Abstract
Congenital long QT syndrome type 3 (LQT3) is the third in frequency compared to the 15 forms known currently of congenital long QT syndrome (LQTS). Cardiac events are less frequent in LQT3 when compared with LQT1 and LQT2, but more likely to be lethal; the likelihood of dying during a cardiac event is 20% in families with an LQT3 mutation and 4% with either an LQT1 or an LQT2 mutation. LQT3 is consequence of mutation of gene SCN5A which codes for the Nav1.5 Na+ channel α-subunit and electrocardiographically characterized by a tendency to bradycardia related to age, prolonged QT/QTc interval (mean QTc value 478 ± 52 ms), accentuated QT dispersion consequence of prolonged ST segment, late onset of T wave and frequent prominent U wave because of longer repolarization of the M cell across left ventricular wall.
Keywords: Long QT syndrome, Long QT syndrome-type-3, Torsade de Pointes, Electrocardiogram
1. Introduction
LQT3 Romano-Ward (OMIM number #600163) is an autosomal dominant channelopathy (exceptionally can be with autosomal recessive inheritance) responsible for 7–10% of total LQTS that affect the chromosome 3(3 (3p21-24)) consequence of mutation of gene SCN5A which codes for the Nav1.5 Na+ channel α-subunit, and electrocardiographically characterized by a tendency to bradycardia related to age, prolonged QT/QTc interval (mean QTc value 478 ± 52 ms), accentuated QT dispersion consequence of prolonged ST segment, late onset of T wave and frequent prominent U wave because of longer repolarization of the M cell across left ventricular wall. The late Na+current (INa+) is due to the failure of the channel to remain inactivated. Therefore, it can enter a bursting mode, during which significant current enters abruptly when it should not. Transmural dispersion of repolarization is greatly amplified in LQTS. Disproportionate prolongation of the M-cell action potential (AP) contributes to the development of long QT intervals, wide-based or notched T waves, and a large transmural dispersion of repolarization, which provides the substrate for the development of a polymorphic ventricular tachycardia (PVT) closely resembling Torsade de Pointes (TdP). An early afterdepolarization (EAD)-induced triggered beat is thought to provide the premature ventricular contraction (PVC) that precipitates TdP. The T waves increases in bradycardias and in pauses and it may present alternating polarity with augmented risk of cardiac events (CEs) (mean 46%) of a bradycardia-triggered PVT called TdP by the French researcher François Dessertenne as well as for atrial fibrillation (AF). These CEs may result in recurrent, palpitation, syncope, seizure, sudden cardiac arrest (SCA) or sudden cardiac death (SCD), which occur predominantly at rest or during sleep without emotional arousal (≈65% of cases). These events can be treated gene-specific therapy for LQT3 with Na+ channel blocking agents of Class IB (mexiletine, lidocaine); Class IC (flecainide) and the piperazine derivate ranolazine (Ranexa®) that may provide protection from the induction of TdP by inhibition persistent or late inward Na+ current (INa) of a gain of function in the cardiac voltage-gated Na+. In symptomatic patients receiving therapy, even after excluding patients who had a SCA before therapy, presence of macro-wave T alternans especially when present despite proper β-blocker therapy, and biallelic pathogenic variants or heterozygosity variants have indication for ICD implantation.
LQTS prevalence: The estimated prevalence of LQTS is approximately 1:2000 (0.05%) to 1:5000 in the general population [1]. This prevalence may be higher because 37% of genotype-confirmed LQTS patients may have concealed form (a normal-range QTc). LQT3, which is the third most common LQTS (7–10% of all cases of LQTS) represents ≈100 to 200 in general population.
LQTS incidence: In the United States, the incidence of congenital LQTS is estimated to be one in 7000–10,000 [2]. Consequently; if LQT3 represents 7–10% of all LQTS cases, this variant has an incidence of ≈720.
Sex: risk is higher among male LQT3 patients with a mutation than among female. Among LQTS patients, the risk of CEs is higher in males until puberty and higher in females during adulthood. The annual incidence of a first SCAUDDEN or SCD is highest among male patients with a mutation at the LQT3 locus (0.96 per year). Prolonged QTc and syncope predispose patients with LQT3 to life-threatening CEs. β-blocker therapy reduces the risk in females. Efficacy in males could not be determined conclusively yet [3]. This pattern is observed in both boy and girl. Wilde et al. described that there is not much difference and actually the number of lethal events is very small. According to the authors, that is the reason that the efficacy of beta blockers could not be found. Therefore, there is not a consensus about it yet.
Age: For patients older than 40 years, LQT3 patients have significantly more cumulative lethal arrhythmic events (35%) than LQT1 (14%), LQT2 (24%) and genotype-negative patients (10%) [4]. LQT3 carriers have infrequent CEs below age of 10 years. Murphy et al. [5] presented a SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia. The fetus presented with episodes of ventricular ectopy progressing to incessant VT and hydrops fetalis. Genetic analysis disclosed a novel, de novo heterozygous mutation in SCN5A (L409P) and a homozygous common variant (R558).
Electrocardiographically concealed LQTS (ecLQTS): this term is used to indicate individuals with genotype of LQTS and a phenotype with normal QT interval (corrected QT interval ≤ 440 ms). They are usually detected on family screening of those with manifest LQTS [6]. ecLQTS represents ≈20–40% of all cases of LQTS. The risk of SCD or ACA is ten times higher in those with ecLQTS than the unaffected family members (4% vs four tenths of a percent). This is not withstanding the finding that those with manifest LQTS had a much higher risk of SCD or ACA at 15%. In ecLQTS, the risk of SCD or ACA is higher in those in LQT1 and LQT3 genotypes than in LQT2 genotype [7]. But unlike in manifest LQTS, females were not shown to be at higher risk in concealed LQTS.
Mechanism: The basic defect in LQT3 or LQTS-type-3 - which is the third most common LQTS - is caused by an excessive inflow of late Na+ current during the plateau, dome or phase 2 of the AP caused by gain-of-function mutations in the SCN5A cardiac Na+ channel gene which mediates the fast Nav1.5 current during AP initiation and also late in phase 2 of AP causing an accelerated recovery from inactivation of Na+ current as well as AP prolongation, especially at low stimulation rates. and for improving treatment efficacies. Late inward Na+ current (INa). It is an integral part of the Na+ current, which persists long after the fast-inactivating component: the larger and transient peak INa. The magnitude of the late INa+ is relatively small in all species and in all types of cardiomyocytes as compared with the amplitude of the fast Na+ current of phase 0, but it contributes significantly to the shape and duration of the AP and surface ECG. This late component had been shown to increase in several acquired or congenital conditions, including hypoxia, oxidative stress, and heart failure, or due to mutations in SCN5A, which encodes the α-subunit of the Na+ channel, as well as in channel-interacting proteins, including four β-subunits and anchoring proteins. Patients with enhanced late I Na+ exhibit the LQT3 variant characterized by high propensity for the life-threatening ventricular arrhythmias, such as TdP, as well as for AF. There are several distinct mechanisms of arrhythmogenesis due to abnormal late INa+ including abnormal automaticity, induced trigger activity both early and delayed after depolarization (EAD and DAD), and dramatic increase of transmural ventricular dispersion of repolarization. Many local anesthetic and antiarrhythmic agents have a higher potency to block late INa+ as compared with fast. In summary, Na+ channels open and inactivate rapidly during depolarization (phase 0 of AP) and reopen during the phase 2 plateau/dome phase, carrying ‘persistent’ or ‘late’ inward current (late INa). Maltsev et al. found INaL was activated at a membrane potential of −60 mV with maximum density at −30 mV in cardiomyocytes of both normal and failing hearts. The steady-state availability was sigmoidal, with an averaged midpoint potential of −94 ± 2 mV and a slope factor of 6.9 ± 0.1 mV. The current was reversibly blocked by the Na+ channel blockers tetrodotoxin and saxitoxin in a dose-dependent manner. Both inactivation and reactivation of INaL had an ultraslow time course (0.6 s) and were independent of voltage. The amplitude of INaL was independent of the peak transient Na+ current.
Malan et al. [8] observed in LQT3 hiPSC models, a high incidence of EADs which is a trigger mechanism for arrhythmia in LQT3. EADs predisposes to ventricular arrhythmias by exaggerating the dispersion of refractoriness throughout the myocardium and increasing the probability of EAD, a phenomenon caused largely by reactivation of calcium channels during the AP plateau. Administration of specific Na+ channel inhibitors was found to shorten AP durations in a dose-dependent manner. These findings were in full agreement with the pharmacological response profile of the underlying patient and of other patients from the same family. Thus, these observations demonstrate the utility of patient-specific LQT3 hiPSCs for assessing pharmacological responses to putative drugs. Brugada syndrome mutations cause a reduced Na+ current, while LQT3 mutations are associated with a gain of function (mirror image) consequently these allelic syndromes result from opposite molecular effects. Phenotypic overlap may exist between the BrS and LQT3. Na+ channel blockade by antiarrhythmic drugs improves the QT interval prolongation in LQT3 but worsens the BrS ST-segment elevation. Although Na+ channel blockade has been proposed as a treatment for LQT3, flecainide also evokes “Brugada-like” ST-segment elevation in LQT3 patients.
Using noninvasive mapping with electrocardiographic imaging (ECGI) to map the cardiac electrophysiological substrate LQTS patients display regions with steep repolarization dispersion caused by localized AP duration (APD) prolongation. This defines a substrate for reentrant arrhythmias, not detectable by surface ECG. Steeper dispersion in symptomatic patients suggests a possible role for ECG imaging in risk stratification.
Intracellular Ca2+ contributes to the regulation of INaL conducted by NaV1.5 mutants and, during excitation-contraction coupling, elevated intracellular Ca2+ suppresses mutant channel INaL and protects cells from delayed repolarization. This is a plausible explanation for the lower arrhythmia risk in LQT3 subjects during sinus tachycardia.
Iyer et al. present the first direct experimental evidence that Purkinje cells are uniquely sensitive to LQT3 mutations, displaying electrophysiological behavior that is highly pro-arrhythmic. Additionally, abnormalities in Purkinje cell repolarization were reversed with exposure to mexiletine [9].
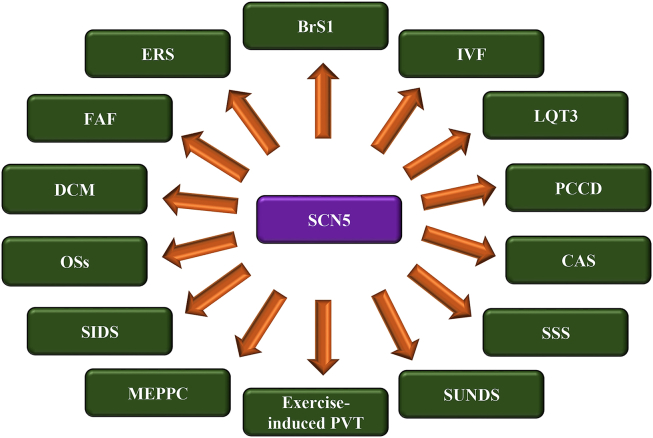
Mutations in SCN5A gene can originate numerous cardiac Na+ channelopathies phenotypes (Fig. 1).
Fig. 1.
BrS1: Brugada Syndrome 1; CAS: Congenital Atrial Standstill; DCM: Dilated Cardiomyopathy; ERS: Early Repolarization Syndrome; FAF: Familial Atrial Fibrillation; IVF: Idiopathic Ventricular Fibrillation; LQT3: Long QT syndrome 3; MEPPC: Multifocal Ectopic Purkinje Premature Contraction; OSs: Overlapping Syndromes; PCCD: Progressive Cardiac Conduction Defect; PVT: Polymorphic Ventricular Tachycardia; SIDS: Sudden Infant Death Syndrome; SSS: Sick Sinus Syndrome; SUNDS: Sudden Unexplained Nocturnal Death Syndrome.
2. Clinical presentation
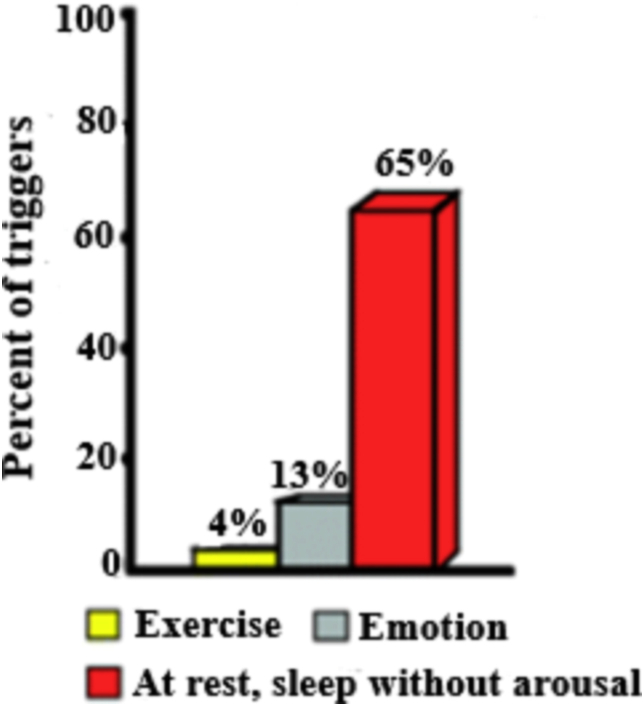
Manifest with syncope, seizures or SCD. In LQT3, majority of arrhythmic events occur during sleep or rest in ≈65% of cases (bradycardia-triggered arrhythmias) (Fig. 2).
Fig. 2.
Triggers for lethal events in LQT3.
Excessive APDs at low heart rates (HRs) predisposes individuals with LQT3 to fatal arrhythmias, typically at rest or during sleep without emotional arousal. Approximately 30% experienced at least one CE: syncope, ACA, or SCD related 25% suffered from LQT3-related ACA/SCD at rest or during sleep, and usually without warning. In some instances, TdP degenerates to ventricular fibrillation (VF) and causes ACA (if the individual is defibrillated) or SCD. Murine hearts bearing an LQT3 mutation show abnormalities in atrial electrophysiology and subtle changes in atrial dimension, including an atrial arrhythmogenic phenotype on provocation.
3. Diagnosis
Genetic testing for common mutations can confirm suspected cases. These testing are considered the gold standard for LQTS diagnosis, unfortunately they are time-consuming and costly when all the 15 candidate genes are screened [10] specific genetic testing for KCNQ1, KCNH2 and SCN5A be performed for any patient who fulfills the following criteria: where a cardiologist has established a strong suspicion for LQTS based on clinical examination, where a patient has asymptomatic QT prolongation in the absence of other clinical conditions that may prolong the QT interval, where a patient is asymptomatic, with QTc values > 460 ms (prepuberty) or >480 ms (adults) on serial 12-lead ECGs and when an LQTS-causative mutation is identified in an index case, mutation-specific genetic testing is recommended for the family members.
4. Electrocardiographic features
They have Long QT interval by ST segment prolongation correspondent plateau, dome or phase 2 of AP by persistent Na+ inflow (gain of function) and delayed onset of T-wave and peaked.
Heart rate: tendency to bradycardia related to age and in some cases, decrease during rising efforts has been observed. When HR increases, the QT interval shortens more in LQT3 than in LQT1 and LQT2. Na+ channel mutations displaying a persistent inward current or a negative shift in inactivation may account for the bradycardia seen in LQT3 patients, whereas SA node pauses or SCA may result from failure of sinoatrial (SA) node cells to repolarize under conditions of extra net inward current [11].
ST segment/T wave: significant prolongation. Consequence: late appearance of T wave. The deltaKPQ mutation causes a small and persistent inflow of Na+ in phase 2 with late reopening, which explains QT interval prolongation. Overlapping existed among the repolarization patterns of 3 genotypes, and one third of LQT3 gene carriers had repolarization patterns similar to those of LQT1 gene carriers. The sensitivities and specificities were higher with family-grouped analysis.
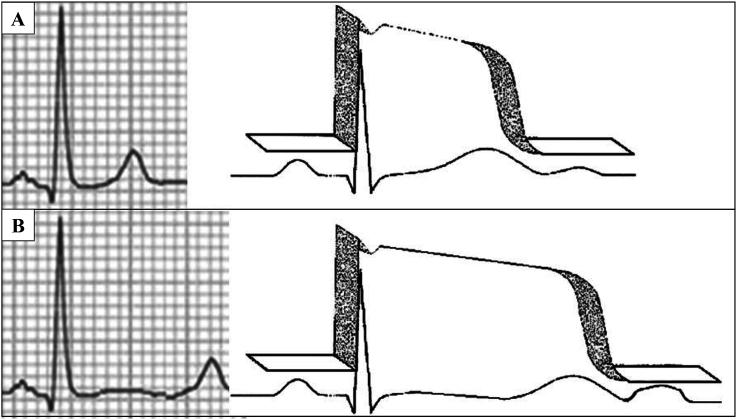
QT interval: In the LQT3 variant it is usually longer than in LQT1 and LQT2. Additionally, it is observed a significant QT interval dependence of the heart rate. The HR increases, the QT interval shortens more in LQT3 than in LQT1 and LQT2 (Fig. 3). Sinus bradycardia with sinus pauses has been reported in up to one third of the patients especially in patients with LQT3 variant [12]. In newborn with a very prolonged QT interval and 2:1 atrioventricular block is frequently caused by V411M, in a voltage-dependent manner. Incorporation of V411M kinetics into atrial and ventricular AP models reproduced prolonged AP repolarization [13]. Unfortunately, there are many examples with 2:1 block in neonates with many other mutations [14]. Zhou et al. firstly reported a heterozygote missense mutation (SCN5A-V411M) in a Chinese family. V411M induced “gain of function” of sodium channel and formed the basis of LQT3. Genetic testing could help to increase the diagnostic accuracy, and facilitate clinical assessment and appropriate therapy to prevent SCD of individuals with SCN5A-V411M mutation [15].
Fig. 3.
Normal (A) and LQT3 (B) ECG and action potential.
5. Polymorphic/polymorphous ventricular tachycardia Torsade de Pointes type characteristic
PVT is a form of VT in which there are multiple ventricular foci with the resultant QRS complexes varying in amplitude, axis, morphology and duration. The common cause of PVT is myocardial ischemia.
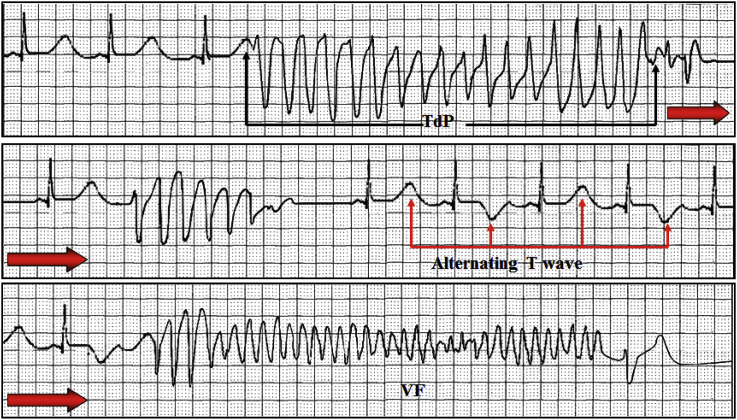
TdP is a specific form of PVT occurring in the context of prolonged QT interval (rarely in normal QT interval) recognized by a continuously changing QRS configuration form beat to beat, indicating a changing ventricular activation sequence. The “polymorphic” nature does not define an arrhythmia mechanism. In the TdP the coupling of the initial PVC is belatedly or telediastolic, the heart rate is high (from 200 to 250 bpm) and characteristically the QRS axis of VT changes suddenly 180° “twisting” around the isoelectric line.
TdP is often short lived and self-terminating, however can be associated with hemodynamic instability and collapse. TdP may also degenerate into may precede development of ventricular fibrillation. This may occur as part of the congenital or acquired LQTS. The last one is usually consequence of drug and/or electrolyte abnormalities, or may be because of reentry in a patient with structural heart disease diagnosed, the patient has to have evidence of both PVT and QT prolongation.
Finally, there is another PVT called bidirectional polymorphic VT, most commonly associated with digoxin toxicity or catecholaminergic polymorphic ventricular tachycardia (CPVT) (Table 1).
Table 1.
Differential diagnosis between Torsade de Pointes and true polymorphic ventricular tachycardia/polymorphous ventricular tachycardia.
| Torsade de Pointes (TdP) |
True polymorphic ventricular tachycardia (PVT) | |
|---|---|---|
| Etiology | Congenital or acquired | Congenital or acquired |
| Related to Sinus Bradycardia | Yes | No |
| Pauses prior to events | Yes | No |
| Electrolytic Disorders | Frequent | No |
| Coupling of the first triggering PVC | Delayed or telediastolic. Very rarely short coupled variant is observed [16] | Initiated by closely coupled beats |
| QT/QTc interval | Prolonged rare normal | Normal [17] |
| U wave | Frequent great voltage | Normal voltage |
Echocardiogram: Increasing evidence supports the notion that LQTS is not purely an “electrical” disease but rather an “electro-mechanical” disease with regionally heterogeneously impaired electrical and mechanical cardiac function. Subclinical cardiomyopathic changes were found in nearly 20% of LQTS patients. Left atrial enlargement is the most common finding and is associated with prolonged QTc and CEs. These changes may stem from underlying contraction abnormalities caused by ion channel dysfunction, structurally normal heart on echocardiography [18], DCM was observed in missense mutation-p.Q371E [19].
Exercise stress testing: Takahashi et al. [20] investigated QT dynamics during exercise testing in LQT3. The study included 37 subjects, comprising 16 genotyped LQTS patients and 21 unrelated healthy subjects without QT prolongation. LQTS patients were divided into LQT3 and non-LQT3 groups. During exercise tests using a modified Bruce protocol, 12-lead ECG monitoring was performed using a novel multifunctional electrocardiograph. QT intervals were automatically measured. The QT/HR relationship was visualized by plotting the beat-to-beat confluence of the recorded data. A linear regression analysis was performed to determine the QT/HR slope and intercept. Estimated QT intervals at HR 60 bpm (QT60) were calculated by the regression line formula. QT/HR slopes were steeper for each LQTS group than for the control group. QT60 values demonstrated a moderate correlation with QT intervals at rest for both groups. The QTc at 4 min of recovery were significantly longer in the non-LQT3 group than in the control group but were not different between the LQT3 and the control groups. Abnormal QT dynamics during exercise testing were observed in both LQT3 patients and other LQTS subtypes. This method may be useful for directing genetic testing in subjects with minimal prolonged QT intervals.
6. Ajmaline challenge
Structural heart disease in association: Kimura et al. [19] described a 16-year-old boy with LQT3 admitted for decompensated heart failure resulting from dilated cardiomyopathy (DCM). His brother was also diagnosed with LQT3 and DCM. A comprehensive genetic analysis identified a novel SCN5A missense mutation-p.Q371E-in these 2 affected living family members. It might be important to suspect the coexistence of DCM and LQT3.
Genotype-specific risk factors for arrhythmic cardiac events: constellation of clinical, electrocardiographic, and genetic factors.
-
1.
Sex: The risk of syncope during β-blocker therapy is higher in women than in men.
-
2.
Age (time dependence): pre-specified age groups, including the fetus, infant, childhood, adolescence, adulthood, and post-40 periods. The risk of syncope during β-blocker therapy is high during childhood and highest in young children. Additionally, no trend toward age and gender dependency of the risk for CEs in patients with LQT3. The lethality of CEs in LQT3 genotype carriers is higher before age 40 years [22]. In genetically tested individuals, the LQT3 genotype is the most powerful predictor of fatal or near-fatal CEs after age 40 [23]. LQT3 genotype carriers exhibited nearly a 5-fold increase in the risk of ACA or death compared with genotype-negative subjects. Clearly, patients with LQTS remain at increased risk of lethal events after 40 years of age, indicating that continuous, age-independent awareness for QT prolongation is essential, and LQTS-3 were found to be the most important predictors of death from any cause or SCA.
-
3.Clinical manifestation:
-
➢The patients in the very high-risk group (5-year Kaplan-Meier cumulative estimate rate of ACA/SCD of 14%) are those with a history of ACA/SCD and/or spontaneous TdP. These patients require an ICD implantation for secondary prevention of SCD.
-
➢The lowest risk was found in patients with only 1 syncopal episode occurring before the start of β-blocker therapy. Patients experiencing syncope after starting β-blocker therapy had a 3.6-fold increase in the risk of severe CEs. Multiple syncopal episodes occurring before initiation of β-blocker therapy were associated with an intermediate risk [24].
-
➢Asthma comorbidity: Asthma Is an independent associated significant increased risk of CEs in affected LQTS. Longer QTc duration was associated with higher incidence of asthma. CEs is diminished after initiation of β-blocker therapy, suggesting a possible role of β-receptor modulation underlying asthma-LQTS association [25].
-
➢
-
4.
Resting heart rate (HR): The QT interval in LQT3 is prolonged during bradycardia. In all age groups, HR at rest tended to be lower in carriers than in non-carriers, and QT is longer in carriers than in non-carriers. Both electrocardiographic characteristics of LQT3 and BrS show age-dependent penetrance. Good tools for clinical diagnosis of LQT3 in this family are QTc at the lowest HR and DeltaQT after a pause in a Holter, even at very young age [26].
-
5.
QTc for heart rate: The high-risk group 5- year Kaplan-Meier cumulative estimate rate of ACA/SCD of 3% includes subjects with QTc of over≥ 500 ms
-
6.
QTc variations mutations: LQTS patients with a wider variation in QTc duration are associated with increased risk of CEs. These findings appear to be genotype-specific, with a pronounced effect among patients with the LQT1 genotype [27].
-
7.
Vectorcardiographic (VCG) parameters: ECG-derived VCG repolarization parameters have significant diagnostic and prognostic value in patients with LQTS with prolonged QTc values [28]. For SCD risk assessment we use the spatial mean QRS-T angle (degrees), spatial peaks QRS-T angle (degree), QT-peak, Tpeak-Tend, and T-wave Eigenvalues (TwEVs). Symptomatic ecLQTS patients are differentiated from asymptomatic patients by the 4th TEV4. QT peak significantly. The optimum QT-peak cut-off value distinguishing all ecLQTS patients from controls is 364.5 ms with a low sensitivity and high specificity. The 2nd-4th TwEVs significantly differentiated the ecLQTS patients from controls. VCG may facilitate not only pregenetic test anticipation regarding status of family members with normal QTc values but also the selection of ecLQTS patients who may most benefit from prophylactic beta blocker therapy.
-
8.
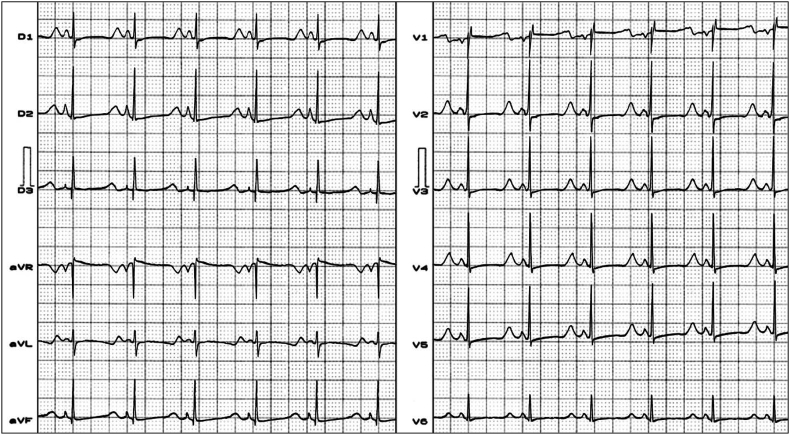
Holter recording: this method adequately quantifies the complexity of ventricular repolarization in the case of LQT3 variant the very prolonged ST segment and delayed (Fig. 4)T wave and may become a useful noninvasive diagnostic tool in LQTS and risk stratification [28]. Continuous Holter monitoring in LQT3 with A1180V cardiac Na+ channel mutation showed prolonged QTc and biphasic T waves, multiple episodes of TdP (Fig. 5) [29].
-
9.
Prior syncope: The high-risk group includes subjects with history of prior syncope.
-
10.
Events lethality: The lethality of CEs was highest in LQT3 males and females (19% and 18%) The lethality of CEs was highest in LQT3 males and females [30].
-
11.
Pregnancy: the pregnancy time is associated with a reduced risk of CEs in LQTS individuals [31].
-
12.
The postpartum period: the 9-month postpartum time had an increased ris. especially among women with the LQT2 genotype. After the 9-month postpartum period, the risk was like the period before the first conception [31]. The cardiac event risk during the high-risk postpartum period was reduced among women using β--blocker therapy.
-
13.
Mutation location: biophysical function, type, and location of the ion-channel mutation.
-
14.
Mutation number: mutations involving two functional defects (i.e., mutations leading to both late Na+ currents and window Na+ currents) had a 2.5- fold increased risk for ACA/SCD as compared with mutations involving only one functional defect.
-
15.
Mutation type: Patients with the KPQ mutation had a 2.4-fold higher risk for CEs from birth through age 40 years compared to patients with the D1790G mutation, and this effect is independent of QTc duration, demonstrating the importance of knowing the specific mutation in risk stratification of LQT3 patients [32].
-
16.
The biophysical function of the mutation: The LQT3-like biophysical phenotype for S1787N depends on both the SCN5A splice variant and on the intracellular pH. The splice variant and environmental factors affect the molecular phenotype of LQT3, with implications for the clinical phenotype, and may provide insight into acidosis-induced arrhythmia mechanisms [33]. The phenotype of LQT3 mutation is dependent on the biophysical alteration induced as well as tissue type. The canonical ΔKPQ mutation causes severe AP prolongation in both tissue types. For LQT3 mutation F1473C, characterized by shifted channel availability, a more severe phenotype is seen in Purkinje fiber cells with AP prolongation and EADs. The LQT3 mutation S1904L demonstrated striking effects on AP duration restitution and more severe AP prolongation in Purkinje fiber cells at higher heart rates [34].
-
17.
Response to β-blockers: β-blocker therapy in LQT3 patients reduces the risk in females; efficacy in males could not be determined conclusively [3]. β-blockers have greater benefit among patients with LQT1 with the greatest benefit among those with cytoplasmic loops mutations. Specific β-blocker agents may provide greater protection than other agents in specific LQTS genotypes.
-
18.
Sodium channel blockers: mexiletine, lidocaine flecainide, and ranolazine are treatment options only for LQT3.
Fig. 4.
ECG example of LQT3. This ECG belongs to a newborn baby with LQT3. Clear ST segment prolongation and delayed appearance of T wave. Affected gene: SCN5A, 3p21-24 mutation in chromosome 3, AP phase: plateau, dome or phase 2 by persistent sodium Na+ inflow (gain-of-function mutations in the SCN5A cardiac Na+ channel gene).
Fig. 5.
Congenital LQT3 QTc: 670 ms Run of TdP after macro wave T alternans. T-wave alternans is a diagnostic feature of the LQT and reflects an enhanced electrical instability during repolarization [21].
7. Treatment
The approach of LQTS has genotype-specific treatment strategies, however most of works are based on QTc [35]. The duration of the QT interval is only a gross estimate of repolarization. Besides, its limited accuracy and reproducibility does not provide information about the T-wave morphology; thus, T shape alterations such as notches, T-wave macro alternans, Tp/Tend, can be only qualitatively described but not objectively quantified.
Triggers and therapeutic management of LQTS arrhythmias have been shown to differ in a manner that depends strikingly on the gene that is mutated. Additionally, β-blockers, effective in the management of LQT1, have been thought to be potentially proarrhythmic in the treatment of LQT3 because of concomitant slowing of heart rate that accompanies decreased adrenergic activity.
7.1. Drugs
β-blocker therapy: these drugs are clinically indicated in all asymptomatic individuals meeting diagnostic criteria, including those who have a pathogenic variant on molecular testing and a normal QTc interval. Although β-blockers are the primary treatment modality for patients with LQTS, these drugs are not completely effective in some patients. CEs continue to occur while patients are taking the prescribed β-blockers, especially in symptomatic patients; 32% of symptomatic patients will have a cardiac event over 5 years, and 14% of patients with a prior SCA will have a recurrence within 5 years [36] are the mainstay of therapy for LQTS, including asymptomatic individuals with prolonged QT intervals and individuals who have a pathogenic variant on molecular testing with a normal QTc interval [37]. Inadequate β-blocker dosing is prevented by regular adjustments in growing children with evaluation of the efficacy of dose by assessment of the exercise ECG or ambulatory ECG. β-blocker propranolol interacts with wild type (WT) and LQT3 mutant Na+ channels in a manner that resembles the actions of local anesthetic drugs. Propranolol blocks Na+ channels in a use-dependent manner; that propranolol efficacy is dependent on the inactivated state of the channel; that propranolol blocks late non-inactivating current more effectively than peak Na+ current; and mutation of the local anesthetic binding site greatly reduces the efficacy of propranolol block of peak and late Na+ channel current. In both the F1473C LQT3 mutation and canonical LQT3 mutation (ΔKPQ), propranolol also shares this important hallmark of local anesthetic (LA) family of drugs due to preferential inhibition of INaL [38]. On the other hand, recent study of Wilde et al. [3] suggest that β-blockers are not effective at all and is clearly in contrast with previous studies.
-
•
β-blockers are less effective clinically in LQT3 than in other LQTS variants [37]. Prolonged QTc and syncope predispose patients with LQT3 to life-threatening CEs. However, β-blocker therapy reduces this risk in females; efficacy in males could not be determined conclusively because of the low number of events [3]. Individuals with LQT3 are most likely to have symptoms at rest. LQT3 is less well treated with β-blocker therapy than other types of LQTS, but β-blocker therapy is still effective in many LQT3 patients, and its use is recommended. LQT3 patients with symptoms may need to take another medication such as mexiletine, flecainide and ranolazine in addition to β-blockers. Na+ channel blockers such as mexiletine, flecainide, ranolazine and the experimental novel late Na+ current inhibitor, GS-6615 (eleclazine) could be treatment options in LQT3. In a large LQT3 population β-blocker therapy was associated with an 83% reduction in cardiovascular events in females but not in males (who had many fewer events), with a significant sex × β-blocker interaction. Each 10-ms increase in QTc duration up to 500 ms was associated with a 19% increase in CEs. Prior syncope doubled the risk for life-threatening events. Prolonged QTc and syncope predispose patients with LQT3 to life-threatening CEs. However, β-blocker therapy reduces this risk in females; efficacy in males could not be determined conclusively because of the low number of events [3]. Preliminary data from the International LQT3 group has shown that β-blockers reduce the risk for CEs in LQT3.
-
•
Some patients may also need an ICD.
-
•
QT-prolonging drugs are not administered to persons with LQT3 without careful consideration of risk versus benefit by the individual(s) and physician(s).
-
•
β-blockers should be given to patients who have QTc-interval prolongation (>460 ms in women and >440 ms in men) and are recommended (class IIa) for patients with a normal QTc interval.
Mexiletine: is a non-selective voltage-gated sodium channel which belongs to the Class IB antiarrhythmic group. Mexiletine blocks the rapid inward Na+ current responsible for phase 0 of cardiac AP. Mexiletine in the mutant Na+ channel the gating is affected which permits abnormal repetitive reopening leading to a sustained ‘‘late’’ inward current and prolonged cardiac AP. Na+ channel blockers can be useful as additional pharmacologic therapy for patients with a QTc interval >500 ms. Mexiletine significantly shortened QTc and reduced the percentage of patients with arrhythmic events (from 22 to 3%), the mean number of arrhythmic events per patient, and the annual rate of arrhythmic events (from 10.3 to 0.7%). Besides shortening QTc interval, mexiletine caused a major reduction of life-threatening arrhythmic events in LQT3 [39]. Ruan et al. provided evidence that mexiletine may facilitate trafficking of mutant proteins, thus producing QT prolongation. These data suggest that caution should be used when recommending this class of drugs to carriers of mutations with undefined electrophysiological properties [40].
Mexiletine infusion test/lidocaine: Funasako et al. analyzed response in ECG parameters measured in II or V5 with i.v. mexiletine infusion (2 mg/kg) during sinus rhythm among 31 genotype-positive LQTS patients. Change in QTc interval after mexiletine was compared between LQT3 LQT1 and LQT2. QTc interval was shortened with mexiletine, degree of QTc shortening (ΔQTc) was significantly larger in LQT3 than in LQT1/LQT2 patients. The sensitivity, specificity and predictive accuracy of mexiletine infusion test for differentiating LQT3 from LQT1/LQT2 were 86.7%, 81.3% and 81.3%, respectively, and the optimal cut-off for ΔQTc was 69 ms on receiver operating characteristic analysis. The authors concluded that pronounced shortening of QT interval with mexiletine may facilitate genetic testing in patients with LQT3variant [41]. In neonates with bradycardia, 2:1 atrioventricular block, prolonged QT interval, and T wave alternans we can begin with IV lidocaine infusion with progressive shortening of the QT interval. This positive lidocaine challenge prompted clinical suspicion of LQT3 and early initiation of mexiletine therapy in cases suspected or confirmed of LQT3 [42]. D1790G (DG), mutation in LQT-3 variant has higher sensitivity to lidocaine EC(50). The combined lidocaine/K+ infusion had a sensitivity, specificity, and accuracy of 88%, 100%, and 94%, respectively, in diagnosing LQTS. When the QTc interval is mildly prolonged. A simplified sequential lidocaine/K+ challenge is accurate in diagnosing LQTS among patients with borderline QTc prolongation. Mexiletine significantly shortened the QT interval in LQT3 patients but not in LQT2 patients. When examined the response to an increase in heart rate, we found that LQT3 patients have a more shortened QT interval in response to heart rate changes than LQT2 patients, and than healthy controls [43].
Flecainide: Low-dose, oral flecainide consistently shortened the QTc interval and normalized the repolarization T-wave pattern in five LQT3 patients with SCN5A:DeltaKPQ mutation. Low-dose flecainide is a promising therapeutic agent for LQTS patients with the SCN5A:DeltaKPQ Na+ channel mutation [44].
Flecainide may induce ST segment elevation in LQT3 patients, raising concerns about the safety of flecainide therapy and demonstrating the existence of an intriguing overlap between LQT3 and BrS [45]. In a Danish family with LQTS, a novel missense mutation in SCN5A, 1786 (L1786Q), was found to be present in heterozygous form co-segregating with long QT interval. Oral Flecainide treatment showed type 1 Brugada ECG pattern in all mutation carriers. Electrophysiological investigations of the mutant in HEK293 cells indicated a reduced peak current, a negative shift in inactivation properties and a positive shift in activation properties, compatible with BrS. Furthermore, the sustained INaL tetrodotoxin-sensitive Na+ current was found to be drastically increased, explaining the association between the mutation and LQTS. The L1786Q mutation is associated with overlapping syndrome LQT3 and concealed BrS phenotype explained by gating characteristics of the mutated ion channel protein. Hence, Na+ channel blockade should be considered in clinical evaluation of apparent LQT3 patients.
Note: functional heterogeneity accounts for the variable response of mutations to the administration of Na+ channel blockers and explains why not all LQT3 patients benefit from treatment with these drugs. SCN5A mutation (D1790G) significantly responded to flecainide therapy yet did not respond to lidocaine.
Ranolazine (Ranexa®): Ranolazine is a piperazine derivative clinically approved for treatment of angina pectoris and is a potential candidate for antiarrhythmic, antiepileptic, and analgesic applications. The drug inhibits persistent or late inward Na+ current (INa) in heart muscle in a variety of voltage-gated Na+ channels. Inhibiting that current leads to reductions in elevated intracellular calcium levels. The drug exerted a concentration-dependent block of INaL of the SCN5A-D1790G channel without reducing peak INa significantly. In the clinical study, among 8 patients with LQT3 and confirmed D1790G mutation, ranolazine had no effects on the sinus rate or QRS width but shortened the QTc from 509 ± 41 to 451 ± 26 ms, a mean decrease of 56 ± 52 ms (10.6%; P = 0.012). The QT-shortening effect of ranolazine remained effective throughout the entire study period of 22.8 ± 12.8 months. Ranolazine reduced the QTc at all heart rates but less so during extreme nocturnal bradycardia. The novel antianginal agent ranolazine, which shows a marked selectivity for late versus peak Na+ current, may represent a novel drug archetype for targeted reduction of I(NaL). Ranolazine at therapeutic concentrations shortened a prolonged QTc interval and improved diastolic relaxation in patients with the LQT3-Delta KPQ mutation, a genetic disorder that is known to cause gain-of-function mutations in the SCN5A in late Na+ current. LQT3 patient carried a mutation in the SCN5A gene in which the cysteine was substituted for a highly-conserved tyrosine (Y1767C) located near the cytoplasmic entrance of the Na(v)1.5 channel pore is blocked by ranolazine but not by many class I antiarrhythmic drugs [46].
8. Experimental drugs
The novel late Na+ current inhibitor, 4 GS-6615 (eleclazine): The novel, potent phase 2 agent with demonstrated preclinical anti-ischemic and antiarrhythmic properties and selective late Na+ current inhibitor, GS-6615 with potential to use for the treatment of LQT3 hypertrophic cardiomyopathy (HCM), and ventricular tachycardia-ventricular fibrillation (VT-VF) [47]. This drug shortens the ventricular APD, monophasic APD (MAPD) and QT/QTc intervals, and decrease to the incidence of PVT/TdP type. To mimic the electrical phenotype of LQT3, late INa was increased using the sea anemone toxin (ATX-II). This drug induced prolongation of APD, MAPD, QT interval, and decreased spatiotemporal dispersion of repolarization and ventricular arrhythmias. Inhibition by GS-6615 of ATX-II enhanced late INa+ was strongly correlated with shortening of myocyte APD and isolated heart MAPD. In contrast to flecainide, GS-6615 had the minimal effects on peak INa. GS-6615 did not decrease the maximal upstroke velocity of the AP in phase 0 (Vmax) nor widen QRS intervals. GS-6615 is a selective inhibitor of late INa+, stabilizes the ventricular repolarization and suppresses arrhythmias in a model of LQT3. The concentrations at which the electrophysiological effects of GS-6615 are observed are comparable to plasma levels associated with QTc shortening in patients with LQT3, indicating that these effects are clinically relevant. Additionally, eleclazine, a new selective cardiac late Na+ current inhibitor, confers concurrent protection against autonomically induced atrial premature beats, T-wave repolarization alternans and heterogeneity, and AF in an intact porcine model.
Triazolopyridine 4 h GS-458967: a recently introduced Gilead Sciences compound, is a selective late INa inhibitor with an IC50 of 200 nM. 4 h improved anti-arrhythmic activity relative to ranolazine. Compound 4 h represents initial foray into a 2nd generation Late INa inhibitor program that improved efficacy and potency relative to ranolazine and is an important proof-of-concept compound. The 4 h compound was shown to cause modest abbreviation of APD and to prevent or abolish both ATX-II and E-4031-induced (i.e., models of LQT3 and LQT2, respectively) VTss in rabbit hearts.
4 h GS-458967 abolish EADs and EAD-induced triggered activity elicited by exposure of canine Purkinje fibers to ATX-II, increased extracellular calcium, and isoproterenol. GS-458967 may be useful to confirm the pathologic roles of late INa and to investigate physiologic and pathologic effects of inhibiting late INa in other excitable tissues.
8.1. Video-assisted thoracoscopic left cardiac sympathetic denervation (LCSD)
LCSD is recommended for high-risk patients with LQTS in whom ICD therapy is refused or contraindicated and/or in whom β-blockers are either not effective, not tolerated, not accepted, or severe asthma, orthostatic hypotension, depression, and diabetes mellitus because these entities may be exacerbated by treatment with β-blockers. LCSD can be useful in individuals who experience events while on therapy with β-blockers or ICD. Despite significant morbidity (dryness on left side, harlequin-type unilateral facial flush, contralateral hyperhidrosis, differential hand temperatures, permanent and transient ptosis, thermoregulation difficulties, sensation of left arm paresthesia and sympathetic flight/fright response loss) resulting from LCSD, patients with LQTS and CPVT have high levels of postoperative satisfaction. Additionally, the recently introduced procedure video-assisted thoracoscopic LCSD is associated with short hospital stays and low morbidity. Indications for LCSD included β-blocker intolerance, nonadherence, symptoms on therapy, QTc>520 ms, competitive sports participation, family history of SCD. LCSD may reduce the wide gap between life-long β-blocker medication and ICD implantation. Although LCSD is highly effective in prevention of CEs in patients with LQTS and CPVT, it is rarely used.
8.2. Implantable cardioverter-defibrillator (ICD) placement
The international guidelines are not always followed, and risk stratification may be based on genotype rather than individual risk profile. In the Swedish ICD & Pacemaker Registry 70% of patients who received ICD treatment met the 2006 Class I or Class IIa recommendations for LQTS treatment. 31% of the LQT3 patients received ICD treatment despite being asymptomatic. Recommendations by the American College of Cardiology, American Heart Association, and Heart Rhythm Society for ICD placement in patients with LQTS include:
-
1)
Survival of a cardiac arrest (class I)
-
2)
Patients with syncope despite β-blocker therapy (class IIa; ICD is reasonable)
-
3)
High-risk categorization, such as LQT3 (class IIb; ICD may be considered): individuals with a QTc interval >500 ms; individuals with QTc interval >600 ms are at extremely high risk. Overt T-wave alternans with short-long cardiac sequences, especially when present despite proper β-blocker therapy, is also associated with a higher risk of CEs; LQTS associated with biallelic pathogenic variants or heterozygosity for pathogenic variants in two different genes (i.e., digenic pathogenic variants) is generally associated with a more severe phenotype with longer QTc interval and a higher incidence of CEs.
-
4)
Possible ICD and/or LCSD for those with β-blocker-resistant symptoms, inability to take β blockers, severe asthma. In general, ICD implantation is not indicated for individuals with LQTS who are asymptomatic and who have not been tried on β-blocker therapy.
-
5)
Prophylactic ICD therapy can be considered for individuals with LQTS who are asymptomatic but suspected to be at very high risk (e.g., those with ≥2 pathogenic variants on molecular testing). Appropriate ICD therapies were predicted by age <20 years at implantation, a QTc >500 ms, prior CA, and CEs despite therapy; within 7 years, appropriate shocks occurred in no patients with none of these factors and in 70% of those with all factors. Refined criteria for implantation, reassessment of pros and cons, ICD reprogramming, and consideration for other existing therapeutic options are necessary [48].
-
6)
Schwartz et al. [48] showed that the majority of adverse effects related to ICD implant occurred in LQT3 patients.
Catheter ablation was the effective method to eliminate the fatal arrhythmias through ablation of the triggering PVCs in the present LQT3 patient with CDI implanted.
8.3. Pacemakers/pacing
Patients with LQT3 are at higher risk at lower heart rates and potentially may benefit from pace maker therapy. LQT3 patients may be more likely to benefit from Na+ channel blockers and from cardiac pacing because they would be at higher risk of arrhythmia at slow heart rates. The usefulness of implanted cardiac pacemakers is based on the premise that pacing eliminates arrhythmogenic bradycardia, decreases heart-rate irregularities (eliminating short-long-short sequences), and decreases repolarization heterogeneity, diminishing the risk of TdP. Pacemakers are particularly helpful in patients with documented pause-bradycardia–induced TdP and in patients with LQT3.
However, data indicate that CEs continue to occur in high-risk patients with cardiac pacing. Because newer models of ICDs include a cardiac pacing function, cardiac pacing (without defibrillators) is unlikely to be used in patients with LQTS. Pacing alone may be used in low-risk patients with LQT3. Cardiac pacemakers may be beneficial for patients with LQT2 or LQT3 and for those with pause-dependent TdP. More important is to recognize that device programming for preventing tachyarrhythmias in patients with long QT differs from the standard pacemaker programming. Prolongation of repolarization also acts as a primary step for the generation of EADs. The focal EAD induced triggered beat(s) can infringe on the underlying substrate of inhomogeneous repolarization to initiate polymorphic reentrant VT, sometimes having the characteristic twisting QRS configuration known as TdP. Both pacing and treatment with mexiletine may reduce TdP in patients with LQT3, but it is not fully understood how these interventions could prevent TdP. Bradycardia, increased dispersion of APD and EADs provoke ventricular ectopy and PVT in deletion SCN5A-Tg hearts. Ventricular pacing reduces APD dispersion, suppresses EADs and prevents PVT in deletion SCN5A-Tg hearts. These effects provide a pathophysiological rationale for pacing in LQT3.
Management of Athletes with LQTS: Prohibition of competitive exercise is considered a strict contraindication to athletic participation. Detection and management of LQTS in the athletes is crucial given the possibility of adverse outcomes with the physical stress. Preparticipation screening examinations should include a thorough clinical and family history, screening ECGs may display key findings consistent with LQTS and genetic testing (can confirm the diagnosis). Additionally, avoidance of QT-prolonging drugs are important issues in life-style modification. All patients with LQTS should avoid drugs that prolong the QT interval or that reduce their serum potassium or magnesium level. Potassium and magnesium deficiency should be corrected. Drugs that cause further prolongation of the QT interval or provoke TdP; competitive sports/activities associated with intense physical activity and/or emotional stress for most individuals.
Patients who have history of LQTS-related symptoms, have QTc >470 ms (males) or 480 (females) or who have an ICD should be limited to Class 1A sports. Asymptomatic genotype-positive/phenotype-negative patents can play, but are not at zero-risk.
Screening for a family history of premature (<40 years) cardiovascular disease and SCD should occur at a young age, because most SCDs in young athletes are caused by inherited cardiac disease.
Unexplained syncope/near-syncope judged not to be of neurocardiogenic (vasovagal) origin; of particular concern when occurring during or after physical exertion.
Premature death (sudden and unexpected or otherwise) before 50 y of age attributable to heart disease in 1 relative.
All patients who have LQTS, symptomatic or asymptomatic, are disqualified from all competitive sports.
Recommended using QTc values of >440 ms (males) or > 460 ms (females) as a trigger for further evaluation. Schnell et al. considered a corrected QT interval ≥500 ms as dangerous [49].
Johnson and Ackerman [50] presented the largest published cohort of athletes with LQTS (130 athletes with LQTS) who have chosen to remain engaged in competitive sports and observed only one athlete experiencing CEs. Guidelines for competitive athletic participation, particularly those disqualifying genotype-positive/phenotype-negative patients (the European Society of Cardiology guidelines), may be excessive for LQTS patients. Comprehensive evaluation, counselling, risk stratification, medical management and preparation must take place prior to allowing. Table 2 summarizes the LQT3 variant characteristics.
Table 2.
Summary of LQT3 variant characteristics.
| Effect of the mutation in the SCN5A cardiac Na+ channel gene | Gain-of-function mutations in the SCN5A cardiac Na+ channel gene which mediates the fast Nav1.5 current during AP initiation and also late in phase 2 of AP causing an accelerated recovery from inactivation of Na+ currents as well as AP prolongation, especially at low stimulation rates |
|---|---|
| Chromosome affected locus | 3 (3p21-24) |
| Inheritance | Autosomal dominant. Very rarely recessive |
| OMIM number | (OMIM#600163) A number sign (#) is used with this entry because LQT3 is caused by mutation in the gene encoding the α-polypeptide of voltage-gated Na+ channel type V. |
| Gene/Locus MIM number | 600163 |
| Relative frequency to other variants | 7-10% of total |
| Phenotype mapping key | 3 |
| Mean penetrance | 79% |
| Events | 46% |
| Event numbers | Fewer than LQT1 and LQT2 |
| Lethality of events | Highest in LQT3 males and females (19% and 18%) than LQT2/LQT3 |
| Life-threatening events at perinatal periods | Mostly those with LQT2, LQT3, or no known mutations |
| Trigger mechanism for arrhythmia | Early After Depolarizations (EADs) |
| Gene affected | SCN5A which codes for the Nav1.5 Na+ channel α -subunit |
| Predominant moment of the events | ≈65 at rest or during sleep without arousal |
| Triggers characteristics | Bradycardia-triggered arrhythmias. Excessive prolongation of the AP and QTc at low heart rates |
| Heart rate (HR) | Tendency to bradycardia related to age and in some cases. Highest risk of arrhythmia during sleep or during periods of slow HR. |
| Response to HR increase | The QTc interval shortens more than in LQT1 and LQT2 variants. |
| QTc mean value | 478 ± 52 ms |
| QT interval dependence to HR | Significant |
| QTc interval duration | It is usually longer than in LQT1 and LQT2. |
| QT dispersion | Accentuated. It is a risk marker for the appearance of arrhythmias |
| LQT3 gene carriers | have repolarization patterns similar to those of LQT1 gene carriers |
| U wave | It could be prominent in many cases because of longer repolarization of the M cell. It increases in bradycardias and in pauses and it may present alternating polarity. |
| Gene-specific therapy for LQT3 with Na+ channel blocking agents Class Ib antiarrhythmic lidocaine/mexiletine and RSD1235 |
In severe forms of LQT3 (Missense mutation, M1766L) in infants, mexiletine partially 'rescued' the defective expression. Higher sensitivity to lidocaine. |
| Gene-specific therapy for LQT3 with Na+ channel blocking agents of Class IC (flecainide) and IB (mexiletine and lidocaine) | SCN5A mutation (D1790G) significantly responded to flecainide therapy yet did not respond to lidocaine. |
| Ranolazine | May provide protection from the induction of TdP by inhibition of a gain of function in the cardiac voltage-gated Na+ |
| High risk patient for events (CA or SCD) In these patients, ICD is indicated. |
QTc interval ≥500 ms in patients receiving therapy Macro-wave T alternans, mainly when present despite proper β-blocker therapy Patient who had CA Biallelic pathogenic variants Heterozygosity for pathogenic variants |
Abnormal QT dynamics during exercise testing are observed in both LQT3 patients and other LQTS subtypes. Exercise stress testing may be useful for directing genetic testing in subjects with borderline prolonged QT intervals.
9. Conclusion
Congenital LQT3 variant of LQTS is responsible of 7–10% of all cases of LQTS. It is caused by SCN5A gene defect. LQT3 variant has high propensity for the TdP, as well as for atrial fibrillation. LQT3 is the third most common LQTS with 7–10% of all cases. The variant has a genotype-specific risk stratification and management with efficacy of class IB (mexiletine/lidocaine) and IC (flecainide) antiarrhythmic and the specific inhibitor of late sodium channel late I(Na) during the phase 2 of AP ranolazine. An ICD should be used in survivors of SCA/SCD and is recommended (class IIa) for patients with syncope while receiving β-blockers; ICD therapy can be considered (class IIb) for primary prevention in patients with characteristics that suggest high risk such as QTc interval >500 ms T-wave alternans, especially when present despite proper β-blocker therapy or patients with biallelic pathogenic variants or heterozygosity for pathogenic variants in two different genes. The international guidelines are not always followed, and risk stratification may be based on genotype rather than individual risk profile. Are considered high at risk for SCA or SCD: QTc ≥500 ms in patients receiving therapy, even after excluding patients who had a cardiac arrest before therapy; presence of macro-wave T alternans especially when present despite proper β-blocker therapy, biallelic pathogenic variants or heterozygosity for pathogenic variants in two different genes (i.e., digenic pathogenic variants). These groups have indication for ICD implantation.
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Refsgaard L., Holst A.G., Sadjadieh G., Haunso S., Nielsen J.B., Olesen M.S. High prevalence of genetic variants previously associated with LQT syndrome in new exome data. Eur J Hum Genet. 2012;20:905–908. doi: 10.1038/ejhg.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent G.M. The molecular genetics of the long QT syndrome: genes causing fainting and sudden death. Annu Rev Med. 1998;49:263–274. doi: 10.1146/annurev.med.49.1.263. [DOI] [PubMed] [Google Scholar]
- 3.Wilde A.A., Moss A.J., Kaufman E.S., Shimizu W., Peterson D.R., Benhorin J. Clinical aspects of type 3 long-QT syndrome: an international multicenter study. Circulation. 2016;134:872–882. doi: 10.1161/CIRCULATIONAHA.116.021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss A.J., Goldenberg I. Importance of knowing the genotype and the specific mutation when managing patients with long QT syndrome. Circ Arrhythm Electrophysiol. 2008;1:213–226. doi: 10.1161/CIRCEP.108.796599. discussion 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy L.L., Moon-Grady A.J., Cuneo B.F., Wakai R.T., Yu S., Kunic J.D. Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia. Heart Rhythm. 2012;9:590–597. doi: 10.1016/j.hrthm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg I., Horr S., Moss A.J., Lopes C.M., Barsheshet A., McNitt S. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011;57:51–59. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez D., Bos J.M., Ackerman M.J. Vectorcardiography identifies patients with electrocardiographically concealed long QT syndrome. Heart Rhythm. 2017;14:894–899. doi: 10.1016/j.hrthm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Malan D., Zhang M., Stallmeyer B., Muller J., Fleischmann B.K., Schulze-Bahr E. Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res Cardiol. 2016;111:14. doi: 10.1007/s00395-016-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer V., Roman-Campos D., Sampson K.J., Kang G., Fishman G.I., Kass R.S. Purkinje cells as sources of arrhythmias in long QT syndrome type 3. Sci Rep. 2015;5:13287. doi: 10.1038/srep13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y., Liu W., Li C., Qiu X., Qin X., Guo B. Common genotypes of long QT syndrome in China and the role of ECG prediction. Cardiology. 2016;133:73–78. doi: 10.1159/000440608. [DOI] [PubMed] [Google Scholar]
- 11.Veldkamp M.W., Wilders R., Baartscheer A., Zegers J.G., Bezzina C.R., Wilde A.A. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res. 2003;92:976–983. doi: 10.1161/01.RES.0000069689.09869.A8. [DOI] [PubMed] [Google Scholar]
- 12.Molnar J., Zhang F., Weiss J., Ehlert F.A., Rosenthal J.E. Diurnal pattern of QTc interval: how long is prolonged? possible relation to circadian triggers of cardiovascular events. J Am Coll Cardiol. 1996;27:76–83. doi: 10.1016/0735-1097(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 13.Horne A.J., Eldstrom J., Sanatani S., Fedida D. A novel mechanism for LQT3 with 2:1 block: a pore-lining mutation in Nav1.5 significantly affects voltage-dependence of activation. Heart Rhythm. 2011;8:770–777. doi: 10.1016/j.hrthm.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Landstrom A.P., Boczek N.J., Ye D., Miyake C.Y., De la Uz C.M., Allen H.D. Novel long QT syndrome-associated missense mutation, L762F, in CACNA1C-encoded L-type calcium channel imparts a slower inactivation tau and increased sustained and window current. Int J Cardiol. 2016;220:290–298. doi: 10.1016/j.ijcard.2016.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H., Li Z., Ali Raza G., Zhu W., Zhou Q., Shen Y. High incidence of sudden cardiac death in one family with type-3 long QT syndrome: molecular genetics and electrophysiology mechanism analysis. Zhonghua Xin Xue Guan Bing Za Zhi. 2015;43:1046–1050. [PubMed] [Google Scholar]
- 16.Leenhardt A., Glaser E., Burguera M., Nurnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of torsade de pointes. a new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg S.J., Scheinman M.M., Dullet N.K., Finkbeiner W.E., Griffin J.C., Eldar M. Sudden cardiac death and polymorphous ventricular tachycardia in patients with normal QT intervals and normal systolic cardiac function. Am J Cardiol. 1995;75:687–692. doi: 10.1016/s0002-9149(99)80654-7. [DOI] [PubMed] [Google Scholar]
- 18.Haugaa K.H., Johnson J.N., Bos J.M., Phillips B.L., Eidem B.W., Ackerman M.J. Subclinical cardiomyopathy and long QT syndrome: an echocardiographic observation. Congenit Heart Dis. 2013;8:352–359. doi: 10.1111/chd.12011. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M., Kohno T., Aizawa Y., Inohara T., Shiraishi Y., Katsumata Y. A novel SCN5A mutation found in a familial case of long QT syndrome complicated by severe left ventricular dysfunction. Can J Cardiol. 2017;33:554. doi: 10.1016/j.cjca.2016.10.010. e555-554 e557. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K., Nabeshima T., Nakayashiro M., Ganaha H. QT Dynamics during exercise in asymptomatic children with long QT syndrome type 3. Pediatr Cardiol. 2016;37:860–867. doi: 10.1007/s00246-016-1360-4. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz P.J., Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 22.Zareba W., Moss A.J., Schwartz P.J., Vincent G.M., Robinson J.L., Priori S.G. Influence of the genotype on the clinical course of the long-QT syndrome. international long-QT syndrome registry research group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg I., Moss A.J., Bradley J., Polonsky S., Peterson D.R., McNitt S. Long-QT syndrome after age 40. Circulation. 2008;117:2192–2201. doi: 10.1161/CIRCULATIONAHA.107.729368. [DOI] [PubMed] [Google Scholar]
- 24.Jons C., Moss A.J., Goldenberg I., Liu J., McNitt S., Zareba W. Risk of fatal arrhythmic events in long QT syndrome patients after syncope. J Am Coll Cardiol. 2010;55:783–788. doi: 10.1016/j.jacc.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Rosero S.Z., Zareba W., Moss A.J., Robinson J.L., Hajj Ali R.H., Locati E.H. Asthma and the risk of cardiac events in thelong QT syndrome. long QT syndrome investigative group. Am J Cardiol. 1999;84:1406–1411. doi: 10.1016/s0002-9149(99)00586-x. [DOI] [PubMed] [Google Scholar]
- 26.Beaufort-Krol G.C., van den Berg M.P., Wilde A.A., van Tintelen J.P., Viersma J.W., Bezzina C.R. Developmental aspects of long QT syndrome type 3 and Brugada syndrome on the basis of a single SCN5A mutation in childhood. J Am Coll Cardiol. 2005;46:331–337. doi: 10.1016/j.jacc.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 27.Mathias A., Moss A.J., Lopes C.M., Barsheshet A., McNitt S., Zareba W. Prognostic implications of mutation-specific QTc standard deviation in congenital long QT syndrome. Heart Rhythm. 2013;10:720–725. doi: 10.1016/j.hrthm.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Priori S.G., Mortara D.W., Napolitano C., Diehl L., Paganini V., Cantu F. Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome. Circulation. 1997;96:3006–3012. doi: 10.1161/01.cir.96.9.3006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Wang J., Chang S., Zhou N., Xing H., Wang L. The SCN5A mutation A1180V is associated with electrocardiographic features of LQT3. Pediatr Cardiol. 2014;35:295–300. doi: 10.1007/s00246-013-0773-6. [DOI] [PubMed] [Google Scholar]
- 30.Zareba W., Moss A.J., Daubert J.P., Hall W.J., Robinson J.L., Andrews M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol. 2003;14:337–341. doi: 10.1046/j.1540-8167.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 31.Seth R., Moss A.J., McNitt S., Zareba W., Andrews M.L., Qi M. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–1098. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Liu J.F., Moss A.J., Jons C., Benhorin J., Schwartz P.J., Spazzolini C. Mutation-specific risk in two genetic forms of type 3 long QT syndrome. Am J Cardiol. 2010;105:210–213. doi: 10.1016/j.amjcard.2009.08.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu R.M., Tan B.H., Tester D.J., Song C., He Y., Dovat S. Arrhythmogenic biophysical phenotype for SCN5A mutation S1787N depends upon splice variant background and intracellular acidosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer V., Sampson K.J., Kass R.S. Modeling tissue- and mutation- specific electrophysiological effects in the long QT syndrome: role of the Purkinje fiber. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz P.J., Ackerman M.J. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34:3109–3116. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 36.Moss A.J., Zareba W., Hall W.J., Schwartz P.J., Crampton R.S., Benhorin J. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 37.Priori S.G., Napolitano C., Schwartz P.J., Grillo M., Bloise R., Ronchetti E. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 38.Bankston J.R., Kass R.S. Molecular determinants of local anesthetic action of beta-blocking drugs: implications for therapeutic management of long QT syndrome variant 3. J Mol Cell Cardiol. 2010;48:246–253. doi: 10.1016/j.yjmcc.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzanti A., Maragna R., Faragli A., Monteforte N., Bloise R., Memmi M. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome type 3. J Am Coll Cardiol. 2016;67:1053–1058. doi: 10.1016/j.jacc.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan Y., Denegri M., Liu N., Bachetti T., Seregni M., Morotti S. Trafficking defects and gating abnormalities of a novel SCN5A mutation question gene-specific therapy in long QT syndrome type 3. Circ Res. 2010;106:1374–1383. doi: 10.1161/CIRCRESAHA.110.218891. [DOI] [PubMed] [Google Scholar]
- 41.Funasako M., Aiba T., Ishibashi K., Nakajima I., Miyamoto K., Inoue Y. Pronounced shortening of QT interval with mexiletine infusion test in patients with type 3 congenital long QT syndrome. Circ J. 2016;80:340–345. doi: 10.1253/circj.CJ-15-0984. [DOI] [PubMed] [Google Scholar]
- 42.Howley L.W., M D.I.M., Bailey A., Schaffer M.S. Neonatal long QT syndrome type 3 predicted by positive lidocaine challenge. Pacing Clin Electrophysiol. 2010;33:377–379. doi: 10.1111/j.1540-8159.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 43.Priori S.G., Cantu F., Schwartz P.J. The long QT syndrome: new diagnostic and therapeutic approach in the era of molecular biology. Schweiz Med Wochenschr. 1996;126:1727–1731. [PubMed] [Google Scholar]
- 44.Windle J.R., Geletka R.C., Moss A.J., Zareba W., Atkins D.L. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A: deltaKPQ mutation. Ann Noninvasive Electrocardiol. 2001;6:153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priori S.G., Napolitano C., Schwartz P.J., Bloise R., Crotti L., Ronchetti E. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation. 2000;102:945–947. doi: 10.1161/01.cir.102.9.945. [DOI] [PubMed] [Google Scholar]
- 46.Huang H., Priori S.G., Napolitano C., O'Leary M.E., Chahine M. Y1767C, a novel SCN5A mutation, induces a persistent Na+ current and potentiates ranolazine inhibition of Nav1.5 channels. Am J Physiol Heart Circ Physiol. 2011;300:H288–H299. doi: 10.1152/ajpheart.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zablocki J.A., Elzein E., Li X., Koltun D.O., Parkhill E.Q., Kobayashi T. Discovery of Dihydrobenzoxazepinone (GS-6615) late sodium current inhibitor (Late INai), a phase II agent with demonstrated preclinical anti-ischemic and antiarrhythmic properties. J Med Chem. 2016;59:9005–9017. doi: 10.1021/acs.jmedchem.6b00939. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz P.J., Spazzolini C., Priori S.G., Crotti L., Vicentini A., Landolina M. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT syndrome Implantable cardioverter-defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 49.Schnell F., Riding N., O'Hanlon R., Axel Lentz P., Donal E., Kervio G. Recognition and significance of pathological T-wave inversions in athletes. Circulation. 2015;131:165–173. doi: 10.1161/CIRCULATIONAHA.114.011038. [DOI] [PubMed] [Google Scholar]
- 50.Johnson J.N., Ackerman M.J. Return to play? athletes with congenital long QT syndrome. Br J Sports Med. 2013;47:28–33. doi: 10.1136/bjsports-2012-091751. [DOI] [PubMed] [Google Scholar]