasion and adhesion and their high levels are usually associated with an aggressive character of tumor cells. The presence of the above receptors was described on different cancer cells of the gastrointestinal duct, including GC (36,37), and they were usually upregulated during metastasis (38–42).
The level of mucin-positive cells was low (3% of GC1401, 5% of GC1415 and 10% of GC1436). At the same time CD10 expression was relatively high and varied from 50% in GC1401 to 80% in GC1415 and up to 90% in GC1436 cells. Previously, expression of CD10 was observed in KATO-III cell line (43). Recently, combined expression of CD10 and MUC was employed to distinguished between gastric and an intestinal types of GC (44,45). According to this classification, the expression of CD10 and the absence of MUC-1 (or other proteins from MUC family) may suggest intestinal type of GC. Moreover, expression of c-MET and Her-2 may also confirm this histological type (46). c-MET and Her-2/neu, the members of receptor tyrosine kinase family, involved in tumor growth and survival, were present on all cells of each cell line, as detected at both the protein and mRNA level. Their overexpression is usually associated with tumor metastasis and poor prognosis (47,48). Strong expression of EMMPRIN as described by Zheng et al (49), may contribute to enhanced growth, invasion and angiogenesis of gastric carcinoma. New, EMMPRIN-positive cell lines, may help to evaluate the angiogenesis process.
One of the intriguing questions in this study was the lack of in vivo metastasis in NOD/SCID mice after subcutaneous engrafting of cancer cells. It has been already observed that although human cancer cells proliferate after injection into nude or SCID mice and form tumors in situ, their ability to form local or distal metastases is rare (50,51). In the case of the presented GC cell lines the lack of metastasis may have arisen from several reasons:
i) Heterotopic human-mice model of subcutaneous engraftment of human GC in NOD/SCID mice did not reconstruct the conditions for growth and metastasis of human cancer in human microenvironment (52).
ii) Presence of a capsule may hamper metastasis (53).
iii) Low levels or lack of important agents, such as cytokines/chemokines and/or their receptors (e.g., low expression of chemokine receptors in the case of presented GC cell lines).
iv) Lack of or very low level of cancer stem cells (CSC) (presented GC cell lines express CD44 but lack the expression of CD54 (54) or CD133+ (55) markers characteristic for CSC).
v) Very low levels of ALDH activity. The expression of ALDH1A3 and ALDH2 was detected by western blotting assay, but surprisingly very low amount of cells (app. 1%) exhibiting ALDH activity, as judged by the ALDEFLUOR assay, were observed. This may be due to the lack of ALDH1A1 and ALDH1A2 which are potentially major contributors of ALDH1 activity in different types of cancer e.g., breast and lung cancer (56,57).
Many studies have shown elevated expression of ALDH isoforms other than ALDH1A1, but they do not directly prove that they are the cause of ALDEFLUOR activity in cancers (58–61).
The role of the ALDH1A3 isoform in GC progression still needs to be elucidated. High levels of this protein were observed in normal stomach tissue, however, its mRNA overexpression was detected in patients with GC, and was correlated with worse patient survival (62). ALDH2 is constitutively expressed in a variety of tissues (63), however, its role in tumor progression is limited to genetic polymorphism (rs671) and correlated with alcohol consumption (64). Our data may suggest that the low, but noticeable, activity of ALDH may come from ALDH1A3 or ALDH2 isoforms, which may play a role in tumorigenesis in a tissue-specific manner.
In summary, cancer metastasis is a complicated, multi-step process, influenced by many factors. Despite the presence of some prometastatic determinants (eg. CD29, CD40, CD44, c-MET, Her-2/neu, composed karyotype) and soluble factors such as IL-8, VEGF (data not shown), the lack of others (chemokine receptors, insufficient levels of CSC, activity of ALDH1A1) may disrupt the progression of metastasis leading to its inhibition.
The mortality of GC is very high due to its high heterogeneity even within the same tumor where cell subpopulations may show a diverse potential to growth and metastasis. In consequence, conventional therapies are not fully effective, as subpopulations of cells may differ in response to them. Three novel cell lines, established and characterized in our laboratory, may provide models for studies on biological heterogeneity of human GC cells.
Acknowledgements
This study was supported by the National Science Centre (grant no. UMO-2012/07B/NZ6/03499).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia A, Santaquilani M, Sant M. Survival for cancer patients in Europe. Ann Ist Super Sanita. 2009;45:315–324. [PubMed] [Google Scholar]
- 3.Fidler IJ. Biological heterogeneity of cancer: Implication to therapy. Hum Vaccines Immunother. 2012;8:1141–1142. doi: 10.4161/hv.19643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato M, Shimada Y, Tanaka H, Hosotani R, Ohshio G, Ishizaki K, Imamura M. Characterization of six cell lines established from human pancreatic adenocarcinomas. Cancer. 1999;85:832–840. doi: 10.1002/(SICI)1097-0142(19990215)85:4<832::AID-CNCR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Park JG, Frucht H, LaRocca RV, Bliss DP, Jr, Kurita Y, Chen TR, Henslee JG, Trepel JB, Jensen RT, Johnson BE, et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990;50:2773–2780. [PubMed] [Google Scholar]
- 6.Chun YH, Kil JI, Suh YS, Kim SH, Kim H, Park SH. Characterization of chromosomal aberrations in human gastric carcinoma cell lines using chromosome painting. Cancer Genet Cytogenet. 2000;119:18–25. doi: 10.1016/S0165-4608(99)00217-4. [DOI] [PubMed] [Google Scholar]
- 7.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, Allan AL. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2009;13:2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu X, Patel S, Mektepbayeva D, Mahjoub A, Huard J, Weiss K. Retinal targets ALDH positive cancer stem cell and alters the phenotype of highly metastatic osteosarcoma cells. Sarcoma. 2015;2015:784954. doi: 10.1155/2015/784954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajani JA, Wang X, Song S, Suzuki A, Taketa T, Sudo K, Wadhwa R, Hofstetter WL, Komaki R, Maru DM, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 2014;8:142–149. doi: 10.1016/j.molonc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XS, Xu Q, Fu XY, Luo WS. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer. 2014;14:705. doi: 10.1186/1471-2407-14-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer LG, McGowan-Jordan J, Schmid M. ISCN 2013 An International System for Human Cytogenetic Nomenclature. S. Karger; Basel: 2013. [Google Scholar]
- 13.Szatanek R, Drabik G, Baran J, Kolodziejczyk P, Kulig J, Stachura J, Zembala M. Detection of isolated tumor cells in the blood and bone marrow of patients with gastric cancer by combined sorting, isolation and determination of MAGE-1, −2 mRNA expression. Oncol Rep. 2008;19:1055–1060. [PubMed] [Google Scholar]
- 14.Iwanuma Y, Chen FA, Egilmez NK, Takita H, Bankert RB. Antitumor immune response of human peripheral blood lymphocytes coengrafted with tumor into severe combined immunodeficient mice. Cancer Res. 1997;57:2937–2942. [PubMed] [Google Scholar]
- 15.Alama A, Barbieri F, Favre A, Cagnoli M, Noviello E, Pedullà F, Viale M, Foglia G, Ragni N. Establishment and characterization of three new cell lines derived from the ascites of human ovarian carcinomas. Gynecol Oncol. 1996;62:82–88. doi: 10.1006/gyno.1996.0194. [DOI] [PubMed] [Google Scholar]
- 16.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doak SH. Aneuploidy in upper gastro-intestinal tract cancers-a potential prognostic marker? Mutat Res. 2008;651:93–104. doi: 10.1016/j.mrgentox.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Leal MF, Martins do Nascimento JL, da Silva CE, Vita Lamarão MF, Calcagno DQ, Khayat AS, Assumpção PP, Cabral IR, de Arruda Cardoso Smith M, Burbano RR. Establishment and conventional cytogenetic characterization of three gastric cancer cell lines. Cancer Genet Cytogenet. 2009;195:85–91. doi: 10.1016/j.cancergencyto.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Buffart TE, Carvalho B, Mons T, Reis RM, Moutinho C, Silva P, van Grieken NC, Vieth M, Stolte M, van de Velde CJ, et al. DNA copy number profiles of gastric cancer precursor lesions. BMC Genomics. 2007;8:345. doi: 10.1186/1471-2164-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David S, Meltzer SJ. Stomach-genetic and epigenetic alterations of preneoplastic and neoplastic lesions. Cancer Biomark. 2010;9:493–507. doi: 10.3233/CBM-2011-0169. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Noguchi T, Kawahara K, Kashima K, Daa T, Yokoyama S. Genetic alterations in 102 primary gastric cancers by comparative genomic hybridization: Gain of 20q and loss of 18q are associated with tumor progression. Mod Pathol. 2004;17:1328–1337. doi: 10.1038/modpathol.3800180. [DOI] [PubMed] [Google Scholar]
- 22.Wu CW, Chen GD, Fann CS, Lee AF, Chi CW, Liu JM, Weier U, Chen JY. Clinical implications of chromosomal abnormalities in gastric adenocarcinomas. Genes Chromosom Cancer. 2002;35:219–231. doi: 10.1002/gcc.10106. [DOI] [PubMed] [Google Scholar]
- 23.Koo SH, Kwon KC, Shin SY, Jeon YM, Park JW, Kim SH, Noh SM. Genetic alterations of gastric cancer: Comparative genomic hybridization and fluorescence In situ hybridization studies. Cancer Genet Cytogenet. 2000;117:97–103. doi: 10.1016/S0165-4608(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 24.Sakakura C, Hagiwara A, Taniguchi H, Yamaguchi T, Yamagishi H, Takahashi T, Koyama K, Nakamura Y, Abe T, Inazawa J. Chromosomal aberrations in human hepatocellular carcinomas associated with hepatitis C virus infection detected by comparative genomic hybridization. Br J Cancer. 1999;80:2034–2039. doi: 10.1038/sj.bjc.6690638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stocks S, Pratt N, Sales M, Johnston DA, Thompson AM, Carey FA, Kernohan NM. Chromosomal imbalances in gastric and esophageal adenocarcinoma: Specific comparative genomic hybridization-detected abnormalities segregate with junctional adenocarcinomas. Genes Chromosomes Cancer. 2001;32:50–58. doi: 10.1002/gcc.1166. [DOI] [PubMed] [Google Scholar]
- 26.Göhring G, Michalova K, Beverloo HB, Betts D, Harbott J, Haas OA, Kerndrup G, Sainati L, Bergstraesser E, Hasle H, et al. Complex karyotype newly defined: The strongest prognostic factor in advanced childhood myelodysplastic syndrome. Blood. 2010;116:3766–3769. doi: 10.1182/blood-2010-04-280313. [DOI] [PubMed] [Google Scholar]
- 27.Orozco JJ, Appelbaum FR. Unfavorable, complex, and monosomal karyotypes: The most challenging forms of acute myeloid leukemia. Oncology (Williston Park) 2012;26:706–712. [PubMed] [Google Scholar]
- 28.Höglund M, Frigyesi A, Säll T, Gisselsson D, Mitelman F. Statistical behavior of complex cancer karyotypes. Genes Chromosomes Cancer. 2005;42:327–341. doi: 10.1002/gcc.20143. [DOI] [PubMed] [Google Scholar]
- 29.Sakakura C, Hagiwara A, Nakanishi M, Shimomura K, Takagi T, Yasuoka R, Fujita Y, Abe T, Ichikawa Y, Takahashi S, et al. Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Brit J Cancer. 2002;87:1153–1161. doi: 10.1038/sj.bjc.6600580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washington K, Gottried MR, Telen MJ. Expression of the cell adhesion molecule CD44 in gastric adenocarcinomas. Hum Pathol. 1994;25:1043–1049. doi: 10.1016/0046-8177(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Ura H, Yasoshima T, Shishido T, Denno R, Hirata K. Establishment and characterization of a human gastric carcinoma cell line that is highly metastatic to lymph nodes. J Exp Clin Cancer Res. 2000;19:113–120. [PubMed] [Google Scholar]
- 32.Mayer B, Lorenz C, Babic R, Jauch KW, Schildberg FW, Funke I, Johnson JP. Expression of leukocyte cell adhesion molecules on gastric carcinomas: Possible involvement of LFA-3 expression in the development of distant metastases. Int J Cancer. 1995;64:415–423. doi: 10.1002/ijc.2910640611. [DOI] [PubMed] [Google Scholar]
- 33.Wakatsuki K, Yamada Y, Narikiyo M, Ueno M, Takayama T, Tamaki H, Miki K, Matsumoto S, Enomoto K, Yokotani T, Nakajima Y. Clinicopathological and prognostic significance of mucin phenotype in gastric cancer. J Surg Oncol. 2008;98:124–129. doi: 10.1002/jso.21093. [DOI] [PubMed] [Google Scholar]
- 34.Xin Y, Grace A, Gallagher MM, Curran BT, Leader MB, Kay EW. CD44V6 in gastric carcinoma: A marker of tumor progression. Appl Immunohistochem Mol Morphol. 2001;9:138–142. doi: 10.1097/00129039-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer. 2014;14:15. doi: 10.1186/1471-2407-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani H, Nakayama T, Yoshie O. In situ expression of the CCL20-CCR6 axis in lymphocyte-rich gastric cancer and its potential role in the formation of lymphoid stroma. Pathol Int. 2011;61:645–651. doi: 10.1111/j.1440-1827.2011.02717.x. [DOI] [PubMed] [Google Scholar]
- 37.Arigami T, Natsugoe S, Uenosono Y, Yanagita S, Arima H, Hirata M, Ishigami S, Aikou T. CCR7 and CXCR4 expression predicts lymph node status including micrometastasis in gastric cancer. Int J Oncol. 2009;35:19–24. doi: 10.3892/ijo_00000308. [DOI] [PubMed] [Google Scholar]
- 38.Jöhrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, Gander H, Höltl L, Bartsch G, Greil R, Thurnher M. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- 39.Rubie C, Oliveira V, Kempf K, Wagner M, Tilton B, Rau B, Kruse B, Konig J, Schilling M. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumor Biol. 2006;27:166–174. doi: 10.1159/000092777. [DOI] [PubMed] [Google Scholar]
- 40.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugasawa H, Ichikura T, Tsujimoto H, Kinoshita M, Morita D, Ono S, Chochi K, Tsuda K, Seki S, Mochizuki H. Prognostic significnce of expression of CCL5/RANTES receptors in patients with gastric cancer. J Surg Oncol. 2008;97:445–450. doi: 10.1002/jso.20984. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Du L, Yang X, Qu A, Zhang X, Zhou C, Wang C. Aberrant CCR4 expression is involved in tumor invasion of lymph node-negative human gastric cancer. PLoS One. 2015;10:e0120059. doi: 10.1371/journal.pone.0120059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Röcken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol. 2004;25:1223–1232. [PubMed] [Google Scholar]
- 44.Namikawa T, Hanazaki K. Mucin phenotype of gastric cancer and clinicopathology of gastric-type differentiated adenocarcinoma. World J Gastroenterol. 2010;16:4634–4639. doi: 10.3748/wjg.v16.i37.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barresi V, Vitarelli E, Grosso M, Tuccari G, Barresi G. Relationship between immunoexpression of mucin peptide cores MUC1 and MUC2 and Lauren's histologic subtypes of gastric carcinomas. Eur J Histochem. 2006;50:301–309. [PubMed] [Google Scholar]
- 46.Yalcin S, Yildiz Y, Sokmensuer C. Frequency of c-Met, HGF, and HER-2 expression and evaluation of their association with clinicopathologic and prognostic factors in gastric cancer. J Clin Oncol. 2015;33(3 Suppl):S88. doi: 10.1200/jco.2015.33.3_suppl.88. [DOI] [Google Scholar]
- 47.Teng L, Lu J. cMET as a potential therapeutic target in gastric cancer (Review) Int J Mol Med. 2013;32:1247–1254. doi: 10.3892/ijmm.2013.1531. [DOI] [PubMed] [Google Scholar]
- 48.Zhu GJ, Xu CW, Fang MY, Zhang YP, Li Y. Detection of Her2/neu expression in gastric cancer: Quantitative PCR versus immunohistochemistry. Exp Ther Med. 2014;8:1501–1507. doi: 10.3892/etm.2014.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng HC, Takahashi H, Murai Y, Zui ZG, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: A good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fidler IJ. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 51.Sharkey FE, Fogh J. Metastasis of human tumors in athymic nude mice. Int J Cancer. 1979;24:733–738. doi: 10.1002/ijc.2910240605. [DOI] [PubMed] [Google Scholar]
- 52.Zheng MJ, Wang J, Chen YW, Xu L, Xue DD, Fu W, Zhang YF, Du Q, Zhao Y, Ling LJ, et al. A novel mouse model of gastric cancer with human gastric microenvironment. Cancer Lett. 2012;325:108–115. doi: 10.1016/j.canlet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Kyriazis AP, DiPersio L, Michael GJ, Pesce AJ, Stinnett JD. Growth patterns and metastatic behavior of human tumors growing in athymic mice. Cancer Res. 1978;38:3186–3190. [PubMed] [Google Scholar]
- 54.Chen T, Yang K, Yu J, Meng W, Yuan D, Bi F, Liu F, Liu J, Dai B, Chen X, et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2012;22:248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, Sentani K, Oue N, Yasui W. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62:112–119. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu S, Xue W, Huang X, Yu X, Luo M, Huang Y, Liu Y, Bi Z, Qiu X, Bai S. Distinct prognostic values of ALDH1 isoenzymes in breast cancer. Tumor Biol. 2015;36:2421–2426. doi: 10.1007/s13277-014-2852-6. [DOI] [PubMed] [Google Scholar]
- 57.You Q, Guo H, Xu D. Distinct prognostic values and potential drug targets of ALDH1 isoenzymes in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:5087–5097. doi: 10.2147/DDDT.S87197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia J, Parikh H, Xiao W, Hoskins JW, Pflicke H, Liu X, Collins I, Zhou W, Wang Z, Powell J, et al. An integrated transcriptome and epigenome analysis identifies a novel candidate gene for pancreatic cancer. BMC Med Genomics. 2013;6:33. doi: 10.1186/1755-8794-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong B, Wu W, Cheng T, Schlitter AM, Qian C, Bruns P, Jian Z, Jäger C, Regel I, Raulefs S, et al. A subset of metastatic pancreatic ductal adenocarcinomas depends quantitatively on oncogenic Kras/Mek/Erk-induced hyperactive mTOR signalling. Gut. 2016;65:647–657. doi: 10.1136/gutjnl-2014-307616. [DOI] [PubMed] [Google Scholar]
- 60.Saw YT, Yang J, Ng SK, Liu S, Singh S, Singh M, Welch WR, Tsuda H, Fong WP, Thompson D, et al. Characterization of aldehyde dehydrogenase isozymes in ovarian cancer tissues and sphere cultures. BMC Cancer. 2012;12:329. doi: 10.1186/1471-2407-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3; Proc Natl Acad Sci USA; 2013; pp. 8644–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li K, Guo X, Wang Z, Li X, Bu Y, Bai X, Zheng L, Huang Y. The prognostic roles of ALDH1 isoenzymes in gastric cancer. Onco Targets Ther. 2016;9:3405–3414. doi: 10.2147/OTT.S102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goedde HW, Agarwal DP. Pharmacogenetics of aldehyde dehydrogenase (ALDH) Pharmacol Ther. 1990;45:345–371. doi: 10.1016/0163-7258(90)90071-9. [DOI] [PubMed] [Google Scholar]
- 64.Hidaka A, Sasazuki S, Matsuo K, Ito H, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Inoue M, Tsugane S, JPHC Study Group Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: The Japan Public Health Center-based prospective study. Carcinogenesis. 2015;36:223–231. doi: 10.1093/carcin/bgu244. [DOI] [PubMed] [Google Scholar]