Abstract
B16F10 murine melanoma cells are frequently used for the study of cancer and melanogenesis. The cells are usually cultured in Dulbecco's Modified Eagle Medium, with the addition of 20 µM pyridoxal (PL) or pyridoxine (PN) for vitamin B6. The difference between these vitamin B6 compounds is thought not to affect cell proliferation, whereas their influence on other physiological effects is poorly understood. In the present study, the effects of PL and PN on cell proliferation and melanogenesis in B16F10 cells were compared. At 500 µM PL significantly suppressed cell growth but the growth inhibitory effect of PN was weak. Although neither of the vitamin B6 compounds affected cell growth at 20 µM, melanogenesis was suppressed by 20 µM PL compared with the effect of PN. In addition, the expression levels of tyrosinase, which is the rate-limiting enzyme, correlated with the melanin content. The results of the present study indicate that PL may be more useful for melanoma therapy and suppression of skin pigmentation than PN. The results also signify the importance of medium selection for cell culture.
Keywords: pyridoxal, pyridoxine, B16F10 cells, cell growth, melanogenesis
Introduction
Cell culture is important for the evaluation of cell physiology and cellular responses to pharmaceutical compounds (1,2). Many cell lines have been established and cultured in appropriate media such as minimum essential medium (MEM), Dulbecco's Modified Eagle Medium (DMEM), and RPMI-1640. Culture media may include glucose, amino acids, vitamins, inorganic salts, and serum; each medium comprises different kinds and quantities of components. To perform precise evaluations, researchers must select the medium appropriate for the cells in their research.
Vitamin B6 comprises pyridoxine (PN), pyridoxal (PL), pyridoxamine, and phosphorylated forms, such as pyridoxine-5′-phosphate, pyridoxal-5′-phosphate (PLP) and pyridoxamine-5′-phsphate (3). It acts as a coenzyme for amino acid metabolism. In general, DMEM is used with 20 µM PN or PL. Although it is suggested that the difference between these vitamin B6 compounds does not affect cell proliferation, high concentration of vitamin B6 did inhibit cell growth in several cancer cells, and the effect of PL was stronger than that of PN (4–7). Conversely, the influence of optimal concentrations on other cell physiological effects is poorly understood. In this study, we evaluated the effects of PL and PN on cell growth and melanogenesis in B16F10 murine melanoma cells.
Materials and methods
Materials
PL hydrochloride (P6155) and PN hydrochloride (P9755) were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMEM without vitamin B6 was manufactured by Funakoshi (Tokyo, Japan). Hoechst 33342 and propidium iodide (PI) were purchased from Dojindo Molecular Technologies, Inc., (Kumamoto, Japan) and Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany), respectively. 3-isobutyl-1-methylxanthine (IBMX) was obtained from Sigma-Aldrich; Merck KGaA. Block Ace was purchased from Dainippon Sumimoto Pharma Co., Ltd., (Osaka, Japan). Antibodies to tyrosinase (sc-7834), PARP (no. 9542), and β-actin (AC-15, A-5441) were obtained from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA), Cell Signaling Technology, Inc., (Danvers, MA, USA), and Sigma-Aldrich, respectively. ECL Prime Western Blotting Detection Reagent was purchased from GE Healthcare (Chicago, IL, USA).
Cell culture
B16F10 cells were kindly gifted by Prof. Naoto Oku (School of Pharmaceutical Sciences, University of Shizuoka, Japan). The cells were maintained in DMEM without vitamin B6 and supplemented with 10% heat-inactivated fetal bovine serum (FBS) under 5% CO2 at 37°C. They were cultured in DMEM without vitamin B6 for more than 1-week before being subjected to analysis.
Cell proliferation and viability assay
Cell proliferation and viability assays examined the effect of vitamin B6 on B16F10 cells. To assess the effect of vitamin B6, the cells were seeded at 1×105 cells/ml medium into 96-well plates in the presence of PL or PN at 20–500 µM for 72 h. The cells were then counted using trypan blue staining. To analyze the cell survival rate, both attached and detached cells were counted; the ratio of attached cell numbers was calculated as viable cells. To examine cell survival in detail, Hoechst-PI staining was performed. Hoechst and PI were used at 2 µg/ml.
To analyze the effect of hydrogen peroxide (H2O2) on cell proliferation, B16F10 cells were seeded at 1×105 cells/ml medium into 96-well plates in the presence of PL or PN at 20 µM for 24 h. The cells were then added with H2O2 at concentrations of 1–10 µM. After 24 h treatment, survival cells were counted by trypan blue staining.
Western blot analysis
Western blot analysis was performed as previously described (8,9). The cells were treated with 100 µM IBMX for 24 h. The proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 4% Block Ace solution. Anti-β-actin, anti-PARP, and anti-tyrosinase antibody were used at 1:10,000, 1:1,500, and 1:250, respectively. The membrane was next incubated with HRP-conjugated secondary antibody. ECL Prime Western Blotting Detection Reagent and LAS-3000 (Fuji-Film, Tokyo, Japan) were used for detection. Finally, the expression levels of tyrosinase were assessed by the Scion Image Software (Scion, Frederick, MD, USA) (n=3).
Measurement of melanin contents
B16F10 cells were seeded at 1×105 cells/ml medium into 6-well plates in the presence of 20 µM PL or PN. Following 24 h incubation, cells were treated with 100 µM IBMX and cultured further for 48 h. To analyze melanin contents, 1.5×106 cells were dissolved in 2N NaOH for 2 h at 80°C. The absorbance at 450 nm was measured to determine melanin content.
Statistical analysis
To assess statistical significance, the cell growth rate was determined by Student's t-test and the results were confirmed by one-way analysis of variance (ANOVA). The dead cell rate was analyzed by one-way ANOVA with Dunnett's post hoc test for the comparison of three groups. The melanin content and tyrosinase expression were evaluated by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
The effect of vitamin B6 compounds on cell growth in B16F10 melanoma cells
To investigate the effect of PL and PN on cell proliferation in B16F10 cells, the cells were treated with different concentrations (20–500 µM) of both PL and PN. PL at 50–500 µM significantly suppressed cell growth compared with DMEM without vitamin B6 (Fig. 1A). At 500 µM, PL showed 95.1% inhibition of cell proliferation, whereas PN showed only 11.4% inhibition (Fig. 1A). The dead cell ratio among VB6 free, PL, and PN was no significant difference (Fig. 1B). We further confirmed the effects of vitamin B6 by Hoechst 33342 and PI staining. Hoechst 33342 stains both viable and dead cells, and the condensed chromatin of apoptotic cells are observed more brightly than the nuclei of viable cells. PI stains dead cells such as apoptotic cells and necrotic cells. At 500 µM PL, although the number of Hoechst-stained cells was significantly decreased, PI-stained cells were scarcely increased (Fig. 1C). The slightly dead cells were detected the condensed chromatin of apoptotic cells (data not shown). The cleavage of PARP, a hallmark of apoptosis, was hardly observed at 500 µM PL or PN (Fig. 1D). On the other hand, cell morphology by PL or PN did not change (data not shown). These results showed that 500 µM PL strongly suppressed cell growth, but did not induce cell death. Otherwise, 20 µM of both vitamin B6 compounds, which are the normal concentration of DMEM, did not affect cell proliferation (Fig. 1A).
Figure 1.
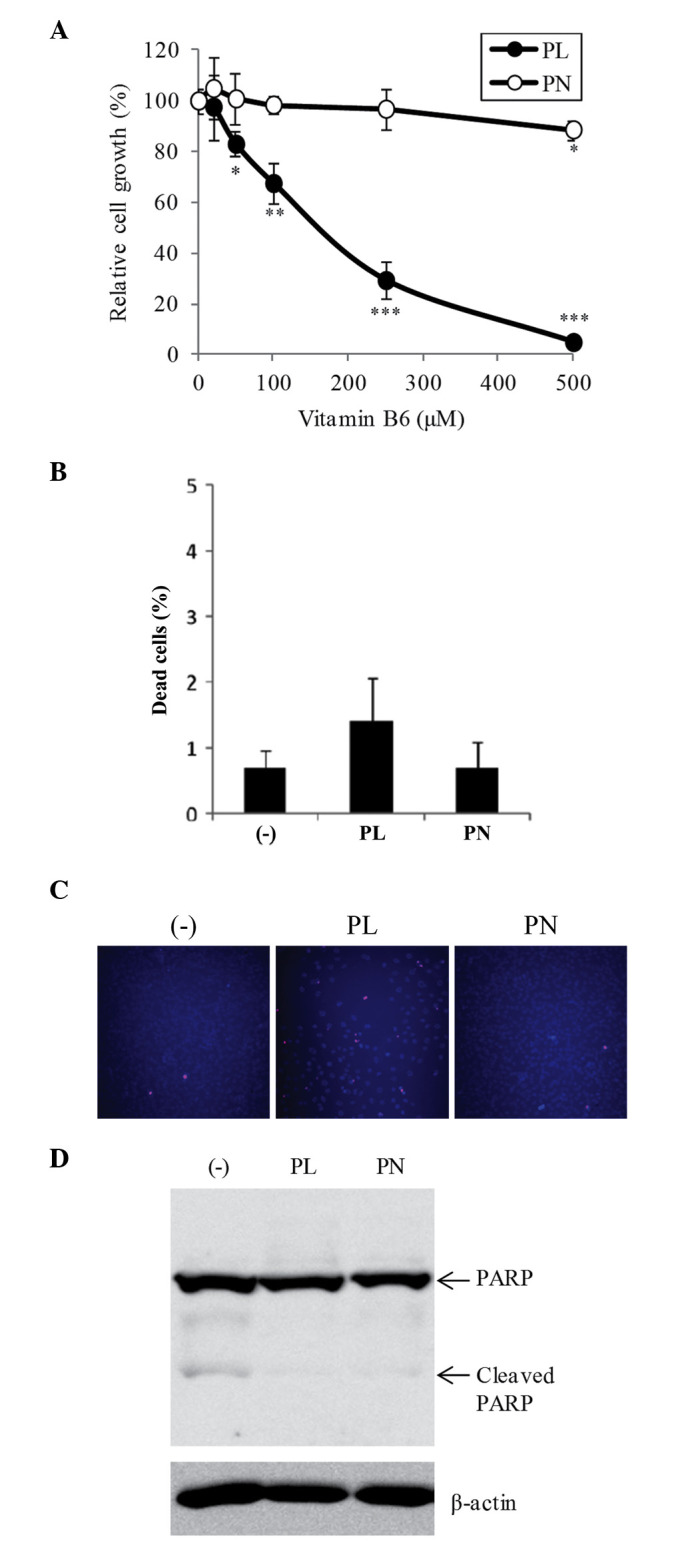
Effects of PL and PN on cell proliferation and viability in B16F10 cells. B16F10 cells were cultured in DMEM with PL or PN (20–500 µM) or DMEM without vitamin B6 for 72 h. Cell growth (A) and dead cells (B) were analyzed by cell counting based on trypan blue staining. (C) Hoechst-PI staining was blue (Hoechst staining) and pink (merged image of Hoechst and PI staining). (D) Cleaved-PARP was examined by western blot analysis at 72 h. The data represent mean ± SD (n=3). *P<0.05, **P<0.01, and ***P<0.001 indicate significant differences compared to DMEM without vitamin B6. DMEM, Dulbecco's modified Eagle's medium; PARP, Poly (ADP-ribose) polymerase; PL, pyridoxal; PN, pyridoxine.
Melanogenesis in B16F10 cells
The effect of 20 µM PL and PN on IBMX-stimulating melanogenesis in B16F10 cells was examined. The total melanin content was increased in both PL and PN by IBMX stimulation, whereas the melanin content in the presence of PL was 19.9% lower than that in PN (Fig. 2A and B). The melanin content in PN was similar to that in the IBMX-only cells without the addition of PL/PN (data not shown). Western blot analysis showed that tyrosinase expression in PL is lower when compared with PN (Fig. 2C). The oxidation also involved melanogenesis. We confirmed that the cell resistance to oxidative stress by H2O2 is not different media containing PL or PN (Fig. 3).
Figure 2.
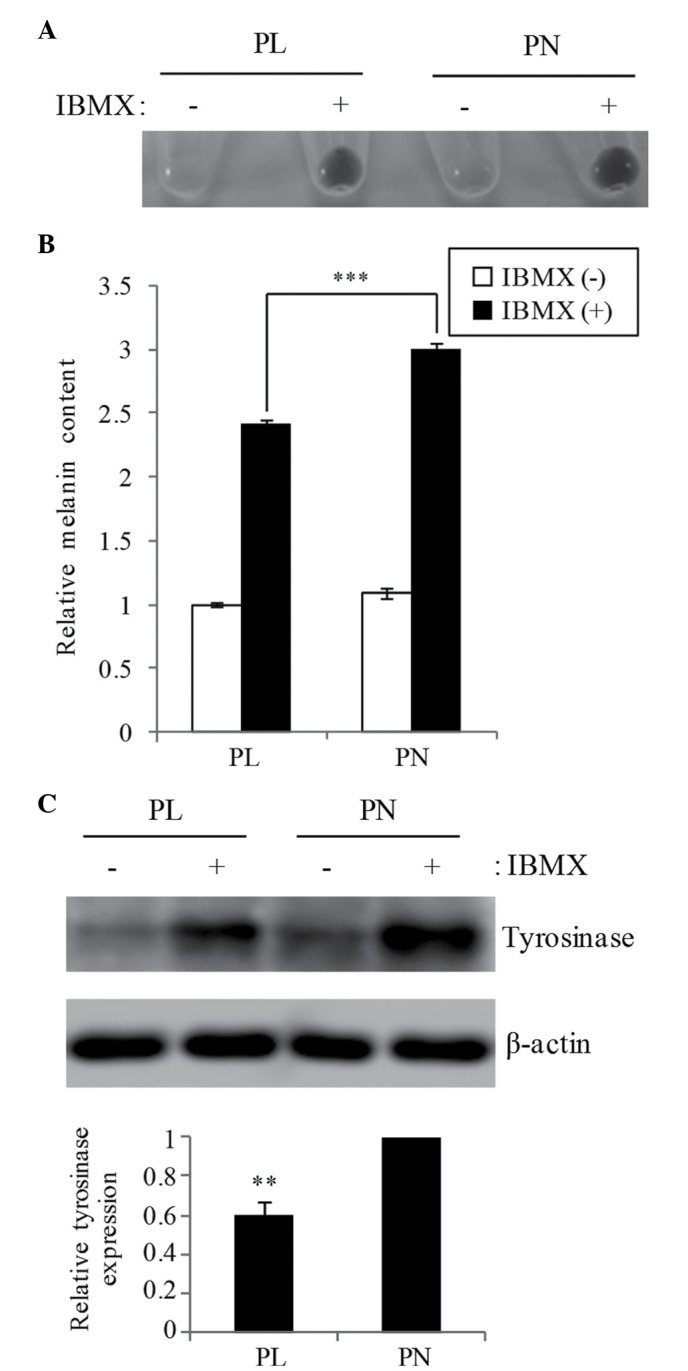
Effect of PL and PN on melanogenesis in B16F10 cells. Cells were cultured with 20 µM PL or PN for 24 h and then treated with 100 µM IBMX for 48 h. PL and PN were contained in the medium in total for 72 h. Melanin content of cultured cells were evaluated for (A) visualization and (B) absorbance at 450 nm. The data represent mean ± SD (n=4). Statistically significant difference is indicated as ***P<0.001. (C) Tyrosinase expression was examined by western blot analysis (upper panel) and showed a graphical representation of the relative signal intensity of tyrosinase (lower panel). The data represent mean ± SD (n=3). **P<0.01 indicates significant difference compared to PN. PL, pyridoxal; PN, pyridoxine; IBMX, 3-isobutyl-1-methylxanthine.
Figure 3.
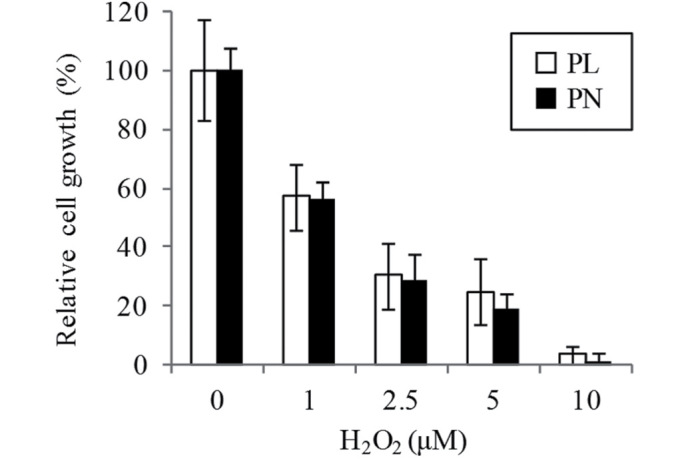
Effect of PL and PN on cell resistance to oxidative stress. Cells were cultured with 20 µM PL or PN for 24 h and then treated with 1–10 µM H2O2 for 24 h. Cell proliferation was assessed by cell counting. The data represent mean ± SD (n=3). PL, pyridoxal; PN, pyridoxine.
Discussion
In DMEM, either PL or PN is used as a vitamin B6 compound. Although the difference between these vitamin B6 compounds does not affect cell growth, the influence on other physiological effects is poor understood. In the present study, we compared the effects of PL and PN in DMEM on cell proliferation and IBMX-stimulating melanogenesis in B16F10 cells. We clarified 500 µM PL inhibits cell proliferation of B16F10 cells. The cleavage of PARP was hardly observed, presumably because few apoptotic cells were observed. The purity of PL is ensured more than 99%, and the other 1% components include less than 0.5% water. In addition, high concentration of PL is also reported to inhibit cell growth through the reduction of nuclear acid precursor uptake in B16 and human melanoma cells (5,6). We could think the effect by PL is not for other impurities. In an animal model, vitamin B6 suppressed colon tumorigenesis by reducing cell growth (10). Epidemiologic research reported that vitamin B6 can protect colon against cancer (11). B16F10 cells are highly malignant compared to B16 cells. Thus, PL might be effective in melanoma therapy. Conversely, the inhibitory effect of PN on cell growth in B16F10 cells is less than that in PL. In MCF-7 cells, growth suppression by PN was weak compared with PL (6,7). In general, vitamin B6 functions after its conversation to an active form, PLP. Although PL is converted to PLP by pyridoxal kinase, conversion of PN to PLP requires two enzymes, pyridoxal kinase and pyridoxamine oxidase (3). It has been reported that intracellular levels of PLP increased in cells exposed to pharmacologic PL concentrations (0.05–0.5 mM), but not PN in M21-HPB human melanoma cells (5). Therefore, the antitumor effect of PL appears to be strong. However, in RAW264.7 cells, the addition of PL could not increase intracellular PLP concentration (12). The cell inhibitory effect of PL might not be dependent on PLP. Further studies are required to clarify this mechanism.
The synthesis of melanin initiates the conversion of L-tyrosine to L-dopa and then to dopaquinone by tyrosinase, which is the rate-limiting enzyme (13). Dopaquinone spontaneously proceeds to melanin by auto-oxidation. We found that the inhibition of melanogenesis by PL is stronger than that by PN. Although anti-oxidative effect of between PL and PN is not different, tyrosinase expression in the presence of PL was lower than that in PN by correlation with melanin contents. PN did not suppress the melanogenesis compared with vitamin B6 free condition. A previous study showed that PL suppressed lipopolysaccharide-stimulating COX-2 expression more strongly than PN in RAW264.7 murine macrophage cells (12). PL might inhibit IBMX-stimulating tyrosinase expression compared with PN. However, melanogenesis is affected by the media type, pH, and glucose concentration (14–16). Therefore, we speculated that the difference of cellular metabolism, such as amino acids by PL and PN, might change the cellular environment, and therefore, alter melanogenesis and tyrosinase expression. Additionally, the present study lacks the melanin quantification in normal cells. To understand the inhibitory mechanism of melanogenesis by vitamin B6 in detail, we need to analyze using not only other melanoma cells but also normal melanocytes.
In conclusion, we showed the different effects of PL and PN on B16F10 cells. PL might help melanoma therapy and suppression of skin pigmentation compared to PN. In addition, we expect that our results offer the researchers an opportunity to consider the selection of an effective medium for cell culture.
Acknowledgements
The authors thank Prof. Naoto Oku (School of Pharmaceutical Sciences, University of Shizuoka, Japan) for the gift of B16F10 cells.
References
- 1.Nema R, Khare S. An animal cell culture: Advance technology for modern research. Adv Biosci Biotechnol. 2012;3:219–226. doi: 10.4236/abb.2012.33030. [DOI] [Google Scholar]
- 2.Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, Goubar A, Martins I, Michels J, Kratassiouk G, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2:257–269. doi: 10.1016/j.celrep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Vacchelli E, Michels J, Garcia P, Kepp O, Senovilla L, Vitale I, Kroemer G. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene. 2013;32:4995–5004. doi: 10.1038/onc.2012.623. [DOI] [PubMed] [Google Scholar]
- 4.DiSorbo DM, Nathanson L. High-dose pyridoxal supplemented culture medium inhibits the growth of a human malignant melanoma cell line. Nutr Cancer. 1983;5:10–15. doi: 10.1080/01635588309513773. [DOI] [PubMed] [Google Scholar]
- 5.Shultz TD, Santamaria AG, Gridley DS, Stickney DR, Slater JM. Effect of pyridoxine and pyridoxal on the in vitro growth of human malignant melanoma. Anticancer Res. 1988;8:1313–1318. [PubMed] [Google Scholar]
- 6.Minamino M, Oka T, Kanouchi H. Growth suppression and cell death by pyridoxal is dependent on p53 in the human breast cancer cell line MCF-7. Biosci Biotechnol Biochem. 2015;79:124–129. doi: 10.1080/09168451.2014.952618. [DOI] [PubMed] [Google Scholar]
- 7.Nakari M, Kanouchi H, Oka T. High dose of pyridoxine induces IGFBP-3 mRNA expression in MCF-7 cells and its induction is inhibited by the p53-specific inhibitor pifithrin-α. J Nutr Sci Vitaminol (Tokyo) 2011;57:280–284. doi: 10.3177/jnsv.57.280. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo T, Yamamoto T, Katsuda C, Niiyama K, Yamamoto A, Yamazaki N, Ohkura K, Kataoka M, Shinohara Y. Substitution of certain amino acids in a short peptide causes a significant difference in their immunoreactivities with antibodies against different epitopes: Evidence for possible folding of the peptide on a nitrocellulose or PVDF membrane. Biologicals. 2009;37:44–47. doi: 10.1016/j.biologicals.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo T, Komatsu M, Yoshimaru T, Kiyotani K, Miyoshi Y, Sasa M, Katagiri T. Involvement of B3GALNT2 overexpression in the cell growth of breast cancer. Int J Oncol. 2014;44:427–434. doi: 10.3892/ijo.2013.2187. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu SI, Watanabe H, Oka T, Tsuge H, Nii H, Kato N. Vitamin B-6-supplemented diets compared with a low vitamin B-6 diet suppress azoxymethane-induced colon tumorigenesis in mice by reducing cell proliferation. J Nutr. 2001;131:2204–2207. doi: 10.1093/jn/131.8.2204. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara J, Otani T, Inoue M, Iwasaki M, Sasazuki S, Tsugane S, Japan Public Health Center-based Prospective Study Group Low intake of vitamin B-6 is associated with increased risk of colorectal cancer in Japanese men. J Nutr. 2007;137:1808–1814. doi: 10.1093/jn/137.7.1808. [DOI] [PubMed] [Google Scholar]
- 12.Kanouchi H, Shibuya M, Tsukamoto S, Fujimura Y, Tachibana H, Yamada K, Oka T. Comparisons of uptake and cell surface binding among pyridoxal, pyridoxine, and pyridoxamine in RAW264.7 cells. Nutrition. 2010;26:648–652. doi: 10.1016/j.nut.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, Bae H. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20:921–934. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 14.Wolnicka-Glubisz A, Nogal K, Żądło A, Płonka PM. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch Dermatol Res. 2015;307:89–98. doi: 10.1007/s00403-014-1523-1. [DOI] [PubMed] [Google Scholar]
- 15.Laskin JD, Mufson RA, Weinstein IB, Engelhardt DL. Identification of a distinct phase during melanogenesis that is sensitive to extracellular pH and ionic strength. J Cell Physio. 1980;103:467–474. doi: 10.1002/jcp.1041030312. [DOI] [PubMed] [Google Scholar]
- 16.Nakayasu M, Saeki H, Tohda H, Oikawa A. Effects of sugars on melanogenesis in cultured melanoma cells. J Cell Physiol. 1977;92:49–55. doi: 10.1002/jcp.1040920107. [DOI] [PubMed] [Google Scholar]


