Abstract
Background:
Long-term healing tissue engineering scaffolds must hold its full mechanical strength at least for 12 weeks. Nano-micro scaffolds consist of electrospinning nanofibers and textile microfibers to support cell behavior and mechanical strength, respectively.
Methods:
The new nano-micro hybrid scaffold was fabricated by electrospinning poly 3-hydroxybutyrate-chitosan-multi-walled carbon nanotube (MWNT functionalized by COOH) solution on knitted silk in a random manner with different amounts of MWNT. The physical, mechanical, and biodegradation properties were assessed through scanning electron microscopy, Fourier-transform infrared (FTIR) spectroscopy, water contact angle test, tensile strength test, and weight loss test. The scaffold without MWNT was chosen as control sample.
Results:
An increase in the amount of MWNT up to 1 wt% leads to better fiber diameter distribution, more hydrophilicity, biodegradation rate, and higher tensile strength in comparison with other samples. The porosity percentage of all scaffolds is more than 80%. According to FTIR spectra, the nanofibrous coat on knitted silk did not have any effect on silk fibroin crystallinity structures, and according to tensile strength test, the coat had a significant effect on tensile strength in comparison with pure knitted silk (P ≤ 0.05). The average fiber diameter decreased due to an increase in electrical conductivity of the solution and fiber stretch in electrical field due to MWNTs. The scaffold containing 1 wt% MWNT was more hydrophilic due to the presence of many COOH groups of functionalized MWNT, thus an increase in the hydrolysis and degradation rate of this sample.
Conclusions:
High intrinsic tensile strength of MWNTs and improvement of nano-micro interface connection lead to an increase in tensile strength in scaffolds containing MWNT.
Keywords: Carbon nanotube, knitted silk, long-term healing tissue engineering, nano-micro scaffold
Introduction
Compact connective tissue consists of collagen I and performs movement and stability of tissues. Tissues such as tendon, ligament and articular cartilage are connective tissues which their healing process are slowly due to the lack in blood vessels and neurons.[1] Tissue engineering is a promising manner which can regenerate and heal the damaged tissues by replacement it with engineered tissue.[2] This process is combination of material science and cell technology.[3] First step in tissue engineering is to design a biodegradable scaffold which could simulate bioenvironmental conditions. The function of scaffold in tissue engineering is to simulate extracellular matrix (ECM) of natural tissue and be a biomimetic of it, so the scaffold should provide an appropriate environment for cell survival and regulate cell behavior and function.[2] An ideal scaffold should have a 3-dimensional porous structure with interconnected porosity, biocompatibility, biodegradability, and harmonic degradation rate with growing new tissue and also must have controllable mechanical properties during degradation process inside the body.[4] ECM consists of regular nanostructure which surrounds cells inside the natural tissues. The usage of nanofibrous scaffolds provides an appropriate environment for cell attachment and proliferation; in addition, nanofibers have high aspect ratio as well as appropriate biomimetic of ECM architecture.[5] Electrospinning is one of the most efficient technics to fabricate nanofibrous scaffolds, because of its facility of usage, 3-dimensional and porous architectural formation. This procedure consists of high-voltage power supply, collector, and syringe dosage pump. The high-voltage power supply has two electrodes that provide an electromagnetic field between the solution and the collector. In this state, the fluid is became charged and stretched from the tip of needle to collector sheet.[6,7]
Bioresorbable polymers are appropriate choice in tissue engineering scaffold production. Synthesis polymers provide good mechanical properties whereas they could not have good cell interaction because of their hydrophobicity properties.[8] Alloying and coating are procedures to enhance polymer properties.[8] Poly 3-hydroxybutyrate (P3HB) is a synthesis thermoplastic polyester that its source is microorganisms. It is a biodegradable, biocompatible, nontoxic, and hydrophobia polymer with high crystallinity degree and slow degradation rate which provide appropriate mechanical properties.[4,9] The use of natural polymers can present high hydrophilicity properties. Chitosan is a biodegradable biopolymer with high degradable rate, biocompatible, available, and cost-effective, but it does not have adequate mechanical properties; thus, usage of chitosan in combination with P3HB is useful.[9,10] In a study, P3HB/chitosan scaffold is prepared by electrospun for cartilage tissue engineering applications; the presence of chitosan in the composition increased degradation rate and hydrophilicity properties versus pure P3HB.[11] According to other study, P3HB plus chitosan polymeric composition provides inadequate mechanical properties in long-term healing tissues; therefore, the use of a ceramic reinforcement can increase the strength of the composition. The use of nanomaterial in preparing scaffolds can mimic the nanostructure of ECM. Nowadays, functionalized carbon nanotubes (CNTs) in two form single-walled carbon nanotube and multi-walled carbon nanotube (MWNT) are used as biomaterial to enhance the features and function of tissue engineering scaffolds. CNTs have high length-to-diameter ratio and surface-to-volume ratio, low density, high mechanical strength, high flexibility, and electrical and thermal conductivity.[12] Functionalization of CNTs leads to increase biocompatibility, hydrophilicity, uniform distribution, and its desirable connection with polymeric matrix.[13] These features cause that it could be applicable as fiber reinforcement in low-density composite materials such as polymeric-based composites.[14] In another study about P3HB-chitosan/MWNT scaffold for cartilage tissue engineering, the scaffold which included more MWNT content had lower degradation rate and possessed higher mechanical strength than pure P3HB and P3HB/chitosan scaffolds which is appropriate for cartilage tissue engineering.[15] According to the studies, regeneration of the long-term healing tissues last for 6 months, so nanofibrous scaffolds in this field of tissue engineering should preserve initial strength for at least 3 months and show half of that after 6 months.[2] Nanofibers could just mimic tissue surface properties (surface tension, topography, etc.) and improve cell behavior.[16] Texture scaffolds have similar mechanical behavior with connective tissues such as tendon, ligament, and cartilage. Texture scaffolds are developing for long-term healing tissue engineering including woven, wire rope, and knitted.[1] Texture microfibers could provide adequate mechanical strength until the tissue heals.[2] Recently, the use of silk as microfibers in texture scaffolds is attended. Silk is an organic biomaterial that has a fibroin core surrounded with a gummy protein called sericin. Semi-crystalline structure of fibroin has high strength and toughness as well as flexibility. Fibroin is attended also because of its biocompatibility, hydrophilicity, and slow degradation rate. Sericin next to the fibroin shows toxic and adverse features. A process to eliminate sericin from silk called degumming is an important section to produce silky texture scaffolds.[1] Nano-micro hybrid scaffolds that consist of a texture microfiber substrate coated with electrospun nanofibers in a two-step procedure cause optimum mechanical properties due to microfibers as well as desire cell response due to nanofibers.[1] In a study that conducted by Tuzlakoglu et al., on the starch-based hybrid scaffold with poly ɛ-caprolactone (PCL), electrospun nanofibers have been shown that nanofibers are look like nanobridges between microfibers which are very similar to architecture of ECM.[16] Sahoo et al. reported that electrospinning poly (lactic-co-glycolic) (PLGA) on knitted PLGA microfibers causes good mechanical strength and integration; moreover, the presence of nanofibers shows good cell behavior.[17] In another study conducted by the same group, about PLGA electrospun nanofibers on knitted silky microfibers reported that the presence of silk causes lower degradation rate and higher mechanical strength during regeneration of long-term healing tissues.[2] In a study, about P3HB or PCL electrospun nanofibers on a silky knitted microfiber substrate concluded that hybrid scaffolds provide adequate mechanical strength.[1] Creation, a hybrid scaffold from P3HB-chitosan electrospun nanofibers on knitted silk microfibers, showed higher mechanical strength than P3HB/silk hybrid scaffold and pure knitted silk texture; also, cell studies on chondrocytes for the hybrid scaffold present the highest growth, attachment, and proliferation of cells in comparison with control samples.[18]
The use of knitted silky texture and presence of MWNT in P3HB-chitosan-MWNT/silk nano-micro hybrid scaffold are a novel method in this study to increase the mechanical properties and improve mechanical support in long-term healing tissue engineering in the field of nano-micro scaffolds production. In this study, physical and mechanical properties of this novel nano-micro hybrid scaffold were investigated by scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), contact angle, and tensile strength test.
Materials and Methods
Materials
P3HB (Mw = 300,000 g/mol) and chitosan (Mw = 1526.454 g/mol, deacetylation degree = 75%–85%) were purchased from Sigma-Aldrich (USA). MWNTs (inner diameter = 3–5 nm, outer diameter = 5–15 nm, length = 50 μm, and purity percent = 95%) functionalized by carboxyl group (COOH) were purchased from US Nano Co (USA). Trifluoroacetic acid (TFA = CF3COOH, Mw = 169.87 g/mol, density = 1.49 g/ml, and purity percent = 99%) and chloroform (CHCl3, Mw = 119.38 g/mol and purity percent = 99.5%) were purchased from Merck (Germany). Phosphate-buffered saline (PBS) solution was purchased from Bioidea (Iran). Silk fibroin from Bombyx mori silk fibers was purchased from Kiashahr Co. (Iran). Raw silk yarns consisted of 3 twisted filaments, each one with 63 filaments, so the total number of filaments in each yarn was equal to 189 filaments.
Fabrication of knitted silk substrate
Knitted ribbon silk (width = 1 cm) was fabricated using the knitting machine (Passap Duomatic, Isfahan University of Technology, Iran). Degumming process was performed on obtained knitted silk scaffold.
Degumming of knitted silk scaffold
According to previous studies,[1,2] the knitted silk scaffold was boiled in a degumming solution of 0.25% sodium carbonate (Na2CO3) solution at 94°C–98°C for 30 min. Then, degumming solution was changed with fresh degumming solution, and the process was repeated for another 60 min. Finally, knitted silk rinsed with distilled water. Changing the color from yellow to white is the end point of this process to recognize that the sericin was completely removed, according to the literatures.[19,20]
Preparing the electrospinning solution
According to previous studies, 9 wt% optimal concentration of P3HB was dissolved and stirred in TFA for 45 min at 50°C; after that, 20 wt% optimal concentration of chitosan was added to P3HB solution and stirred and dissolved in 30 min.[11,15,18] Different concentration of MWNT in 0, 0.5, and 1 wt% was added to P3HB/chitosan solution and was stirred for 5 min. Sonication was used 12 s to distribute MWNT uniformly.
Fabrication of nano-micro scaffold
Knitted silk of 1 cm × 20 cm saturated to 5 wt% P3HB in chloroform as mediate solution in order to enhance the interface connection between electrospun nanofibers and silk microfiber, and then, knitted silk was fixed on collector. After mediate solution saturated silk dried, P3HB-chitosan/MWNT solution was electrospinned on knitted silk randomly. Optimal electrospun parameters are reported in Table 1. In all of analyses on P3HB-chitosan-x% MWNT/silk nano-micro scaffolds, the control sample is the one without MWNT.
Table 1.
Optimized parameters of electrospinning process

Physical properties
To investigate surface, nano/micro interface, and porosity of scaffolds, SEM (AIS 2300 C, SEI; Japan) was used. Surface of the scaffolds was coated with gold by sputter-coating machine (SC7620). Sectional imaging was done for determining nano/micro interface of scaffolds.[18] Average and distribution of fiber diameters was calculated by measuring 20 fibers of each sample image that obtained by SEM using ImageJ software (ImageJ, National Institutes of Health, USA). Porosity percentage was evaluated in 3 layers by SEM analyzing by MATLAB (MATLAB-8.1.0.604).[18] To ensure the complete sericin renovation of silk fibroin structure in degumming process, FTIR-attenuated total reflectance (FTIR-ATR, JASCO, 6300, Japan) was done on the knitted silk; also for evaluating chemical structure of scaffolds in presence and absence of MWNT, the same analysis was done. For determining the exist peaks in MWNT-included scaffolds, FTIR was done on pure MWNT powder. All of the IR spectroscopies were done in wavenumber range 4000–400 cm−1.[18] To determine the wettability and hydrophilicity of scaffolds, water contact angle analysis was used according to the standard ASTM D7334.[21] Briefly, a distilled water droplet was dropped down on the surface of scaffolds and a photo was taken after 10 s by camera (KSV Cam 200 Instrument, Finland). Then, the angle of the water droplet measured by the image analyzing software (Image J, National Institutes of Health, USA) (n = 5).[15,18]
Mechanical properties
Tensile strength test was applied for evaluating mechanical properties of the nano-micro structure by MTS testing machine (MTS system, model 1M/H). The test was done until rupturing in 5 mm/min strain rate (n = 3).[15,18]
Biodegradation properties
Biodegradation properties of scaffolds were assessed through weight loss test in PBS solution during 52 days. This test was done according to ASTM-F1635 (n = 3).[15,22]
Statistical analysis
All of the tests were done using the statistical software (IBM SPSS statistics version 24 release 24.0.0.0 2016 64-bit edition) to assess the statistical signification of samples. One-way ANOVA was chosen for this purpose.
Results
Morphology of nano-micro scaffolds
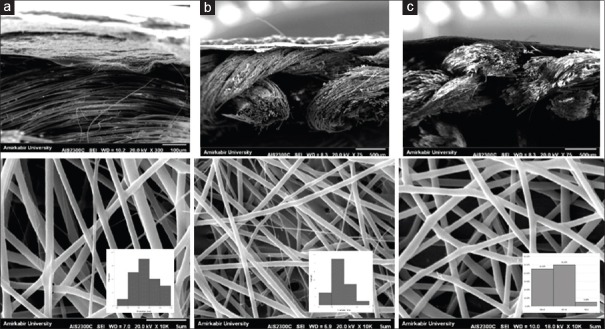
As shown in Figure 1 and Table 2, the scaffold containing 1 wt% MWNT with 544 ± 68.21 nm average diameter had the best fiber diameter distribution. Fiber diameter average in the sample with 1 wt% MWNT had significant difference in comparison with the sample with 0.5 wt% MWNT and control sample (P ≤ 0.05). An increase in the amount of MWNT leads to a reduction in the average fiber diameter, and the scaffold that includes 1 wt% MWNT with 544 ± 68.21 nm had the least average diameter (P ≤ 0.05). Figure 2 shows that the scaffolds with 1 wt% MWNT had better connection in interface of nano/micro phases in comparison with other samples. Figure 2 shows the SEM image of final nano-micro scaffold morphology.
Figure 1.
SEM images from interface and surface of nano-micro scaffolds: PHB-chitosan - 0% MWNT/silk (a), PHB-chitosan- 0.5% MWNT/silk (b), PHB-chitosan- 1% MWNT/silk (c). SEM: Scanning electron microscopy, PHB: Polyhydroxybutyrate, MWNT: Multi-walled carbon nanotube
Table 2.
Average fiber diameter of scaffolds

Figure 2.

SEM image of P3HB-chitosan-MWNT/Silk hybrid nano-micro scaffold. SEM: Scanning electron microscopy, MWNT: Multi-walled carbon nanotube
Porosity
Porosity is one of important parameters for choosing a scaffold because of its significant effect on cell behavior in cell culturing. The result of porosity percentage is shown in Figure 3. There was no significant difference in the porosity percentage in the first layer between nano-micro scaffolds included different amount of MWNT (P ≥ 0.05); also, the difference between interconnected porosity percentage average in second and third layers was not significant (P ≥ 0.05).
Figure 3.
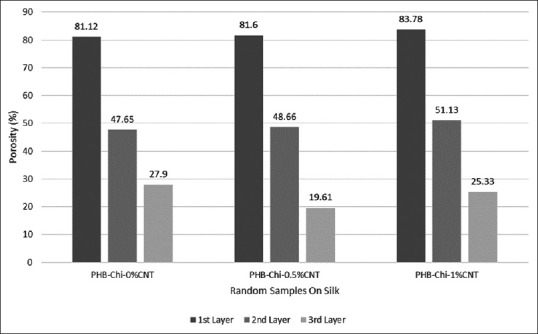
Porosity percentage charts of nano-micro scaffolds in three layers
Fourier-transform infrared analyses
Figures 4 and 5 show FTIR-ATR spectrum of degummed knitted silk, P3HB-chitosan-0.0% MWNT/silk and P3HB-chitosan-1% MWNT/silk, and also FTIR spectrum of pure MWNT. Degummed knitted silk spectrum includes N-H stretching bond in 3274 cm−1 and C-H stretching bond in 2922 cm−1; also, clear existence of amide I bonds in 1620 cm−1 (C=O stretching), amide II in 1508 cm−1 (C-N stretching plus N-H in-plane bending), and amide III in 1223 cm−1 ensures the main fibroin structure in degummed silk.[23] Existence of amide I bond in 1620 cm−1 shows the change from α-helix to β-sheet conformation in silk structure.[23,24] P3HB stretching bonds include 1725 cm−1 (C=O), 1181 and 1284 cm−1 (C-O-C), and 1455 cm−1 (CH2). As for chitosan spectrum, stretching bonds include 1055 cm−1 (C-O), amide I in 1682 cm−1 (C=O) and amide II in 1540 cm−1 (N-H) were observed; also, the peak in 1104 cm−1 was related to ester formation from free carboxylic functional group inside P3HB.[18] Vibrating state of CNTs functionalized by COOH was observed in 1430 cm−1 and 1622 cm−1 (C-C), 3437 cm-1 (O-H), and 807 cm-1 and 1151 cm-1 (C-O); also COOH functional group of MWNT was observed in 2965 cm−1. The peak in 3437 cm-1 in the sample containing 1 wt% MWNT is related to the existence of OH group of the MWNTs. The peak in 1720 cm−1 could be indicated RCONHR’ connection between free chitosan amine group and MWNT carboxylic group or could be related to amide bond inside chitosan structure.[15]
Figure 4.
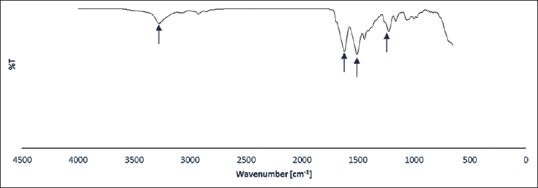
Fourier-transform infrared spectrum of degummed silk
Figure 5.
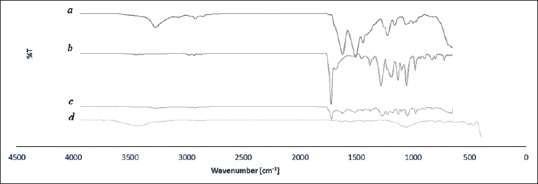
FT-IR spectrum of pure silk (a), PHB-chitosan - 0% MWNT/silk (b), PHB-chitosan - 1% MWNT/silk (c), pure MWNT powder (d). FTIR: Fourier-transform infrared, PHB: Polyhydroxybutyrate, MWNT: Multi-walled carbon nanotube
Contact angle measurement
Table 3 shows the results obtained from contact angle measurement on P3HB-chitosan-x% MWNT/silk nano-micro scaffolds. Water contact angle in all of the scaffolds was lower than 90°. The scaffold containing 1 wt% MWNT had the highest hydrophilicity in comparison with other samples. The differences between the scaffolds containing MWNT and control sample are significant (P ≤ 0.05) while there was no significant difference between the scaffolds containing 0.5 and 1 wt% MWNT (P > 0.05).
Table 3.
Water contact angles of scaffolds

According to the results obtained from assessing the morphology, fiber diameter distribution, porosity and hydrophilicity, the scaffold containing 1 wt% MWNT was chosen as optimum sample and the tensile strength test was done on this scaffold in comparison with the control sample (without MWNT). and finally, the results were compared with the control sample (without MWNT).
Tensile strength test
The results of tensile strength test were shown in Tables 4. Scaffold with 1 wt% MWNT had the highest tensile strength in comparison with control sample and pure knitted silk. The Presence of P3HB-Chitosan/1 wt% MWNT nanofibrous electrospun coat on microfibrous knitted silk led to an increase in tensile strength 1.64 times in comparison with pure knitted silk (microfibers without coat). There was no significant statistical difference between 1 wt% included MWNT and control sample (P > 0.05), but this difference was significant between nanofiber-coated samples and pure knitted silk without nanofibers (P ≤ 0.05).
Table 4.
Average tensile strength of scaffolds and degummed knitted silk

Biodegradation test
As shown in Figure 6, there was an increase in weight loss in the sample containing MWNT in comparison with control sample during 52 days. Weight loss percentage for the scaffold containing MWNT was 22.69% while it was 5.03% for control sample.
Figure 6.
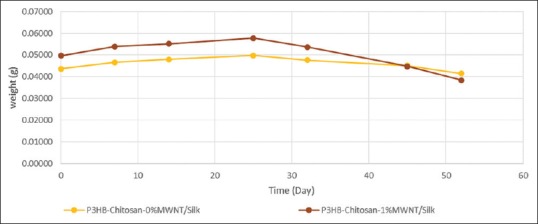
Biodegradation process of scaffolds in phosphate buffered saline during 52 days
Discussion
Increasing MWNTs up to of 1 wt% lead to an increase in electrical conductivity due to an increase in the amount of MWNT in polymeric solution and connection between nano/micro interfaces and decrease the fiber diameter in scaffolds. Increasing MWNT up to optimal content lead to overcome MWNT electrical conductivity effect on increasing the viscosity related to MWNT and this cause the reduction of fibers diameter and more fibers stretch in electrical field of electrospinning process. The presence of COOH group in MWNT and carbonyl group (C=O) in P3HB mediated solution could be a justification for better connection between nano/micro phase interfaces; also high aspect ratio and high reactivity of MWNT could provide a better connection with the silk substrate. In other hand sonication of MWNT cause appropriate distribution of MWNT among the polymeric chains and prevent agglomeration; so this could be effective in enhancement of diameter distribution, morphology and reduction in average fibers diameter. Moreover, high length-to-diameter ratio of CNTs leads to their placement and orientation along the polymeric chains, so this issue increases the electrical conductivity and fiber diameter reduction.
The presence of nanoparticle often leads to a reduction in the porosity percentage of polymeric composite scaffolds,[15,25] while in this study, there was no undesirable effect on porosity percentage. Increasing the amount of MWNT from 0.5-1 wt% the porosity percentage was still more than 80% and appropriate for tissue engineering applications.[26] Results concluded from FTIR indicate β-sheets remaining in silk fibroin structure and P3HB-chitosan/1% MWNT solution electrospinning cause no change in chemical structure of silk.
Presence of chitosan in the scaffolds lead to disorder P3HB crystalline phase; consequently the peaks move toward higher wavelength (lower wave number); this movement is because of intermolecular bond formation between amino acid group of chitosan and carbonyl group of P3HB and consequently reduces crystallinity of P3HB.[18]
Hydrophilicity of the scaffold without MWNT is because of amine group (NH2) in chitosan. An increase in carboxylic group (COOH) due to increase in the amount of MWNT up to 1 wt% in P3HB-chitosan hydrophilic polymeric solution leads to a decrease in the water contact angle.
Increasing in the tensile strength of the sample containing MWNT could be known because of high intrinsic strength of carbon nanotubes and improvement of nano/micro interface connection in this scaffold. The presence of nanofibers coat on knitted silk had a significant effect on increasing tensile strength in comparison with pure knitted silk (P ≤ 0.05). CNTs shows a high strength along its longitude.[27] Mattioli-Belmonte et al. in a study about PCL/CNT reported that with increasing the amount of CNT, at first strength increased and then decreased subsequently because of agglomeration.[28] In this study, adding 1wt% MWNT to P3HB-chitosan composition did not have any undesirable effect on mechanical strength and this content did not cause agglomeration. In the study conducted by Jalal et al. is reported that adding nanomaterial to a polymeric matrix as reinforcement leads to a reduction in porosity percentage, thus, an increase in the strength due to the filled pores;[29] in this study, adding MWNT up to 0.5 and 1 wt% to P3HB-chitosan solution leads to retain the desirable limit of porosity for tissue engineering applications (more than 80%) and did not fill the pores while tensile strength in the sample containing 1 wt% MWNT was increased; Therefore, the P3HB-chitosan-1% MWNT/silk can be appropriate for long-term healing tissue engineering applications mechanically.
Higher degradation rate of the scaffold containing MWNT in comparison with control sample can be due to water absorption of silk[18,30] and release H+ from carboxylic group of MWNT in the degradation solution. These facts lead to acidification of environment and intensification of hydrolysis. In other word, nanofibers could be influenced under two hydrolysis layers mutual; swelled silk and acidic solution.
In general, according to the result obtained, the P3HB-chitosan-1%MWNT/silk nano-micro scaffold can be appropriate for long-term healing tissue engineering applications.
Conclusion
The results obtained from SEM showed that the reduction in average fiber diameter of the samples containing MWNT was because of an increase in electrical conductivity of the solution and fiber stretch in electrical field due to MWNTs; this reduction was significant in 1 wt% MWNT samples (P ≤ 0.05); also, this scaffold had better diameter distribution and nano-micro interface connection in comparison with other samples. The scaffolds containing MWNT had still more than 80% porosity percentage. Water contact angle test indicated the scaffold containing 1 wt% MWNT is more hydrophilic due to COOH group of functionalized MWNT. Tensile strength of the scaffold containing 1 wt% MWNT was more than scaffold without MWNT and pure knitted silk scaffold; electrospun nanofibers coat had a significant effect on pure knitted silk without coating mechanically. High intrinsic along length strength of CNTs and enhancement of nano/micro interface connection lead to an increase in the tensile strength of the scaffold containing 1 wt% MWNT. According to the results, P3HB-chitosan-1%MWNT/silk scaffold had appropriate physical and mechanical properties for long-term healing tissue engineering applications.
Financial support and sponsorship
This study is funded by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Naghashzargar E, Farè S, Catto V, Bertoldi S, Semnani D, Karbasi S, et al. Nano/micro hybrid scaffold of PCL or P3HB nanofibers combined with silk fibroin for tendon and ligament tissue engineering. J Appl Biomater Funct Mater. 2015;13:e156–68. doi: 10.5301/jabfm.5000216. [DOI] [PubMed] [Google Scholar]
- 2.Sahoo S, Toh SL, Goh JC. PLGA nanofiber-coated silk microfibrous scaffold for connective tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:19–28. doi: 10.1002/jbm.b.31678. [DOI] [PubMed] [Google Scholar]
- 3.Kasoju N, Bhonde RR, Bora U. Fabrication of a novel micro-nano fibrous nonwoven scaffold with antheraea assama silk fibroin for use in tissue engineering. Mater Lett. 2009;63:2466–9. [Google Scholar]
- 4.Güven EÖ, Demirbilek M, Saglam N, Karahaliloglu Z, Erdal E, Bayram C, et al. Preparation and characterization of polyhydroxybutyrate scaffolds to be used in tissue engineering applications. Hacet J Biol Chem. 2008;36:305–11. [Google Scholar]
- 5.Chen SH, Chang Y, Lee KR, Lai JY. A three-dimensional dual-layer nano/microfibrous structure of electrospun chitosan/poly (d, l-lactide) membrane for the improvement of cytocompatibility. J Membr Sci. 2014;450:224–34. [Google Scholar]
- 6.Zheng Y, Monty J, Linhardt RJ. Polysaccharide-based nanocomposites and their applications. Carbohydr Res. 2015;405:23–32. doi: 10.1016/j.carres.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30:4094–103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiankang H, Dichen L, Yaxiong L, Bo Y, Hanxiang Z, Qin L, et al. Preparation of chitosan-gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009;5:453–61. doi: 10.1016/j.actbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Wang A, Jing D, Gong Y, Zhao N, Zhang X, et al. Novel biodegradable films and scaffolds of chitosan blended with poly(3-hydroxybutyrate) J Biomater Sci Polym Ed. 2005;16:1379–94. doi: 10.1163/156856205774472308. [DOI] [PubMed] [Google Scholar]
- 10.Giretova M, Medvecky L, Stulajterova R, Sopcak T, Briancin J, Tatarkova M, et al. Effect of enzymatic degradation of chitosan in polyhydroxybutyrate/chitosan/calcium phosphate composites on in vitro osteoblast response. J Mater Sci Mater Med. 2016;27:181. doi: 10.1007/s10856-016-5801-7. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi D, Karbasi S, Razavi S, Mohammadi S, Shokrgozar MA, Bonakdar S. Electrospun poly (hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J Appl Polym Sci. 2016;133:44171. [Google Scholar]
- 12.O'Connell MJ. USA: CRC Press; 2006. Carbon Nanotubes: Properties and Applications. [Google Scholar]
- 13.Li QH, Zhou QH, Dan D, Yu QZ, Li G, Gong KD, et al. Enhanced thermal and electrical properties of poly (D, L-lactide)/multi-walled carbon nanotubes composites by in-situ polymerization. Trans Nonferrous Metals Soc China. 2013;23:1421–7. [Google Scholar]
- 14.Ma Y, Zheng Y, Wei G, Song W, Hu T, Yang H, et al. Processing, structure, and properties of multiwalled carbon nanotube/poly (hydroxybutyrate-co-valerate) biopolymer nanocomposites. J Appl Polym Sci. 2012;125:620. [Google Scholar]
- 15.Karbasi S, Alizadeh ZM. Effects of multi-wall carbon nanotubes on structural and mechanical properties of poly (3-hydroxybutyrate)/chitosan electrospun scaffolds for cartilage tissue engineering. Bull Mater Sci. 2017;6:1–7. [Google Scholar]
- 16.Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL, et al. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J Mater Sci Mater Med. 2005;16:1099–104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo S, Cho-Hong JG, Siew-Lok T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed Mater. 2007;2:169–73. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- 18.Karbasi S, Fekrat F, Semnani D, Razavi S, Zargar EN. Evaluation of structural and mechanical properties of electrospun nano-micro hybrid of poly hydroxybutyrate-chitosan/silk scaffold for cartilage tissue engineering. Adv Biomed Res. 2016;5:180. doi: 10.4103/2277-9175.194802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teh TK, Toh SL, Goh JC. Optimization of the silk scaffold sericin removal process for retention of silk fibroin protein structure and mechanical properties. Biomed Mater. 2010;5:35008. doi: 10.1088/1748-6041/5/3/035008. [DOI] [PubMed] [Google Scholar]
- 20.Farè S, Torricelli P, Giavaresi G, Bertoldi S, Alessandrino A, Villa T, et al. In vitro study on silk fibroin textile structure for anterior cruciate ligament regeneration. Mater Sci Eng C Mater Biol Appl. 2013;33:3601–8. doi: 10.1016/j.msec.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 21.PA: ASTM International West Conshohocken; 2008. Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. [Google Scholar]
- 22.PA: ASTM International West Conshohocken; 2000. Standard Test Method for In Vitro Degradation Testing of Poly (L-lactic Acid) Resin and Fabricated Form for Surgical Implants. [Google Scholar]
- 23.Mobini S, Hoyer B, Solati-Hashjin M, Lode A, Nosoudi N, Samadikuchaksaraei A, et al. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J Biomed Mater Res A. 2013;101:2392–404. doi: 10.1002/jbm.a.34537. [DOI] [PubMed] [Google Scholar]
- 24.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammadian M, Haghi A. Systematic parameter study for nano-fiber fabrication via electrospinning process. Bulg Chem Commun. 2014;46:545–55. [Google Scholar]
- 26.Ghasemi-Mobarakeh L, Semnani D, Morshed M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Appl Polym Sci. 2007;106:2536–42. [Google Scholar]
- 27.Spinks GM, Shin SR, Wallace GG, Whitten PG, Kim SI, Kim SJ. Mechanical properties of chitosan/CNT microfibers obtained with improved dispersion. Sens Actuators B Chem. 2006;115:678–84. [Google Scholar]
- 28.Mattioli-Belmonte M, Vozzi G, Whulanza Y, Seggiani M, Fantauzzi V, Orsini G, et al. Tuning polycaprolactone-carbon nanotube composites for bone tissue engineering scaffolds. Mater Sci Eng. 2012;32:152–9. [Google Scholar]
- 29.Jalal M, Fathi M, Farzad M. Effects of fly ash and TiO 2 nanoparticles on rheological, mechanical, microstructural and thermal properties of high strength self compacting concrete. Mech Mater. 2013;61:11–27. [Google Scholar]
- 30.Kundu S. Netherlands: Elsevier Science; 2014. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. [Google Scholar]