Abstract
Galectin-3 (Gal-3), a β-galactoside-binding protein, has been implicated in cell proliferation, cell adhesion, and the progression and metastasis of various types of cancer. The present study investigated the involvement of Gal-3 in the tumorigenesis and progression of pituitary tumors using three rat pituitary tumor cell lines. Following transfection with Gal-3 expression and interference vectors, the impact of Gal-3 on proliferation, apoptosis and migration of pituitary tumor cells was been investigated. Meanwhile, BCL2 associated X, apoptosis regulator (Bax), caspase-3 and matrix metalloproteinase 7 (MMP7) protein expression levels were analyzed by western blotting. The results of the present study revealed that Gal-3 expression in GH3 and GH4C1 cells was higher than in RC-4B/C cells. Furthermore, Gal-3 was demonstrated to promote the proliferation and migration of GH3 and GH4C1 cells, and inhibit cell apoptosis. Caspase-3 and MMP7 protein expression was also increased by Gal-3, while Bax expression was decreased. These results suggested that Gal-3 serves an important function in the tumorigenesis and development of pituitary tumors, and it may be a useful target for the treatment of pituitary tumors in the future.
Keywords: pituitary adenomas, galectin-3, proliferation, apoptosis, migration
Introduction
Pituitary adenomas are one of the most common intracranial tumors; having the third highest morbidity rate of all intracranial tumors following brain gliomas and meningiomas (1). Pituitary adenomas are divided into different classifications through tumor size and hormone secretion function (2). According to tumor size, pituitary adenomas are divided into microadenomas and macroadenomas by an arbitrary cutoff size of 10 mm (2). According to hormone secretion, the tumors are divided into hormone-secreting pituitary adenomas and nonfunctional adenomas (2). Although the majority of pituitary adenomas are benign and rarely progress to malignancies, they are usually aggressive due to hormone excessive or insufficiency (1). Due to the partial pressure of the tumor and disordered pituitary hormone secretion, pituitary adenomas are characterized by a series of clinical symptoms which seriously affect the quality of life of the patients. The aim of the present study was to identify objective molecular markers to predict the aggressive potential of tumors, and guide strategies for surgery and medical treatment.
In our previous study, gene arrays were used to screen for differentially expressed genes in aggressive pituitary adenomas and a series of upregulated genes, including galectin-3 (Gal-3) were identified, indicating that Gal-3 may be involved in the invasive development of pituitary adenomas (3,4). Gal-3, a β-galactoside-binding protein, is one of the most frequently investigated members of the galectin family. Gal-3 is involved in normal biological processes including cell growth, differentiation and cell adhesion, but is also involved in the progression of a number of types of cancer, including pituitary, thyroid, colon and breast cancer (5–8). However, the mechanisms underlying the involvement of Gal-3 in the tumorigenesis and development of pituitary adenomas remain unknown. In the present study, Gal-3 expression was detected in three different rat pituitary tumor cell lines (GH3, GH4C1 and RC-4B/C). Expression and knockdown vectors of Gal-3 were utilized to investigate the involvement of Gal-3 in the tumorigenesis and progression of pituitary tumors, and provide a theoretical basis for treatment of pituitary tumors.
Materials and methods
Cell culture
Three kinds of rat pituitary tumor cell lines GH3 was obtained from BeNa Culture Collection (Kunshan, China), and GH4C1 and RC-4B/C were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 15% horse serum (Gibco, Thermo Fisher Scientific, Inc.), 2.5% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin, and were maintained at 37°C in a 5% CO2 humidified environment.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis of Gal-3
Total cellular RNA was extracted from pituitary tumor cells using TRIzol reagent (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer's instructions. Then, RNA was reverse-transcribed into cDNA using PrimeScript™ RT reagent kit with gDNA Eraser, and cDNA amplification was performed using a SYBR Green PCR assay (both from Takara Bio, Inc., Dalian, Japan). The transcriptional levels of genes were calculated using the 2−ΔΔCq method (9). The PCR reaction (20 µl total volume) contained 10 µl 2X SYBR Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan), 0.40 µmol/l each primer and 0.2±0.02 µg cDNA template. The following three-step RT-qPCR reaction was performed: Pre-denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 56–60°C for 20 sec and elongation at 72°C for 20 sec. The sequences for the primers are as follows: Gal-3 sense, 5′-CCCCGCTTCAATGAGA-3′ and antisense, 5′-GAATGGTTTGCCGCTC-3′; β-actin sense, 5′-CGTTGACATCCGTAAAGAC-3′ and antisense, 5′-TAGGAGCCAGGGCAGTA-3′.
Construction of expression and interference vectors and cell transfection
The Gal-3 gene was amplified using primers (Gal-3 forward, 5′-ATAGGATCCATGGCAGACGGCTTCTCACTT-3′ and reverse, 5′-ATCGAAGACTTAGATCATGGCGTGGGAAG-3′) and was inserted into the corresponding site of the pGPU6/GFP/Neo vector (GenePharma Co., Ltd., Shanghai, China) according to the manufacturer's instructions to construct the overexpression vector pGPU6/GFP/Neo-Gal-3-Homo-1. Negative control pGPU6/GFP/Neo-shNC (target sequence, 5′-GTTCTCCGAACGTGTCACGT-3′), and Gal-3 interference vectors pGPU6/GFP/Neo-shGal-3-Homo-A (target sequence, 5′-AGCCTTCCCAGGGCAACCTGG-3′) were purchased from GenePharma Co., Ltd. Cells were seeded in cell plates with DMEM and cultured until the cells reached 70–80% confluence, and then were transfected with 800 ng/well expression plasmids or interference plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The untransfected cells served as the control group.
MTT assay
Cell proliferation was measured using an MTT assay. GH3 and GH4C1 cells were seeded in 96-well plates and transfected with interference vectors, expression vectors or empty control vectors. At the indicated time-points following transfection (24, 48 and 72 h), 20 µl MTT (5 mg/ml; Bioswamp; Wuhan Beinglay Biological Technology Co., Wuhan, China) was added to each well. Following incubation for 4 h, the medium was removed and 150 µl DMSO was added to each well. Following shaking at low speed for 10 min, the absorbance of converted the dye was measured at a wavelength of 490 nm.
Cell apoptosis assay
Apoptosis analysis was performed using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) flow cytometry kit (BD Biosciences, Franklin Lakes, CA, USA) according to the manufacturer's instructions. GH3 and GH4C1 cells were transfected with Gal-3 interference vectors, empty vectors or expression vectors using Lipofectamine 2000. Cells were washed 48 h after this three times with ice-cold PBS and resuspended in 200 µl of binding buffer at a concentration of 1×106 cells/ml. Annexin V-FITC (10 µl) and PI (10 µl) were added and cells were incubated for 30 min at 4°C in the dark. Finally, 300 µl binding buffer was added and the cells were analyzed using flow cytometry (Cytomics FC 500) within 1 h and the results analyzed using CXP 2.1 Software (both from Beckman Coulter, Brea, CA, USA).
Cell migration assay
Cell migration and invasion were assessed by Transwell assays. GH3 and GH4C1 cells from different groups were starved with serum-free DMEM medium for 24 h. Cells were diluted to 1×105 cells/ml and 200 µl cell suspension was added in the upper Transwell chambers (Corning Inc., Corning, NY, USA), and 500 µl DMEM medium supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in the lower chambers, this was repeated three times for every group. Following incubation for 48 h in 24-well plates at 37°C, cells were fixed for 10 min using 1 ml/well 4% paraformaldehyde (Bioswamp; Wuhan Beinglay Biological Technology Co.), stained by 0.5% crystal violet (Bioswamp; Wuhan Beinglay Biological Technology Co.) for 30 min at room temperature and washed 3 times using 1X PBS. Finally, the Transwell plates were wiped carefully with a cotton swab to remove non-migrated cells, and viewed under an optical microscope (Nikon Corporation, Tokyo, Japan) at magnification of ×200 to count the number of cells.
Western blot analysis
Antibodies against Gal-3 (cat. no. ab2785; dilution 1:1,000), BCL2 associated X apoptosis regulator (Bax; cat. no. ab32503; dilution 1:2,000), caspase-3 (cat. no. ab90437; dilution 1:1,000), matrix metalloproteinase 7 (MMP7; cat. no. ab5706; dilution 1:1,000) and β-actin (cat. no. ab8227; dilution 1:2,000) were all purchased from Abcam (Cambridge, UK). Western blot analysis was performed as previously described (10). Briefly, cells were washed with PBS and lysed in lysis buffer (20 mmol/l Tris-HCL pH 7.4, 150 mmol/l NaCl, 0.5% Nonidet P-40, 1 mmol/l EDTA, 50 µg/ml leupeptin, 30 µg/ml aprotinin, and 1 mmol/l PMSF). The protein concentration was determined by BCA kit (Bioswamp; Wuhan Beinglay Biological Technology Co.) Equal amounts of protein (30 µg) were separated by 10% SDS-PAGE and then transferred onto PVDF membrane (EMD Millipore, Billerica, MA, USA). The membranes were blocked for 2 h at room temperature with 5% skim milk in TBS (20 mmol/l Tris, 500 mmol/l NaCl, and 0.05% Tween-20). Subsequently, the membrane was incubated with primary antibodies overnight at 4°C. Then, the membranes were washed twice with TBS and incubated in biotinylated goat anti-rabbit IgG secondary antibody (cat. no. W4011; 1:3,000 dilution; Promega Corporation, Madison, WI, USA) for 2 h at room temperature. Immunoreactivity was visualized by colorimetric reaction using ECL substrate buffer (EMD Millipore). Membranes were scanned with Gel Doz EZ imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation. Data were analyzed by one-way analysis of variance using SPSS 19.0 software (IBM Corp, Armonk, NY, USA), followed by Dunnett's or Duncan's tests for multiple comparisons. P<0.05, P<0.01 and P<0.001 were considered to indicate a statistically significant difference.
Results
Expression of Gal-3 in rat pituitary tumor cells
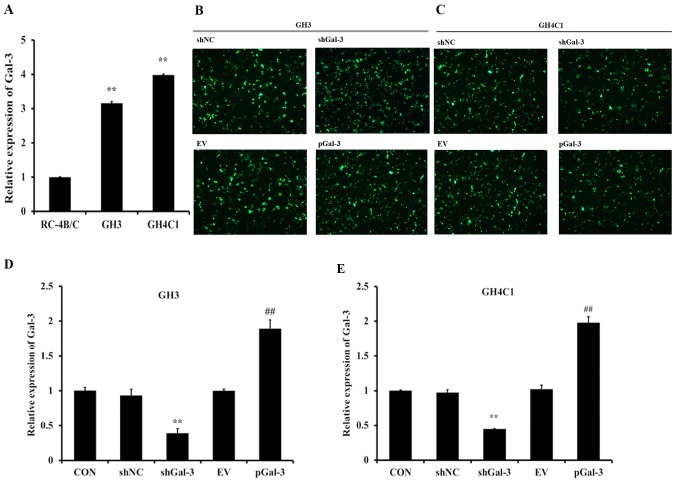
To explore the expression levels of Gal-3 in rat pituitary tumor cells, three cell lines were selected to undergo RT-qPCR, and the results revealed that Gal-3 expression levels were increased in GH3 and GH4C1 cells compared with RC-4B/C cells (Fig. 1A). As presented in Fig. 1B and C, the interference vector (shGal-3) and expression vector (pGal-3) were successfully expressed in GH3 and GH4C1 cells. Then, the expression efficiency of the vectors was assessed with RT-qPCR, which revealed that shGal-3 significantly reduced the expression of Gal-3 and pGal-3 markedly increased the expression level of Gal-3 compared with their respective controls (Fig. 1D and E).
Figure 1.
Expression of Gal-3 in rat pituitary tumor cell lines. (A) The mRNA levels of Gal-3 in three different cell lines (GH3, GH4C1 and RC-4B/C) were detected by RT-qPCR. (B) GH3 cells and (C) GH4C1 cells transfected with shNC, shGal-3, EV and pGal-3 and detected by fluorescence microscopy (magnification, ×200). The mRNA expression levels of Gal-3 in (D) GH3 cells and (E) GH4C1 cells transfected with shNC, shGal-3, EV and pGal-3 **P<0.01 vs. RC-4B/C or shNC; ##P<0.01 vs. shGal-3. Gal-3, galectin-3; shNC, negative control interference vector; shGal-3, galectin-3 interference vector; EV, empty vector control; pGal-3, galectin-3 overexpression vector; CON, control.
The effect of Gal-3 on rat pituitary tumor cell proliferation
Cell proliferation was measured by MTT. There were five groups: CON (control), shNC (cells transfected with the negative control interference vector), shGal-3 (cells transfected with shGal-3), EV (cells transfected with an empty vector) and shGal-3+pGal-3 (cells transfected with shGal-3 initially, and then the Gal-3 expression vector). As presented in Fig. 2, cell proliferation in the shGal-3 group was decreased compared with the shNC group at 24, 48 and 72 h. Cell proliferation in the shGal-3+pGal-3 group was also significantly increased in a time-dependent manner compared with the shGal-3 group (Fig. 2). The results indicated that Gal-3 promoted the proliferation of GH3 and GH4C1 cells.
Figure 2.
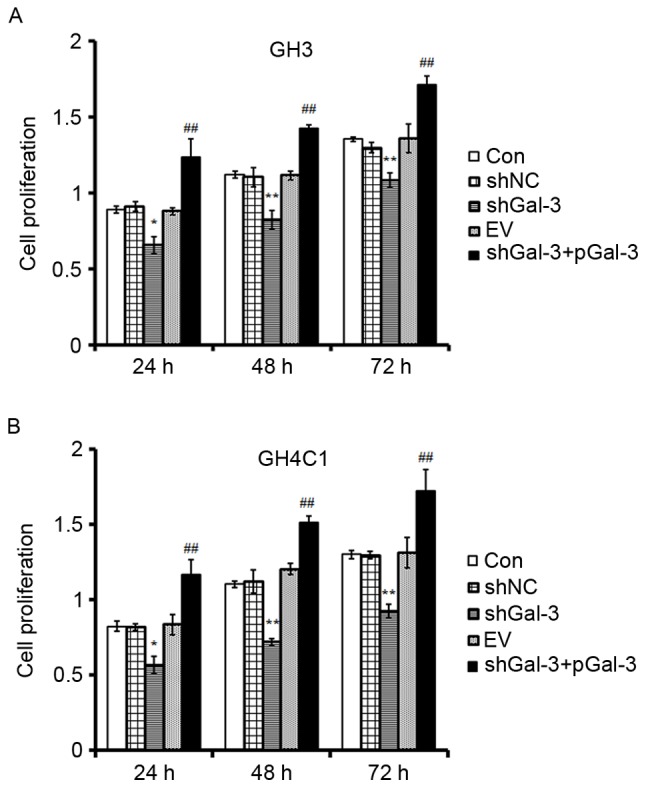
Gal-3 promoted GH3 and GH4C1 cell proliferation, as assessed using MTT assays. (A) GH3 and (B) GH4C1 cell proliferation following transfection with shGal-3 was decreased compared with the shNC group, while the proliferation was increased in the shGal-3+pGal-3 group compared with the shGal-3 group. **P<0.01 vs. shNC; ##P<0.01 vs. shGal-3. Gal-3, galectin-3; shNC, negative control interference vector; shGal-3, galectin-3 interference vector; EV, empty vector control; pGal-3, galectin-3 overexpression vector; CON, control.
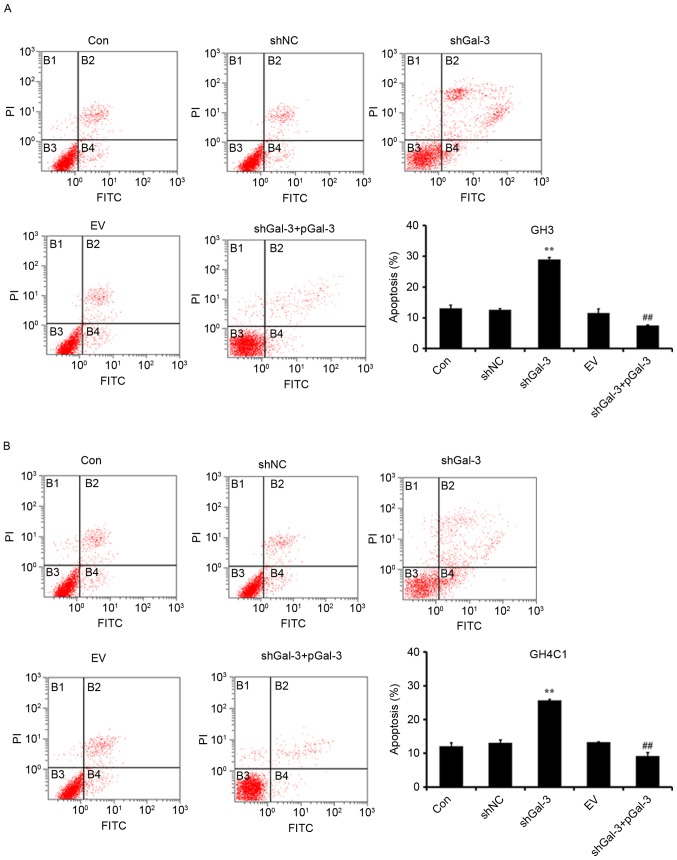
Effect of Gal-3 on rat pituitary tumor cell apoptosis
Cell apoptosis was measured by Annexin V-FITC/PI flow cytometry. As presented in Fig. 3, transfection with shGal-3 significantly increased the percentage of apoptotic cells compared with the shNC group, while the shGal-3+pGal-3 group had decreased apoptosis compared with the shGal-3 group. These results indicated that Gal-3 inhibited the apoptosis of GH3 and GH4C1 cells.
Figure 3.
Gal-3 reduces apoptosis in rat pituitary tumor cell lines. Apoptosis in (A) GH3 and (B) GH4C1 cells was assessed using flow cytometry. **P<0.01 vs. shNC; ##P<0.01 vs. shGal-3. Gal-3, galectin-3; shNC, negative control interference vector; shGal-3, galectin-3 interference vector; EV, empty vector control; pGal-3, galectin-3 overexpression vector; CON, control; FITC, fluorescein isothiocyanate; PI, propidium iodide.
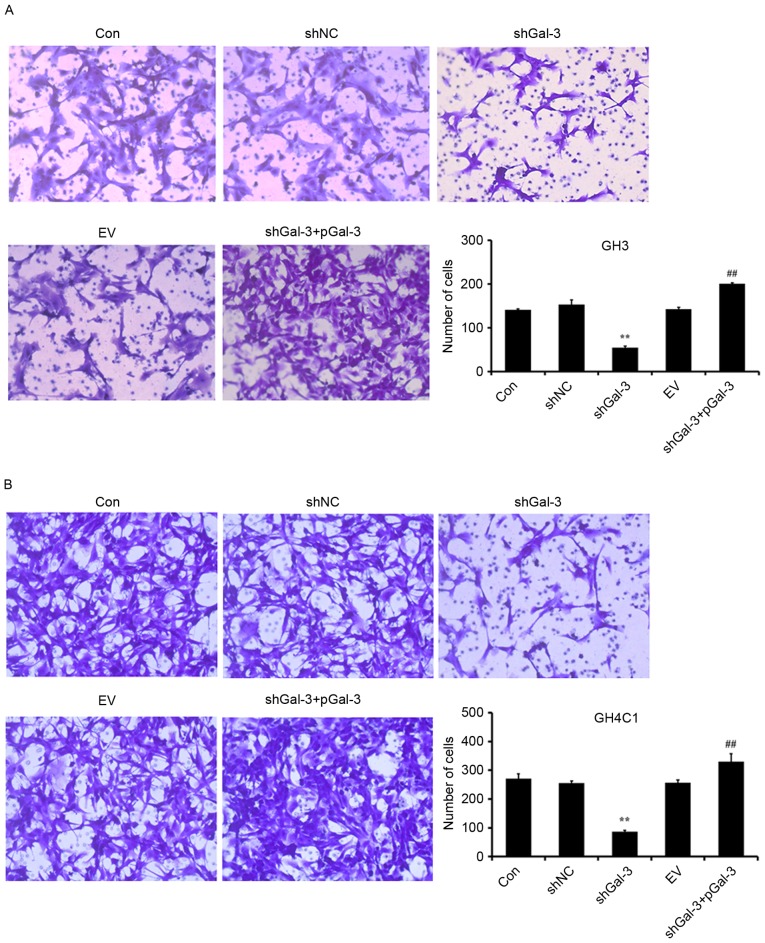
Effect of Gal-3 on rat pituitary tumor cell migration
Cell migration was assessed using a Transwell test. As presented in Fig. 4, transfection with shGal-3 significantly decreased the number of migrating cells compared with the shNC group, while shGal-3+pGal-3 groups significantly increased the migration of cells compared with shGal-3. These results indicated that Gal-3 increased the migration of GH3 and GH4C1 cells.
Figure 4.
Gal-3 promoted the migration of rat pituitary tumor cell lines. Migration was assessed in (A) GH3 and (B) GH4C1 cells by Transwell assays (magnification, ×200). **P<0.01 vs. shNC; ##P<0.01 vs. shGal-3. Gal-3, galectin-3; shNC, negative control interference vector; shGal-3, galectin-3 interference vector; EV, empty vector control; pGal-3, galectin-3 overexpression vector; CON, control.
Effect of Gal-3 on apoptosis-associated genes and oncogenes
In order to further understand the effect of Gal-3 on apoptosis-associated genes and oncogenes, the expression of Bax, caspase-3 and MMP7 were detected by Western blot. As presented in Fig. 5, in GH3 and GH4C1 cells, the protein expression levels of caspase-3 and MMP7 were decreased while Bax expression was increased in the shGal-3 group compared with the controls. In addition, caspase-3 and MMP7 protein expression were increased following overexpression of Gal-3, while Bax expression was decreased (Fig. 5).
Figure 5.
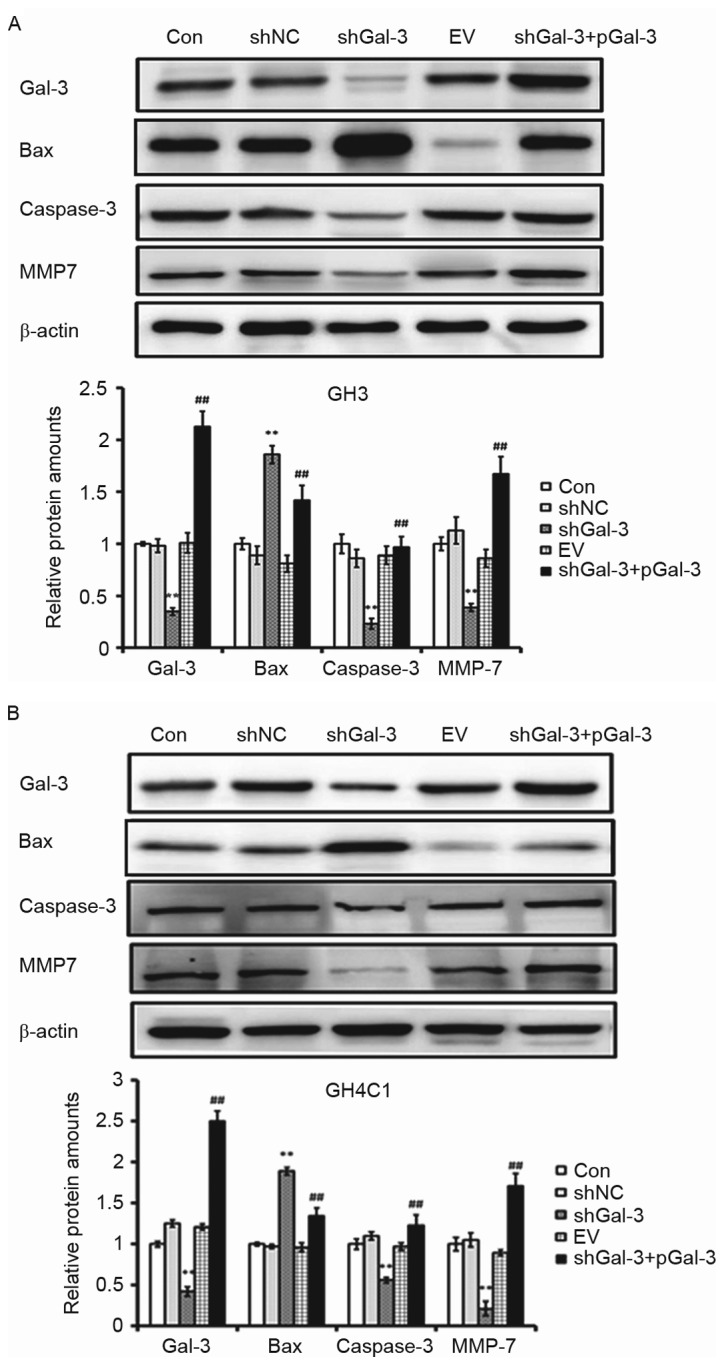
Effect of Gal-3 on the expression of apoptosis-associated genes and oncogenes. The expression of apoptosis-associated genes in (A) GH3 and (B) GH4C1 cells was detected by western blotting. Representative blots and the fold changes in each protein to β-actin ratio are presented. **P<0.01 vs. shNC; ##P<0.01 vs. shGal-3. Gal-3, galectin-3; shNC, negative control interference vector; shGal-3, galectin-3 interference vector; EV, empty vector control; pGal-3, galectin-3 overexpression vector; CON, control; Bax, BCL2-associated X, apoptosis regulator; MMP7, matrix metalloproteinase 7.
Discussion
Galectins are a family of animal lectins with diverse biological functions. They are involved in extracellular and intracellular processes: Extracellularly, by interacting with cell-surface and extracellular matrix glycoproteins and glycolipids, and intracellularly, by interacting with cytoplasmic and nuclear proteins to regulate signaling pathways (11). Previous research has demonstrated that galectins serve vital functions in cancer and that they contribute to neoplastic transformation, the maintenance of transformed phenotypes, tumor cell adhesion and survival, angiogenesis and tumor metastasis (12). Among the different members of the galectin family, Gal-3 has been reported to be involved in various biological processes, including cell proliferation and differentiation, tumor cell adhesion, apoptosis, tumor progression and metastasis.
In the present study, Gal-3 expression levels in GH3 and GH4C1 cells were increased compared with RC-4B/C cells. Then, two pituitary tumor cell lines were selected for further study concerning the role of Gal-3 on cell proliferation, cell apoptosis, cell migration and the expression of apoptosis-associated genes and oncogenes. In a previous study, inhibition of Gal-3 gene expression by RNA interference decreased HP75 cell proliferation and increased apoptosis (13). The present study demonstrated that inhibition of Gal-3 expression following transfection with RNA interference vectors decreased cell proliferation, migration and the expression of caspase-3 and MMP7 in GH3 and GH4C1 cells, but increased cell apoptosis and expression of the apoptosis-associated gene Bax. Furthermore, following interference with Gal-3 expression, overexpression of Gal-3 repaired the reduction of cell proliferation, migration and expression of caspase-3 and MMP7, and inhibited cell apoptosis and Bax protein expression. These results indicated that Gal-3 contributes to the tumorigenesis and progression of pituitary tumors.
Gal-3 is expressed in a number of types of tumor, and the intensity of its expression and localization depend on tumor progression, invasiveness and metastatic potential (14). Previous evidence suggests that Gal-3 may be a useful biomarker for differential diagnosis of brain tumors, including various glioneuronal tumors, pituitary adenomas, meningiomas and schwannomas (15). The present study is focused on the involvement of Gal-3 on pituitary adenomas. Given the function of Gal-3 in pituitary tumor cell proliferation and apoptosis, Gal-3 may be a potential target for the treatment of aggressive pituitary tumors. However, the precise mechanisms by which Gal-3 promotes the tumorigenesis and progression of pituitary tumors require further study.
Acknowledgements
The present study was supported by grants from Project of Health and Family Planning Commission of Hubei Province (grant nos. WJ2015MA012 and WJ2015MB118).
References
- 1.Jiang X, Zhang X. The molecular pathogenesis of pituitary adenomas: An update. Endocrinol Metab (Seoul) 2013;28:245–254. doi: 10.3803/EnM.2013.28.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatzellis E, Alexandraki KI, Androulakis II, Kaltsas G. Aggressive pituitary tumors. Neuroendocrinology. 2015;101:87–104. doi: 10.1159/000371806. [DOI] [PubMed] [Google Scholar]
- 3.Gong J, Diao BO, Yao GJ, Liu Y, Xu GZ. Analysis of regulatory networks constructed based on gene coexpression in pituitary adenoma. J Genet. 2013;92:489–497. doi: 10.1007/s12041-013-0299-y. [DOI] [PubMed] [Google Scholar]
- 4.Huang CX, Hou YH, Liu YS. Expression of galectin-3 correlates with apoptosis in pituitary adenoma cells. Neurosci Bull. 2008;24:34–38. doi: 10.1007/s12264-008-1029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arfaoui-Toumi A, Kria-Ben Mahmoud L, Ben Hmida M, Khalfallah MT, Regaya-Mzabi S, Bouraoui S. Implication of the galectin-3 in colorectal cancer development (about 325 tunisian patients) Bull Cancer. 2010;97:E1–E8. doi: 10.1684/bdc.2010.1032. [DOI] [PubMed] [Google Scholar]
- 6.Manivannan P, Siddaraju N, Jatiya L, Verma SK. Role of pro-angiogenic marker galectin-3 in follicular neoplasms of thyroid. Indian J Biochem Biophys. 2012;49:392–394. [PubMed] [Google Scholar]
- 7.Yamaki S, Fujii T, Yajima R, Hirakata T, Yamaguchi S, Fujisawa T, Tsutsumi S, Asao T, Yanagita Y, Iijima M, Kuwano H. Clinicopathological significance of decreased galectin-3 expression and the long-term prognosis in patients with breast cancer. Surg Today. 2013;43:901–905. doi: 10.1007/s00595-012-0378-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang CX, Zhao JN, Zou WH, Li JJ, Wang PC, Liu CH, Wang YB. Reduction of galectin-3 expression reduces pituitary tumor cell progression. Genet Mol Res. 2014;13:6892–6898. doi: 10.4238/2014.August.29.11. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Na YJ, Jeon YJ, Suh JH, Kang JS, Yang KH, Kim HM. Suppression of IL-8 gene expression by radicicol is mediated through the inhibition of ERK1/2 and p38 signaling and negative regulation of NF-kappaB and AP-1. Int Immunopharmacol. 2001;1:1877–1887. doi: 10.1016/S1567-5769(01)00113-8. [DOI] [PubMed] [Google Scholar]
- 11.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 12.Newlaczyl AU, Yu LG. Galectin-3-a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Riss D, Jin L, Qian X, Bayliss J, Scheithauer BW, Young WF, Jr, Vidal S, Kovacs K, Raz A, Lloyd RV. Differential expression of galectin-3 in pituitary tumors. Cancer Res. 2003;63:2251–2255. [PubMed] [Google Scholar]
- 14.Righi A, Jin L, Zhang S, Stilling G, Scheithauer BW, Kovacs K, Lloyd RV. Identification and consequences of galectin-3 expression in pituitary tumors. Mol Cell Endocrinol. 2010;326:8–14. doi: 10.1016/j.mce.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Min HS, Kim B, Myung J, Paek SH. Galectin-3: A useful biomarker for differential diagnosis of brain tumors. Neuropathology. 2008;28:497–506. doi: 10.1111/j.1440-1789.2008.00909.x. [DOI] [PubMed] [Google Scholar]